Introduction
The key clinical features of rheumatoid arthritis
(RA), an inflammatory autoimmune disease, are synovial inflammation
and chronic corrosive damage to joints; however, its pathogenesis
is unknown at present (1–3). In patients with RA, synovial
hyperplasia and tissue inflammation may cause joint and surrounding
tissue damage, resulting in joint deformity and dysfunction
(4,5). Furthermore, lesions may also affect all
joints with synovial membranes, with those of the hands and feet
being the most common (4,5). Several studies have indicated that
inflammatory cytokines and chemokines, including tumor necrosis
factor (TNF)-α, interleukin (IL)-6 and IL-1β serve key roles in the
development and progression of autoimmune diseases, including
systemic lupus erythematosus, systemic vasculitis, scleroderma and
RA (1–3). Humanized anti-TNF-α (4) and anti-IL-6 receptor (5) monoclonal antibodies, and inflammatory
cytokines or chemokines antagonist drugs are widely used to treat
RA in clinic; these treatments demonstrate good clinical
therapeutic effects on RA (5). In
addition, certain therapeutic drugs that target immune cells have
also been developed and applied clinically, including the
CTLA4-IgG1 fusion protein, which was developed by Repligen and was
approved for clinical use in the treatment of graft-versus-host
responses (6). Furthermore, CD2
receptor antibodies and anti-T-cells are also at different stages
of clinical research (7,8).
Therefore, it is feasible to treat RA by inhibiting
the inflammatory response.
Tanshinone IIA (Tan IIA), the main active ingredient
in tanshinone, can effectively inhibit the lipopolysaccharide
(LPS)-induced expression of pro-inflammatory molecules in human
peripheral blood mononuclear cells (PBMCs) (9), downregulate serum levels of
inflammatory cytokine in coronary syndromes (10) and induces apoptosis in
fibroblast-like synoviocytes in RA (11). However, the effect of Tan IIA on the
inflammatory response in patients with RA and the corresponding
mechanism of action remain unknown. Therefore, the aim of the
current study was to investigate the inhibitory effect of Tan IIA
on the inflammatory response in patients with RA and explore the
corresponding mechanism by investigating the effect of Tan IIA on
the LPS-induced expression of associated proteins [sirtuin-1
(SIRT-1) p65 and β-arrestin2] in PBMCs, and TNF-α and IL-6 levels.
The current study also aimed to assess whether Tan IIA inhibits the
inflammatory response in patients with RA by inhibiting the
expression of various inflammatory factors (including TNF-α and
IL-6) in PBMCs.
Materials and methods
Clinical data
The diagnosis of RA was performed according to the
ACR/EULAR 2010 classification criteria (12). A total of 50 randomly selected
patients with RA were recruited from the Department of
Rheumatology, Third Affiliated Hospital of Zhejiang Chinese Medical
University (Hangzhou, China) between January and June 2016. A total
of 35 patients with RA, including 10 males and 25 females, with a
mean age of 50.3±9.8 years and a mean progression time of 6.9±2.3
years were recruited as the research group. A total of 15 patients
with RA, including 5 males and 10 females, with a mean age of
49.7±7.6 years and a mean progression time of 6.8±1.9 years were
recruited as the control group. No significant differences in the
age, gender, progression time and other general data were
identified between the research and control groups. The current
study was approved by the Ethics Committee of The Third Affiliated
Hospital of Zhejiang Chinese Medical University. Written informed
consent was obtained from each patient. The exclusion criteria were
as follows: i) Patients with RA who also had other rheumatism
diseases, immune system diseases, tumors and liver and kidney
function deficiencies; ii) patients with RA who were pregnant,
lactating or had mental diseases; and iii) patients with RA who
used immunomodulatory drugs within half a year.
Clinical drugs and experimental
materials
Methotrexate (product ID: H31020644) and meloxicam
(product ID: H20030486) were purchased from Shanghai Sine Pharma
Co., Ltd. (Shanghai, China) and Simcere Pharmaceutical Co., Ltd.
(Nanjing, China), respectively. A Tan IIA sulfonate sodium
injection (product ID: H31022558) and leflunomide (product ID:
H20080420) were purchased from Shanghai No. 1 Biochemical &
Pharmaceutical Co., Ltd. (Shanghai, China) and Jiangsu Yabang
Aipusen Pharmaceutical Co., Ltd. (Yancheng, China), respectively.
Tan IIA was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). TNF-α (cat. no. KLC103a.96) and IL-6 (cat. no. AF-200-06)
ELISA kits were purchased from Shanghai Kang Lang Biological
Technology Co., Ltd. (Shanghai, China) and Shanghai Institute of
Biological Products Co., Ltd. (Shanghai, China), respectively. A
rat TNF-α ELISA kit (cat. no. K1052-100) was purchased from Amyjet
Scientific Inc. (Wuhan, China). A rat IL-6 ELISA kit (cat. no.
KS12244) was purchased from Shanghai Keshun Biological Technology
Co., Ltd. (Shanghai, China). An Animal tissue/cell total protein
extraction kit (cat. no. SD-001/SN-002) was purchased from Amyjet
Scientific, Inc.
Cell transfection
An siRNA negative control
(F:5′-CCCAUUCAUUGUUGUCACUTT-3′, R:5′-AGUGACAACAAUGAAUGGGTT-3′), a
β-arrestin 2 siRNA oligo sequence (oligo1,
5′-ACCUUUUCGUCUUUUGCUCCC-3′; oligo2, 5′-GAGCAAAAGACGAAAAGGUUG−3′)
were synthesized by Shanghai Gene Pharma Co., Ltd. (Shanghai,
China). These were directly transferred into cells via the
Lipofectamine™ 2000 transfection reagent (cat. no. 11668019;
Invitrogen; Thermo Fisher Scientific, Inc.). The final
concentration of siRNA used was 50 nM.
