Introduction
Pituitary tumors account for 10% of intracranial
cancer, which is derived from the remnant cells in anterior
pituitary, posterior pituitary and craniopharyngeal epithelium, and
represents 10–15% of all intracranial tumors. Each patient with
pituitary cancers should be given individually selected treatment
approach (1). Normally, pituitary
surgery is one of the biggest series including transcranial,
transsphenoidal, and endoscopic techniques. Transsphenoidal surgery
is usually considered as the first-choice therapy for most patients
with pituitary tumors, which is an effective and safe treatment
(2). Comprehensive therapy is
gradually important among endocrinologists, ophthalmologists, and
radiologists (3). Thus, it is
helpful to clarify the pathogenesis and progression of pituitary
tumors. The newly discovered reports are important to give us a
clue to clarify the molecular mechanism of pituitary tumors. For
example, histone deacetylases (HDAC) 11 had a key function in
multiple cellular activities and HDAC11 suppressed P53 expression
in pituitary tumor cells. Inhibition of HDAC11 had a therapeutic
potential in the treatment of pituitary tumors (4). MiRNAs were involved in regulating the
progression of pituitary tumors. MiRNA153 inhibited MMQ cell growth
and promoted cell apoptosis by down-regulating Skp protein
(5). Enhancer of zeste homolog 2
(EZH2) levels were associated with the invasiveness, proliferation,
angiogenesis, hormone function in pituitary adenomas, which could
be a useful diagnostic marker and pharmacotherapeutic target for
malignant pituitary tumors (6).
Thus, it is important to demonstrate the pathogenesis and
progression of pituitary tumors and find an effective antitumor
drug candidate for pituitary tumors.
To date, Chinese herb are widely used for thousands
of human cancers in clinical therapy. Matrine is a major active
compound and extracted from the dry roots of Sophora
flavescens, which has been reported to possess potent antitumor
activity in various human cancer cells. Matrine inhibited cell
proliferation and induced apoptosis of human colon cancer cells
in vitro by upregulating Bax, and downregulating Bcl-2 and
releasing Cyto C from the mitochondria to the cytosol and
activating caspase-3 and caspase-9 (7). This was consistent with the founding in
breast cancers, which was found that matrine inhibited the growth
and induced apoptosis of breast carcinoma MCF-7 cells (8). Moreover, matrine showed the suppression
activity in the proliferation and metastasis of highly-metastatic
breast cancer MDA-MB-231 cells via EGF/VEGF-VEGFR1-Akt-NF-kappaB
signaling pathway (9). Matrine
suppressed gastric cancer cell line MNK45 in a dose-dependent
manner and the anti-tumor activity was associated with the
modulation of the NF-kappaB, XIAP, CIAP, and p-ERK proteins
expression in MNK45 cells (10,11).
Autophagy, a new identified programmed cell death, played an
important role in tumor progression. Zhang, J. found that matrine
suppressed the proliferation of SGC-7901 gastric cancer cells and
induced G1-phase cell cycle arrest by activating both autophagy and
apoptosis in the therapy of gastric cancers (12). The microRNAs, a class of small,
non-coding, regulatory RNAs were reported to be involved in the
tumorigenesis of human cancers. In gastric cancer cell line
SGC7901, matrine treatment induced considerable changes in the
miRNA expression profiles of SGC7901 cells, which was involved in
57 identified enrichment pathways in tumorigenesis (13). In non-small lung cancer cell lines,
matrine induced the cell arrest at G1/G0 phase and cell apoptosis
by uperegulating the expression of miR126 (14).
Matrine, an alkaloid extracted from the Chinese herb
Sophora flavescens Ait, may be a promising candidate drug in
the therapy of human various cancers. However, the molecular
mechanisms of matrine in pituitary cancer cells was not clearly
clarified and the involved anti-tumor mechanism need to be further
explored.
