Introduction
Coronary artery bypass grafting (CABG) has become
one of the most common and effective strategies for managing
coronary heart disease in the world (1). Unfortunately, CABG can induce ischemic
injury, especially for patients having poor cardiac contractile
function (2). Intervention methods,
for example, anesthetics, before and after myocardial ischemia,
enables to lessen myocardial ischemic damage to some extent
(3,4). Importantly, both sevoflurane and
propofol are frequently used anesthetics in clinical settings
(5,6). Sevoflurane, as an inhalation
anesthetic, plays a myocardial protective role on low risk patients
treated with CABG (7). Sevoflurane
has been found to exert a more remarkable effect on gene expression
in patients undergoing CABG compared with propofol (8). The myocardial protection effect of
sevoflurane might be achieved by downregulating platelet
endothelial cell adhesion molecule-1 (9) and/or troponin I (10). On the contrary, propofol has
performed better in cardiovascular instability, severe ischemia or
acute/urgent surgery patients (11).
Additionally, intravenous anesthetics propofol has been implicated
to attenuate myocardial lipid peroxidation and systemic
inflammation in CABG surgery (12,13).
Growing evidence has demonstrated that sevoflurane as well as
propofol exert important functions in cardio protection.
Nevertheless, the potential cardio-protection mechanisms of these
anaesthetics still remain unclear, and which anesthetic is
appropriate also needs to be investigated.
Microarray profiles provide a basic starting point
in the detection of mechanistic causes. One standard approach is
extracting differentially expressed genes (DEGs). However, this
approach provides only limited information on the biological role
of the DEGs. Moreover, there is frequently little overlap between
microarray studies (14,15), but pathway analysis can conquer the
weaknesses of the current single-locus approaches. It has been
demonstrated that pathway-based analysis has the ability of
enhancing power and robustness, as well as extracting biological
interaction among gene pairs (16,17).
Investigating biological pathways based on system biology
techniques can provide comprehensive insights into the components
of pathways, thereby facilitating the inference of novel targets
for diseases (18). It has been
demonstrated that network of gene-gene functional interactions is
useful in predicting biological functions (19,20).
There are many approaches to predict function based on gene-gene
networks. Significantly, the most common method used in the studies
is some variation of the guilt by association (GBA) (21,22). It
has been suggested that GBA can predict pathways in all kinds of
biological networks such as gene co-expression network (19).
Hence, in the present study, in order to provide the
foundation to select the appropriate anesthetic, network-based
method and GBA principle were used to further identify the optimal
pathways using the known pathway data and microarray profile.
E-GEOD-4386 data were used to identify DEGs. Then, a differential
co-expressed network (DCN) was constructed using DEGs, and sub-DCN
was extracted relying on the weight values which were computed
using Spearman's correlation coefficient (SCC). The KEGG pathways
for CABG were collected on the basis of the known confirmed
database as well as DEGs and the seed pathways were predicted
through GBA principle according to the area under the curve (AUC)
of pathway categories, and the pathway terms with AUC >0.9 were
defined as the seed pathways. KEGG pathway enrichment analysis was
conducted for the DEGs based on the DAVID tool to detect the
significant pathways. The final optimal pathways were identified
based on the traditional pathway analysis and network-based pathway
inference approach. These pathways may be helpful for the
appropriate selection of propofol or sevoflurane, thus improving
the clinical outcomes of patients undergoing CABG surgery.
Materials and methods
Gene expression profile and
pre-treatment
The expression data of E-GEOD-4386 was downloaded
from the A-AFFY-44 - Affymetrix GeneChip Human Genome U133 Plus 2.0
platform of ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/). The E-GEOD-4386
dataset includes data of 40 samples: 10 patients who underwent CABG
with intravenous anesthetic propofol treatment, 10 patients who
underwent CABG with anesthetic gas sevoflurane treatment and 20
baseline samples (23). All the
patients underwent the same procedure except for the anesthesia.
The baseline samples were of the same patients before CABG. The
atrial samples were obtained prior to as well as after CABG to
investigate gene expression. The microarray profiling of the 40
atrial samples were downloaded to further detect DEGs.
Before analysis, E-GEOD-4386 was pre-processed,
including background correction using robust multi-array average
(RMA) (24), quartile normalization
(25), and perfect match
(PM)/mismatch (MM) correction by means of MAS 5.0 package (26). Finally, probe data were mapped to
human gene symbols using annotate package (27). Finally, 20,514 genes were obtained
for further investigation.
Identification of DEGs
The LIMMA package (28) with t-test was used in our work to
compare the gene data before and after sevoflurane or propofol
treatment to further achieve DEGs between two samples. SPSS 17.0
(SPSS, Inc., Chicago, IL, USA) was used to process the raw data
through Log2 transformation. Then, multiple test was used to adjust
the original P-values relying on Benjamini & Hochberg (29) according to false discovery rate
(FDR). The cut-off criteria of DEGs were regarded to be FDR
<0.05 as well as |log fold change (FC)| ≥0.5.
Establishment of DCN
After identification of DEGs, the construction of
DCN was performed based on DEGs using Cytoscape software. Then, SCC
was applied to measure the co-expressed strength of each edge in
the DCN. In the current study, the absolute value of SCC of one
edge was defined as the weight of the corresponding interaction.
The greater the weight score was, the more relevant the interaction
was to the disease or clinical effect. Hence, the edges having
weight scores >0.8 were selected to establish the sub-DCN which
was also visualized using Cytoscape software.
