|
1
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabanayagam C, Yip W, Ting DS, Tan G and
Wong TY: Ten emerging trends in the epidemiology of diabetic
retinopathy. Ophthalmic Epidemiol. 23:209–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michels RG: Proliferative diabetic
retinopathy: Pathophysiology of extraretinal complications and
principles of vitreous surgery. Retina. 1:1–17. 1981.PubMed/NCBI
|
|
4
|
Chang W, Lajko M and Fawzi AA:
Endothelin-1 is associated with fibrosis in proliferative diabetic
retinopathy membranes. PLoS One. 13:e01912852018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abu El-Asrar AM, De Hertogh G, van den
Eynde K, Alam K, Van Raemdonck K, Opdenakker G, Van Damme J, Geboes
K and Struyf S: Myofibroblasts in proliferative diabetic
retinopathy can originate from infiltrating fibrocytes and through
endothelial-to-mesenchymal transition (EndoMT). Exp Eye Res.
132:179–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Y, Feng B, Chen S, Chu Y and
Chakrabarti S: Mechanisms of endothelial to mesenchymal transition
in the retina in diabetes. Invest Ophthalmol Vis Sci. 55:7321–7331.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardali E, Sanchez-Duffhues G,
Gomez-Puerto MC and Ten Dijke P: TGF-β-induced
