Introduction
Brain injuries caused by transient or permanent
focal cerebral ischemia develop according to a series of
pathological mechanisms that include free radical release,
blood-brain barrier (BBB) disruption, microglial activation,
inflammation and neuronal apoptosis (1). Additionally, post-ischemic inflammation
mediates the pathological processes associated with ischemic brain
injury (2) Toll-like receptors
(TLRs) serve a central role in innate immunity and are implicated
in a range of inflammatory diseases (3). Several studies have demonstrated that
TLR4 expression is elevated following cerebral ischemia (4) and that the degree of ischemic brain
injury and neuroinflammation is significantly lower in
TLR4-deficient mice compared with wild-type mice (5). Furthermore, nuclear factor (NF)-κB,
which is a key downstream factor of the TLR4 signaling pathway, is
activated following cerebral ischemia, to promote inflammatory
reactions and produce inflammatory molecules that further aggravate
ischemic brain injury (5–7). TLR4 signaling is a promising
therapeutic target for the treatment of ischemic stroke (8) because the downregulation of TLR4
expression inhibits NF-κB and reduces the expression of
inflammatory molecules; this ultimately leads to the attenuation of
ischemic brain injury.
Resveratrol is a polyphenol that is abundantly
expressed in a wide variety of plant species and has been reported
to possess cardioprotective (9),
anticancer (10), anti-inflammatory
(11) and neuroprotective properties
(12). Although a study demonstrated
that resveratrol reduces ischemia-induced brain damage due to its
anti-oxidative properties (13), its
potential underlying molecular mechanisms remain unknown.
Therefore, the present study investigated whether resveratrol
downregulates activity in the TLR4 signaling pathway in a rat model
of cerebral ischemia.
Materials and methods
Induction of ischemia-reperfusion
A total of 140 adult Sprague-Dawley rats weighing
250–300 g were obtained from the Shanghai Laboratory Animal Center
(Shanghai, China). All animal experiments were approved by the
Institutional Animal Care and Use Committee of Hubei University of
Medicine (Shiyan, China). Rats were housed in a colony room under
controlled temperature (22°C), a humidity of 40–70% and a 12-h
light/dark cycle, with free access to food and water. All surgical
procedures were performed using sterile techniques in accordance
with institutional guidelines. Following the induction of
anesthesia with 5% isoflurane in 70/30 medical air/oxygen, all
animals were trans-orally intubated while a small rodent respirator
was used to maintain adequate respiration; 3% isoflurane in 70/30
medical air/oxygen was used to maintain anesthesia.
Next, the rats were subjected to
ischemia-reperfusion (IR), as described by Shi et al
(14) with minor revisions. Briefly,
the right common carotid artery, external carotid artery and
internal carotid artery were exposed and a nylon monofilament
suture with a distal cylinder (diameter: 0.32 mm) was inserted from
the external carotid artery into the internal carotid artery and
then gently advanced to occlude the origin of the right middle
cerebral artery; the suture was withdrawn 2 h following occlusion.
In the sham-operated rats, the external carotid artery was prepared
for insertion of the suture but it was not inserted. During the
surgical procedure, rectal temperature was maintained at 37.0±0.5°C
with a thermostatically controlled infrared lamp.
Experimental groups
The rats were separated into four groups as follows:
i) The sham group (n=30), which was subjected to the sham
operation; ii) the middle cerebral artery occlusion (MCAO) group
(n=36), which was subjected to IR and treated with a normal saline;
iii) the R10 group (n=30), which was subjected to IR and treated
with 10 mg/kg of resveratrol [intraperitoneal (i.p.)] the R100
group (n=36), which was subjected to IR and treated with 100 mg/kg
of resveratrol (i.p.). Resveratrol was obtained from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) placed in normal saline containing
20% hydroxypropyl β-cyclodextrin and intraperitoneally injected at
2 h following the onset of ischemia.
Assessment of neurological deficit
scores
At 24 h following the cerebral IR procedure,
neurological deficit scores were assessed according to the method
described by Bederson et al (15) with minor revisions, as follows: 0=no
observable deficit; 1=contralateral forelimb flexion; 2=decreased
resistance to lateral push without circling; and 3=circling to the
contralateral side.
Infarct volume analysis
At 24 h following the cerebral IR procedure, the
animals were anesthetized and sacrificed by rapid decapitation. The
brains were removed, immersed in a cold saline solution for 10 min
and then sectioned into standard coronal slices (2 mm thick) using
a brain matrix slicer. The slices were placed in the vital dye
2,3,5-triphenyltetrazolium chloride (2% TTC; Sigma-Aldrich; Merck
KGaA) at 37°C under dark conditions for 20 min. Following this
staining procedure, infarct regions appear white, whereas
non-infarct regions appear red. The infarct areas in each brain
slice were measured using ImageJ software (version 1.46; National
Institutes of Health, Bethesda, MD, USA) and infarct volume was
calculated according to the following formula: V=t × (A1 + A2 + …
An), where ‘V’ is the infarct volume, ‘t’ is the slice thickness
and ‘A’ is the infarct area.
Histopathological analysis
At 24 h following the cerebral IR procedure, the
animals were anesthetized and perfused with 4% paraformaldehyde.
The brains were removed, fixed with 4% paraformaldehyde at 4°C for
24 h and embedded in paraffin. Next, coronal sections (4 µm thick)
were deparaffinized with xylene, rehydrated with a graded alcohol
series and stained with hematoxylin and eosin (HE) at room
temperature for 3 min. The sections were visualized with a light
microscope at a magnification of ×400.
Assessment of cerebral water
content
Briefly, 24 h following the cerebral IR procedure,
the rats were sacrificed and the brains were quickly removed. The
ischemic hemispheres were immediately weighed on an electronic
balance to ascertain the wet weight (WW) and then dried to constant
weight for 24 h in a 100°C oven to obtain the dry weight (DW).
Cerebral water content was calculated using the following equation:
H2O (%)=(WW-DW)/WW×100%.
Biochemical analysis
Myeloperoxidase (MPO) activity was assessed to
determine the extent of inflammation. At 24 h following the
cerebral IR procedure, the rats were anesthetized and ischemic
brain samples (1.0 mm from bregma to −3.0 mm from bregma) were
collected. MPO activity in the ischemic brain was measured with an
assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer's protocol; the results are
expressed as U/g of tissue.
Measurement of BBB permeability
The BBB permeability was assessed using measurement
of Evans blue (EB) extravasation. The EB dye (2% in saline, 4 ml/kg
for each rat) was injected into the left jugular vein at 23 h
following ischemia (1 h prior to sacrifice) and then the rats were
transcardially perfused with PBS to remove the intravascular dye.
