Introduction
Prostate cancer (PCa) is the leading cause of
cancer-associated mortality in men worldwide (1). Approximately 60,300 new PCa cases are
diagnosed annually, and ~26,600 die of aggressive PCa in China
annually (2). Although many
approaches have been explored to improve the diagnosis and
treatment of PCa, the incidence and mortality of PCa has still
increased in recent years (1,3).
Furthermore, the morphological features and grading system
evaluated by pathologists are still the most valuable diagnostic
criteria and predictors for PCa patients (4). Hence, novel biomarkers contributing to
the diagnosis and prognosis predictions of PCa patients need to be
identified.
Eukaryotic translation initiation factor (EIF) 5A2
is one of two isoforms in the EIF5A family that mainly acts as an
elongation factor during the mRNA translation step (5). The EIF5A2 gene is located on the human
chromosome at 3q26, where many other candidate oncogenes exist
(6,7). EIF5A2 is only found in testes, brain
and tumor tissues (8). A series of
studies have shown that overexpression of EIF5A2 is closely
associated with tumor growth, metastasis and chemoresistance in
diverse cancers and may serve as a potential prognostic marker
(9,10). Our previous study also indicated that
EIF5A2 overexpression predicts tumor metastatic potential in
patients with localized invasive bladder cancer treated with
radical cystectomy (11). Previous
studies have shown that inhibition of EIF5A2 activity by
N1-guanyl-1,7-diaminoheptane (GC7) has strong anti-tumor effects on
human cancer cells (12,13), indicating that EIF5A2 could be a
potential target for anticancer therapy. However, the expression
pattern and clinical significance of EIF5A2 in PCa have not yet
been elucidated.
The present study investigated EIF5A2 expression and
characterized its clinicopathological significance in a large
cohort of PCa tissues. It was identified in the present that EIF5A2
expression was upregulated at both mRNA and protein levels in PCa
tumor compared with non-tumor tissues. EIF5A2 overexpression was
associated with aggressive clinicopathological features. Notably,
elevated expression of EIF5A2 predicted unfavorable
progression-free survival and could be an independent prognostic
biomarker for PCa patients.
Materials and methods
Human tissue specimens and
patients
Three clinical specimens including cancer and
corresponding adjacent non-tumor tissues, were collected during
radical resection to detect the mRNA and protein levels of EIF5A2.
In addition, 72 formalin-fixed, paraffin-embedded PCa tumor
specimens, and 20 matched adjacent non-tumor tissue specimens were
collected to perform immunohistochemical staining. The median age
of the patients was 65 years (range, 48–87 years). The samples in
the present study were collected from The First Affiliated
Hospital, Sun Yat-sen University, Guangdong, China, Jiangmen
Central Hospital, Guangdong, China and Affiliated Yantai
Yuhuangding Hospital, Qingdao University, Shandong, during January
2005 to December 2008, after a distinctive pathological diagnosis
of prostate adenocarcinoma. Most of the patients (69/72) received
radical prostatectomy, while only few of them (3/72) underwent
transurethral resection prostate as they were diagnosed with benign
prostate hyperplasia before surgery. None of the patients received
immunotherapy or radiotherapy before surgical treatment. All the
patients were staged using the classification of the American Joint
Committee on Cancer staging system for prostate cancer (14). Biochemical recurrence was defined as
Prostate specific antigen (PSA) levels >0.4 ng/ml. The last
follow-up update was June 2017, and the median follow-up time was
100 months. All the samples were collected with the patient's
written informed consent after approval from the Institute Research
Medical Ethics Committee of the Hospitals.
RNA extraction and semi-quantitative
reverse transcription polymerase chain reaction (SqRT-PCR)
Total RNA was isolated from three resected fresh
tumors and matched with adjacent on-tumor tissues using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). RNA purity and concentration were determined by a standard
ultraviolet spectrophotometric assay. RT-PCR was performed using
PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol. GAPDH was used as an
internal control. The primers used were as follows: EIF5A2 forward,
5′-AAGATGGTTACCTTTCCCTG-3′ and reverse,
5′-TACAGCATATTCTTCACTCATTG-3′; GAPDH forward,
(5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min, followed by 35
cycles of denaturation at 95°C for 1 min, annealing at 55°C for 1
min, extension at 72°C for 1 min and elongation for 7 min at 72°C.
Samples were then resolved on a 2% agarose gel, stained with
ethidium bromide for 30 min at room temperature and visualized
using a high-performance ultraviolet transilluminator (Analytik
Jena US LLC; Upland, CA, USA).
Western Blot analysis
Resected fresh tumor tissue and matched adjacent
non-tumor tissue were homogenized and lysed in
radioimmunoprecipitation assay buffer on ice. Protein
concentrations were determined using a BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.) standardized with bovine
serum albumin (Invitrogen; Thermo Fisher Scientific, Inc.).