KEGG annotation for DEGs
KEGG provides a reference knowledge base for better
understanding the biological processes. In the current study,
overall 300 background pathways covering 6,919 genes were collected
from KEGG database. Next, the identified DEGs were mapped to the
300 pathway terms to extract the DEG-related pathways. Finally, the
pathway slim set was required in propofol treatment, and
sevoflurane treatment groups, consisting of 87 DEGs and 64 pathways
in propofol treatment, and 182 DEGs as well as 84 pathways in
sevoflurane group.
Seed pathways using ‘GBA’
prediction
Then, GBA method was employed for the DCN to further
predict significant biological pathways in patients undergoing CABG
procedure. In detail, 3-fold cross-validation was utilized to
obtain a gene list sorted using the ranked scoring in the DCN as to
how these genes participated in the known pathway categories. With
regard to every gene of the DCN, we mapped all neighbouring genes
of this gene to each pathway term, and we then calculated the
multifunctionality (MF) value for each gene enriched in the pathway
term.
Afterwards, we calculated the AUC value for each
pathway category using support vector machine (SVM), and the mean
value of the AUC across all pathway categories was obtained. Thus,
the pathway terms were ranked relying on the AUC scores. In the
literature related to the gene functions, AUC values >0.7 were
considered good (30). In this
study, the pathway terms of AUC >0.9 were regarded to be the
seed pathways.
Pathway analyses for DEGs using
DAVID
KEGG provides a reference knowledge base for better
understanding biological processes. DAVID (http://david.abcc.ncifcrf.gov/) is an analytical tool
used to analyze a large number of genes (31). To further explore the biological
functions of DEGs, DAVID was utilized to conduct the traditional
pathway analysis using the Expression Analysis Systematic Explorer
(EASE) test (32) based on KEGG
pathway database. The significant pathways were extracted when FDR
was set at 0.001, and gene count >5.
Results
Sevoflurane influences more DEGs
compared with propofol
Before DCN construction, DEGs in propofol and
sevoflurane treatment were screened out. When the threshold was set
at FDR <0.05 and |log FC| ≥0.5, compared with the baseline
group, there were respectively 87 and 182 DEGs in patients treated
with propofol and sevoflurane (Fig.
1). The expression levels of the DEGs in propofol and
sevoflurane groups are available in Tables SI and SII, respectively (supplementary material).
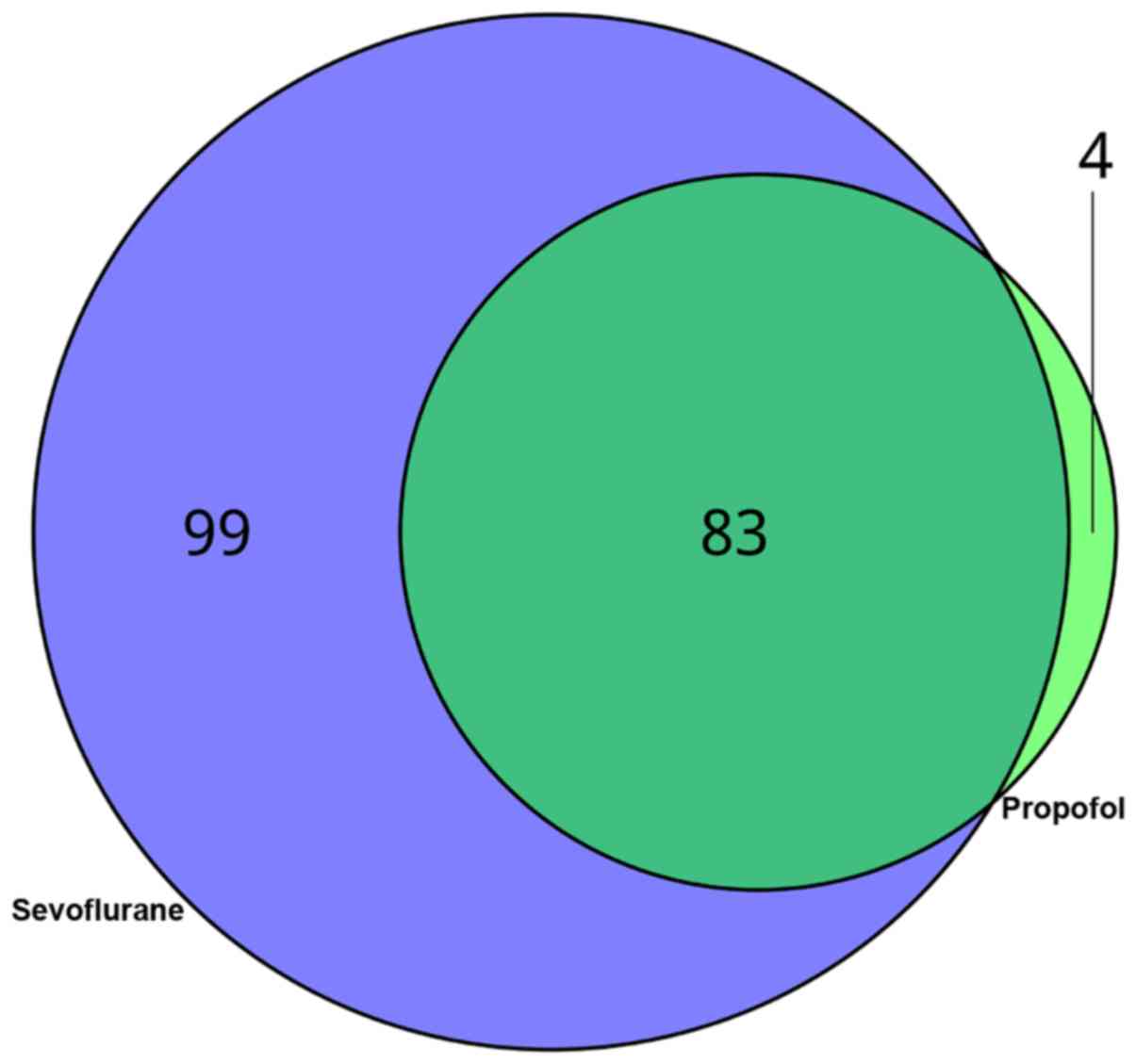
Then, it was found that 83 DEGs were common between the two groups.