Rheumatoid rat model
A total of 60 male Sprague Dawley rats (age, 5–6
weeks; weight, 200–220 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). Animals were housed
at a temperature of 20–24°C and a humidity of 60% with a 12 h
light/dark cycle for one week. Rats were able to drink and eat
freely prior to and during the experiment. A total of 10 rats were
selected as the control group. The skin of left posterior foot on
each rat was sterilized with 75% alcohol and iodophor disinfectant,
and 0.1 ml saline was injected. The other 50 rats were selected as
the model group. The procedure for the model group was as follows:
Heat inactivated bacillus Calmette-Guérin and Freund's adjuvant
(cat. no. YS-XQ0496; Shanghai Yansheng Biochemical Reagent Co.,
Ltd., Shanghai, China) in a l0 mg/ml oil and water emulsion was
obtained from an ice bath, and then the skin of left posterior foot
on each rat was sterilized with 75% alcohol and iodophor
disinfectant and 0.1 ml prepared emulsion was injected. After 14
days, the rats in model group were again injected with 0.1 ml
prepared emulsion.
Experimental animal administration and
collection of specimens
Following the establishment of the RA model, the
rats were fed for 1 week. Then the RA model rats were separated
into the following three groups: Control model (RA), routine
treatment (RT) and Tan IIA-treated (Tan IIA). The control group and
RA groups were provided a normal diet. Saline (1 ml/l00 g) was
given by gavage administration once daily for 3 weeks. The RT group
was fed a normal diet; the rats were also administered with
methotrexate tablets dissolved in physiological saline by gavage
(0.51 mg/kg/day), once every 3 days for 3 weeks. The Tan IIA group
was fed a normal diet; the rats were also administered with Tan IIA
dissolved in physiological saline by gavage (0.50 mg/kg/d), once
every 3 days for 3 weeks.
After 3 weeks, the weights of the rats was recorded
prior to and following the 12-h fasting state. Rats were
anesthetized with chloral hydrate (400 mg/kg, intraperitoneal;
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and blood
was extracted from the heart of each rat. Rats were subsequently
sacrificed by cervical dislocation. Blood (5 ml) was extracted
using a vacuum chamber without anticoagulants for 30 min in a 37°C
constant temperature water bath. The blood was then centrifuged at
a speed of 500 × g for 10 min at room temperature to separate the
serum. The serum was then divided into 100 µl tubes and preserved
at 4°C.
General examination of different
groups
Three variables were measured 0, 14, 21, 28, 35 and
42 days after the establishment of the RA model. The variables were
as follows: Weight, perimeter of the left posterior ankle and
posterior plantar metatarsal thickness.
Clinical treatment methods and
detection indices
Patients in the research and control groups orally
received conventional drug treatment, including 5 mg methotrexate
once every 2 weeks and 7.5 mg meloxicam and 10 mg leflunomide once
daily. Patients in the research group received a Tan IIA injection
of 40 mg via an intravenous drip. Prior to treatment and following
1 week of continuous treatment, 10 ml venous blood was obtained
from all patients; the blood was centrifuged at 500 × g for 10 min
at room temperature to obtain serum. TNF-α and IL-6 serum levels
were determined using ELISA kits following the manufacturer's
protocol.
Separation and culture of PBMCs
Prior to treatment, venous blood was obtained from
patients with RA in the control group and used to obtain PBMCs by
density gradient centrifugation (1,000 × g for 25 min at room
temperature). Two PBMCs samples (3–5×106 cells) were
obtained from each patient and cultured at 37°C in RPMI-1640 (cat.
no. 12491–15; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; cat. no. 10100-147;
Thermo Fisher Scientific, Inc.). Subsequently, one cultured PBMC
sample was treated with 1 µg/ml LPS (cat. no. 00-4976-93; Thermo
Fisher Scientific, Inc.) for 48 h at 37°C. The other sample was
treated with 1 µg/ml LPS for 24 h at 37°C, then 0.75 µg/ml Tan IIA
was added for a further 24 h of induction at 37°C. Subsequently,
both PBMCs samples were centrifuged (at 500 × g for 5 min at room
temperature) to obtain the supernatant and PBMCs. TNF-α and IL-6
serum levels were determined using ELISA kits in accordance with
the manufacturer's protocol. The total proteins of PBMCs were
extracted using total cell protein extraction kit and stored at
−80°C prior to analysis.
siRNA interference assay
A total of 5 ml PBMCs (1.0–1.5×106/ml)
were conventionally cultured and centrifuged (500 × g for 5 min at
room temperature) to remove medium. Following one wash of the cells
in PBS, PBMCs were treated with Lipofectamine 2000 transfection
reagent kit (cat. no. 11668019; Thermo Fisher Scientific, Inc.) to
transfect β-arrestin 2 siRNA (100 nM). PBMCs treated with
β-arrestin 2 siRNA were regarded as the control group. After
transfecting the cells for 4 h, the medium (RPMI 1640 and 10% FBS)
was refreshed. After incubating the cells for 24 h, 1 µg/ml LPS was
added to the medium for another 24 h. Then 0.75 µg/ml Tan IIA was
or was not added to the PBMCs for another 24 h. Subsequently, the
PBMCs samples were centrifuged (at 500 × g for 5 min at room
temperature) to obtain supernatant and PBMCs. TNF-α and IL-6 serum
levels were determined using ELISA kits. The total proteins of
PBMCs were extracted using total cell protein extraction kit and
stored at −80°C until analysis.