Different proteins were separated by 10% SDS-PAGE and transferred
to polyvinylidene difluoride membranes. The membranes were blocked
with 5% (W/V) nonfat-dry milk in TBST (25 mM Tris HCl, pH 7.5; 150
mM NaCl; 0.05% Tween-20) for 1 h at room temperature followed by
probing with primary antibodies against EIF5A2 (1:1,000; cat. no.
ab150439; Abcam, Cambridge) or GAPDH (1:1,000; cat. no. ab181602;
Abcam, Cambridge, UK) for 2 h at room temperature and then washed 3
times with TBST, followed by incubating for 1 h at room temperature
with horse radish peroxidase-conjugated secondary antibodies
(1:2,000; cat. no. ab150077; Abcam). Immunoreactive protein was
detected using enhanced chemiluminescence detection reagents (GE
Healthcare, Chicago, IL, USA) according to the manufacturer's
protocol.
Immunohistochemical staining and
evaluation
A total of 72 formalin-fixed paraffin-embedded tumor
tissue samples and 20 corresponding samples of non-tumor tissues
were used for EIF5A2 immunohistochemical staining. The experiment
was performed using a standard streptavidin-biotin-peroxidase
complex protocol as described previously (11). The slides were incubated at 4°C in a
moist chamber overnight with a primary antibody against human
EIF5A2 (1:200; cat. no. ab150439; Abcam). Staining with PBS instead
of the primary antibody against EIF5A2 was used as the negative
control; ovarian tumor tissue with positive EIF5A2 expression was
used as the positive control.
For evaluation of EIF5A2 in different prostate
tissues, a semiquantitative scoring method was used, according to
our previous study (11). Briefly, a
staining index (values 0–12) was obtained as the intensity of
EIF5A2 staining (negative=0, weak=1, moderate=2, or strong=3