Significantly, 99 DEGs were unique to sevoflurane treatment, while
4 DEGs were specific to the patients who received propofol
treatment.
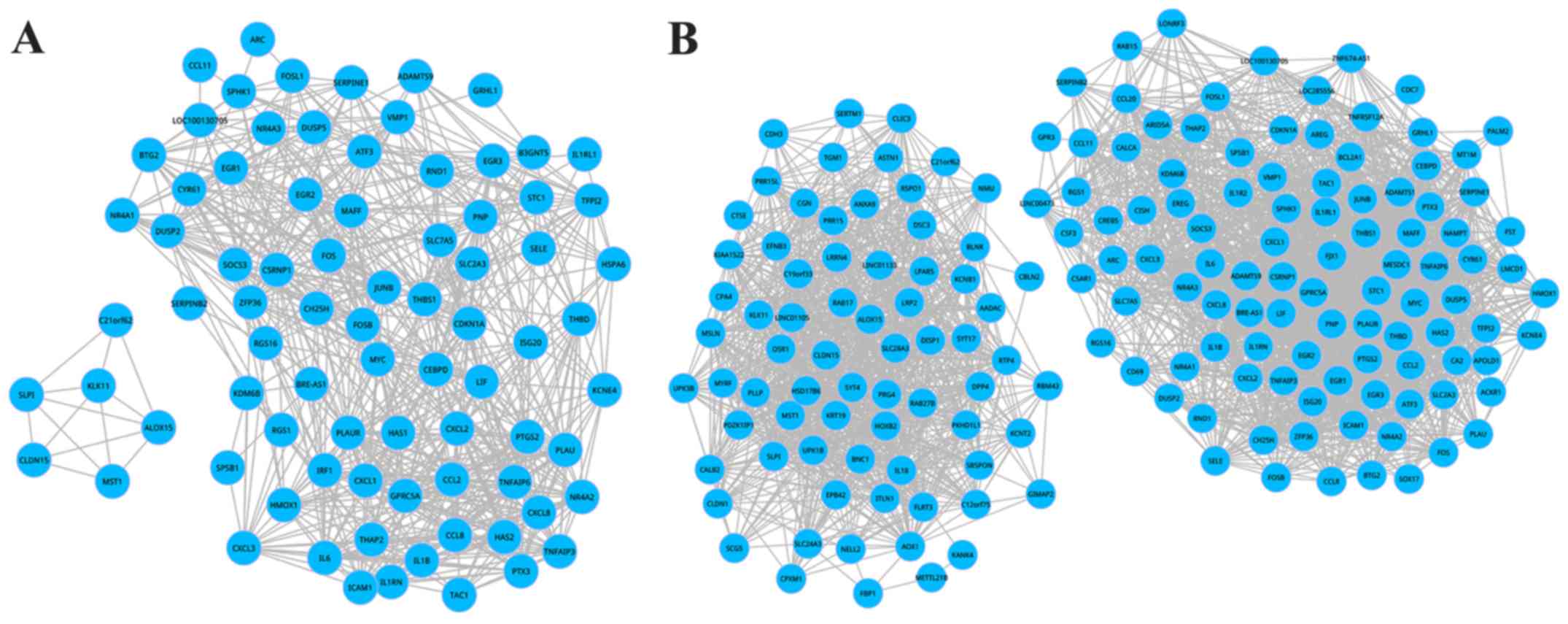
To further reveal the DEG-biological activities, a
DCN was established for propofol and sevoflurane groups relying on
the above-identified DEGs. There were 87 nodes within the DCN of
propofol group and 182 DEGs in the DCN of the sevoflurane group.