endothelial-mesenchymal transition in fibrotic diseases. Int J Mol
Sci. 18:E21572017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piera-Velazquez S, Li Z and Jimenez SA:
Role of endothelial-mesenchymal transition (EndoMT) in the
pathogenesis of fibrotic disorders. Am J Pathol. 179:1074–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Q, Qiu F, Zhou K, Matlock HG,
Takahashi Y, Rajala RVS, Yang Y, Moran E and Ma JX: Pathogenic Role
of microRNA-21 in diabetic retinopathy through downregulation of
PPARα. Diabetes. 66:1671–1682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao S, Li T, Li J, Lu Q, Han C, Wang N,
Qiu Q, Cao H, Xu X, Chen H and Zheng Z: miR-23b-3p induces the
cellular metabolic memory of high glucose in diabetic retinopathy
through a SIRT1-dependent signalling pathway. Diabetologia.
59:644–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mortuza R, Feng B and Chakrabarti S:
miR-195 regulates SIRT1-mediated changes in diabetic retinopathy.
Diabetologia. 57:1037–1046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin LL, An MX, Liu YL, Xu HC and Lu ZQ:
MicroRNA-126: A promising novel biomarker in peripheral blood for
diabetic retinopathy. Int J Ophthalmol. 10:530–534. 2017.PubMed/NCBI
|
|
14
|
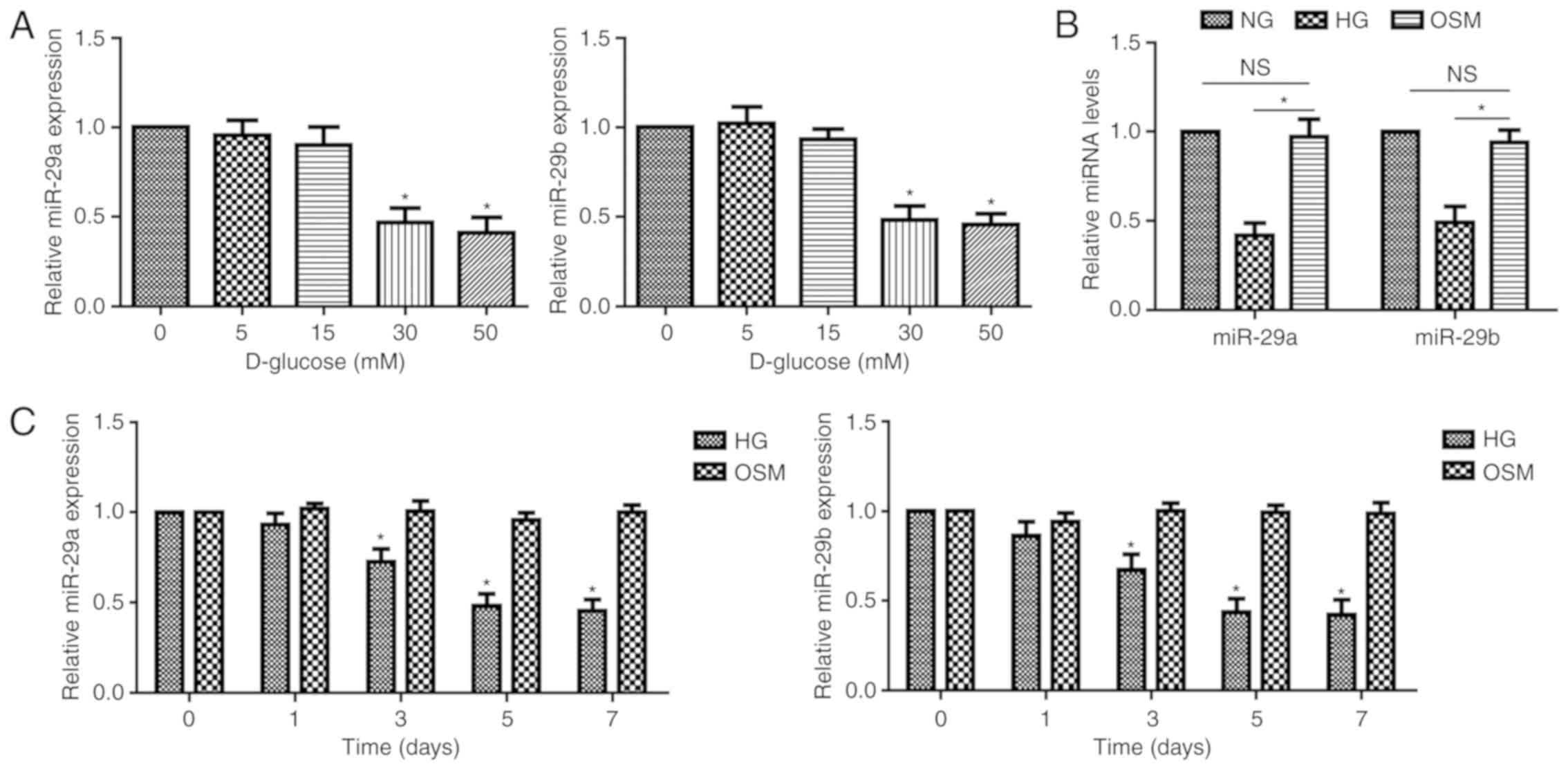
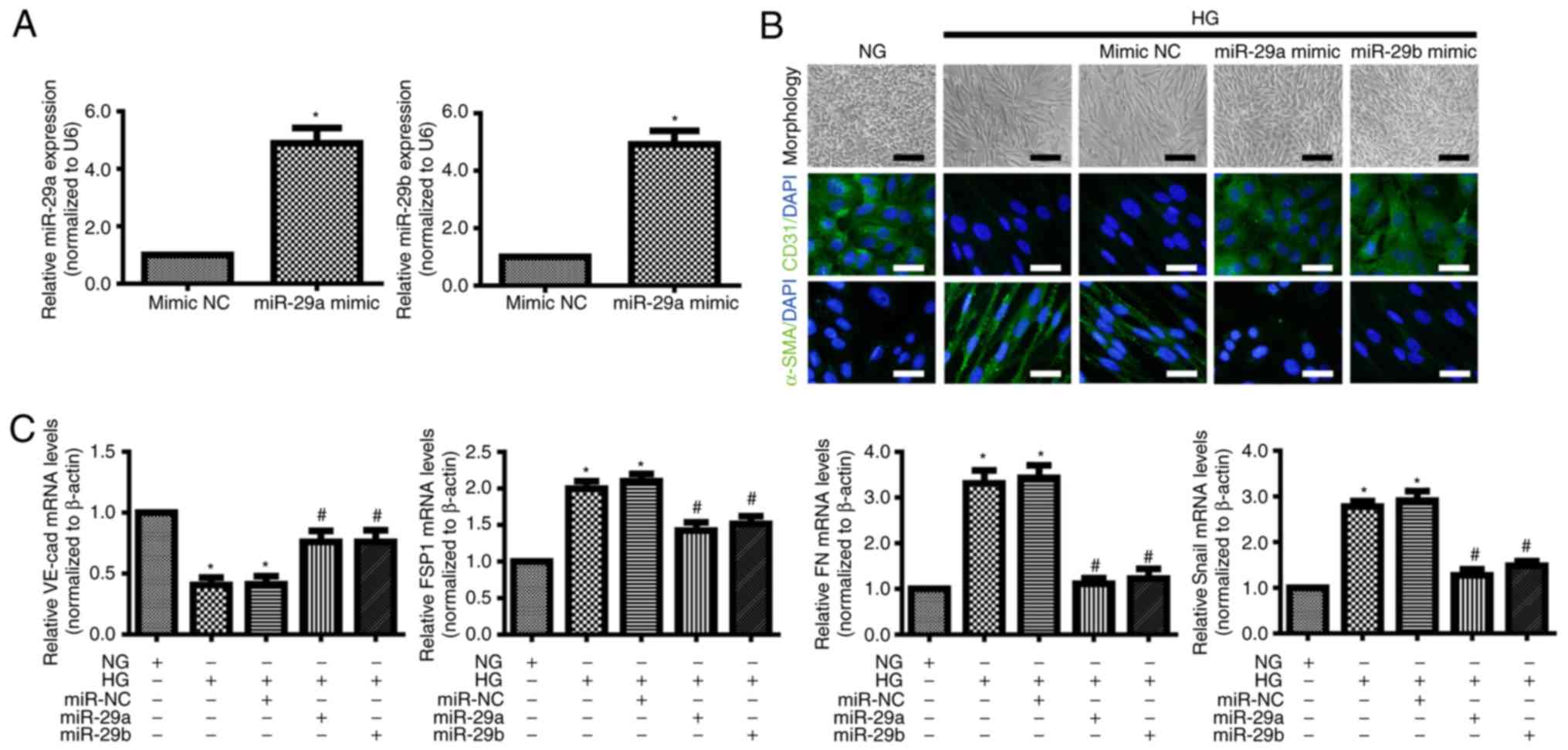
Zhang J, Wu L, Chen J, Lin S, Cai D, Chen
C and Chen Z: Downregulation of MicroRNA 29a/b exacerbated diabetic
retinopathy by impairing the function of muller cells via forkhead
box protein O4. Diab Vasc Dis Res. 15:214–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Chen M, Chen J, Lin S, Cai D,
Chen C and Chen Z: Long non-coding RNA MIAT acts as a biomarker in
diabetic retinopathy by absorbing miR-29b and regulating cell
apoptosis. Biosci Rep. 37:BSR201700362017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He Y, Huang C, Lin X and Li J: MicroRNA-29
family, a crucial therapeutic target for fibrosis diseases.
Biochimie. 95:1355–1359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cushing L, Kuang P and Lu J: The role of
miR-29 in pulmonary fibrosis. Biochem Cell Biol. 93:109–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM
and Lan HY: miR-29b as a therapeutic agent for angiotensin
II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol
Ther. 22:974–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perez L, Munoz-Durango N, Riedel CA,
Echeverria C, Kalergis AM, Cabello-Verrugio C and Simon F:
Endothelial-to-mesenchymal transition: Cytokine-mediated pathways
that determine endothelial fibrosis under inflammatory conditions.