Western blot analysis
Protein was extracted using RIPA (cat. no. P0013C;
Beyotime Institute of Biotechnology, Haimen, China) and the
concentration of total PBMC protein was determined using a BCA
protein assay kit. Then total protein (70 µg/lane) was separated
using SDS-PAGE (5% gel for concentration and 12% gel for
separation) and transferred to a polyvinylidene difluoride membrane
(cat. no. LC2002; Thermo Fisher Scientific, Inc.). Following
blocking at room temperature with 5% bovine serum albumin for 1 h
(cat. no. 30036727; Thermo Fisher Scientific, Inc.), the membrane
was incubated with primary and second antibodies. Following
incubation with BeyoECL plus (cat. no. p0018M; Beyotime Institute
of Biotechnology), the protein bands were analyzed by ImageJ
software (National Institutes of Health, Bethesda, MD, USA). The
primary antibody included anti-β-arrestin 2 (cat. no. ab54790),
anti-NAD-dependent protein deacetylase SIRT1 (19A7AB4; cat. no.
ab110304; both 1:2,000), anti-transcription factor p65 (p65; cat.
no. ab16502; 1:1,000) and anti-β-actin (cat. no. ab8227; 1:2,000;
Abcam, Cambridge, UK) antibodies. The secondary antibodies included
horseradish-peroxidase-conjugated goat anti-mouse (cat. no. ab6789)
and anti-rabbit (cat. no. ab6721; both 1:2,000; Abcam)
antibodies.
Detection of mRNA expression by
semi-quantitative reverse transcription polymerase chain reaction
(RT-PCR) analysis
Total RNA was extracted from PBMCs using cell RNA
kit (cat. no. DP419; Tiangen Biotech, Co., Ltd., Beijing, China).
cDNA was synthesized from RNA using a One Step PrimeScript miRNA
cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan). The RT protocol
was as follows: 37°C for 60 min and 85°C for 5 sec. PCR was
subsequently performed using SYBR Premix Ex Taq™ II (Takara Bio,
Inc.) and ABI 7500 Fluorescence Quantitative PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in a 20 µl volume. The thermocycling conditions were a
follows: 95°C for 30 sec, 40 cycles of 90°C for 5 sec and 65°C for
30 sec. cDNA (10 µl/lane) was separated in 1% nucleic acid
electrophoresis. 1.2% agarose (cat. no. A9414; Sigma-Aldrich; Merck
KGaA) and 5 µl Goodview nucleic acid dye (cat. no. HGV-2; Beijing
SBS Genetech Co., Ltd., Beijing, China) were used to separate PCR
products, the results of which were observed using a Bio-Rad Gel
Imaging Analyzer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Image J software v1 (National Institutes of Health, Bethesda, MD,
USA) was used to analyze gray values. The relative expression of
each gene was normalized to β-actin. Primer sequences were as
follows: β-arrestin 2 forward (F), 5′-ACCCATCACCCTCTGATCCT-3′ and
reverse (R), 5′-CTGCCCTCTCCTAGTCAGGT-3′; SIRT1 F,
5′-TGCAGGATTTTAGCCCTGGAG-3′ and R, 5′-AAAGGATTTTGAGGCAAAAGAAGA-3′;
p65 F, 5′-CTGTCCCCAAGCCAGGTAAG and R, 5′-AGAGGTGATTTTTGTTCCCCCA-3′;
and β-actin F, 5′-AAGTACTCCGTGTGGATCGG-3′ and R,
5′-TCAAGTTGGGGGACAAAAAG-3′.
Statistical analysis
All data are represented as mean ± standard
deviation. The differences among two groups were analyzed by
independent-samples t-tests and multiple groups were compared using
one-way analysis of variance followed by Duncan's post-hoc test.
Statistical analyses were conducted using SPSS 19.0 (IBM Corp.,
Armonk, NY, USA). P<0.05 indicated that the difference between
groups was statistically significant.
Results
Tan IIA alleviates RA in rats with the
condition
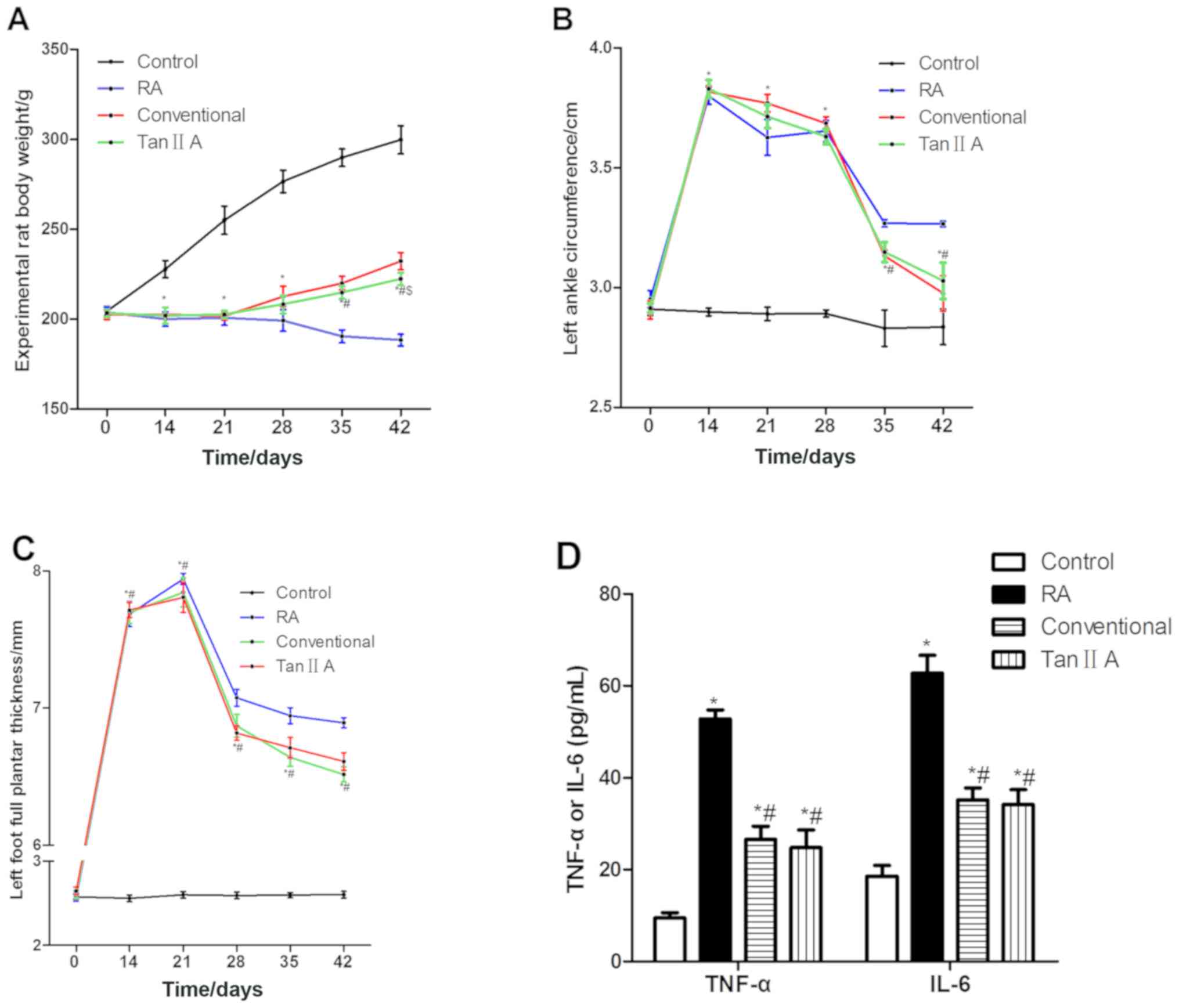
No significant difference was identified in the
weight of rats in each group prior to the establishment of the RA
model (Fig. 1A). On days 14 and 21,
rats in the control group were significantly heavier compared with
those in the Tan IIA group (P<0.05). After 1, 2 and 3 weeks of
treatment (28, 35 and 42 days after model establishment), the Tan
IIA group was significantly lighter compared with the control group
(P<0.05). After 2 and 3 weeks of treatment, the Tan IIA group
was significantly lighter compared with the RT group (P<0.05).