Materials and methods
Cell lines and agents
The mouse pituitary tumor cell line AtT-20 (CRL1795,
ATCC) and rat pituitary GH3 tumor cell line RC-4B/C (cat. no:
JN-C0859; Rongbai biological corporation, Shanghai, China) were
cultured in Ham's F-10 nutrient mixture containing with 15% horse
serum (cat. no: 26050070) and 2.5% fetal bovine serum (cat. no:
10099141), which were purchased from Thermo Fisher Scientific,
Inc., Waltham, MA, USA. Rat pituitary tumor cells (cat. no: GH3
FS-C-0014) was purchased from Fengshou biological corporation
(Shanghai, China). The cells were cultured in DMEM medium
(Invitrogen; Thermo Fisher Scientific, Inc.) with 10% fetal bovine
serum (Trace Scientific Ltd., Melbourne, Australia) at 37°C in an
atmosphere of 95% air and 5% CO2. Matrine (cat. no:
HY-N0164) was obtained from MedChemExpress (Shanghai, China) with
purity of more than 99.80%. MTT agent was obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
MTT assay
Cell viability was determined by MTT assay. Briefly,
the pituitary tumor cells pituitary RC-4B/C and rat pituitary tumor
cells GH3 were plated into 96-well plate and cultured for 6 h. The
cells were treated with different dose of matrine for 24 h. The
concentrations of matrine were 0.1, 0.5 and 2.5 mg/ml,
respectively. Matrine was dissolved with DMSO and the final
concentration of DMSO in medium was 0.1%. The cells treated with
0.1% DMSO were used as negative controls. In the other experiment,
RC-4B/C cells and GH3 cells were treated with 1.0 mg/ml of matrine
for 24, 48 and 72 h, respectively. The cell viability was
determined MTT assay and the survival rate of matrine-treated
pituitary cancer cells was calculated by graphpad 5.0.
Nuclear and cytoplasmic protein
extraction
The RC-4B/C cells were treated with different
concentrations of matrine for 24 h. The total cells in each group
were collected. The nuclear and cytoplasmic proteins were extracted
by nuclear and cytoplasmic protein extraction kit (cat. no: P0027),
which was purchased from Beyotime Institute of Biotechnology,
(Haimen, China). The levels of cytoplasmic and nuclear of Foxo3a
was detected by western blotting. Here, β-tublin was used as the
marker in cytoplasm and lamin B1 was used as the internal reference
in nucleus.
Western blot analysis
The western blotting was performed as described
(15,16). The levels of p-Akt, total Akt,
p-FoxO3a, total Foxo3a, Bim, Bax and Bcl-2 were detected by western
blotting analysis. The antibodies used here were shown as follows:
FoxO3a (75D8; cat. no: #2497) was a rabbit monoclonal antibody for
FoxO3a protein and purchased from Cell Signaling Technology, Inc.,
(Danvers, MA, USA). The p-Akt antibody (cat. no: sc-7985-R, a
rabbit polyclonal IgG) and Akt antibody (BDI111, cat. no: sc-56878,
a mouse monoclonal IgG1) was purchased from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). Anti-Bim antibody [Y36]
(ab32158) was a rabbit monoclonal IgG and purchased from Abcam
Corporation. Bax (D2E11) rabbit mAb no: 5023 was a rabbit IgG and
detected endogenous levels of total Bax protein, which was obtained
from Cell Signaling Technology, Inc. Anti-Bcl-2 antibody [E17]
(ab32124) was a rabbit monoclonal antibody and purchased from Abcam
corporation. β-actin Antibody (C4) (cat. no: sc-47778) was a mouse
monoclonal IgG1 and purchased from Santa Cruz Biotechnology, Inc.
The ‘grey values’ was determined from scans of western blots
according to software protocols. The software used for densitometry
is Image J (v1.48; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
The data was analyzed with SPSS software v13 (SPSS
Inc., Chicago, IL, USA). In the present study, two sets of
independent samples were analyzed with the student's t-test. The
multiple comparisons were analyzed by using ANOVA and Tukey test.
The data was analyzed with t-test. The results were shown as mean ±
standard deviations. P<0.05 was considered to indicate a
statistically significant difference.