scores) and the proportion of immunopositive cells of interest
(<25%=1, 25–50%=2, >50% to <75%=3, and ≥75%=4 scores) was
calculated. The results were observed and assessed by two
independent experienced researchers in a blinded manner.
Statistical analysis
SPSS package (v20.0; IBM Corp., Armonk, NY, USA) was
used for statistical analysis. Image J (v1.8.0; National Institutes
of Health, Bethesda, MD, USA) software was used to calculate the
intensity of western blot bands and SqRT-PCR results. Student's
t-test was employed to compare EIF5A2 expression between the tumor
and adjacent non-tumor tissue. Pearson χ2 tests were
performed to determine the association between EIF5A2 protein
expression and clinicopathologic parameters. Kaplan-Meier analysis
was used for univariate survival analysis and the log-rank test was
applied to compare different survival curves. The multivariate Cox
regression model was used to evaluate the potential independent
prognostic factors and 95% confidence intervals (CI) of the hazard
ratio (HR). P<0.05 was considered to indicate a statistically
significant difference.
Results
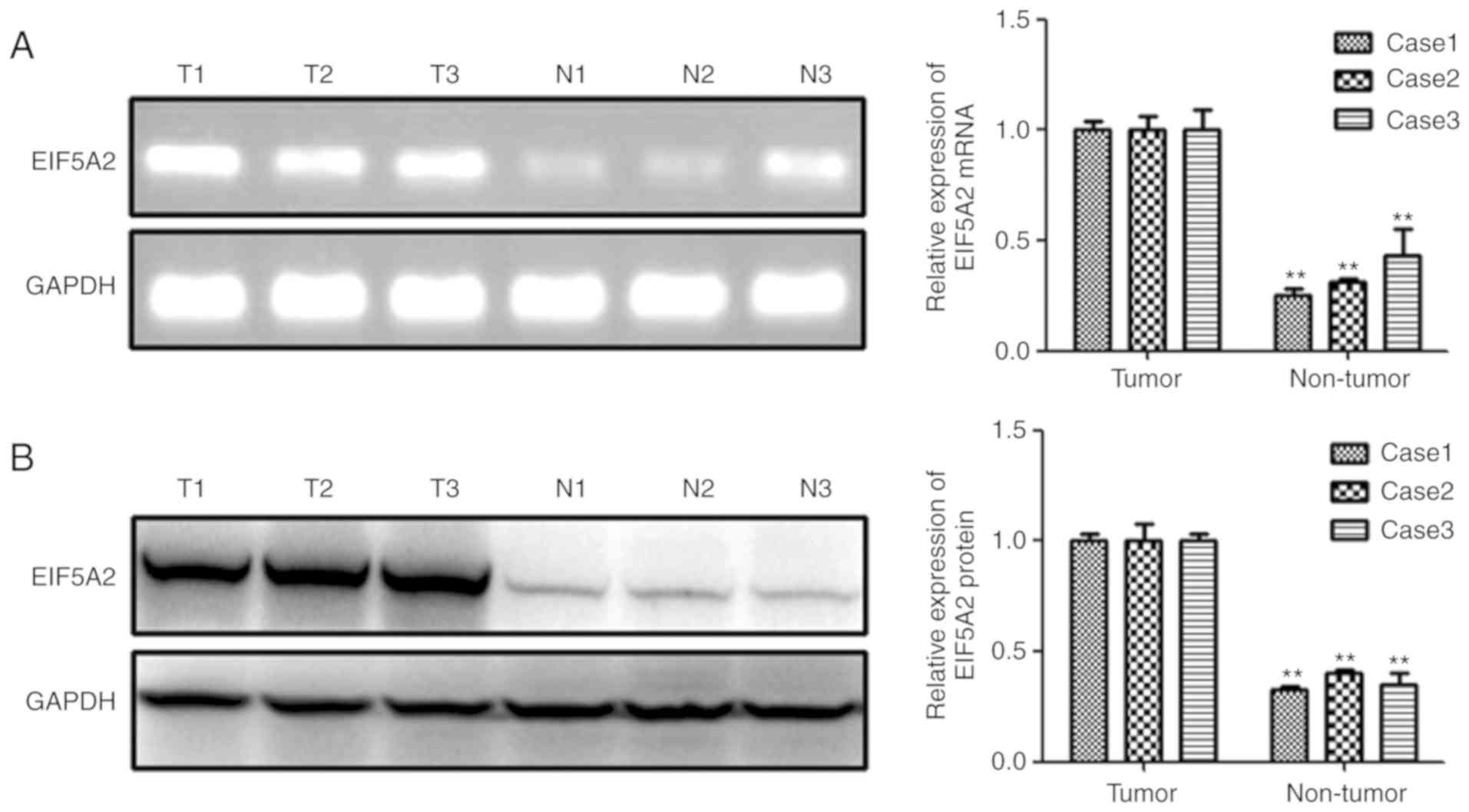
EIF5A2 mRNA and protein levels are
highly expressed in freshly resected PCa specimens
To detect the EIF5A2 mRNA and protein expression
pattern in prostate cancer, RT-PCR and western blot analyses in
three sets of freshly resected, paired specimens were conducted
first. As shown in Fig. 1A, the
SqRT-PCR results indicated that EIF5A2 mRNA levels were
significantly higher in PCa tissues than that in adjacent non-tumor
tissue in all three specimens. Subsequently, the EIF5A2 protein
expression was assessed by Western Blot assay, and consistent with