Cytokine Growth Factor Rev. 33:41–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
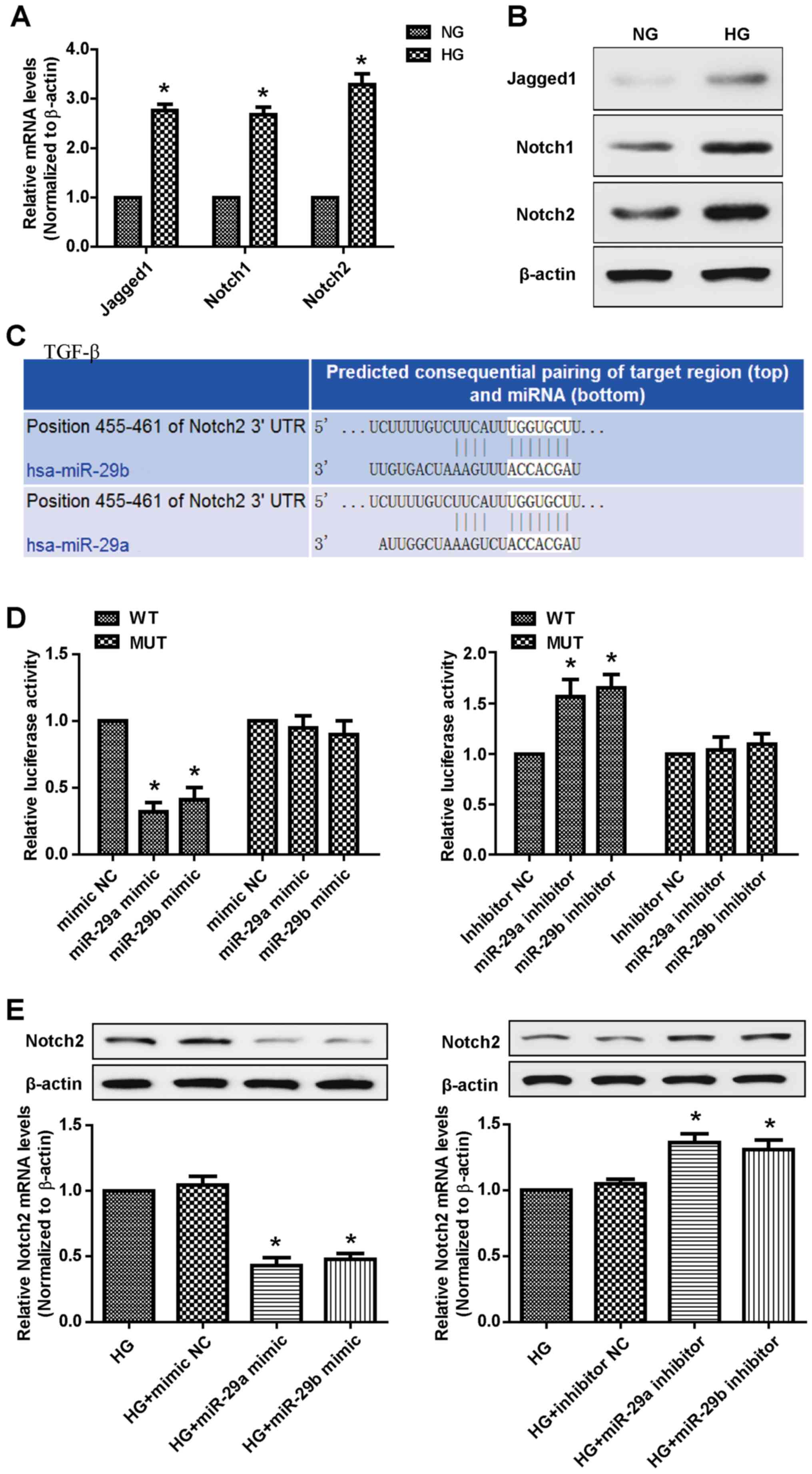
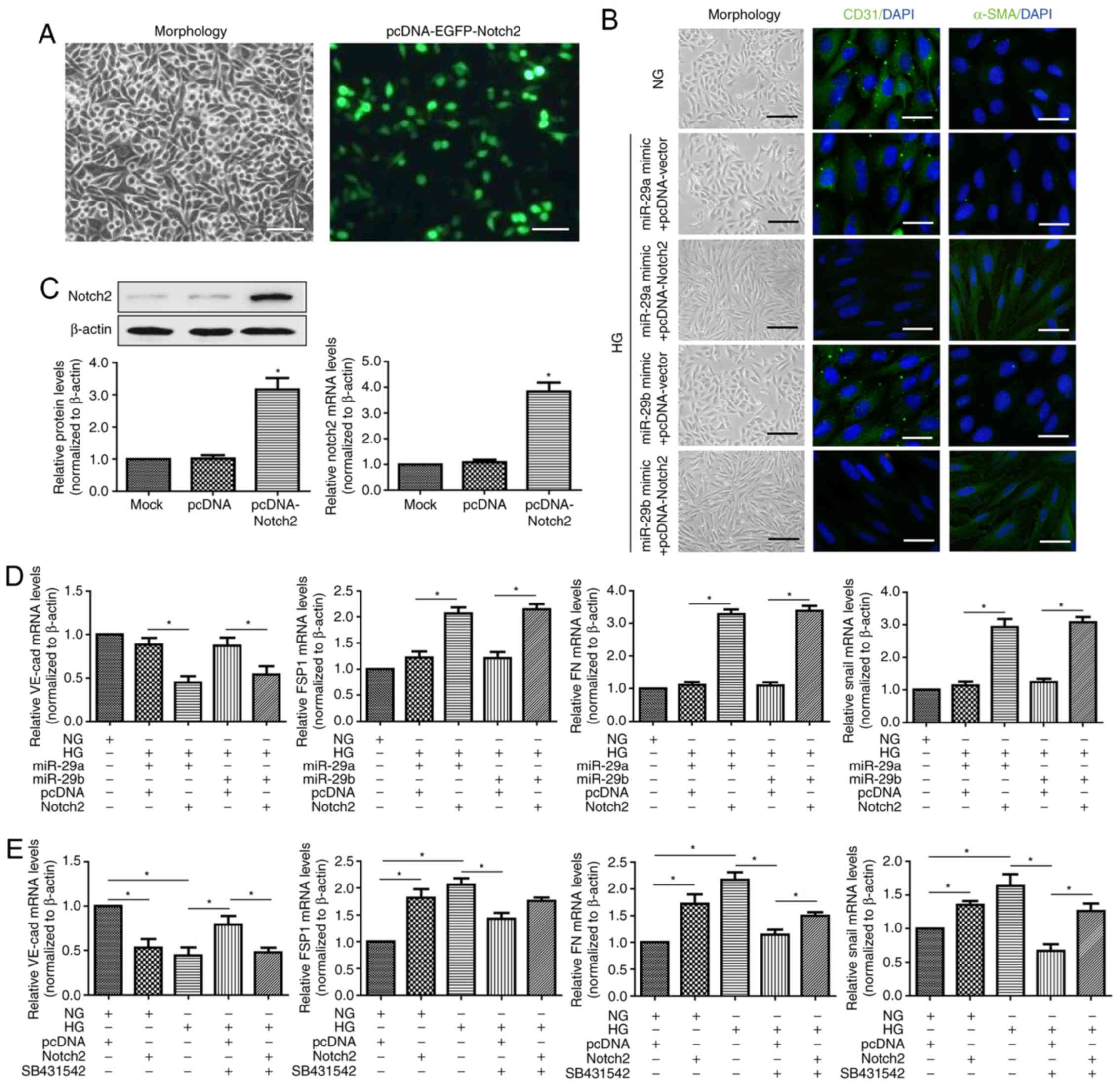
Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu
M, Ye S and Liu Y: Blockade of Jagged/Notch pathway abrogates
transforming growth factor β2-induced epithelial-mesenchymal
transition in human retinal pigment epithelium cells. Curr Mol Med.
