Introduction
Ischemic stroke, the leading cause of mortality and
long-term disability among patients with stroke, accounts for 85%
of all stroke cases (1). The
occlusion of a blood vessel caused by the formation of thrombus or
embolus in the brain are the most common processes resulting in
ischemic stroke (2). For patients
with ischemic stroke, the most effective therapy is to restore the
blood circulation as soon as possible (3). However, reperfusion is associated with
potential risks, including cerebral infarction, cerebral edema,
nerve function deficit and cerebral microcirculatory damage,
collectively known as cerebral ischemia/reperfusion (I/R) injury
(4). Previous studies have indicated
that cerebral I/R injury is accompanied by a series of mechanisms,
including inflammation, oxidative stress and apoptosis, which serve
roles in the subsequent disability and mortality (5,6).
Therefore, alleviation of the inflammatory response, oxidative
stress and cell apoptosis is one of the most important approaches
to promote the recovery from the ischemic stroke.
β-patchoulene (β-PAE; C15H24) extracted from the
Pogostemon cablin is an active natural tricyclic
sesquiterpene (7). The results
obtained in the current study demonstrated that β-PAE may exhibit a
potent anti-inflammatory effect as previously reported (8). Furthermore, a previous study indicated
that β-PAE may serve as an antioxidant in a mouse model (9). The above results indicated that β-PAE
may be used for the treatment of cerebral I/R injury.
Toll-like receptor 4 (TLR4), a germline-encoded
pattern recognition receptor, serves a role in the regulation of
inflammation (10). As an important
target gene of TLR4, nuclear factor-κB (NF-κB) regulates the
production of inflammatory cytokines, including tumor necrosis
factor-α (TNF-α), interleukin (IL)-1β and IL-6 at the
transcriptional level (11). A
previous study revealed that the TLR4/NF-κB signaling pathway was
strongly activated during the development of cerebral I/R injury
(12). Therefore, suppressing the
activity of the TLR4/NF-κB signaling pathway represents a potential
neuroprotective therapeutic strategy for the treatment of cerebral
I/R injury. A previous study indicated that β-PAE may inhibit the
NF-κB pathway in a number of ways (9). Therefore, β-PAE may serve a protective
role in cerebral I/R injury by inactivating the NF-κB pathway.
The current study used a rat model of middle
cerebral artery occlusion (MCAO) to study the effect of β-PAE on
cerebral I/R injury and investigate the underlying mechanisms.
Materials and methods
Experimental animals
A total of 32 specific pathogen free male
Sprague-Dawley rats (age, 8 weeks; weight, 80–120 g) were obtained
from Experimental Animal Center of Hebei Province (Shijiazhuang,
China) and housed in a controlled environment at 25±3°C and 60%
humidity, in a 12-h light/dark cycle with free access to food and
water. The experiment was approved by The Ethics Committee of
Cangzhou Central Hospital (Cangzhou, China). All rats were
maintained in a specific pathogen free environment with free access
to food and water for 7 days.
Group allocation and the animal model
of focal cerebral ischemia
β-PAE was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany; purity >97%). All rats were randomly divided
into four groups (n=8/group): Sham, β-PAE, I/R and I/R + β-PAE.