After 3 weeks of treatment, the Tan IIA group was significantly
heavier compared with the RA group (P<0.05). The weights of the
treated groups were higher compared with those in the RA group.
No significant difference was identified in the
circumference of the left posterior ankle of the rats prior to the
establishment of the RA model (Fig.
1B). On days 14 and 21, the circumference in the control group
was significantly decreased compared with those in the Tan IIA
group (P<0.05). After 1 week of treatment, the circumference was
significantly greater in the Tan IIA group compared with the
control group (P<0.05). After 2 and 3 weeks of treatment, the
Tan IIA group was significantly increased and decreased compared
with the control and RA groups, respectively (P<0.05).
No significant difference was identified in the
posterior plantar metatarsal thickness of the rats in each group
prior to the establishment of the RA model (Fig. 1C). On days 14 and 21, the thickness
of the Tan IIA group was significantly greater compared with the
control group (P<0.05). After 1, 2 and 3 weeks of treatment, the
posterior plantar metatarsal thickness in the Tan IIA group was
significantly increased and decreased compared with the control and
RA groups, respectively (P<0.05). No significant difference was
identified in the posterior plantar metatarsal thickness of the Tan
IIA group compared with the RT group.
After 3 weeks of treatment, the concentration of
TNF-α and IL-6 in the Tan IIA group was significantly lower
compared with those in the RA group (P<0.05; Fig. 1D). No significant difference in
protein expression was identified between the Tan IIA and RT
groups.
Tan IIA injections decrease TNF-α and
IL-6 levels in the serum of patients with RA
Prior to treatment, no significant differences in
TNF-α and IL-6 levels in the serum of patients with RA were
identified between the research and control group (Table I). Following treatment, the TNF-α and
IL-6 levels in research group were significantly lower compared
with those in the research group prior to treatment and those in
the control group following treatment (all P<0.05).
 | Table I.TNF-α and IL-6 levels in the serum of
patients with rheumatoid arthritis prior to and following
treatment. |
Table I.
TNF-α and IL-6 levels in the serum of
patients with rheumatoid arthritis prior to and following
treatment.
|
|
| TNF-α (pg/ml) | IL-6 (pg/ml) |
|---|
|
|
|
|
|
|---|
| Group | Number | Prior to
treatment | Following
treatment | Prior to
treatment | Following
treatment |
|---|
| Research | 35 | 45.37±8.15 |
38.14±6.76a,b | 8.72±3.45 |
4.46±1.38a,b |
| Control | 15 | 44.82±6.97 | 42.67±5.42 | 8.67±2.87 | 8.23±1.22 |
Tan IIA treatment decreases TNF-α and
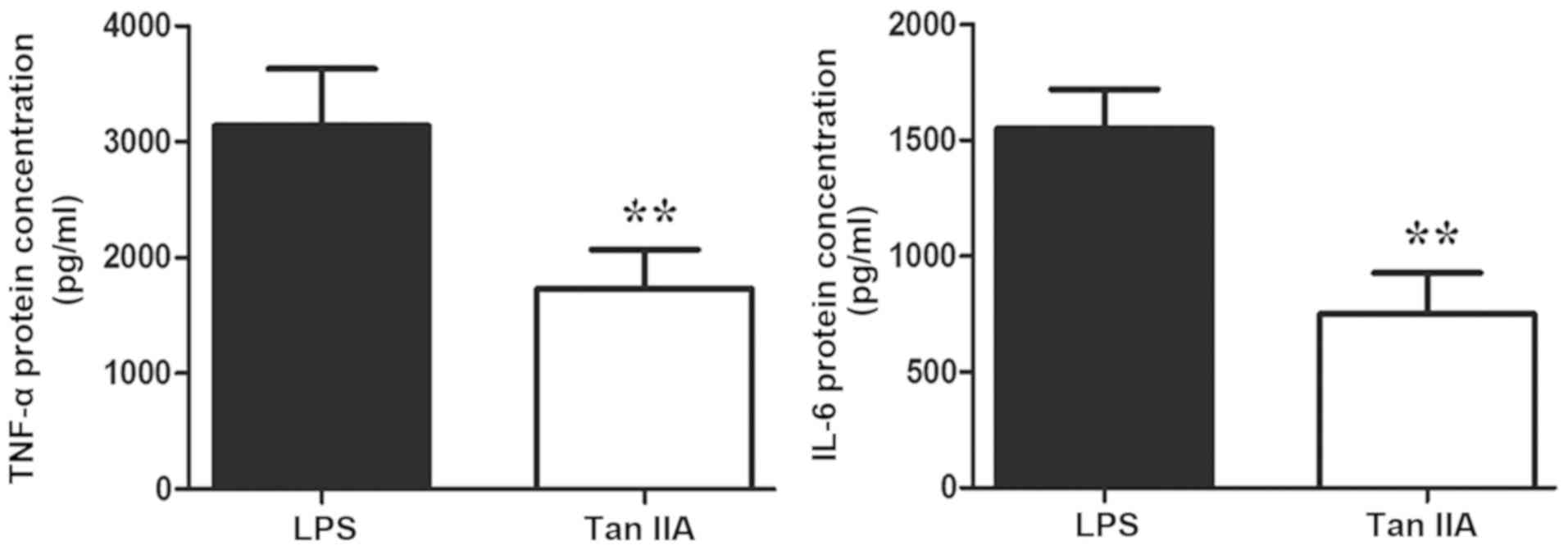
IL-6 levels in PBMCs of patients with RA
Tan IIA significantly inhibited the LPS-induced
secretion of TNF-α and IL-6 in PBMCs of patients with RA (both
P<0.01; Fig. 2).