the mRNA expression, the protein level of EIF5A2 was notably
up-regulated in PCa tissue compared with that in adjacent non-tumor
tissue (Fig. 1B).
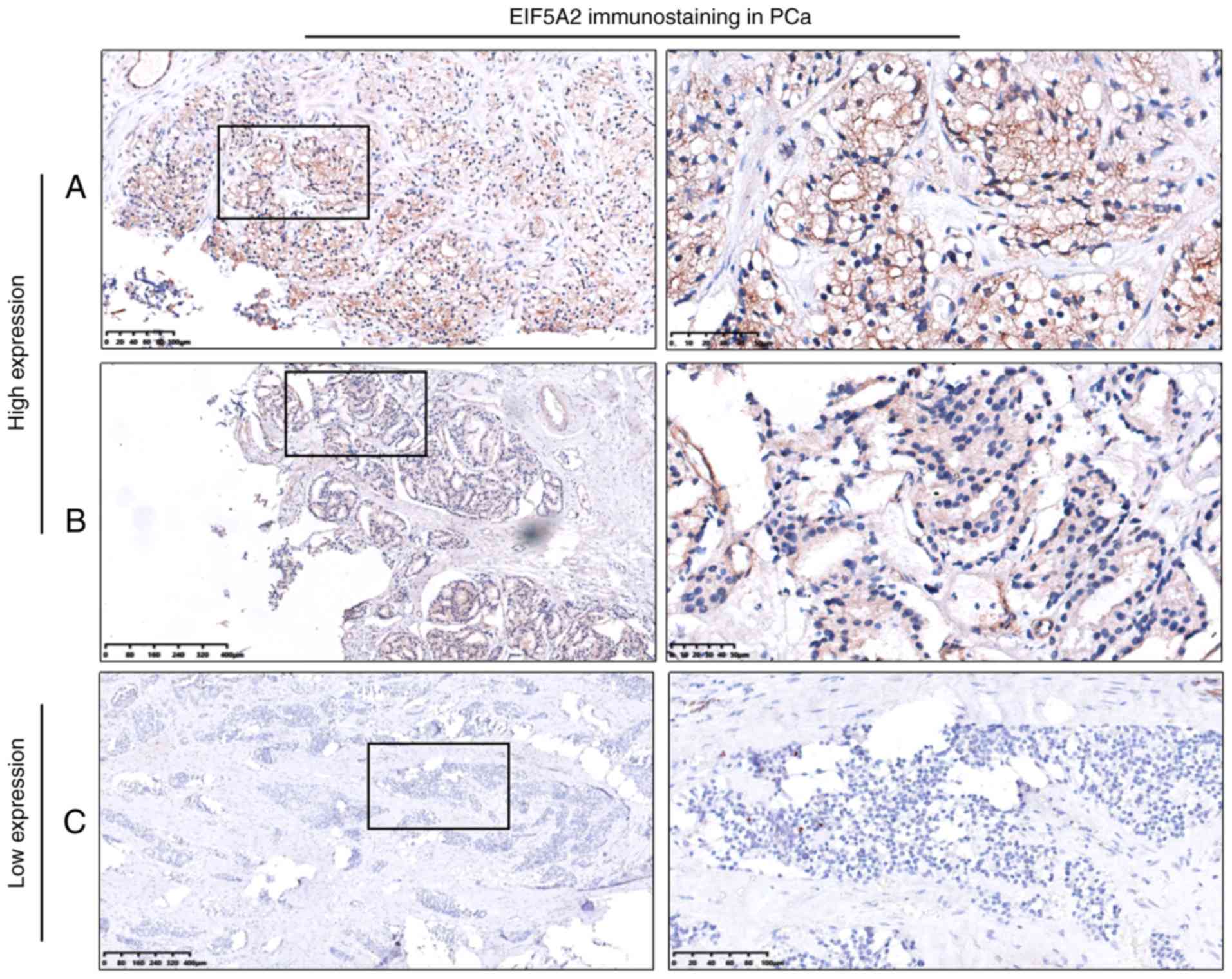
EIF5A2 protein levels are elevated in
paraffin embedded PCa tissues
To further validate the expression level of EIF5A2
protein in PCa, immunohistochemical staining in a large cohort of
PCa tissues including 72 PCa and 20 corresponding non-tumor tissues
was conducted. Samples with a staining index ≥6 (median score of
EIF5A2 expression in PCa tissues) were defined as high expression
and samples with a staining index <6 were defined as low
expression. EIF5A2 overexpression was detected in 53 tumor tissue
samples (73.6%, 53/72), but in only three adjacent non-tumor tissue
samples (15.0%, 3/20; Fig. 2).
EIF5A2 protein expression is
associated with aggressive clinicopathological variables
In order to uncover the clinical significance of
EIF5A2 in PCa, the associations between the expression of EIF5A2
and clinicopathological parameters were analyzed. High or low
expression rates of EIF5A2 protein in PCa with respect to several
standard clinicopathological features are shown in Table I. EIF5A2 overexpression was
associated with tumor stage (P=0.011) and biochemical recurrence
status (P=0.032) However, no significant association was detected
between EIF5A2 overexpression and other clinicopathological
parameters, including age (P=0.964), serum PSA level (P=0.179) and
Gleason Score (P=0.253).
 | Table I.Correlation of EIF5A2 expression with
clinicopathological parameters of PCa patients. |
Table I.
Correlation of EIF5A2 expression with
clinicopathological parameters of PCa patients.