Next, the ischemic hemispheres were homogenized in a tenfold volume
of 50% trichloroacetic acid solution to precipitate the protein and
centrifuged for 10 min at 4°C and 2,000 × g. The resulting
supernatant was diluted with ethanol (1:3) and fluorescence was
measured at 610 nm to determine the absorbance of EB; the results
are expressed as µg/g of brain tissue.
Western blot analysis
At 24 h following the cerebral IR procedure,
ischemic cortical tissue samples were collected and total protein
was extracted using a protein extraction kit (Xiamen Tagene
Biotechnology Co. Ltd., Xiamen, China) according to the
manufacturer's protocol. Briefly, 100 µg samples of protein were
separated on 10% SDS polyacrylamide gels, transferred to
nitrocellulose membranes and then blocked in 5% nonfat dry milk
buffer for 1 h at room temperature. The membranes were incubated at
4°C overnight with either a rabbit polyclonal antibody against TLR4
(1:1,000; cat. no. sc30002) or a mouse monoclonal antibody against
NF-κB p65 (1:500; cat. no. sc71675; both Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and then incubated st room temperature for 2
h with horseradish peroxidase-conjugated goat anti-rabbit (cat. no.
PV9001) mouse (cat. no. PV9002) secondary antibodies (1:1,000;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China).
Protein expression levels were detected with an
electrochemiluminescence detection system (Dalian Meilun
Biotechnology Co., Ltd., Dalian, China) and exposed on X-ray film.
The densities of the protein bands were scanned and analyzed with
an image analyzer and ImageJ software (version 1.46).
Reverse-transcription polymerase chain
reaction (RT-PCR)
For the RT-PCR procedure, the rats were deeply
anaesthetized and transcardially perfused with ice-cold PBS. The
brains were quickly removed, the cortical tissues were dissected
and the samples were stored at −80°C until analysis. Total RNA was