Results
Matrine inhibits cell proliferation of
pituitary cancer cells
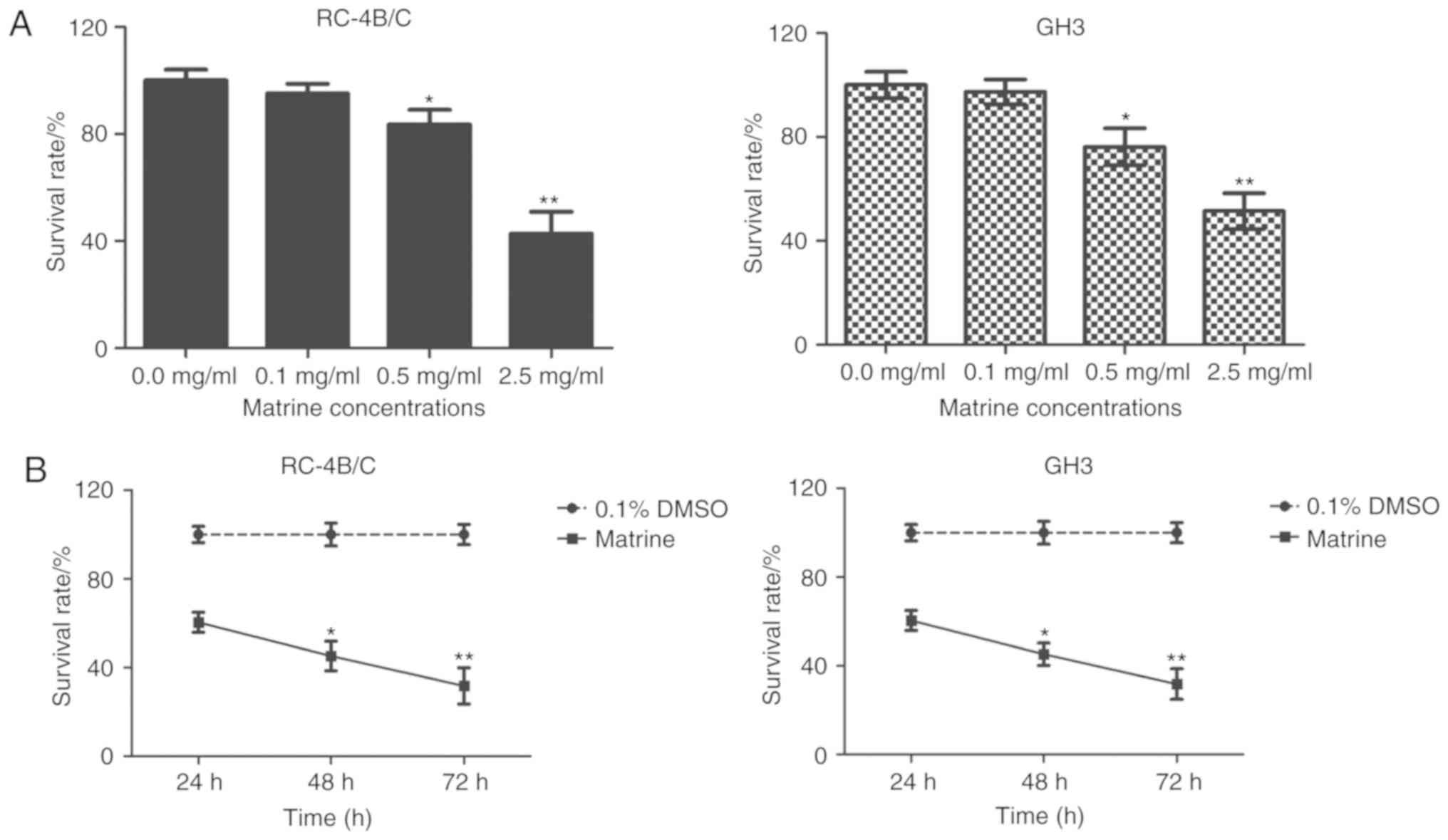
Firstly, we wanted to test whether matrine had
inhibitory effects on the proliferation of pituitary cancer cells.
The pituitary cancer cells RC-4B/C and GH3 were treated with
increasing concentrations of matrine for 24 h and MTT assay was
performed to detect cell viability of matrine-treated pituitary
cancer cells. As shown in Fig. 1A,
the survival rates of RC-4B/C cells and GH3 cells were
significantly decreased in matrine-treated cells than that of 0.1%
DMSO-treated cells (*P<0.05, **P<0.01, compared with 0.1%
DMSO treated pituitary RC-4B/C cells and GH3 cells). There was no
statistical difference between the cells treated with 0.1 mg/ml of
matrine and 0.1% DMSO. Next, 1.0 mg/ml of matrine were used to
treat pituitary cancer cells for 24, 48 and 72 h, respectively. As
shown in Fig. 1B, the survival rates
of RC-4B/C cells and GH3 cells significantly decreased in a
time-dependent manner (*P<0.05, **P<0.01, compared with the
survival rate in 24 h). All the data revealed that matrine
inhibited cell proliferation of pituitary cancer cells in a dose-
and time-dependent manner.