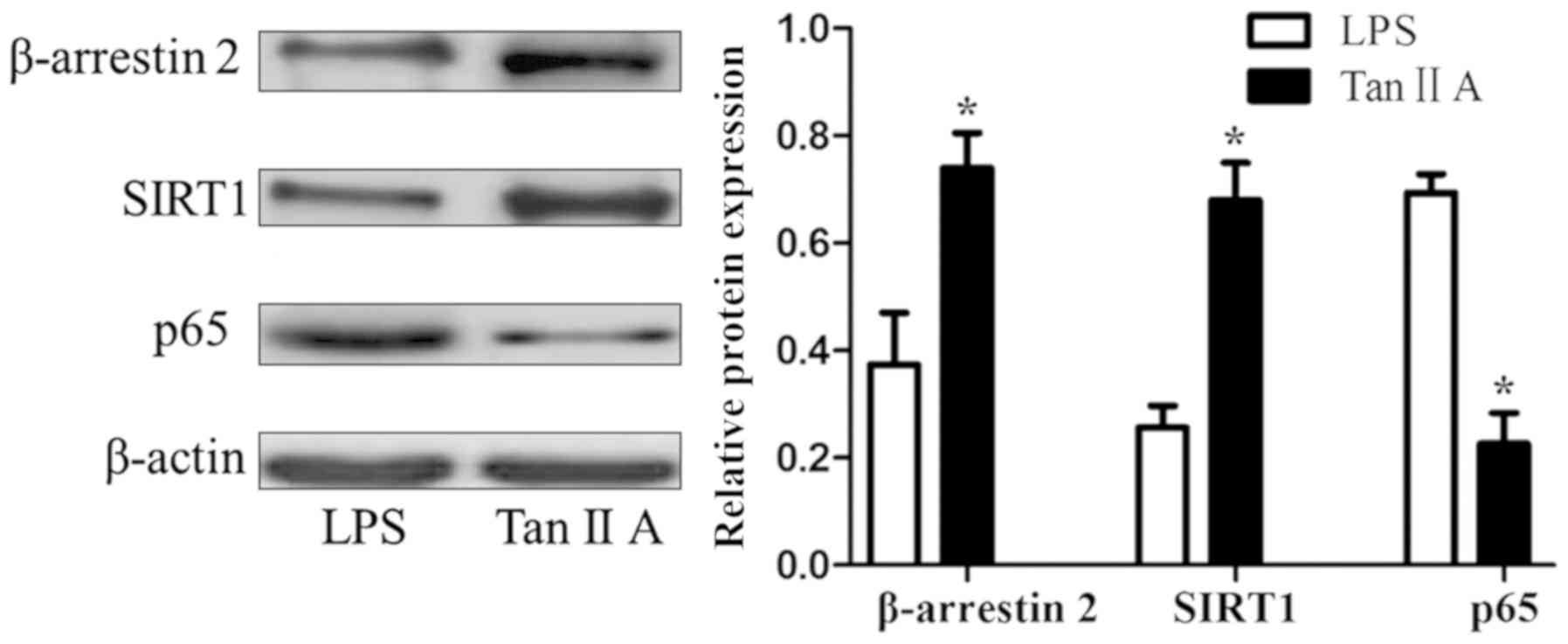
Tan IIA decreases β-arrestin 2 and
SIRT1, and increases p65 proteins in PBMCs of patients with RA
Tan IIA significantly upregulated the LPS-inhibited
expression of β-arrestin 2 and SIRT1 proteins, and downregulated
the LPS-induced expression of the p65 protein in PBMCs of patients
with RA (all P<0.05; Fig. 3).