extracted using TRIzol reagents (Invitrogen; Thermo Fisher
Scientific, Inc.), and then reverse-transcribed at 42°C for 60 min
and at 95°C for 5 min to obtain single-strand cDNA with a Reverse
Transcription System (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol.
Single-strand cDNA was amplified using PCR with a
100 µl reaction mixture containing 50 mM KCl, 10 mM Tris-HCl (pH
9.0), 2 mM MgCl2, 200 µM dNTPs, 0.5 µM of sense and antisense
primers, and 2.5 units of Taq DNA polymerase (Promega Corporation).
The primer sequences were as follows: Cyclooxygenase-2 (COX-2;
sense: 5′-CCATGTCAAAACCGTGGTGAATG-3′; antisense:
5′-ATGGGAGTTGGGCAGTCATCAG-3′; product size: 374 bp), matrix
metalloproteinase-9 (MMP-9; sense: 5′-AAGGATGGTCTACTGGCAC-3′;
antisense: 5′-AGAGATTCTCACTGGGGC-3′; product size: 279 bp) and the
internal standard β-actin, (sense: 5′-CCCATCTATGAGGGTTACGC-3′;
antisense: 5′-TTTAATGTCACGCACGATTTC-3′; product size: 150 bp). The
reactions were initially heated at 94°C for 4 min and then at 94°C
for 40 sec, 58°C for 40 sec, and 72°C for 50 sec over a total of 38
cycles. The reactions were stopped at 72°C for 7 min. The PCR
products (10 µl) were electrophoresed in a 2% agarose gel
containing ethidium bromide and DNA band optical density was
measured with a UVP gel analysis system (Quantity One; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
ELISAs
At 24 h following the cerebral IR procedure, 1 ml
blood samples were drawn from the rat hearts. The samples were
centrifuged at 4°C and 2,000 × g for 10 min and then the
supernatants were collected. The plasma contents of tumor necrosis
factor-α (TNF-α) and interleukin (IL)-1β were measured using rat
TNF-α (cat. no. RTA00) or IL-1β (cat. no. RLB00) ELISA kits
(R&D Systems, Inc., Minneapolis, MN, USA).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used to analyze the data in the current study. All results are