|
|
| EIF5A2
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | N | Low (%) | High (%) | P-valuea |
|---|
| Age |
|
|
| 0.964 |
| <65
y | 42 | 11 (26.2) | 31 (73.8) |
|
| ≥65
y | 30 | 8 (26.7) | 22 (73.3) |
|
| PSA level |
|
|
| 0.179 |
| ≥10 | 57 | 13 (22.8) | 44 (77.2) |
|
|
<10 | 15 | 6 (40.0) | 9 (60.0) |
|
| Tumor stage |
|
|
| 0.011 |
|
T1+T2 | 43 | 16 (37.2) | 27 (62.8) |
|
| T3 | 29 | 3 (10.3) | 26 (89.7) |
|
| Biochemical
recurrence |
|
|
| 0.032 |
|
Positive | 27 | 11 (40.7) | 16 (59.3) |
|
|
Negative | 45 | 8 (17.8) | 37 (82.2) |
|
| Gleason score |
|
|
| 0.253 |
|
<7 | 56 | 13 (23.2) | 43 (76.8) |
|
| ≥7 | 16 | 6 (37.5) | 10 (62.5) |
|
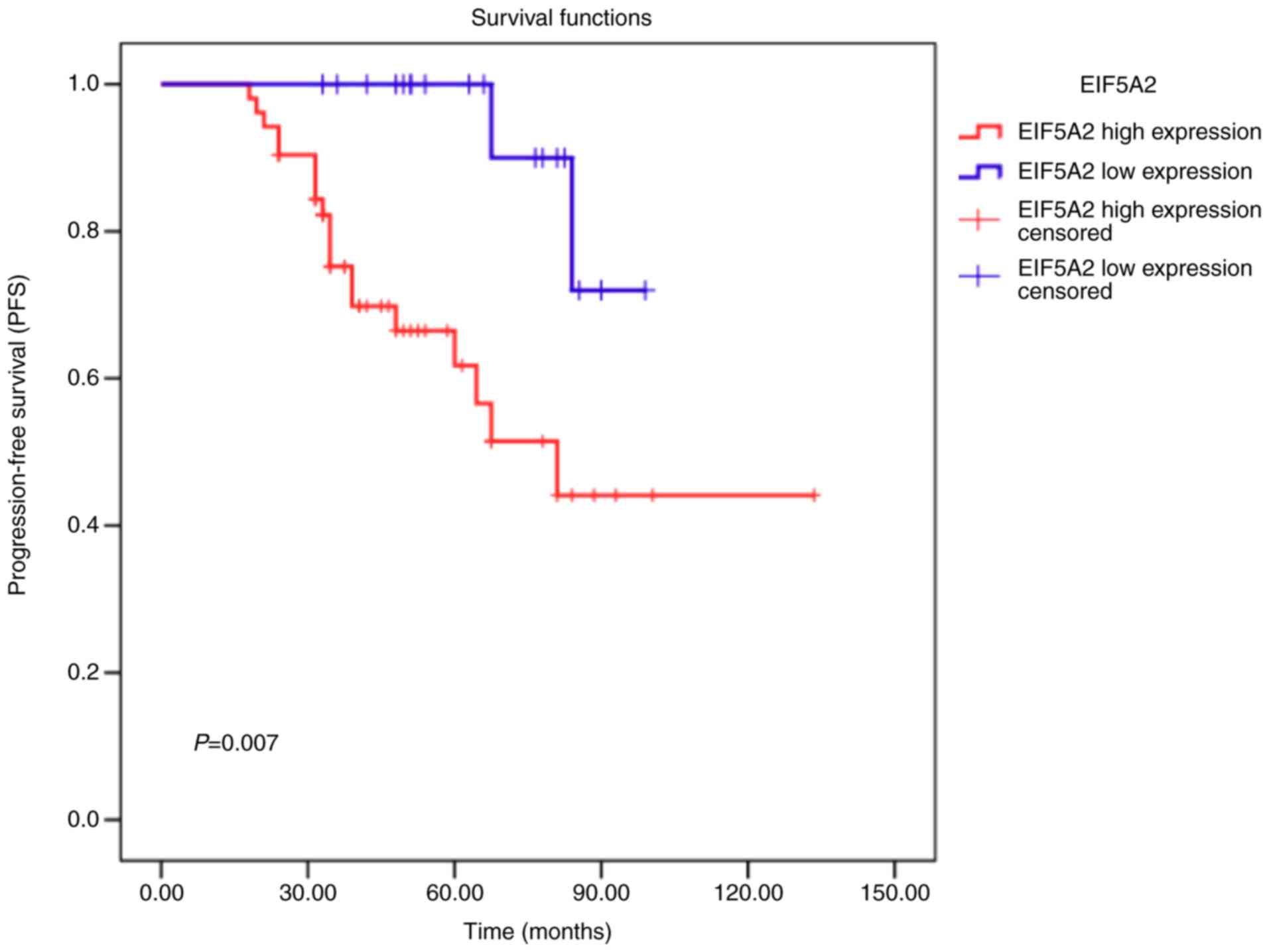
EIF5A2 overexpression predicts
inferior prognosis in PCa patients
To associate the EIF5A2 expression levels to
clinical outcome, Kaplan-Meier survival analysis was used to
analyze the prognostic value of EIF5A2 in PCa patients. As shown in
Fig. 3, PCa patients with high
EIF5A2 expression had a significantly shorter progression-free
survival time than that of those with low EIF5A2 expression
(P=0.007). Subsequently, multivariate Cox regression analysis was
used to evaluate the potential prognostic significance of EIF5A2
expression. The results showed that high expression of EIF5A2 was
an independent prognostic factor for poor overall survival (hazard
ratio, 0.366; 95% confidence interval, 0.349–0460; P=0.021;
Table II).
 | Table II.Multivariate analysis on overall
survival (Cox regression model). |
Table II.