14:523–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dou GR, Wang L, Wang YS and Han H: Notch
signaling in ocular vasculature development and diseases. Mol Med.
18:47–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geng H and Guan J: MiR-18a-5p inhibits
endothelial-mesenchymal transition and cardiac fibrosis through the
Notch2 pathway. Biochem Biophys Res Commun. 491:329–336. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sorrentino FS, Matteini S, Bonifazzi C,
Sebastiani A and Parmeggiani F: Diabetic retinopathy and endothelin
system: Microangiopathy versus endothelial dysfunction. Eye (Lond).
32:1157–1163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang H, Ma X, Liu J, Fan Y and Deng X:
High glucose-induced endothelial progenitor cell dysfunction. Diab
Vasc Dis Res. 14:381–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmitt MJ, Margue C, Behrmann I and Kreis
S: MiRNA-29: A microRNA family with tumor-suppressing and
immune-modulating properties. Curr Mol Med. 13:572–585. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kriegel AJ, Liu Y, Fang Y, Ding X and
Liang M: The miR-29 family: Genomics, cell biology, and relevance
to renal and cardiovascular injury. Physiol Genomics. 44:237–244.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fabbri M, Garzon R, Cimmino A, Liu Z,
Zanesi N, Callegari E, Liu S, Alder H, Costinean S,
Fernandez-Cymering C, et al: MicroRNA-29 family reverts aberrant
methylation in lung cancer by targeting DNA methyltransferases 3A
and 3B. Proc Natl Acad Sci USA. 104:15805–15810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao
Y, Dong Q, Pang Z, Guan Q, Gao L, et al: Significance of serum
microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A
clinical study. Acta Diabetol. 48:61–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pandey AK, Verma G, Vig S, Srivastava S,
Srivastava AK and Datta M: miR-29a levels are elevated in the db/db
mice liver and its overexpression leads to attenuation of insulin
action on PEPCK gene expression in HepG2 cells. Mol Cell
Endocrinol. 332:125–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang B, Komers R, Carew R, Winbanks CE, Xu
B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K,
Gregorevic P, et al: Suppression of microRNA-29 expression by
TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc
Nephrol. 23:252–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Rooij E, Sutherland LB, Thatcher JE,
DiMaio JM, Naseem RH, Marshall WS, Hill JA and Olson EN:
Dysregulation of microRNAs after myocardial infarction reveals a
role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA.
105:13027–13032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou X, Chen X, Cai JJ, Chen LZ, Gong YS,
Wang LX, Gao Z, Zhang HQ, Huang WJ and Zhou H: Relaxin inhibits
cardiac fibrosis and endothelial-mesenchymal transition via the
Notch pathway. Drug Des Devel Ther. 9:4599–4611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noseda M, McLean G, Niessen K, Chang L,
Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead
B, et al: Notch activation results in phenotypic and functional
changes consistent with endothelial-to-mesenchymal transformation.
Circ Res. 94:910–917. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshimatsu Y and Watabe T: Roles of TGF-β
signals in endothelial-mesenchymal transition during cardiac
fibrosis. Int J Inflam. 2011:7240802011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu CH, Suriguga, Gong M, Liu WJ, Cui NX,
Wang Y, Du X and Yi ZC: High glucose induced endothelial to
mesenchymal transition in human umbilical vein endothelial cell.
Exp Mol Pathol. 102:377–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Paolo S, Gesualdo L, Ranieri E,
Grandaliano G and Schena FP: High glucose concentration induces the
overexpression of transforming growth factor-beta through the
activation of a platelet-derived growth factor loop in human
mesangial cells. Am J Pathol. 149:2095–2106. 1996.PubMed/NCBI
|