presented as the mean ± standard deviation. All statistical
analyses were performed using analysis of variance followed by
Student-Newman-Keuls test for multiple group comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
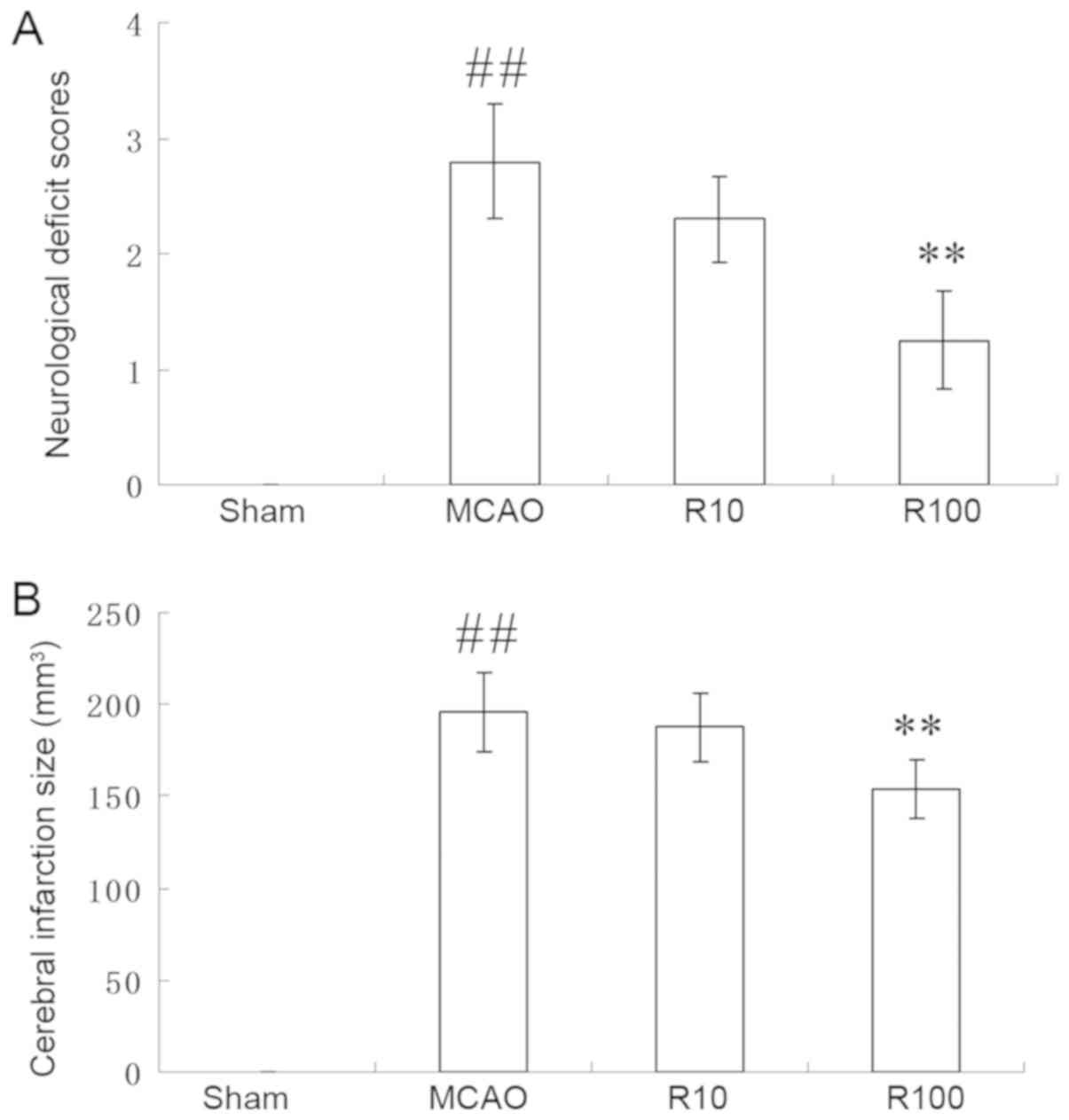
Resveratrol reduces neurological
deficit scores, cerebral infarct size, neuronal injury and brain
edema
Rats subjected to the cerebral IR procedure
exhibited increases in neurological deficit scores, cerebral
infarct size, neuronal injury and cerebral water content. Although
there were no differences between the vehicle-treated group and the
10 mg/kg resveratrol group, 100 mg/kg of resveratrol significantly
reduced the neurological deficit scores (P<0.01; Fig. 1A) and cerebral infarct size
(P<0.01; Fig. 1B) at 24 h
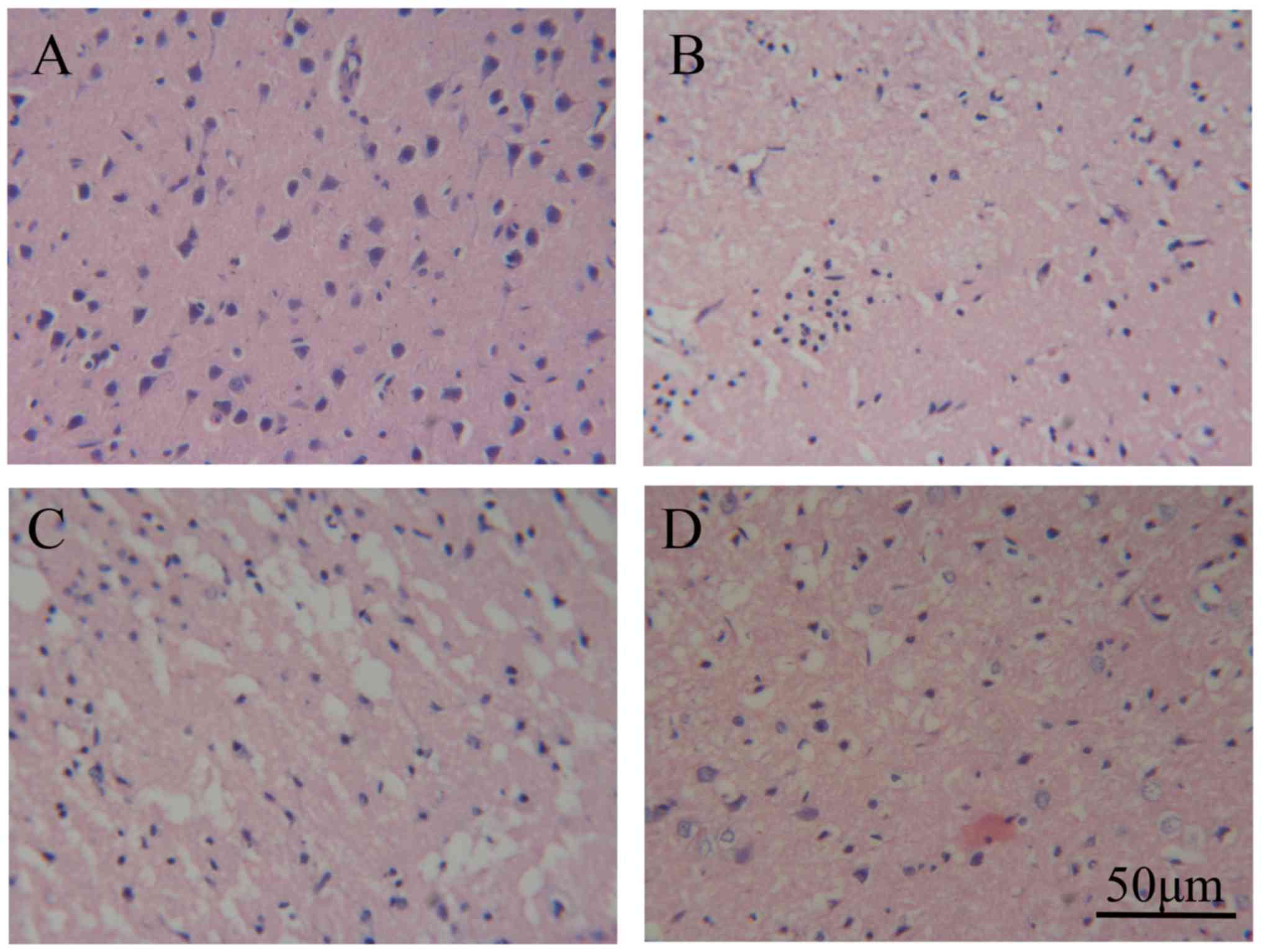
following cerebral IR. Additionally, HE staining revealed that no
injured neurons were identified in the sham-operated group
(Fig. 2A), cerebral IR caused
neuronal injury in the ischemic hemisphere (Fig. 2B). Although 10 mg/kg of resveratrol
(Fig. 2C) did not reduce the extent
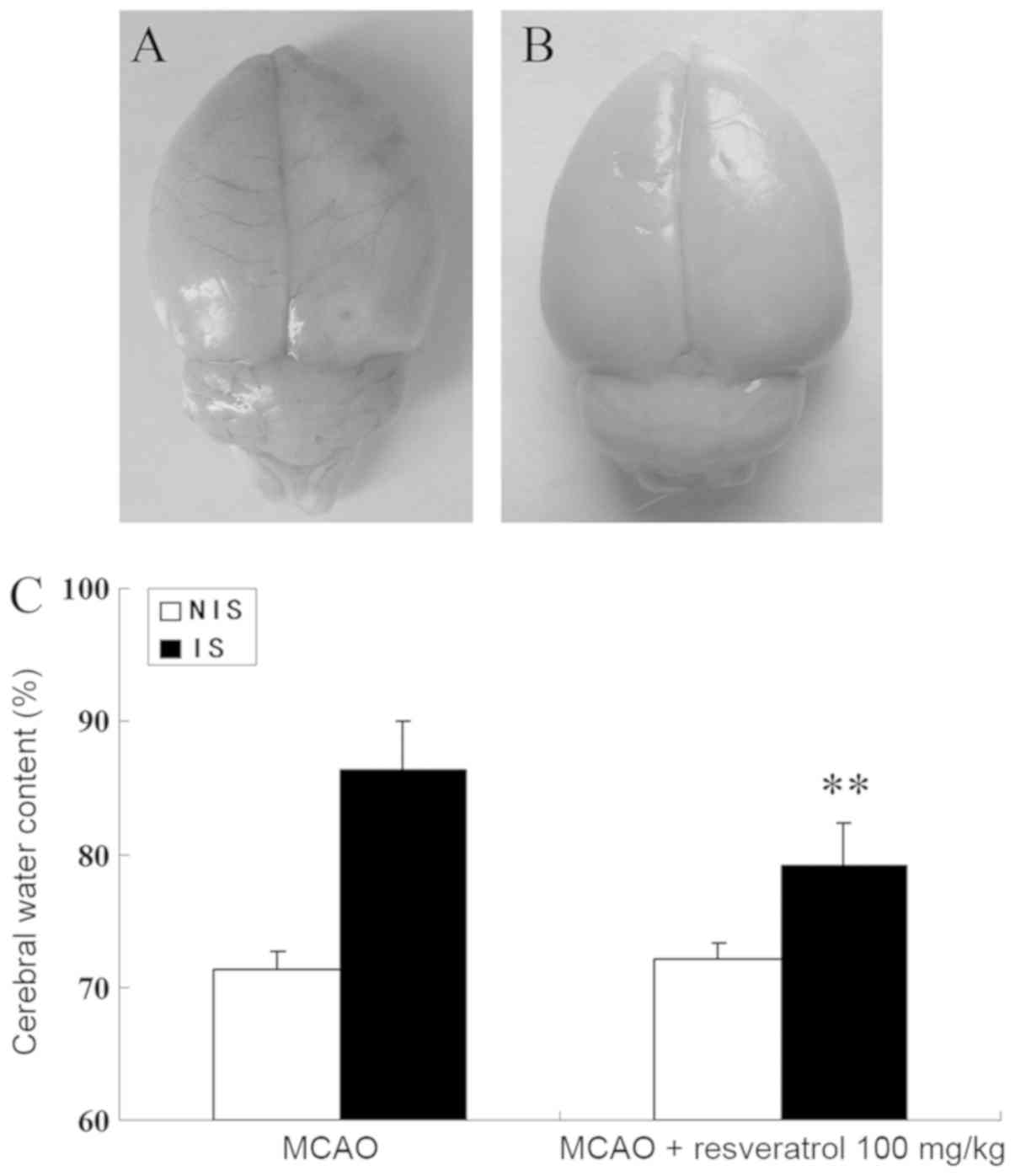
of neuronal injury, 100 mg/kg of resveratrol did (Fig. 2D). Furthermore, 100 mg/kg of
resveratrol significantly reduced the cerebral water content in the
ischemic hemisphere produced by cerebral IR when compared with the
MCAO group (P<0.01; Fig. 3).
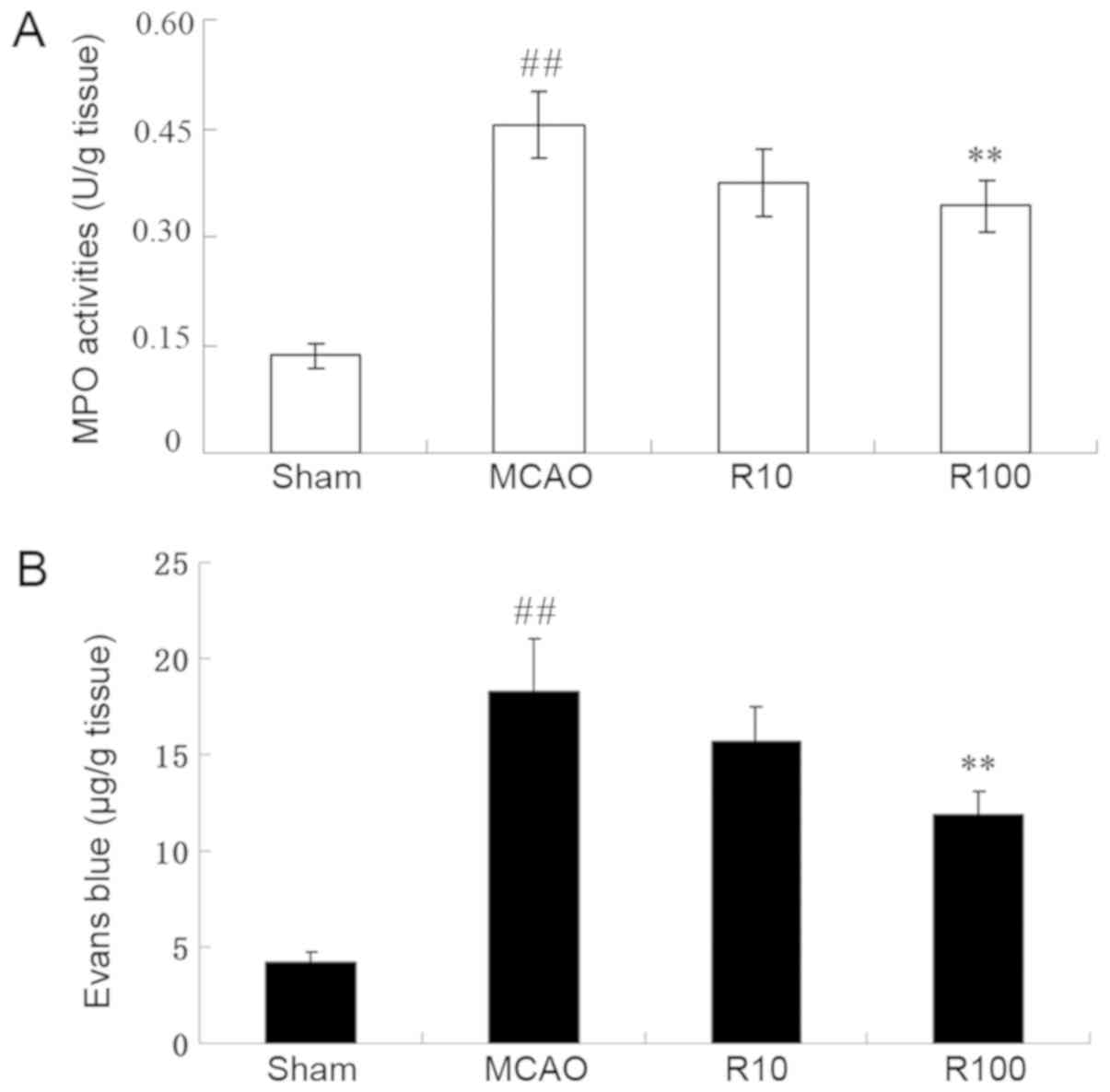
Resveratrol reduces ischemia-induced
inflammation
MPO enzymatic activity was assessed to determine the
extent of inflammation in ischemic brain tissues. The experimental
data demonstrated that MPO activity was significantly elevated at
24 h following cerebral IR compared with the sham-operated group
(P<0.05). However, this elevation exhibited a significant
decrease following treatment with 100 mg/kg of resveratrol
(P<0.01) but not 10 mg/kg of resveratrol (P>0.05; Fig. 4A).