Matrine inhibits the phosphorylation
levels of Akt and Foxo3a in RC-4B/C cells
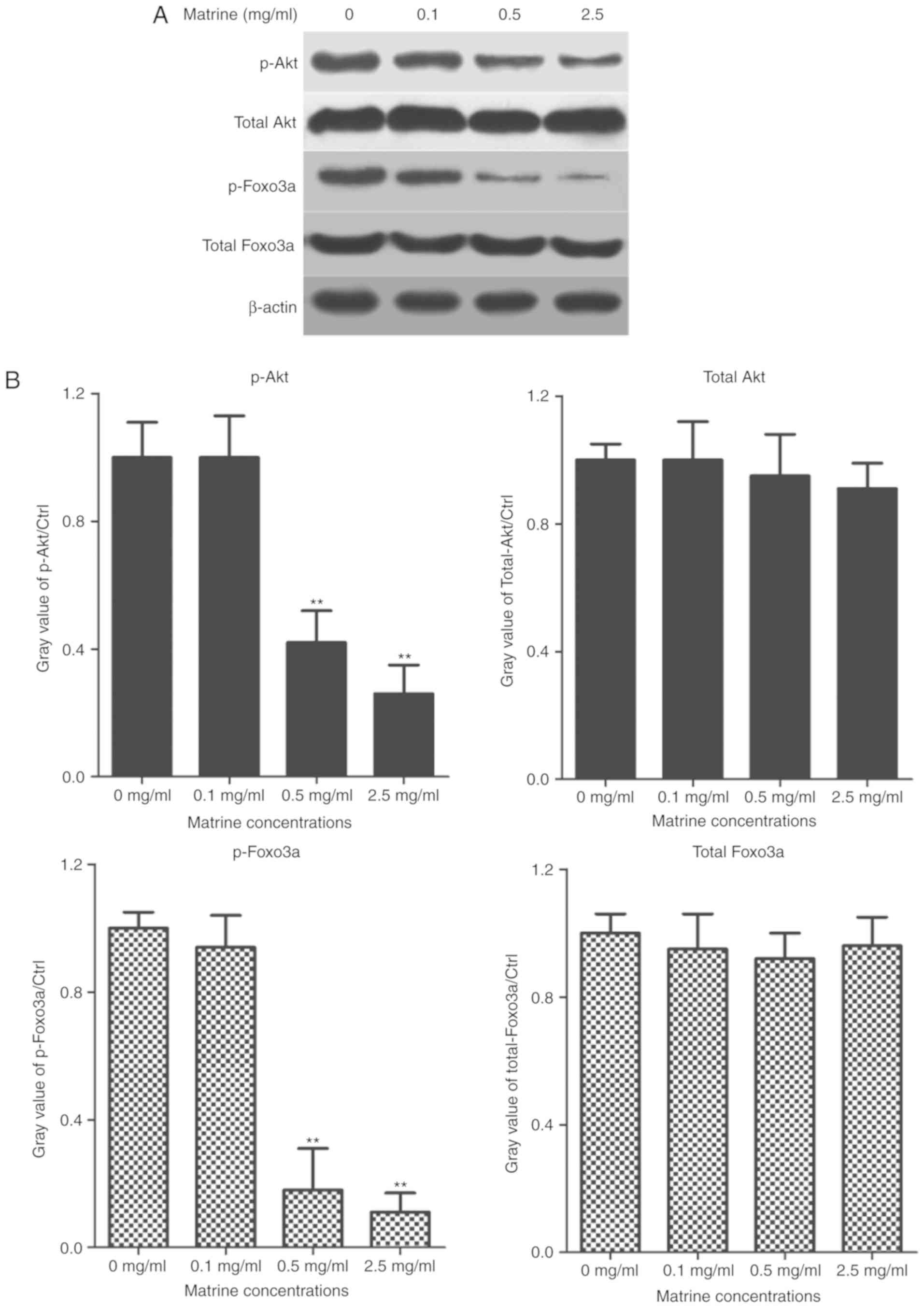
Next, we further investigated the molecular
mechanism that how matrine possessed the anti-tumor effects in
RC-4B/C cells. Western blotting analysis was used to test whether
matrine affected Akt signaling pathway. Briefly, RC-4B/C cells were
treated with 0.1, 0.5 and 2.5 mg/ml of matrine for 24 h and the
cells treated with 0.1% DMSO were used as negative controls. The
levels of phosphorylated Akt, total Akt, phosphorylated Foxo3a and
total Foxo3a were tested by western blotting analysis. As shown in
Fig. 2, as the concentration of
matrine increased, the expression of p-Akt and p-Foxo3a
significantly decreased, while the levels of total Akt and total
Foxo3a were not obviously changed. All the data showed that matrine
regulated the proliferation of pituitary cancer cells probably by
Akt/Foxo3a signaling pathway.