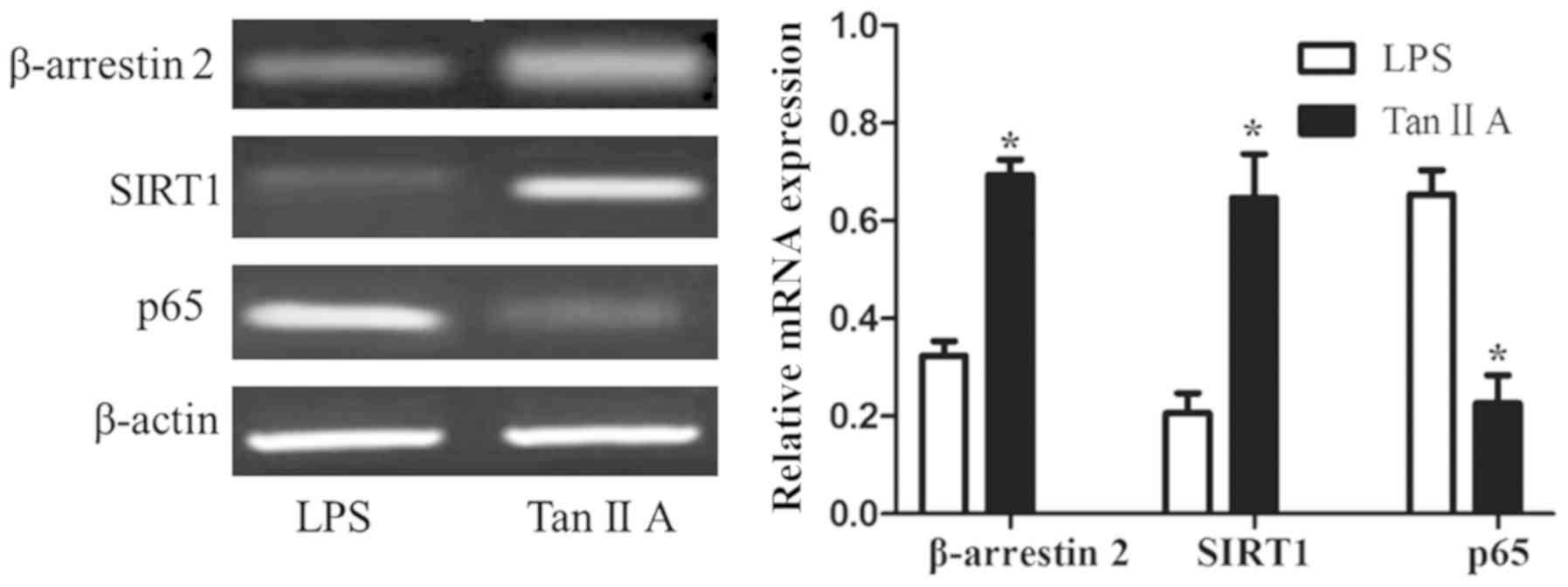
Tan IIA decreases β-arrestin 2 and
SIRT1, and increases p65 mRNA in PBMCs of patients with RA
Tan IIA significantly upregulated the expression of
the β-arrestin 2 and SIRT1 mRNAs in PBMC stimulated by LPS, and
inhibited the expression of p65 mRNA compared with cells only
stimulated with LPS (all P<0.05; Fig.
4).
β-arrestin 2 siRNA increases TNF-α,
IL-6 and p65, and decreases SIRT1 in PBMCs of patients with RA
Compared LPS stimulation for 48 h alone (A group),
TNF-α and IL-6 in PBMCs pretreated with β-arrestin 2 siRNA for 4 h
(C group) increased significantly. Furthermore, SIRT1 protein
expression decreased and p65 protein expression increased (all
P<0.05; Fig. 5). Tan IIA did not
significantly reverse the β-arrestin 2 siRNA-induced changes to
expression.
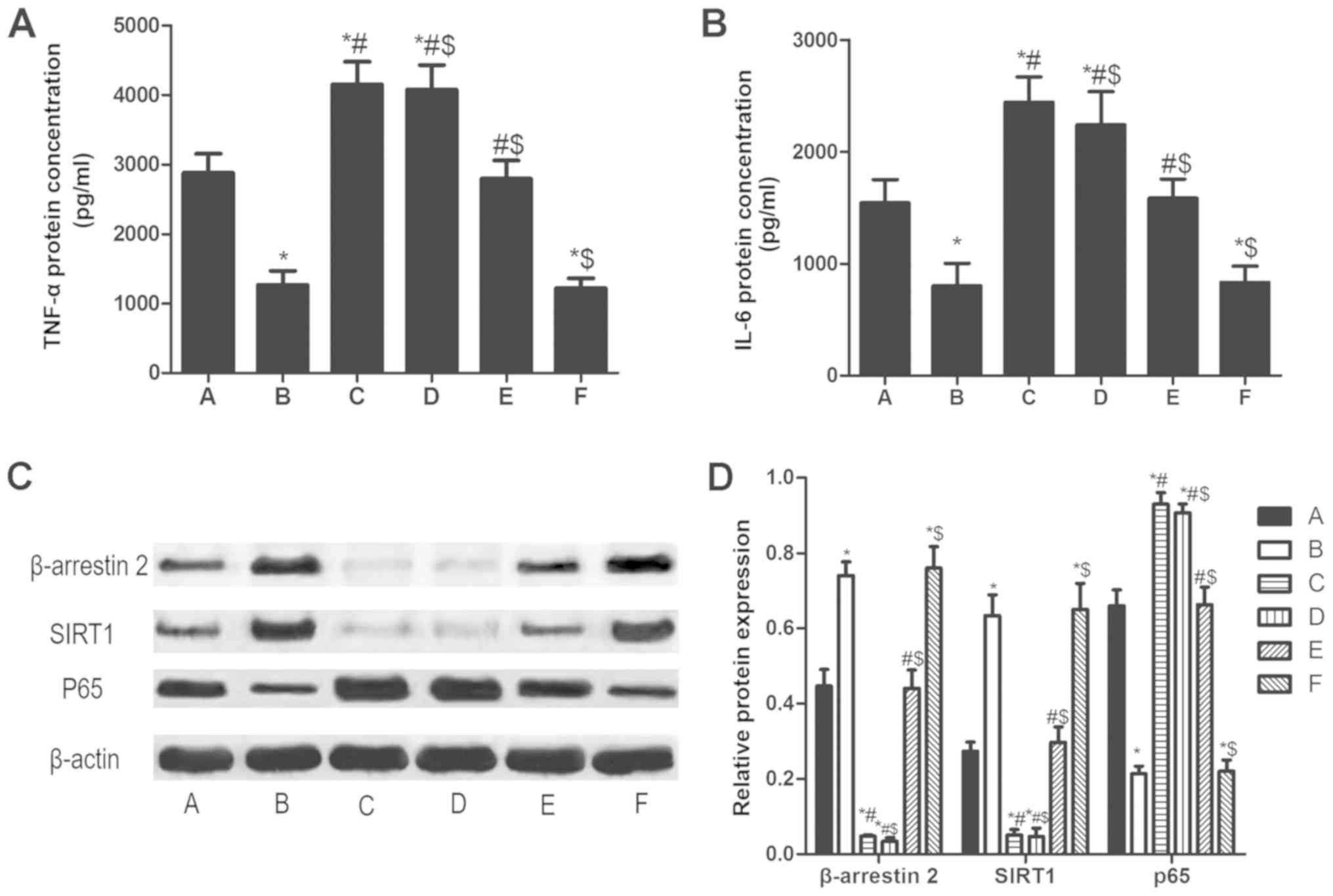 | Figure 5.β-arrestin 2 siRNA increases TNF-α,
IL-6 and p65, and decreases SIRT1 in peripheral blood mononuclear
cells of patients with rheumatoid arthritis. The concentration of
(A) TNF-α and (B) IL-6. (C) Qualitative and (D) quantitative
analysis of western blotting results for β-arrestin 2, SIRT1 and
P65. The groups were as follows: A, LPS stimulation for 48 h; B,
LPS stimulation for 48 h and Tan IIA treatment for another 24 h; C,
β-arrestin 2 siRNA incubation for 4 h and LPS stimulation for 48 h;
D, β-arrestin 2 siRNA incubation for 4 h, LPS stimulation for 48 h
and Tan IIA treatment for another 24 h; E, negative control siRNA
incubation and LPS stimulation for 48 h; E, negative control siRNA
incubation, LPS stimulation for 48 h and Tan IIA treatment for
another 24 h. *P<0.05 vs. A group; #P<0.05 vs. B
group; $P<0.05 vs. C group. si, small interfering;
SIRT1, NAD-dependent protein deacetylase sirtuin-1; p65,
transcription factor p65; TNF, tumor necrosis factor; IL,
interleukin; Tan IIA, tanshinone II A; LPS,
lipopolysaccharides. |
Discussion
TNF-α has been demonstrated to promote the
occurrence of inflammation by inducing T cells to produce a variety
of inflammatory factors (1–3). IL-6, a cytokine produced in monocytes,
macrophages and lymphocytes, can stimulate and be associated with
the immune response in the body by inducing the differentiation of
mononuclear cells and B cells, and enhancing the activity of NK
cells (1–3). A previous study demonstrated that TNF-α
and IL-6 levels in the serum and synovial fluid of patients with RA
were high, indicating that they serve key roles in the pathogenesis
of RA by promoting the inflammatory response of patients with RA
(2). The current study revealed that
TNF-α and IL-6 levels in the serum of patients with RA in the
research group following treatment were significantly lower than
those in the research group prior to treatment and the control
group following treatment, indicating that Tan IIA sulfonate sodium
injections could inhibit the inflammatory response in patients with
RA. Nizamutdinova et al (13)