Multivariate analysis on overall
survival (Cox regression model).
| Variables | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Tumor
stagea | 1.427 | 1.344–2.322 | 0.013 |
| Biochemical
recurrenceb | 1.322 | 1.658–2.763 | 0.001 |
| EIF5A2
expressionc | 0.366 | 0.349–0460 | 0.021 |
Discussion
EIF5A2 is thought to be a candidate oncogene that is
involved in tumor proliferation, invasion, metastasis, cell aging
and drug resistance (9,10). However, the expression pattern and
clinical significance of EIF5A2 in PCa are unclear. The present
study demonstrated that EIF5A2 was upregulated in PCa tissues at
both the mRNA and protein levels; overexpression of EIF5A2 protein
was associated with aggressive pathological parameters including
tumor stage and biomedical recurrence. Moreover, high expression of
EIF5A2 predicted inferior prognosis of PCa patients and may be an
independent prognostic marker.
The expression of EIF5A2 is specific to the tissue
and cell type. In addition to its expression in the normal testis
and brain, EIF5A2 levels are increased in a number of malignancies.
It was reported that EIF5A2 was up-regulated in ovary cancer
(15), nasopharyngeal carcinoma
(16), colorectal carcinoma
(17), hepatocellular carcinoma
(18), gastric cancer (19) and non-small cell lung cancer
(20). A previous study by Li et
al (21), revealed that EIF5A2
was significantly decreased in prostate cancer when Ephrin type-A
receptor 6 was knocked down. However, the expression type of EIF5A2
in prostate cancer cells or tissues, and clinical significance of
EIF5A2 have not yet been investigated. Our previous study indicated
that EIF5A2 protein levels were elevated in bladder cancer tissues
compared with that in adjacent normal bladder tissues by using
immunohistrochemical staining (11).
Consistent with these results, in the present study, EIF5A2 mRNA
and protein levels were shown to be elevated in PCa tissue. These
coherent data suggested that EIF5A2 overexpression might be a
common event in the tumorigenesis and progression of different
cancers. Moreover, the present study demonstrated that upregulation
of EIF5A2 was closely associated with aggressive
clinicopathological features and might be an independent candidate
prognostic marker for PCa patients. These findings are in
accordance with previous studies. For instance, in early-stage
cervical cancer, EIF5A2 overexpression was correlated with higher
International Federation of Gynecology and Obstetrics stage, deep
cervical stromal invasion, lymphovascular space involvement and
pelvic lymph node metastasis, and might be a prognostic factor for
overall survival and disease-free survival (22). In gastric cancer, EIF5A2
overexpression was positively correlated with more advanced pT
stage, more advanced pN stage and positive lymphovascular invasion,
and predicted poor prognosis (19).
However, in nasopharyngeal carcinoma, EIF5A2 expression had no
significant association with clinicopathological characteristics,
such as tumor stage and world health organization classification
(16). These results indicated that,
even though different tumor types share similar prognosis
predictions in regard to EIF5A2 expression, the biological behavior
and anatomical characteristics of EIF5A2 might be distinct among
the tumor subtypes.
Accumulating evidence suggests that EIF5A2 serves a
vital role in the malignant behavior of diverse cancers. For
instance, in hepatocellular carcinoma, EIF5A2 not only promoted
cancer cell proliferation, migration and invasion (18), but also enhanced cell metabolic
reprogramming (23). Furthermore,
ablation of EIF5A2 could induce tumor vasculature remodeling and
improve the tumor response to chemotherapy (24). Recently, Bai et al (25) revealed that EIF5A2 contributes to the
maintenance of Cluster of differentiation 133+ hepatocellular
carcinoma cells, which was the key biomarker to identify and
characterize cancer stem cells. A similar influence of EIF5A2 on
migration, invasion and chemosensitivity was also observed in
esophageal squamous cell carcinoma (26,27) and
non-small cell lung cancer (28,29).
Although the clinical significance of EIF5A2 in PCa has been
demonstrated in the present study, its biological function should
be explored in in vitro and in vivo experiments in
future work.