These demonstrated that all DEGs were mapped to the DCN. In the
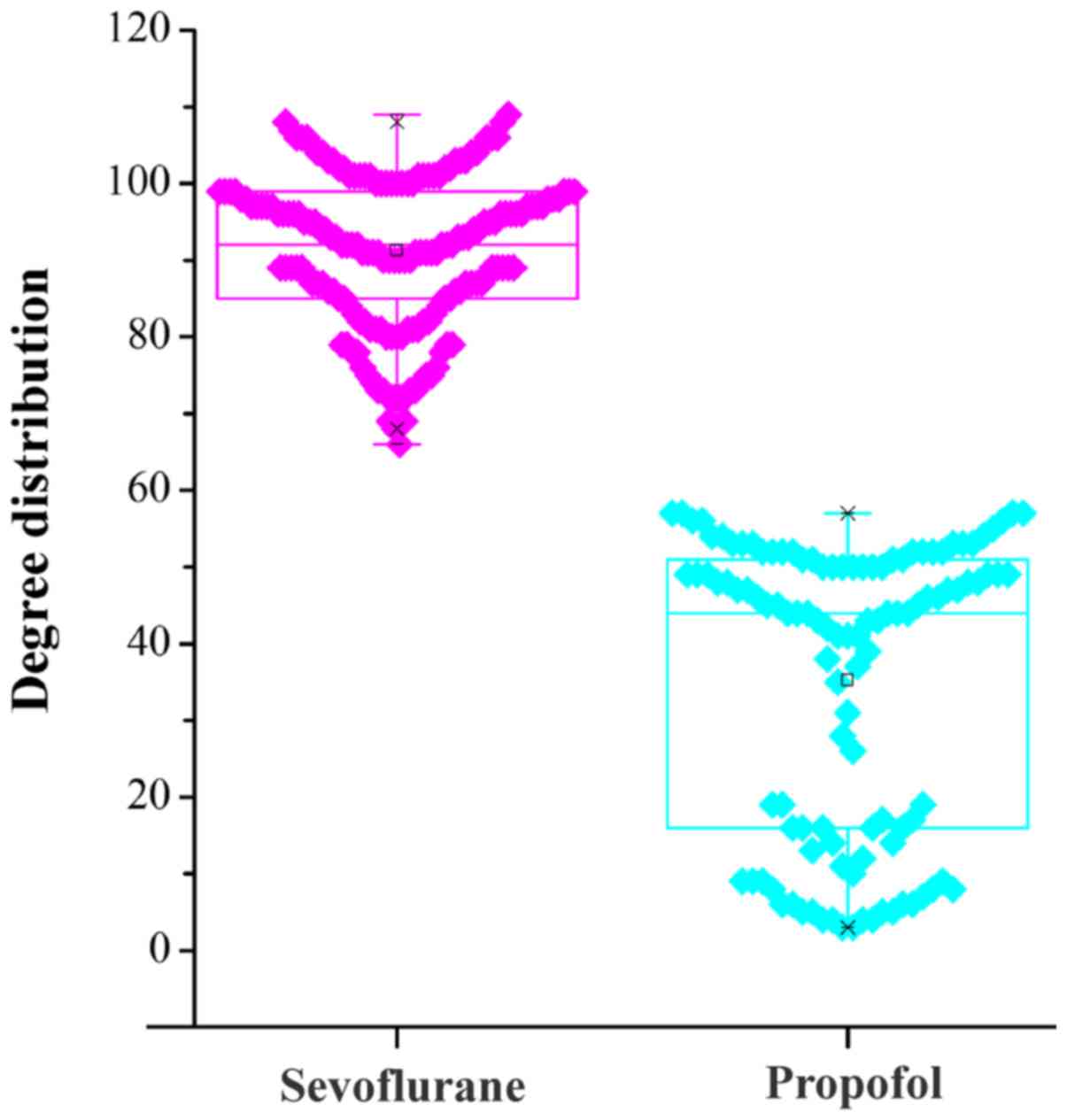
network, degree can explain the network structure. Thus, we
analyzed the topological degree for each node in the DCN, and the
degree distribution for each node is shown in Fig. 2. The degrees in sevoflurane group
were higher than those in propofol group. Then, SCC was utilized to
calculate the weight score of each edge, and the interactions
having weight values >0.8 were selected to build the sub-DCN
(Fig. 3). Overall, 81 nodes and 728
interactions were involved in the sub-DCN of the propofol group,
and 175 nodes and 3,286 interactions were in the sub-DCN of the
sevoflurane group.
Seed pathways using GBA
prediction
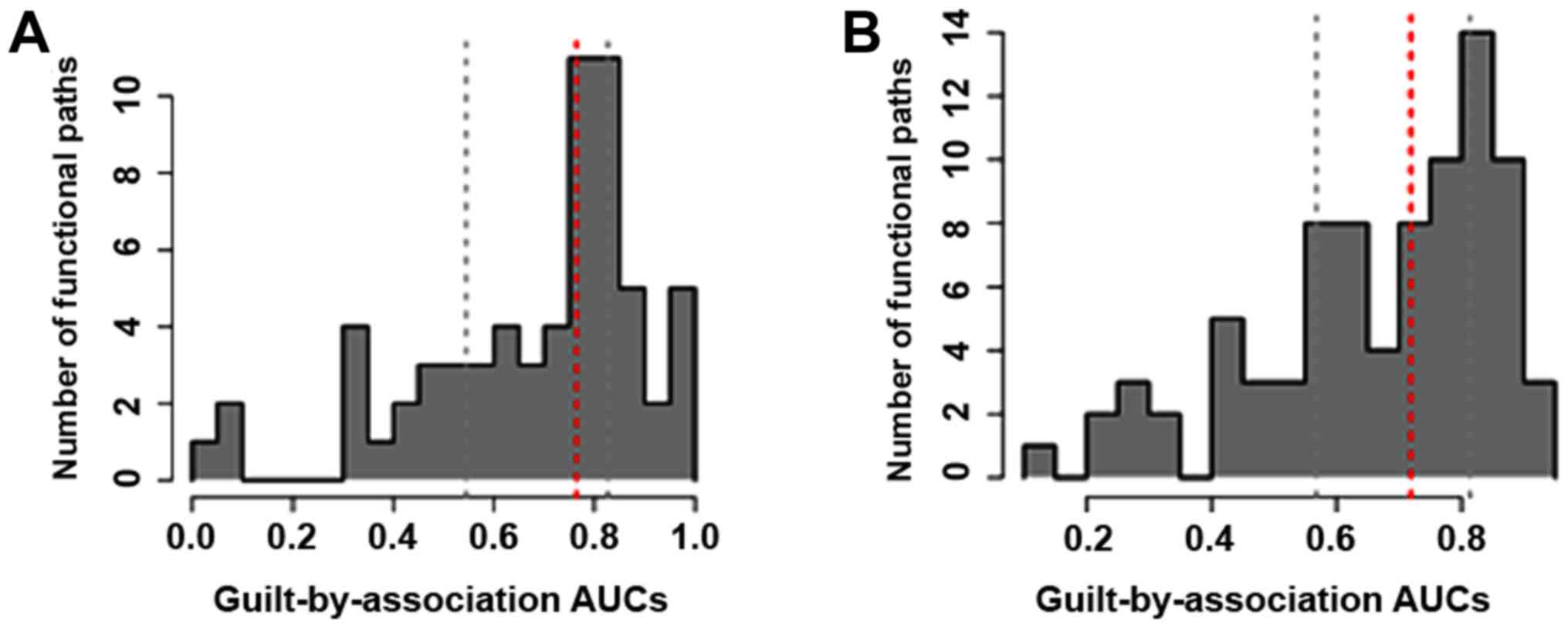
The AUC distribution for pathway terms is displayed
in Fig. 4. The AUC for most pathways
ranged from 0.4 to 0.8 in these two groups. Together, 38 and 28
pathway terms were respectively identified in the propofol and
sevoflurane groups based on AUC >0.7. Significantly, among these
pathways, the AUC value of 8 pathways in the propofol group and 4
pathways in the sevoflurane group was higher than 0.9, and these
pathways were determined as the seed pathways. Specific information
on the seed pathways is listed in Table
I.
 | Table I.The seed pathways in the propofol and
sevoflurane groups. |
Table I.
The seed pathways in the propofol and
sevoflurane groups.
| Propofol-specific
pathways | AUC |
Sevoflurane-specific pathways | AUC |
|---|
| hsa04064:NF-κB
signaling pathway | 0.9921 |
hsa04060:Cytokine-cytokine receptor
interaction | 0.9315 |
| hsa04623-Cytosolic
DNA-sensing | 0.9921 | hsa04064:NF-κB
signaling pathway | 0.9249 |
|
hsa05321-inflammatory bowel disease | 0.9922 | hsa04621:NOD-like
receptor signaling pathway | 0.9181 |
|
hsa05332-graft-versus-host disease | 0.9843 | hsa04668:TNF
signaling pathway | 0.9111 |
|
hsa05133-Pertussis | 0.9715 |
|
|
| hsa05203-Viral
carcinogenesis | 0.9325 |
|
|
| hsa04621-NOD-like
receptor signaling pathway | 0.9104 |
|
|
| hsa04668:TNF
signaling pathway | 0.9002 |
|
|
Pathway enrichment analysis for