Matrine promotes the nuclear Foxo3a
localization and inhibits the levels of cytoplasmic Foxo3a
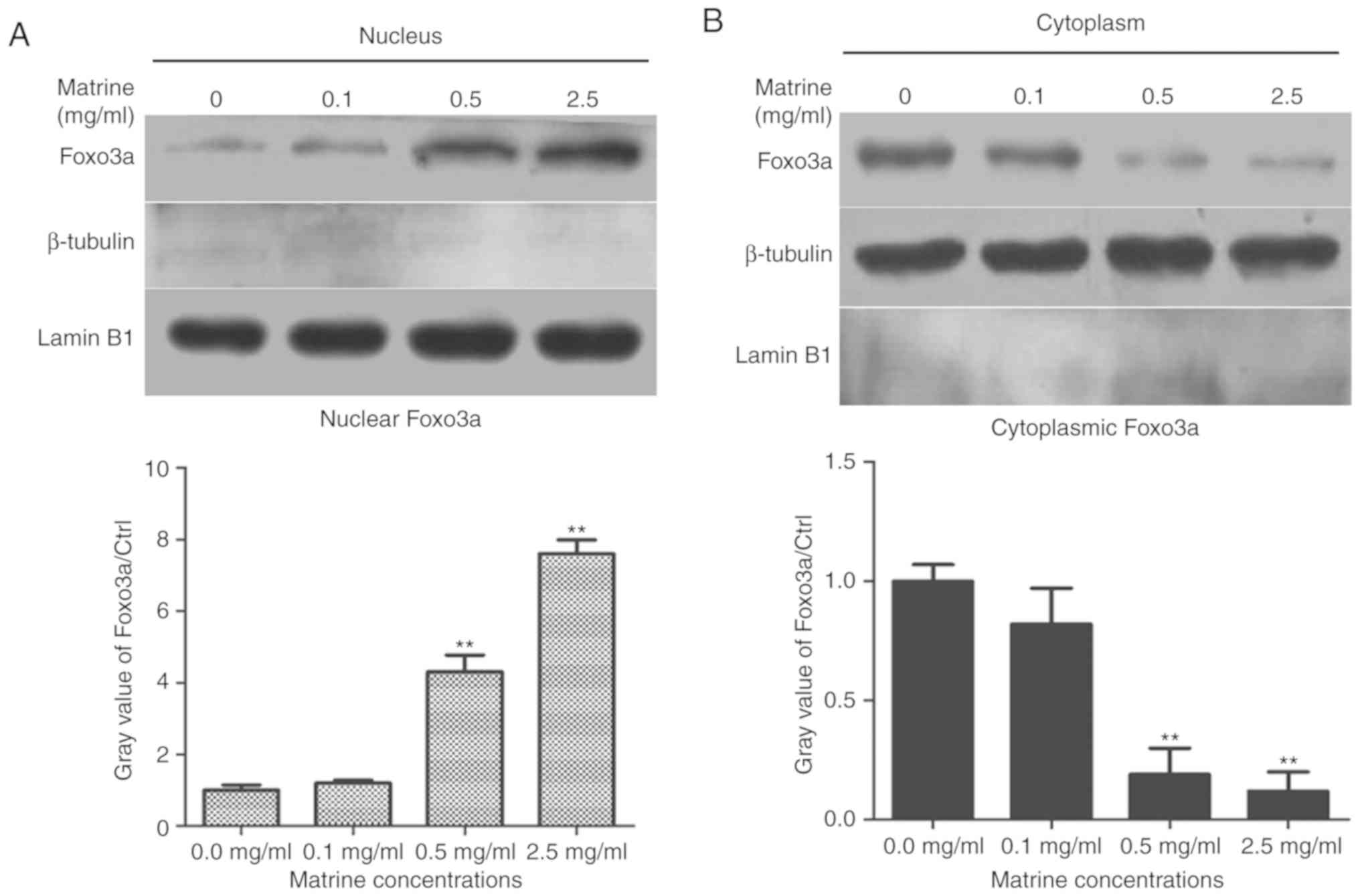
FOXO3A, a transcription factors of the forkhead
family, was phosphorylated and inactivated via PI3K/Akt signaling
pathway. Next, we detected whether matrine induced the
re-localization of Foxo3a in RC-4B/C cells. The cytoplasmic and
nuclear proteins were extracted in matrine-treated RC-4B/C cells,
as shown in Fig. 3, the levels of
Foxo3a in nucleus gradually increased as the concentration of
matrine increased in RC-4B/C cells, while the cytoplasmic Foxo3a
level significantly decreased, suggesting that matrine treatment
promoted the nuclear localization of Foxo3a by inhibiting the
phosphorylation of Foxo3a.