With regard to the mechanisms underlying the role of
EIF5A2 in cancer, a variety of downstream events have been
reported. EIF5A2 induced cancer cell epithelial-mesenchymal
transition contributes to tumor metastasis and/or drug resistance
in hepatocellular carcinoma (18),
colorectal carcinoma (17,30) and esophageal squamous cell carcinoma
(27). In hepatocellular carcinoma,
the c-myelocytomatosis/microRNA-29b axis was involved in the
process of EIF5A2 maintaining the features of cancer stem cell of
hepatocellular carcinoma (25). In
addition, EIF5A2 promoted tumor angiogenesis and relied on vascular
endothelial growth factor, p38 mitogen-activated protein kinases
and the c-Jun N-terminal kinase/c-Jun pathway (24,27). In
summary, EIF5A2 has the capacity to play multiple roles in
tumorigenesis and tumor progression, which are ascribed to its
complicated mechanism. To the best of our knowledge, the present
study reported for the first time, the expression type and clinical
significance of EIF5A2 in prostate cancer, which will be of great
value to link EIF5A2 and progression of prostate cancer. However,
more specimens and more in-depth experiments are needed to get a
complete picture of EIF5A2 in prostate cancer. In conclusion, the
results suggest that EIF5A2 could serve as a novel biomarker for
progression-free survival and a potential therapeutic target for
the treatment of PCa, but this needs to be investigated
further.
Acknowledgements
Not applicable.
Funding
The present study was funded by Guangdong Provincial
Science and Technology Foundation (Grant no. 2015A030310117).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF and JHL designed the current study. JL, HWZ and
YC performed the experiments and collected the data. JHW, ZHC and
ZHF analyzed the data. YH and WC analyzed the data and prepares the
manuscript. JL, HZ and YC contributed equally to this work.
Ethics approval and consent to
participate
All the samples were collected with the patient's
written informed consent after approval from the Institute Research
Medical Ethics Committee of the Hospitals.
Patient consent for publication
Written informed consent for participation and
publication was obtained from all patients prior to enrollment in
the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR, Humphrey PA and Grading Committee: The 2014
international society of urological pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI
|
|
5
|
Benne R, Brown-Luedi ML and Hershey JW:
Purification and characterization of protein synthesis initiation
factors eIF-1, eIF-4C, eIF-4D, and eIF-5 from rabbit reticulocytes.
J Biol Chem. 253:3070–3077. 1978.PubMed/NCBI
|
|
6
|
Wang Z, McGlynn KA, Rajpert-De ME, Bishop
DT, Chung CC, Dalgaard MD, Greene MH, Gupta R, Grotmol T, Haugen
TB, et al: Meta-analysis of five genome-wide association studies
identifies multiple new loci associated with testicular germ cell
tumor. Nat Genet. 49:1141–1147. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davidson MA and Shanks EJ: 3q26-29
Amplification in head and neck squamous cell carcinoma: A review of
established and prospective oncogenes. FEBS J. 284:2705–2731. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jenkins ZA, Haag PG and Johansson HE:
Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved
vertebrate variant of eukaryotic translation initiation factor 5A
with tissue-specific expression. Genomics. 71:101–109. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang FW, Guan XY and Xie D: Roles of
eukaryotic initiation factor 5A2 in human cancer. Int J Biol Sci.
9:1013–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathews MB and Hershey JW: The translation
factor eIF5A and human cancer. Biochim Biophys Acta. 1849:836–844.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei JH, Cao JZ, Zhang D, Liao B, Zhong WM,
Lu J, Zhao HW, Zhang JX, Tong ZT, Fan S, et al: EIF5A2 predicts
outcome in localised invasive bladder cancer and promotes bladder
cancer cell aggressiveness in vitro and in vivo. Br J Cancer.
110:1767–1777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou B, Fan J, Wang K, Chen W, Zhou X,
Zhang J, Lin S, Lv F and Chen Y: N1-guanyl-1,7-diaminoheptane (GC7)
enhances the therapeutic efficacy of doxorubicin by inhibiting
activation of eukaryotic translation initiation factor 5A2 (eIF5A2)
and preventing the epithelial-mesenchymal transition in
hepatocellular carcinoma cells. Exp Cell Res. 319:2708–2717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Liu R, Fu P, Du F, Hong Y, Yao M,
Zhang X and Zheng S: N1-Guanyl-1,7-diaminoheptane sensitizes
estrogen receptor negative breast cancer cells to doxorubicin by
preventing epithelial-mesenchymal transition through inhibition of
eukaryotic translation initiation factor 5A2 activation. Cell
Physiol Biochem. 36:2494–2503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer. (New York, NY). 2010.
|
|
15
|
Yang GF, Xie D, Liu JH, Luo JH, Li LJ, Hua
WF, Wu HM, Kung HF, Zeng YX and Guan XY: Expression and
amplification of eIF-5A2 in human epithelial ovarian tumors and
overexpression of EIF-5A2 is a new independent predictor of outcome
in patients with ovarian carcinoma. Gynecol Oncol. 112:314–318.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang PY, Zeng TT, Ban X, Li MQ, Zhang BZ,
Zhu YH, Hua WF, Mai HQ, Zhang L, Guan XY and Li Y: Expression of
EIF5A2 associates with poor survival of nasopharyngeal carcinoma
patients treated with induction chemotherapy. BMC Cancer.