DEGs
Relying on FDR <0.001 as well as gene count
>5, 8 significant pathways were identified in the propofol
group, and 10 significant pathways were detected in the sevoflurane
group. The list of differential pathways in the two groups is shown
in Table II. From the Table, we
found that most of the differential pathways were the same in these
two groups (NF-κB signaling pathway, NOD-like receptor signaling
pathway and TNF signaling pathway). Only the pathway of
cytokine-cytokine receptor interaction, and African trypanosomiasis
were unique to sevoflurane. However, no pathway was unique to
propofol.
 | Table II.List of the differential pathways in
the propofol and sevoflurane groups. |
Table II.
List of the differential pathways in
the propofol and sevoflurane groups.
| Propofol-specific
differential pathways | FDR | Gene count |
Sevoflurane-specific pathways | FDR | Gene count |
|---|
| hsa04668:TNF
signaling pathway | 2.04E-13 | 14 | hsa04668:TNF
signaling pathway | 4.38E-13 | 16 |
|
hsa05144:Malaria | 1.03E-06 | 7 |
hsa05144:Malaria | 2.65E-09 | 10 |
|
hsa05134:Legionellosis | 1.85E-06 | 7 |
hsa05134:Legionellosis | 2.86E-05 | 7 |
| hsa05132:Salmonella
infection | 2.32E-05 | 7 | hsa05132:Salmonella
infection | 3.71E-05 | 8 |
| hsa05166:HTLV–I
infection | 6.40E-05 | 10 |
hsa04060:Cytokine-cytokine receptor
interaction | 4.76E-05 | 12 |
| hsa04064:NF-κB
signaling pathway | 3.48E-04 | 6 | hsa04064:NF-κB
signaling pathway | 5.04E-05 | 8 |
| hsa05323:Rheumatoid
arthritis | 3.67E-04 | 6 | hsa05323:Rheumatoid
arthritis | 5.42E-05 | 8 |
| hsa04621:NOD-like
receptor signaling pathway | 5.84E-04 | 7 | hsa04621:NOD-like
receptor signaling pathway | 3.60E-04 | 6 |
|
|
| 5 | hsa05143:African
trypanosomiasis | 4.80E-04 | 6 |
|
|
|
| hsa05166:HTLV–I
infection | 5.57E-04 | 11 |
Identifying the optimal pathways
The final optimal pathways were identified based on
the traditional pathway analysis and network-based pathway
inference approach. Finally, 3 optimal pathways were identified in
the propofol group, including NF-κB signaling pathway, TNF
signaling pathway, and NOD-like receptor signaling pathway.