Matrine increases the expression of
pro-apoptotic proteins in RC-4B/C cells
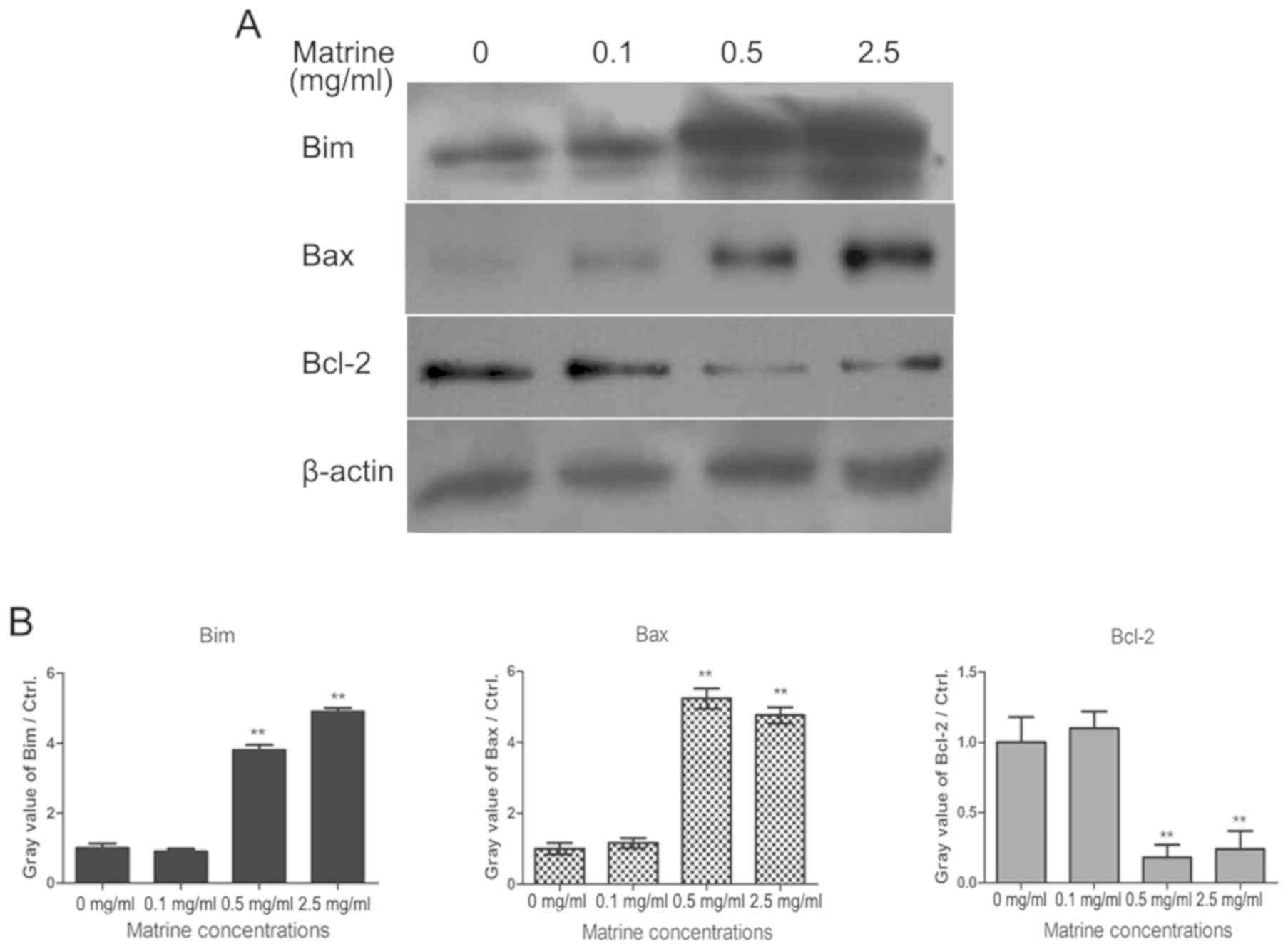
It has reported that activated Foxo3a promoted cell
apoptosis through expression of genes for cell death, such as Fas
ligand (FasL), Bim and tumor necrosis factor related
apoptosis-inducing ligand (TRAIL) etc. Then, we tested the
expression of apoptosis related proteins in matrine-treated
pituitary cancer cells. As shown in Fig.
4, RC-4B/C cells were treated with 0.1, 0.5 and 2.5 mg/ml of
matrine for 24 h, and the levels of Bim, Bax and Bcl-2 were
determined by western blotting analysis. The results demonstrated
that matrine promoted the expression of Bim and increased the ratio
of Bax/Bcl-2.