Prior to surgery, rats in the β-PAE and I/R + β-PAE groups
were pretreated with β-PAE (10 mg/kg body weight in normal saline)
by tail intravenous injection. The dose of 10 mg/kg of β-PAE was
selected based on previously published data. Liu et al
(13) indicated that high dose (100
µmol/l) of β-PAE resulted in significant cytotoxicity to GES-1
cells. Furthermore, according to previous reports, 10 mg/kg of
β-PAE may exert protective effects on a number of pathological
processes, including acute lung injury (14), gastric ulcer (7) and inflammatory disorders (9). Following injection for 1 h, the focal
cerebral ischemia injury model was generated in the I/R and I/R +
β-PAE groups through thread embolism as previously described
(15). Rats were anesthetized with
an intraperitoneal injection of pentobarbital sodium (30 mg/kg body
weight; Sigma-Aldrich; Merck KGaA) prior to MCAO. Rats were
euthanized by intraperitoneal injection of pentobarbital sodium
(200 mg/kg body weight) prior to collecting the brain tissues. The
external carotid artery and left common carotid artery were exposed
via a midline neck incision. A 6-0 nylon monofilament was used to
occlude the origin of the MCA and block the blood flow. After 2 h
of occlusion, reperfusion was accomplished by filament withdrawal,
and the incision was sutured. For sham and β-PAE group rats,
external carotid artery and left common carotid artery were exposed
without MCA occlusion. During surgery, the internal temperature of
rats was maintained at 37±0.5°C using a heating pad. Rats were used
for subsequent experiments after 24 h.
Measurement of the brain infarct
volume
The cerebral infract volume was assessed using the
2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich; Merck
KGaA) staining. Briefly, brain tissues were isolated from rats
(n=8) and fixed in 4% buffered paraformaldehyde at 4°C for 20 min,
embedded in paraffin and 3-µm-thick slides were cut. Following
dewaxing, slides were stained with 2% TTC for 30 min at room
temperature, followed by fixation with 10% formaldehyde neutral
buffer solution overnight at 4°C. Red staining was observed in the
normal healthy areas and the infract areas were unstained. Infarct
volume was evaluated using the ‘Count/Size’ tool of Image-Pro
software (version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA).
Brain water content
The brain of each rat was isolated gently and
rapidly within <2 min. Each brain was weighted immediately using
an electronic balance (Sartorius AG, Göttingen, Germany).
Subsequently, brains were dried at 100°C for 24 h in an oven and
weighed again. Brain water content was calculated using the
following equation: (Wet weight-dry weight)/wet weight ×100%.
Modified Neurological Severity Scores
(mNSS)
Neurological deficit was measured using mNSS as
previously described (16). Higher
scores indicated increased severity of the injury. The following
scoring criteria were used: Score 0, normal behavior, no observable
neurological deficits; score 1, failure to extend contralateral
forelimbs; score 2, contralateral circling when walking; score 3,
contralateral falling over when walking; score 4, inability to walk
and no spontaneous motor activity.
Detection of mitochondrial membrane
potential (MMP)
Chondriosomes were extracted from the hippocampi in
each group as previously described (17). Briefly, the hippocampus was isolated
from each brain gently and homogenized using a Teflon glass
homogenizer at 0°C in a mixed medium including mannitol, 220 mM;
sucrose, 70 mM; 4-(2-hydroxyethyl)-1-piperazineethanesulphonic
acid, 2 mM; EGTA, 0.2 mM and bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA), 0.5 mg/ml; pH 7.2. The samples were
centrifuged twice and the mitochondrial sediment was washed twice
with the isolation medium (Sangon Biotech Co., Ltd., Shanghai,
China) for 10 min at 8,000 × g at 4°C. The mitochondrial pellet was
re-suspended in cold isolation medium without EGTA. Mitochondrial
permeability transition pore (MPTP) was detected using the MMP
colorimetric method kit (cat. no. C2006; Beyotime Institute of
Biotechnology, Haimen, China) with a microplate reader at a
wavelength of 450 nm.
Flow cytometry assay
Rats (n=8) in each group were euthanized by
intraperitoneal injection of pentobarbital sodium (200 mg/kg body
weight) and subjected to perfusion prior to brain collection.
Heparin (0.01%) was injected into the tail vein of the rats.
Subsequently, brains were lavaged by cold normal saline through the
aorta. Afterwards, brains were rinsed with PBS, cut using
ophthalmic scissors, vortexed and filtered through a 200-µm nylon
mesh. Cell suspensions were collected and centrifuged at 3,000 × g
at 4°C for 5 min. The pelleted samples were re-suspended in PBS and
filtered through a 300-µm nylon mesh and centrifuged at 3,000 × g
at 4°C for 5 min to collect the cells. Cells were stained with
Annexin-V allophycocyanin and propidium iodide (PI) using a flow
cytometry kit (cat. no. 00-4300-54; both Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) as previously described (18). Cell apoptosis was detected using a
FACSCalibur flow cytometer (Becton, Dickinson and Company,
Franklin, Lakes, NJ, USA).