Moreover, 4 optimal pathways were identified in the sevoflurane
group, including cytokine-cytokine receptor interaction, NOD-like
receptor signaling pathway, NF-κB signaling pathway, and TNF
signaling pathway. Based on these results, we observed that
NOD-like receptor signaling pathway, NF-κB signaling pathway, and
TNF signaling pathway is the common optimal one in these two
groups. Cytokine-cytokine receptor interaction was unique to
sevoflurane.
Discussion
Previous studies have reported that sevoflurane and
propofol are the two most common and effective anesthetic agents in
CABG surgery (33). Nevertheless,
the underlying cardio-protection mechanisms of the two anaesthetics
still remain unclear, and which anesthetic is appropriate also need
to be investigated. Compared to studying single gene biomarkers,
investigating biological functions appears more promising in
understanding the disease-related insights (34). Network analysis has been broadly
utilized in many diseases to comprehend the biological processes of
diseases, and to further obtain clinical insights (35). In recent years, GBA method has been
proposed to predict gene functions based on the indirect
connections (36,37). Nevertheless, integration of
gene-related pathway prediction and network modeling are sparse.
Therefore, herein, GBA method combined with DCN-based analysis were
used to further investigate the optimal pathways for patients
treated by CABG surgery plus propofol or sevoflurane management,
relying on the known KEGG pathway data and microarray profile. In
the current work, 83 common, 99 sevoflurane-specific as well as 4
propofol-specific DEGs from the expression profile of atrial
samples were found.
Finally, there were 8 and 4 pathway terms having AUC
>0.9 and these pathways were considered as the seed pathways.
Traditional pathway analysis demonstrated that most of the
differential pathways were the same in these two groups. Only
cytokine-cytokine receptor interaction was unique to sevoflurane,
and no pathway was specific to propofol.
The pathways of NF-κB signaling pathway, TNF
signaling pathway, and NOD-like receptor signaling were common in
the protofol and sevoflurane groups, which were associated with
immune responses. Myocardial ischaemia activates NF-κB causing the
generation of inflammatory mediators such as TNF-α and IL-1
(38). Furthermore, sevoflurane has
been demonstrated to reduce the levels of inflammatory cytokines
and to inhibit NF-κB activation (39). Moreover, inhibiting NF-κB has been
suggested to provide the protection against myocardial ischaemia in
sevoflurane preconditioning (40).
Another study has reported that propofol can effectively attenuate
the inflammation through NF-κB signal pathway (41). NOD-like receptor is involved in the
innate immunity and inflammation (42,43). It
has been suggested that cardiac surgery produces a systemic
inflammatory response, which was caused by surgical trauma
(44). Consequently, understanding
the inflammatory condition of patients prior to CABG surgery is
crucially significant to reduce the postoperative
complications.
The pathway of cytokine-cytokine receptor
interaction was unique to sevoflurane in our study. Another study
also has demonstrated that the pathway associated with
cytokine-cytokine receptor interaction is unique to sevoflurane
(8). Cytokines are a group of
molecules that transmit intercellular signals, and they induce
responses through binding to specific receptors on the cell surface
(45). Moreover, injured endothelial
cells give rise to cytokines (for example, interleukin) that
stimulate the expression of adhesion proteins and cell adhesion
molecules (46). It has been
implicated that elevated level of IL-8 is related to an increased
risk of coronary artery disease (47). Accordingly, this result further meant
that the anesthetic sevoflurane might provide the patients with
more protection in inflammatory diseases.
Of course, there were several limitations in this
study. Limited samples were used to predict the pathway biomarkers,
which might result in biased estimates. Moreover, given that only
bioinformatics methods were used in our study, the conclusions have
not been confirmed based on any lab experiment. Despite these
shortcomings, our study provided important implications for the
molecular mechanisms of cardio-protection of sevoflurane and
propofol in CABG surgery, but a further study is needed to validate
our findings relying on lab techniques.
In the current work, the data were recruited from
the E-GEOD-4386 which was produced by Lucchinetti et al
(23). Based on the study of
Lucchinetti et al, DEGs between the two groups were detected
in our study, and pathway enrichment analyses were also conducted
to examine the underlying mechanism of the two anaesthetic agents.
Nevertheless, certain discrepancies also exist. Few studies have
identified potential pathways based on the traditional pathway
analysis and network-based pathway inference approach using AUC
indicator. According to the study of Lucchinetti et al,
further analyses relying on bioinformatics were implemented in the
current study, including the construction of DCN, seed pathway
identification, and AUC calculation. Although Bu et al
(48) and Li et al (8) have also analyzed the effect of
sevoflurane and propofol on gene expression based on the
E-GEOD-4386 dataset, the methods used and the outcomes in their
research are different from our study. A GBA method combined with
DCN-based analysis was used to identify optimal pathways in our
study, while Bu et al (48)
used module topological analysis to evaluate significant
pathway-related modules and Li et al (8) only identified the DEGs and performed GO
and KEGG pathway enrichment analyses for DEGs. Compared with the
previous studies, some novel seed pathways were found in our study,
such as NF-κB signaling pathway, inflammatory bowel disease,
graft-versus-host disease and TNF signaling pathway. Hence, our
results provide new insights into the understanding of
cardio-protection mechanisms of sevoflurane and propofol.