16:6692016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ,
Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, et al: Overexpression
of EIF5A2 promotes colorectal carcinoma cell aggressiveness by
upregulating MTA1 through C-myc to induce
epithelial-mesenchymaltransition. Gut. 61:562–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu
L, Lau SH, Li Y, Li Y and Guan XY: Overexpression of eukaryotic
initiation factor 5A2 enhances cell motility and promotes tumor
metastasis in hepatocellular carcinoma. Hepatology. 51:1255–1263.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng QB, Kang WM, Yu JC, Liu YQ, Ma ZQ,
Zhou L, Cui QC and Zhou WX: Overexpression of eukaryotic
translation initiation factor 5A2 (EIF5A2) correlates with cell
aggressiveness and poor survival in gastric cancer. PLoS One.
10:e1192292015.
|
|
20
|
He LR, Zhao HY, Li BK, Liu YH, Liu MZ,
Guan XY, Bian XW, Zeng YX and Xie D: Overexpression of eIF5A-2 is
an adverse prognostic marker of survival in stage I non-small cell
lung cancer patients. Int J Cancer. 129:143–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Ma Y, Xie C, Wu Z, Kang Z, Fang Z,
Su B and Guan M: EphA6 promotes angiogenesis and prostate cancer
metastasis and is associated with human prostate cancer
progression. Oncotarget. 6:22587–22597. 2015.PubMed/NCBI
|
|
22
|
Yang SS, Gao Y, Wang DY, Xia BR, Liu YD,
Qin Y, Ning XM, Li GY, Hao LX, Xiao M and Zhang YY: Overexpression
of eukaryotic initiation factor 5A2 (EIF5A2) is associated with
cancer progression and poor prognosis in patients with early-stage
cervical cancer. Histopathology. 69:276–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao TT, Lin SH, Fu L, Tang Z, Che CM,
Zhang LY, Ming XY, Liu TF, Tang XM, Tan BB, et al: Eukaryotic
translation initiation factor 5A2 promotes metabolic reprogramming
in hepatocellular carcinoma cells. Carcinogenesis. 38:94–104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang FW, Cai MY, Mai SJ, Chen JW, Bai HY,
Li Y, Liao YJ, Li CP, Tian XP, Kung HF, et al: Ablation of EIF5A2
induces tumor vasculature remodeling and improves tumor response to
chemotherapy via regulation of matrix metalloproteinase 2
expression. Oncotarget. 5:6716–6733. 2014.PubMed/NCBI
|
|
25
|
Bai HY, Liao YJ, Cai MY, Ma NF, Zhang Q,
Chen JW, Zhang JX, Wang FW, Wang CY, Chen WH, et al: Eukaryotic
initiation factor 5A2 contributes to the maintenance of CD133(+)
hepatocellular carcinoma cells via the c-Myc/microRNA-29b axis.
Stem Cells. 36:180–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Li XD, Zhou Y, Ban X, Zeng TT, Li
L, Zhang BZ, Yun J, Xie D, Guan XY and Li Y: Stemness and
chemotherapeutic drug resistance induced by EIF5A2 overexpression
in esophageal squamous cell carcinoma. Oncotarget. 6:26079–26089.
2015.PubMed/NCBI
|
|
27
|
Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J,
Zeng ZL, Chen J, Cao TT, Ban X, et al: Increased expression of
EIF5A2, via hypoxia or gene amplification, contributes to
metastasis and angiogenesis of esophageal squamous cell carcinoma.
Gastroenterology. 146:1701–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Jiang R, Cui EH, Feng WM, Guo HH,
Gu DH, Tang CW, Xue T and Bao Y: N1-guanyl-1,7-diaminoheptane
enhances the chemosensitivity of NSCLC cells to cetuximab through
inhibition of eukaryotic translation initiation factor 5A2
activation. Eur Rev Med Pharmacol Sci. 20:1244–1250.
2016.PubMed/NCBI
|
|
29
|
Xu G, Shao G, Pan Q, Sun L, Zheng D, Li M,
Li N, Shi H and Ni Y: MicroRNA-9 regulates non-small cell lung
cancer cell invasion and migration by targeting eukaryotic
translation initiation factor 5A2. Am J Transl Res. 9:478–488.
2017.PubMed/NCBI
|
|
30
|
Bao Y, Lu Y, Wang X, Feng W, Sun X, Guo H,
Tang C, Zhang X, Shi Q and Yu H: Eukaryotic translation initiation
factor 5A2 (eIF5A2) regulates chemoresistance in colorectal cancer
through epithelial mesenchymal transition. Cancer Cell Int.
15:1092015. View Article : Google Scholar : PubMed/NCBI
|