Resveratrol reduces BBB
permeability
The EB extravasation analyses were performed to
assess the extent of BBB permeability. The experimental data
demonstrated that the EB content was significantly elevated at 24 h
following cerebral IR compared with the sham operation group
(P<0.05). However, this elevation exhibited a significant
decrease following treatment with 100 mg/kg of resveratrol
(P<0.01) but not 10 mg/kg of resveratrol (P>0.05; Fig. 4B).
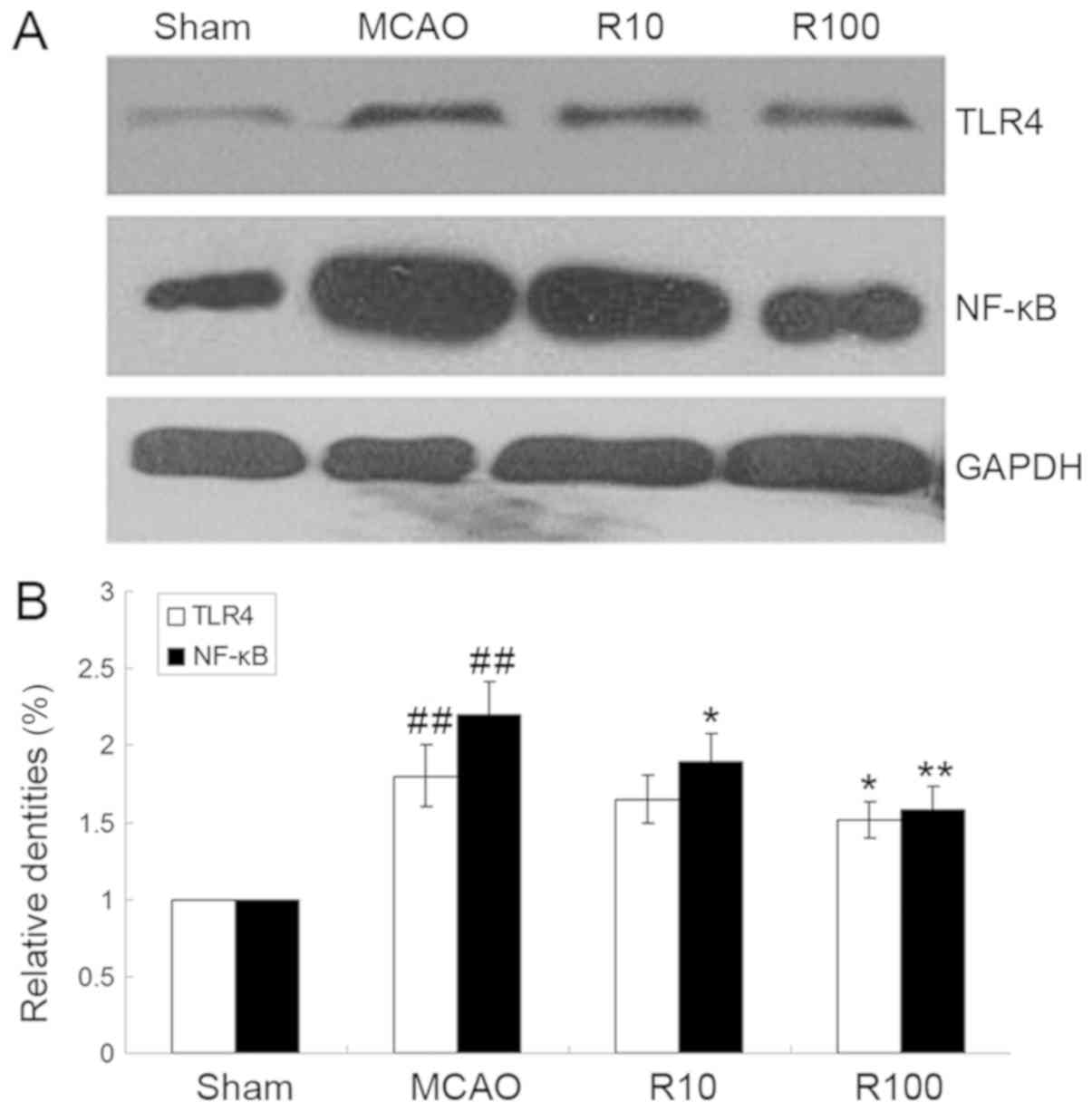
Resveratrol downregulates the protein
expression levels of TLR4 and NF-κB
The protein expression levels of TLR4 and NF-κB p65
in ischemic brain tissues increased at 24 h following cerebral IR
but were significantly downregulated by resveratrol (P<0.05;
Fig. 5).