Discussion
Pituitary cancer is one of the genitourinary
malignancies with high mortality that seriously threatens human
health. The new approaches and drugs are being developed to improve
quality of pituitary cancer patients. One of the important way to
concur pituitary cancers is to clarify the mechanism of
tumorigenesis and proliferation of pituitary cancer cells. In the
present study, we explored the molecular mechanism of matrine in
pituitary cancer cells, which could be used as an effective drug
candidate in the clinical therapy of pituitary cancers.
Firstly, the anticancer activity of matrine in
pituitary cancer cells were tested by MTT assay. We found that
matrine inhibited cell viability of RC-4B/C cells and GH3 cells in
a time-dependent and dose-dependent manner. The results
demonstrated that matrine had potent antitumor activity in
pituitary cancer cells. This was consistent with the results that
matrine suppressed bladder tumor invasion in a rat model, which
could be primarily mediated by regulating the expression of COX-2
and cPLA2 in the bladder (17).
Furthermore, we explored the molecular mechanism of matrine in
pituitary cancer cells by western blotting analysis. It has been
reported that matrine regulated the cell proliferation of cancer
cells by suppressing PI3K/Akt signaling pathways (18,19),
Akt/Foxo pathway (20), Akt/m
TOR/S6K and ERK1/2 signaling (21),
which was detected in pituitary cancer cells. Then, we tested
whether matrine was involved in regulating Akt/Foxo3a signaling
pathway in the cell proliferation of pituitary cancer cells. The
phosphorylated Foxo3a was translocated into cytoplasm and inhibited
the down-stream gene expression, which inhibited cell apoptosis in
various cancer cells. Luckily, the results demonstrated that as the
concentration of matrine increased, the levels of p-Akt
significantly decreased, as well as the levels of pFoxo3a in
matrine-treated RC-4B/C cells. Moreover, we extracted the total
nuclear and cytoplasmic proteins of matrine-treated RC-4B/C cells
and tested the levels of Foxo3a by western blotting analysis. This
result revealed that as the concentration of matrine increased, the
level of Foxo3a in cytoplasm significantly decreased and the level
of foxo3a in nucleus obviously increased. This was consistent with
the expression of the downstream gene, bim, which obviously
increased as the concentration of matrine increased in RC-4B/C
cells.
However, there was a limitation in our study that we
did not successfully identify and isolate the normal counterpart of
pituitary tumor cells. Thus, we does not evaluate the matrine on
normal pituitary tissue, and it remains unclear whether normal and
neoplastic pituitary cells show a differential sensitivity to
matrine. But, we had tested the role of Matrine in ‘normal’ liver
cell line-L02 cells. The results demonstrated that Matrine had
showed a dual effect on the growth of normal cells L02. At a lower
concentration (0.1~0.4 mg/ml) of Matrine, the proliferation of L02
cells were significantly increased, especially being treated for 72
h. However, higher concentration of Matrine (more than 0.8 mg/ml)
showed an increasing dose-dependent cytotoxicity in L02 cells (the
data was unpublished). All the data demonstrated that lower
concentration of Matrine might promote the cell proliferation of
normal cells and higher concentration of Matrine could kill normal
cells as well. Thus, it is an interesting question that how to
increase the sensitivity of tumor cells to matrine but decrease the
cytotoxic effects on normal cells, that would be a perfect
anti-tumor reagent in pituitary tumor therapy. Intracranial
pituitary tumors are generally small in size, deep in position, and
not easy to observe and measure; moreover, the variability of tumor
growth and the large tumor often oppress the lower part of the
hypothalamus, which usually causes sudden death of the animal.
Therefore, next, we would construct animal model of human
transplantable pituitary tumor in nude mice by subcutaneous
transplantation and treat them with different doses of Matrine to
observe the dynamic change of tumor volume, and take the tumor
tissue for molecular In-depth study of mechanism. This would help
to further prove the anti-tumor effects of matrine. In conclusion,
matrine induced cell death of pituitary cancer cells and the
molecular mechanism results showed that the inhibition of cell
proliferation might be involved in Akt/Foxo3a signaling pathway.
Matrine could be used as a novel agent in clinical therapy of
pituitary cancer patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LL and PG performed the literature analysis and
designed the experiment. LL and SC performed the experimental
studies. YS and LK performed data analysis. GL and LK were involved
in the acquisition and interpretation of data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou HJ, Zhan RY, Ma YH, Cao F and Zheng
XJ: Primary sellar melanocytic tumor mimicking hemorrhagic
pituitary macroadenoma: Case report and literature review. Br J
Neurosurg. 29:298–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loyo-Varela M, Herrada-Pineda T,
Revilla-Pacheco F and Manrique-Guzman S: Pituitary tumor surgery:
Review of 3004 cases. World Neurosurg. 79:331–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nasi D, Perano D, Ghadirpour R, Iaccarino
C, Servadei F and Romano A: Primary pituitary neuroendocrine tumor:
Case report and literature review. Surg Neurol Int. 8:1012017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Fu L, Li S, Xu Z and Li X: Histone
deacetylase 11 suppresses p53 expression in pituitary tumor cells.