Taken together, sevoflurane and propofol might
synergistically decrease myocardial reperfusion injury of patients
treated by CABG, because similarity and particularity were all
found in the pathway alterations caused by propofol and
sevoflurane. Our present study deepens the understanding of cardio
protective mechanism of sevoflurane and propofol. The optimal
pathways in our study may be helpful for the appropriate selection
of propofol or sevoflurane, thus promoting improvement in the
clinical outcomes of patients undergoing CABG surgery. Further
efforts will be made to investigate the underlying
cardio-protection mechanisms of anaesthetics in animal models.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZGP and YJD designed this research. ZGP and XZZ
collected the data and prepared the figures. ZGP and ZMZ analyzed
the data. ZGP wrote the manuscript. YJD contributed substantially
to its revision. All the authors have read and approved this
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rezaianzadeh A, Maghsoudi B, Tabatabaee H,
Keshavarzi S, Bagheri Z, Sajedianfard J, Gerami H and Rasouli J:
Factors associated with extubation time in coronary artery bypass
grafting patients. PeerJ. 3:e14142015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Selvanayagam JB, Petersen SE, Francis JM,
Robson MD, Kardos A, Neubauer S and Taggart DP: Effects of off-pump
versus on-pump coronary surgery on reversible and irreversible
myocardial injury: A randomized trial using cardiovascular magnetic
resonance imaging and biochemical markers. Circulation.
109:345–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varadarajan SG, An J, Novalija E and Stowe
DF: Sevoflurane before or after ischemia improves contractile and
metabolic function while reducing myoplasmic Ca(2+) loading in
intact hearts. Anesthesiology. 96:125–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frässdorf J, De Hert S and Schlack W:
Anaesthesia and myocardial ischaemia/reperfusion injury. Br J
Anaesth. 103:89–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hellström J, Öwall A, Bergström J and
Sackey PV: Cardiac outcome after sevoflurane versus propofol
sedation following coronary bypass surgery: A pilot study. Acta
Anaesthesiol Scand. 55:460–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zheng H, Chen CL, Lu W and Zhang
YQ: Sevoflurane at 1 MAC provides optimal myocardial protection
during off-pump CABG. Scand Cardiovasc J. 47:175–184. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin E and Symons JA: Volatile anaesthetic
myocardial protection: A review of the current literature. HSR Proc
Intensive Care Cardiovasc Anesth. 2:105–109. 2010.PubMed/NCBI
|
|
8
|
Li H, Cang J and Zhang X: Sevoflurane
exerts a more marked influence compared with propofol on gene
expression in patients undergoing coronary artery bypass graft
surgery. Exp Ther Med. 11:448–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia C, Julier K, Bestmann L, Zollinger
A, von Segesser LK, Pasch T, Spahn DR and Zaugg M: Preconditioning
with sevoflurane decreases PECAM-1 expression and improves one-year
cardiovascular outcome in coronary artery bypass graft surgery. Br
J Anaesth. 94:159–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao YT and Li LH: Sevoflurane versus
propofol for myocardial protection in patients undergoing coronary
artery bypass grafting surgery: A meta-analysis of randomized
controlled trials. Chin Med Sci J. 24:133–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jakobsen CJ, Berg H, Hindsholm KB, Faddy N
and Sloth E: The influence of propofol versus sevoflurane
anesthesia on outcome in 10,535 cardiac surgical procedures. J
Cardiothorac Vasc Anesth. 21:664–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sayin MM, Özatamer O, Taşöz R, Kilinç K
and Ünal N: Propofol attenuates myocardial lipid peroxidation
during coronary artery bypass grafting surgery. Br J Anaesth.
89:242–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corcoran TB, Engel A, Sakamoto H, O'Shea
A, O'Callaghan-Enright S and Shorten GD: The effects of propofol on
neutrophil function, lipid peroxidation and inflammatory response
during elective coronary artery bypass grafting in patients with
impaired ventricular function. Br J Anaesth. 97:825–831. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louwen F, Muschol-Steinmetz C, Reinhard J,
Reitter A and Yuan J: A lesson for cancer research: Placental
microarray gene analysis in preeclampsia. Oncotarget. 3:759–773.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES, et al: Gene set enrichment analysis: a
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tilford CA and Siemers NO: Gene set
enrichment analysis. Methods Mol Biol. 563:99–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Curtis RK, Oresic M and Vidal-Puig A:
Pathways to the analysis of microarray data. Trends Biotechnol.
23:429–435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin T: Inferring biological knowledge of
pathways from an ontology fingerprint-derived gene network. PhD
dissertation, Medical University of South Carolina. Publication no
AAI3569828 (Charleston, SC, USA). 2012.
|
|
19
|
Peña-Castillo L, Tasan M, Myers CL, Lee H,
Joshi T, Zhang C, Guan Y, Leone M, Pagnani A, Kim WK, et al: A
critical assessment of Mus musculus gene function prediction using
integrated genomic evidence. Genome Biol. 9 (Suppl 1):S22008.