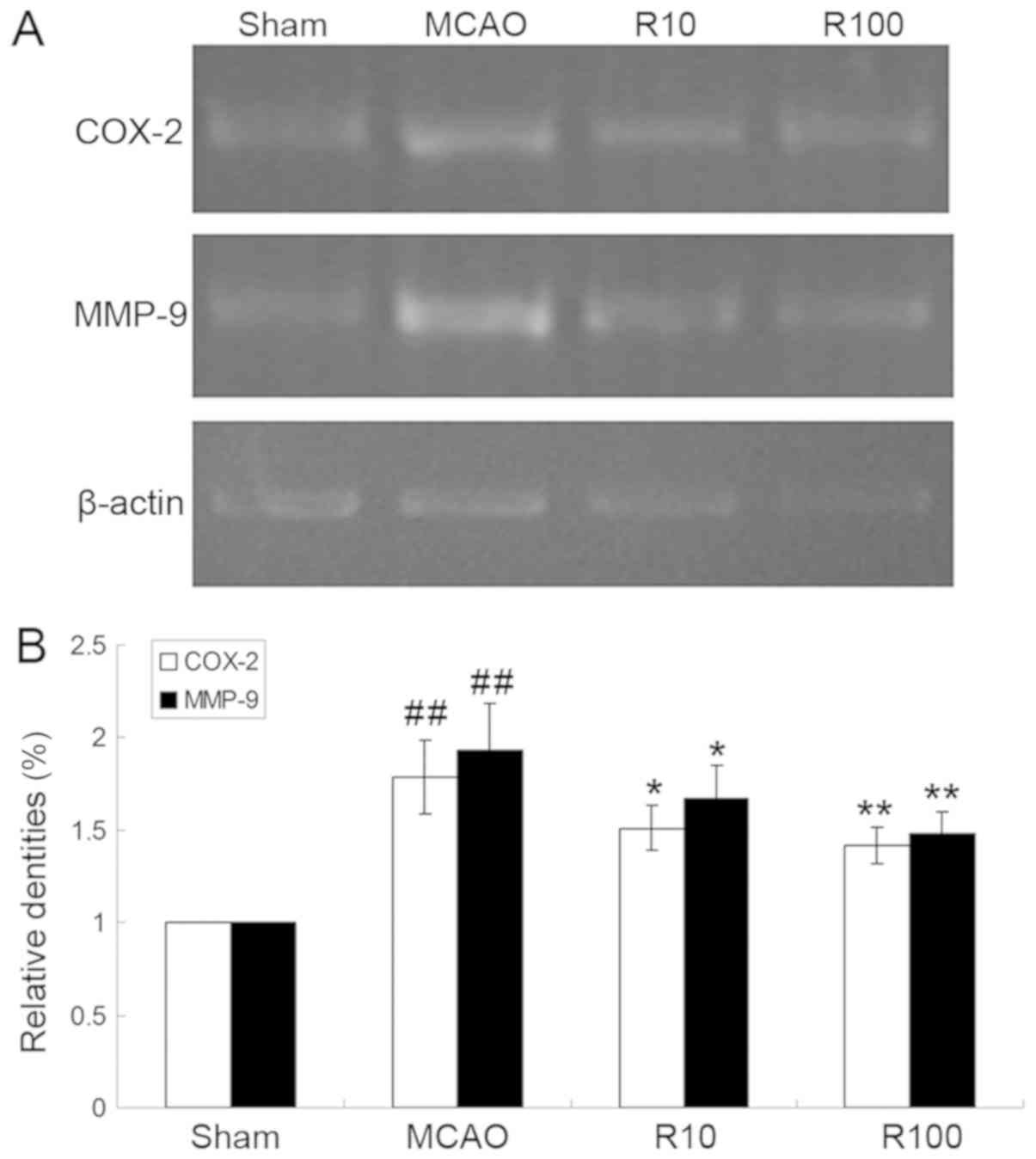
Resveratrol downregulates the mRNA
expression levels of COX-2 and MMP-9
The mRNA expression levels of COX-2 and MMP-9 mRNA
in ischemic brain tissues increased at 24 h following cerebral IR
but were significantly downregulated by resveratrol (P<0.01;
Fig. 6).
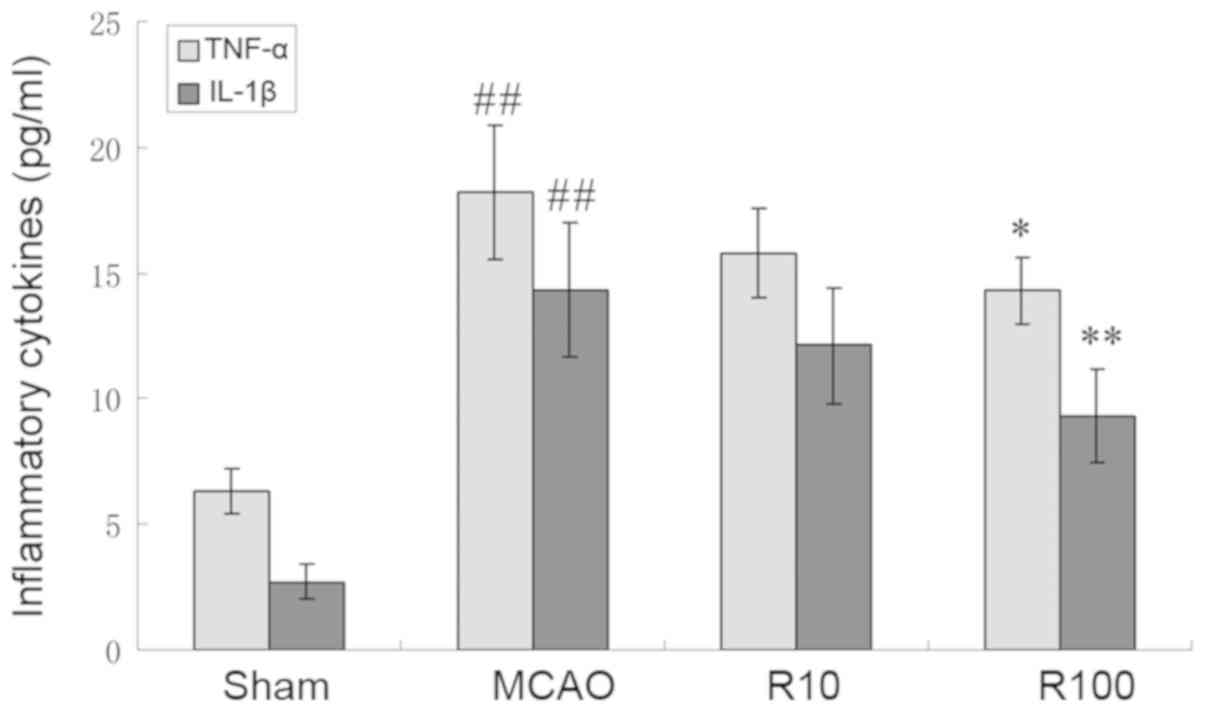
Resveratrol reduces blood levels of
TNF-α and IL-1β
The blood levels of TNF-α and IL-1β increased at 24
h following cerebral IR but were significantly reduced by
resveratrol (P<0.05; Fig. 7).
Discussion
Stroke remains a leading cause of mortality and
neurological disabilities in adults around the world. Currently,
acute ischemic stroke (AIS) is treated using two major therapeutic
strategies: Thrombolytic therapy and neuroprotective therapy
(16) Although tissue-plasminogen
activator is the only FDA-approved therapy for AIS within a 3–4.5 h
time window (17), only 1–2% of
patients have the opportunity to receive thrombolytic therapy due
to the brevity of this window (18).