Cell Biol Int. 41:1290–1295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao ZR, Li M, Shi P and Zhang P: MiRNA153
induces pituitary tumor MMQ cell line apoptosis through
down-regulating Skp protein expression. Eur Rev Med Pharmacol Sci.
21:1270–1275. 2017.PubMed/NCBI
|
|
6
|
Liu B, Pang B, Wang Q, Yang S, Gao T, Ding
Q, Liu H, Yang Y, Fan H, Zhang R, et al: EZH2 upregulation
correlates with tumor invasiveness, proliferation, and angiogenesis
in human pituitary adenomas. Hum Pathol. 66:101–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang C, Liu SP, Fang CH, He RS, Wang Z,
Zhu YQ and Jiang SW: Effects of matrine on the proliferation of
HT29 human colon cancer cells and its antitumor mechanism. Oncol
Lett. 6:699–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Li X, Bai M, Suo Y, Zhang G and Cao
X: Matrine inhibited proliferation and increased apoptosis in human
breast cancer MCF-7 cells via upregulation of Bax and
downregulation of Bcl-2. Int J Clin Exp Pathol. 8:14793–14799.
2015.PubMed/NCBI
|
|
9
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology.
59:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo C, Zhong HJ, Zhu LM, Wu XG, Ying JE,
Wang XH, Lü WX, Xu Q, Zhu YL and Huang J: Inhibition of matrine
against gastric cancer cell line MNK45 growth and its anti-tumor
mechanism. Mol Biol Rep. 39:5459–5464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo C, Zhu Y, Jiang T, Lu X, Zhang W, Jing
Q, Li J, Pang L, Chen K, Qiu F, et al: Matrine induced gastric
cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules
of Bcl-2 family. Toxicology. 229:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Li Y, Chen X, Liu T, Chen Y, He
W, Zhang Q and Liu S: Autophagy is involved in anticancer effects
of matrine on SGC-7901 human gastric cancer cells. Oncol Rep.
26:115–124. 2011.PubMed/NCBI
|
|
13
|
Li H, Xie S, Liu X, Wu H, Lin X, Gu J,
Wang H and Duan Y: Matrine alters microRNA expression profiles in
SGC-7901 human gastric cancer cells. Oncol Rep. 32:2118–2126. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
An Q, Han C, Zhou Y, Li F, Li D, Zhang X,
Yu Z, Duan Z and Kan Q: Matrine induces cell cycle arrest and
apoptosis with recovery of the expression of miR-126 in the A549
non-small cell lung cancer cell line. Mol Med Rep. 14:4042–4048.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Egawa H, Jingushi K, Hirono T, Ueda Y,
Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
The miR-130 family promotes cell migration and invasion in bladder
cancer through FAK and Akt phosphorylation by regulating PTEN. Sci
Rep. 6:205742016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CH, Chang CY, Lee KR, Lin HJ, Chen TH
and Wan L: Flavones inhibit breast cancer proliferation through the
Akt/FOXO3a signaling pathway. BMC Cancer. 15:9582015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao H, Guo Y, Deng N, Fei P, Qiu X, Zheng
P, Feng J and Dai G: Suppressive effect of matrine on tumor
invasion in N-Butyl-N-(4-Hydroxybutyl)Nitrosamine-induced urinary
bladder carcinogenesis. Chemotherapy. 60:119–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai S, Chen T, Yu X, Luo M, Chen X, Lin C,
Lai Y and Huang H: The specific killing effect of matrine on
castration-resistant prostate cancer cells by targeting the
Akt/FoxO3a signaling pathway. Oncol Rep. 37:2819–2828. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang
HH, Liu X, Liang DS, Lu YJ, Shan HL and Jiang HC: Matrine inhibits
breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells.
Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang M, Zhao Y, Liu N, Chen E, Quan Z, Wu
X and Luo C: Overexpression of HepaCAM inhibits bladder cancer cell
proliferation and viability through the AKT/FoxO pathway. J Cancer
Res Clin Oncol. 143:793–805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mu DW, Guo HQ, Zhou GB, Li JY and Su B:
Oleanolic acid suppresses the proliferation of human bladder cancer
by Akt/mTOR/S6K and ERK1/2 signaling. Int J Clin Exp Pathol.
8:13864–13870. 2015.PubMed/NCBI
|