View Article : Google Scholar
|
|
20
|
Mostafavi S, Ray D, Warde-Farley D,
Grouios C and Morris Q: GeneMANIA: A real-time multiple association
network integration algorithm for predicting gene function. Genome
Biol. 9 (Suppl 1):S42008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharan R, Ulitsky I and Shamir R:
Network-based prediction of protein function. Mol Syst Biol. 3:88.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zuberi K, Franz M, Rodriguez H, Montojo J,
Lopes CT, Bader GD and Morris Q: GeneMANIA prediction server 2013
update. Nucleic Acids Res. 41:W115–W122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lucchinetti E, Hofer C, Bestmann L,
Hersberger M, Feng J, Zhu M, Furrer L, Schaub MC, Tavakoli R,
Genoni M, et al: Gene regulatory control of myocardial energy
metabolism predicts postoperative cardiac function in patients
undergoing off-pump coronary artery bypass graft surgery:
Inhalational versus intravenous anesthetics. Anesthesiology.
106:444–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bolstad BM, Irizarry RA, Åstrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pepper SD, Saunders EK, Edwards LE, Wilson
CL and Miller CJ: The utility of MAS5 expression summary and
detection call algorithms. BMC Bioinformatics. 8:2732007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu LJ, Gazin C, Lawson ND, Pagès H, Lin
SM, Lapointe DS and Green MR: ChIPpeakAnno: A bioconductor package
to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics.
11:2372010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smyth GK: Limma: linear models for
microarray data. Bioinformatics and Computational Biology Solutions
Using R and Bioconductor. Gentleman R, Carey VJ, Huber W, Irizarry
RA and Dudoit S: Statistics for Biology and Health. (Springer, New
York, NY). 397–420. 2005. View Article : Google Scholar
|
|
29
|
Benjamini Y, Drai D, Elmer G, Kafkafi N
and Golani I: Controlling the false discovery rate in behavior
genetics research. Behav Brain Res. 125:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gillis J and Pavlidis P: The role of
indirect connections in gene networks in predicting function.
Bioinformatics. 27:1860–1866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ford G, Xu Z, Gates A, Jiang J and Ford
BD: Expression Analysis Systematic Explorer (EASE) analysis reveals
differential gene expression in permanent and transient focal
stroke rat models. Brain Res. 1071:226–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neilsen PM, Cheney KM, Li CW, Chen JD,
Cawrse JE, Schulz RB, Powell JA, Kumar R and Callen DF:
Identification of ANKRD11 as a p53 coactivator. J Cell Sci.
121:3541–3552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ponomarev I, Wang S, Zhang L, Harris RA
and Mayfield RD: Gene coexpression networks in human brain identify
epigenetic modifications in alcohol dependence. J Neurosci.
32:1884–1897. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doig TN, Hume DA, Theocharidis T, Goodlad
JR, Gregory CD and Freeman TC: Coexpression analysis of large
cancer datasets provides insight into the cellular phenotypes of
the tumour microenvironment. BMC Genomics. 14:4692013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chua HN, Sung WK and Wong L: Exploiting
indirect neighbours and topological weight to predict protein
function from protein-protein interactions. Bioinformatics.
22:1623–1630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yip AM and Horvath S: Gene network
interconnectedness and the generalized topological overlap measure.
BMC Bioinformatics. 8:222007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bowie AG and O'Neill LA: Vitamin C
inhibits NF-kappa B activation by TNF via the activation of p38
mitogen-activated protein kinase. J Immunol. 165:7180–7188. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong C, Zhou Y and Liu H: Nuclear factor
kappaB and anesthetic preconditioning during myocardial
ischemia-reperfusion. Anesthesiology. 100:540–546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Konia MR, Schaefer S and Liu H: Nuclear
factor-[kappa]B inhibition provides additional protection against
ischaemia/reperfusion injury in delayed sevoflurane
preconditioning. Eur J Anaesthesiol. 26:496–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tian Y, Guo S, Guo Y and Jian L:
Anesthetic propofol attenuates apoptosis, Aβ accumulation, and
inflammation induced by sevoflurane through NF-κB pathway in human
neuroglioma cells. Cell Mol Neurobiol. 35:891–898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen G, Shaw MH, Kim YG and Nuñez G:
NOD-like receptors: Role in innate immunity and inflammatory
disease. Annu Rev Pathol. 4:365–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanneganti TD, Lamkanfi M and Núñez G:
Intracellular NOD-like receptors in host defense and disease.
Immunity. 27:549–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Levy JH and Tanaka KA: Inflammatory
response to cardiopulmonary bypass. Ann Thorac Surg. 75:S715–S720.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ozaki K and Leonard WJ: Cytokine and
cytokine receptor pleiotropy and redundancy. J Biol Chem.
277:29355–29358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Auer J, Weber T, Berent R, Lassnig E, Lamm
G and Eber B: Genetic polymorphisms in cytokine and adhesion
molecule genes in coronary artery disease. Am J Pharmacogenomics.
3:317–328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boekholdt SM, Peters RJ, Hack CE, Day NE,
Luben R, Bingham SA, Wareham NJ, Reitsma PH and Khaw KT: IL-8
plasma concentrations and the risk of future coronary artery
disease in apparently healthy men and women: The EPIC-Norfolk
prospective population study. Arterioscler Thromb Vasc Biol.
24:1503–1508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bu X, Wang B, Wang Y, Wang Z, Gong C, Qi F
and Zhang C: Pathway-related modules involved in the application of
sevoflurane or propofol in off-pump coronary artery bypass graft
surgery. Exp Ther Med. 14:97–106. 2017. View Article : Google Scholar : PubMed/NCBI
|