Additionally, reperfusion followed by thrombolysis may exacerbate
brain injuries via a series of pathological mechanisms including
inflammation and BBB disruption (19). Therefore, identification of novel
potential neuroprotective agents targeting the pathological
mechanisms underlying cerebral IR is necessary.
The present study demonstrated that resveratrol
protected brains against ischemic stroke in an experimental rat
model, which is consistent with a previous study (20). Several studies have investigated the
potential mechanisms underlying the neuroprotective capabilities of
resveratrol. For example, Sinha et al (21) demonstrated that resveratrol protects
rat brain tissues against focal cerebral ischemia by reducing
oxidative stress, while Tsai et al (22) demonstrated that resveratrol
downregulates the expression of inducible nitric oxide synthase
(NOS) and upregulates the expression of endothelial NOS, which may
explain how resveratrol protects rat brains against focal cerebral
ischemia. Additionally, Li et al (23) reported that resveratrol attenuates
ischemic brain injury and that these effects may be associated with
the inhibition of neuronal apoptosis via the upregulation of
hippocampal Bcl-2.
Another previous study demonstrated that resveratrol
is neuroprotective against cerebral ischemia injury via
anti-oxidant and anti-inflammatory mechanisms (24). Although specific reports demonstrated
that resveratrol does not have beneficial effects on memory and
cognitive dysfunction (25), these
differences may be attributed to the use of different disease
models. Therefore, additional studies will be required to clarify
these discrepancies. The present study demonstrated that
resveratrol decreased the enzymatic activity of MPO, which suggests
that resveratrol possesses anti-inflammatory properties that are
beneficial following ischemic stroke. It is also possible that the
neuroprotective effects of resveratrol against ischemic stroke may
be associated with its anti-inflammatory activities.
TLRs serve critical roles in the induction of innate
and adaptive immunity (26).
Additionally, these receptors possess leucine-rich repeats in their
extracellular region, which are responsible for the recognition of
pathogen-associated molecular patterns and endogenous
‘danger’-associated molecular patterns and a Toll IL-1 receptor
domain in the intracellular region that is required for the
initiation of intracellular signaling (27). Of the TLR family, TLR4 has been of
particular interest because it is the primary receptor recognizing
bacterial infections and endogenous ligands released following
tissue injury. Endogenous ‘danger signals’, including high mobility
group box protein 1 and heat shock proteins activate TLR4
signaling. The activation of TLR4 stimulates IκB-α phosphorylation
and degradation, which results in the nuclear translocation of
NF-κB. Subsequently, NF-κB activation regulates the expression
levels of inflammatory genes that are involved in innate immune
responses and lead to the initiation of inflammation (28).
The TLR4 and NF-κB signaling pathways are widely
considered to mediate ischemic brain injury processes and to be a
promising therapeutic target for ischemic stroke (29,30).
Neurological dysfunction scores and cerebral infarction scores in
TLR4-deficient mice are significantly lower compared with those of
wild-type mice, and the present study demonstrated that resveratrol
downregulated the expression of TLR4 and inhibited the activation
of NF-κB. The low expression of TLR4 following resveratrol
administration could weaken activation of the TLR4/NF-κB signaling
pathway and attenuate inflammation, which in turn would reduce the
ischemic brain injury induced by inflammation.
Zhao et al (31) reported that ischemic stroke leads to
enhanced expression of COX-2, which results in progressive ischemic
brain injury. Nimesulide, a selective COX-2 inhibitor, attenuates
COX-2 activity and ameliorates cerebral ischemia injury (32), which indicates that COX-2 may be an
important therapeutic target for AIS. MMP-9, a proteolytic enzyme,
degrades important structures in the microvascular wall to increase
microvascular permeability and BBB disruption (33). During cerebral ischemia, MMP-9
expression is upregulated, which leads to the degradation of
occludin that, in turn, causes BBB leakage, brain edema, brain
hemorrhages and secondary brain damage (34). In the present study, resveratrol
downregulated the mRNA expression levels of COX-2 and MMP-9
following cerebral IR in rats; this suggests that resveratrol
attenuated inflammatory reactions and BBB disruption, and
furthermore that these activities may be associated with the
inhibition of COX-2 and MMP-9.
During cerebral ischemia, the TLR4/NF-κB pathway
regulates the expression levels of inflammatory cytokines,
including TNF-α and IL-1β, which propagates the inflammatory
cascade reaction and eventually increases brain damage. The blood
levels of TNF-α and IL-1β are increased following cerebral
ischemia, but are decreased by curcumin (29). In the present study, the blood levels
of TNF-α and IL-1β were elevated, but were lowered by resveratrol
treatment. These findings suggest that the anti-inflammatory
properties of resveratrol may be attributable to inhibition of the
TLR4/NF-κB signaling pathway.
In conclusion, the present study demonstrated that
resveratrol reduced neurological dysfunction, neuronal injury,
cerebral infarction and BBB permeability in a rat model of focal
cerebral ischemia, and demonstrated that these activities may be
associated with the downregulation of inflammatory processes and
the TLR4 signaling pathway. These experimental results suggest that
the TLR4 signaling pathway may be an important therapeutic target
for and resveratrol a promising neuroprotective agent against,
ischemic stroke.
Acknowledgements
The authors would like to give thanks for the
technical assistance of Professor Dong-Sheng Li from the Institute
of Life Science at Taihe Hospital, Hubei University of Medicine
(Shiyan, China).
Funding
The Natural Science Foundation of the Fujian
Province of China (grant no. 2017J01204) and the Key Young Talents
Cultivation Project of Health and Family Planning Commission of the
Fujian Province of China (grant no. 2016-ZQN-28) contributed to
this study.
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
JRL and XKT designed the study and wrote the paper.
JRL, YW, DWT and SSS performed the experiments and helped perform
the analysis with constructive discussions. XKT revised the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Hubei University of
Medicine (Shiyan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dirnagl U: Pathobiology of injury after
stroke: The neurovascular unit and beyond. Ann N Y Acad Sci.
1268:21–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu Y, Chen J and Shen J: Herbal medicines
for ischemic stroke: Combating inflammation as therapeutic targets.
J Neuroimmune Pharmacol. 9:313–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lucas K and Maes M: Role of the toll like
receptor (TLR) radical cycle in chronic inflammation: Possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tu XK, Yang WZ, Shi SS, Wang CH, Zhang GL,
Ni TR, Chen CM, Wang R, Jia JW and Song QM: Spatio-temporal
distribution of inflammatory reaction and expression of TLR2/4
signaling pathway in rat brain following permanent focal cerebral
ischemia. Neurochem Res. 35:1147–1155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caso JR, Pradillo JM, Hurtado O, Lorenzo
P, Moro MA and Lizasoain I: Toll-like receptor 4 is involved in
brain damage and inflammation after experimental stroke.