demonstrated that Tan IIA could inhibit the expression of TNF-α by
regulating the phosphatidylinositol 4,5-bisphosphate 3-kinase/RAC-α
serine/threonine-protein kinase, protein kinase C and
tyrosine-protein kinase JAK/signal transducer and activator of
transcription (STAT)3 signaling pathways. The aforementioned study
also revealed that Tan IIA could inhibit the TNF-α-induced ICAM-1
expression. Lin et al (14)
revealed that Tan IIA inhibited the in vitro and in
vivo growth of breast cancer stem cells by downregulating the
IL-6/STAT3/NF-κB signaling pathway, suggesting that Tan IIA
regulated the expression of TNF-α and IL-6 through regulating
multiple signaling pathways.
To further reveal the mechanism behind the Tan
IIA-induced downregulation of TNF-α and IL-6 levels, the effect of
Tan IIA on the expression of associated proteins (including SIRT1,
β-arrestin 2, TNF-α and IL-6) in LPS-stimulated PBMCs was
investigated. The current study demonstrated that Tan IIA inhibited
the LPS-induced secretion of TNF-α and IL-6, upregulated the
LPS-inhibited expression of β-arrestin 2 and SIRT1 proteins, and
downregulated the LPS-induced expression of p65 protein in PBMCs of
patients with RA. However, Tan IIA could not inhibit the β-arrestin
2 siRNA-induced secretion of TNF-α and IL-6 in PBMCs of patients
with RA. These results indicated that Tan IIA inhibited the
expression of TNF-α and IL-6 in patients with RA through
upregulating β-arrestin 2 expression, thus the inflammatory
response in patients with RA was inhibited.
β-arrestin 2 can regulate human immunological
functions by inhibiting activation of the NF-κB signaling pathway,
and regulating the chemotaxis of immune cells and multiple
signaling pathways (1–3). As β-arrestin 2 serves a key role in
regulating human immunological functions, it may be associated with
the development and progression of certain autoimmune associated
diseases (1–3). Li et al (15) demonstrated that β-arrestin 2
inhibited RA progression by inhibiting the inflammatory response in
RA rats; the authors hypothesized that the inhibitory response may
be associated with inhibiting the NF-κB signaling pathway. The
NF-κB signaling pathway is one of the most important signaling
pathways in mammalian cells and a node in multiple cell signaling
pathways (16). Following its
activation, the NF-κB signaling pathway was revealed to regulate
the expression of a variety of downstream inflammatory cytokines,
which can regulate the inflammatory response (17).
The current study revealed that β-arrestin 2
expression in PBMCs of patients with RA was positively associated
with SIRT1 expression and was negatively associated with p65. SIRT1
is a histone deacetylase that is widely expressed in human cells
(18–20). SIRT1 can deacetylate p53, UCP2, NF-κB
or other transcription factors to exert biological functions
(14–20). p65, a key protein in the NF-κB
signaling pathway, is acetylated to exert its biological functions.