Circulation. 115:1599–1608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tu XK, Yang WZ, Shi SS, Chen Y, Wang CH,
Chen CM and Chen Z: Baicalin inhibits TLR2/4 signaling pathway in
rat brain following permanent cerebral ischemia. Inflammation.
34:463–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan T, Liu Q, Qian Y, Yang H, Kong J, Kou
J and Yu B: Ruscogenin reduces cerebral ischemic injury via
NF-κB-mediated inflammatory pathway in the mouse model of
experimental stroke. Eur J Pharmacol. 714:303–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lan L, Tao J, Chen A, Xie G, Huang J, Lin
J, Peng J and Chen L: Electroacupuncture exerts anti-inflammatory
effects in cerebral ischemia-reperfusion injured rats via
suppression of the TLR4/NF-κB pathway. Int J Mol Med. 31:75–80.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mokni M, Hamlaoui S, Karkouch I, Amri M,
Marzouki L, Limam F and Aouani E: Resveratrol provides
cardioprotection after ischemia/reperfusion injury via modulation
of antioxidant enzyme activities. Iran J Pharm Res. 12:867–875.
2013.PubMed/NCBI
|
|
10
|
Kma L: Synergistic effect of resveratrol
and radiotherapy in control of cancers. Asian Pac J Cancer Prev.
14:6197–6208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taguchi A, Wada-Hiraike O, Kawana K, Koga
K, Yamashita A, Shirane A, Urata Y, Kozuma S, Osuga Y and Fujii T:
Resveratrol suppresses inflammatory responses in endometrial
stromal cells derived from endometriosis: A possible role of the
sirtuin 1 pathway. J Obstet Gynaecol Res. 40:770–778. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li
T, Shi JX and Zhang X: Resveratrol prevents neuronal apoptosis in
an early brain injury model. J Surg Res. 189:159–165. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren J, Fan C, Chen N, Huang J and Yang Q:
Resveratrol pretreatment attenuates cerebral ischemic injury by
upregulating expression of transcription factor Nrf2 and HO-1 in
rats. Neurochem Res. 36:2352–2362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi SS, Yang WZ, Tu XK, Wang CH, Chen CM
and Chen Y: 5-Lipoxygenase inhibitor zileuton inhibits neuronal
apoptosis following focal cerebral ischemia. Inflammation.
36:1209–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen F, Qi Z, Luo Y, Hinchliffe T, Ding G,
Xia Y and Ji X: Non-pharmaceutical therapies for stroke: Mechanisms
and clinical implications. Prog Neurobiol. 115:246–269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salam KA, Ummer K, Pradeep Kumar VG and
Noone ML: Intravenous thrombolysis for acute ischemic stroke in the
3 to 4.5-hour window-The Malabar experience. Int J Stroke.
9:426–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bansal S, Sangha KS and Khatri P: Drug
treatment of acute ischemic stroke. Am J Cardiovasc Drugs.
13:57–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan J, Konstas AA, Bateman B, Ortolano GA
and Pile-Spellman J: Reperfusion injury following cerebral
ischemia: Pathophysiology, MR imaging, and potential therapies.
Neuroradiology. 49:93–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu KT, Chiou RY, Chen LG, Chen MH, Tseng
WT, Hsieh HT and Yang YL: Neuroprotective effects of resveratrol on
cerebral ischemia-induced neuron loss mediated by free radical
scavenging and cerebral blood flow elevation. J Agric Food Chem.
54:3126–3131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sinha K, Chaudhary G and Gupta YK:
Protective effect of resveratrol against oxidative stress in middle
cerebral artery occlusion model of stroke in rats. Life Sci.
71:655–665. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW,
Liu HY, Zhang FB and Huang SS: Resveratrol neuroprotective effects
during focal cerebral ischemia injury via nitric oxide mechanism in
rats. J Vasc Surg. 46:346–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Pang L, Fang F, Zhang G, Zhang J,
Xie M and Wang L: Resveratrol attenuates brain damage in a rat
model of focal cerebral ischemia via up-regulation of hippocampal
Bcl-2. Brain Res. 1450:116–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orsu P, Murthy BV and Akula A:
Cerebroprotective potential of resveratrol through anti-oxidant and
anti-inflammatory mechanisms in rats. J Neural Transm (Vienna).
120:1217–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farzaei MH, Rahimi R, Nikfar S and
Abdollahi M: Effect of resveratrol on cognitive and memory
performance and mood: A meta-analysis of 225 patients. Pharmacol
Res. 128:338–344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ulevitch RJ: Therapeutics targeting the
innate immune system. Nat Rev Immunol. 4:512–520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Means TK, Golenbock DT and Fenton MJ: The
biology of Toll-like receptors. Cytokine Growth Factor Rev.
11:219–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carmody RJ and Chen YH: Nuclear
factor-kappaB: Activation and regulation during toll-like receptor
signaling. Cell Mol Immunol. 4:31–41. 2007.PubMed/NCBI
|
|
29
|
Tu XK, Yang WZ, Chen JP, Chen Y, Ouyang
LQ, Xu YC and Shi SS: Curcumin inhibits TLR2/4-NF-κB signaling
pathway and attenuates brain damage in permanent focal cerebral
ischemia in rats. Inflammation. 37:1544–1551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiang HF, Cao DH, Yang YQ, Wang HQ, Zhu
LJ, Ruan BH, Du J and Wang MC: Isoflurane protects against injury
caused by deprivation of oxygen and glucose in microglia through
regulation of the Toll-like receptor 4 pathway. J Mol Neurosci.
54:664–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Patzer A, Herdegen T, Gohlke P and
Culman J: Activation of cerebral peroxisome proliferator-activated
receptors gamma promotes neuroprotection by attenuation of neuronal
cyclooxygenase-2 overexpression after focal cerebral ischemia in
rats. FASEB J. 20:1162–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Candelario-Jalil E, González-Falcón A,
Garcia-Cabrera M, León OS and Fiebich BL: Wide therapeutic time
window for nimesulide neuroprotection in a model of transient focal
cerebral ischemia in the rat. Brain Res. 1007:98–108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei H, Wang S, Zhen L, Yang Q, Wu Z, Lei
X, Lv J, Xiong L and Xue R: Resveratrol attenuates the blood-brain
barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after
cerebral ischemia reperfusion in rats. J Mol Neurosci. 55:872–879.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao
J, Chen L, Cui L and Qiao H: Protective effect of shikonin in
experimental ischemic stroke: Attenuated TLR4, p-p38MAPK, NF-κB,
TNF-α and MMP-9 expression, up-regulated claudin-5 expression,
ameliorated BBB permeability. Neurochem Res. 39:97–106. 2014.
View Article : Google Scholar : PubMed/NCBI
|