SIRT1 can downregulate the acetylation level of the p65 protein in
the inflammatory response, which can inhibit the level of
transcription of downstream inflammatory genes, including TNF-α and
IL-6 (17). TNF-α and IL-6, as two
important inflammatory factors, are not only associated with
regulating the body's inflammatory response (21,22), but
also serve an important role in the development of rheumatoid
diseases (23,24). In summary, the present findings
suggested that Tan IIA inhibited NF-κB activity through
upregulating β-arrestin 2 expression to inhibit the inflammatory
response in PBMCs of patients with RA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW conceived, designed and revised the current
study. JT and SZ analyzed the data and wrote the manuscript. FZ
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of The Third Affiliated Hospital of Zhejiang Chinese
Medical University (Hangzhou, China). A written informed consent
form was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Astry B, Harberts E and Moudgil KD: A
cytokine-centric view of the pathogenesis and treatment of
autoimmune arthritis. J Interferon Cytokine Res Official.
31:927–940. 2011. View Article : Google Scholar
|
|
2
|
Mcinnes IB and Schett G: Cytokines in the
pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7:429–442.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kunz M and Ibrahim SM: Cytokines and
cytokine profiles in human autoimmune diseases and animal models of
autoimmunity. Mediators Inflamm. 2009:9792582009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simsek I: TNF inhibitors for rheumatoid
arthritis-a year in review. Bull NYU Hosp Jt Dis. 69:220–224.
2011.PubMed/NCBI
|
|
5
|
Woodrick RS and Ruderman EM: Interleukin 6
inhibition-RA and beyond. Bull NYU Hosp Jt Dis. 69:225–229.
2011.PubMed/NCBI
|
|
6
|
Kremer JM, Westhovens R, Leon M, Di
Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P,
Nuamah IF, et al: Treatment of rheumatoid arthritis by selective
inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl
J Med. 349:1907–1915. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rao DA, Gurish MF, Marshall JL,
Slowikowski K, Fonseka CY, Liu Y, Donlin LT, Henderson LA, Wei K,
Mizoguchi F, et al: Pathologically expanded peripheral T helper
cell subset drives B cells in rheumatoid arthritis. Nature.
542:110–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Wang D, Lu S, Xu Q, Zhao L, Zhao J,
Song Y and Wang H: Increased circulating follicular treg cells are
associated with lower levels of autoantibodies in patients with
rheumatoid arthritis in stable remission. Arthritis Rheumatol.
70:711–721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu Q, Chen H, Sheng L, Liang Y and Li Q:
Sodium tanshinone IIA sulfonate prolongs the survival of skin
allografts by inhibiting inflammatory cell infiltration and T cell
proliferation. Int immunopharmacol. 22:277–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang Q, Wang H, Li S and Xu H: The effect
of sodium tanshinone IIA sulfate and simvastatin on elevated serum
levels of inflammatory markers in patients with coronary heart
disease: A study protocol for a randomized controlled trial. Evid
Based Complement Alternat Med. 2013:7565192013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jie L, Du H, Huang Q, Wei S, Huang R and
Sun W: Tanshinone IIA induces apoptosis in fibroblast-like
synoviocytes in rheumatoid arthritis via blockade of the cell cycle
in the G2/M phase and a mitochondrial pathway. Biol Pharm Bull.
37:1366–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kay J and Upchurch KS: ACR/EULAR 2010
rheumatoid arthritis classification criteria. Rheumatology
(Oxford):. 51 (Suppl 6):vi5–vi9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nizamutdinova IT, Kim YM, Jin H, Son KH,
Lee JH, Chang KC and Kim HJ: Tanshinone IIA inhibits TNF-α-mediated
induction of VCAM-1 but not ICAM-1 through the regulation of GATA-6
and IRF-1. Int Immunopharmacol. 14:650–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin C, Wang L, Wang H, Yang L, Guo H and
Wang X: Tanshinone IIA inhibits breast cancer stem cells growth in
vitro and in vivo through attenuation of IL-6/STAT3/NF-κB signaling
pathways. J Cell Biochem. 114:2061–2070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li P, Cook JA, Gilkeson GS, Luttrell LM,
Wang L, Borg KT, Halushka PV and Fan H: Increased expression of
beta-arrestin 1 and 2 in murine models of rheumatoid arthritis:
Isoform specific regulation of inflammation. Mol Immunol. 49:64–74.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan W, Petznick A, Heryati S, Rifada M and
Tong L: Nuclear factor-κB: Central regulator in ocular surface
inflammation and diseases. Ocul Surf. 10:137–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng C, Yin Q and Wu H: Structural
studies of NF-κB signaling. Cell Res. 21:183–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thakur BK, Chandra A, Dittrich T, Welte K
and Chandra P: Inhibition of SIRT1 by HIV-1 viral protein Tat
results in activation of p53 pathway. Biochem Biophys Res Commun.
424:245–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao W, Kruse JP, Tang Y, Jung SY, Qin J
and Gu W: Negative regulation of the deacetylase SIRT1 by DBC1.
Nature. 451:587–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuno A, Hori YS, Hosoda R, Tanno M, Miura
T, Shimamoto K and Horio Y: Resveratrol improves cardiomyopathy in
dystrophin-deficient mice through SIRT1 protein-mediated modulation
of p300 protein. J Biol Chem. 288:5963–5972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zelová H and Hošek J: TNF-α signalling and
inflammation: Interactions between old acquaintances. Inflamm Res.
62:641–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neurath MF and Finotto S: IL-6 signaling
in autoimmunity, chronic inflammation and inflammation-associated
cancer. Cytokine Growth Factor Rev. 22:83–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seriolo B, Paolino S, Sulli A, Fasciolo D
and Cutolo M: Effects of anti-TNF-alpha treatment on lipid profile
in patients with active rheumatoid arthritis. Ann NY Acad Sci.
1069:414–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishimoto N, Hashimoto J, Miyasaka N,
Yamamoto K, Kawai S, Takeuchi T, Murata N, van der Heijde D and
Kishimoto T: Study of active controlled monotherapy used for
rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): Evidence of
clinical and radiographic benefit from an × ray reader-blinded
randomised controlled trial of tocilizumab. Ann Rheum Dis.
66:1162–1167. 2007. View Article : Google Scholar : PubMed/NCBI
|