Western blotting
Total protein, nucleoprotein and cytoplasmic protein
were extracted from brain tissues as previously described (19). The concentration of protein was
measured using the bicinchoninic acid protein assay (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). Subsequently,
50 µg of protein samples were separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). After blocking with 5% BSA (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 1 h at room temperature,
membranes loaded with total proteins were incubated with the
following antibodies: Rabbit anti-rat apoptosis regulator BAX (Bax;
cat. no. 14796; 1:1,000), apoptosis regulator Bcl-2 (Bcl-2; cat.
no. 3498; 1:1,000), TNF-α (cat. no. 8184; 1:1,000), IL-1β (cat. no.
12703; 1:1,000), IL-6 (cat. no. 12912; 1:1,000), TLR4 (cat. no.
14358; 1:1,000), IκBα (cat. no. 4812; 1:1,000), β-actin (cat. no.
4970; 1:1,000; all Cell Signaling Technology, Inc., Danvers, MA,
USA) overnight at 4°C. Membranes loaded with nucleoproteins were
incubated with the following antibodies: Rabbit anti-rat NF-κB
(cat. no. 8242; 1:1,000; Cell Signaling Technology, Inc.) and Lamin
B (cat. no. sc-6216; 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C. Membranes loaded with
cytoplasmic proteins were incubated with the following antibodies:
Rabbit anti-rat NF-κB (cat. no. 8242; 1:1,000) and β-actin (cat.
no. 4970; 1:1,000; both Cell Signaling Technology, Inc.) overnight
at 4°C. Subsequently, membranes were incubated with secondary
antibodies (dilution, 1:5,000; Santa Cruz Biotechnology, Inc.) for
1.5 h at room temperature. The immunoreactive bands were observed
using an enhanced chemiluminescence reagent kit (Bio-Rad
Laboratories, Inc.) and analyzed using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
The mRNA expression levels of TNF-α, IL-1β and IL-6
were measured using RT-qPCR. Total RNA was extracted from brain
tissues with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The extracted RNA was reverse transcribed
into cDNA using the ThermoScript™ RT-PCR system at 40°C for 2 h
(Invitrogen; Thermo Fisher Scientific, Inc.). The reaction was
performed at 37°C for 30 min, followed by 42°C for 30 min and 85°C
for 5 min. Transcripts were quantified by qPCR using a Bio-Rad
CFX96 real-time PCR detection system with SYBR-Green IQTM Supermix
(both Bio-Rad Laboratories, Inc.). The PCR reaction was performed
at 95°C for 3 min, followed by 40 cycles of 95°C for 12 sec and
60°C for 40 sec. An internal control (GAPDH) was used to normalize
the expression of the target genes. Quantification of TNF-α, IL-1β
and IL-6 levels was conducted using the CFX96™ Real-Time PCR
detection system (Bio-Rad Laboratories, Inc.). The primers were
purchased from Sangon Biotech Co., Ltd. The following primers were
used: TNF-α forward, 5′-ACATACTGACCCACGGCTTC-3′ and reverse,
3′-TCACCCATCCCATCTCTCTC-5′; IL-1β forward,
5′-CAGGCAGGCAGTATCACTCA-3′ and reverse, 3′-AGGCCACAGGTATTTTGTCG-5′;
and IL-6 5′-forward, GGCGGATCGGATGTTGTGAT-3′ and reverse,
3′-GGACCCCAGACAATCGGTTG-5′. The relative quantification of the gene
expression was calculated using the 2−ΔΔCq method
(20).
Caspase-3 activity measurement
Caspase-3 colorimetric assay kit (cat. no. 610322;
BD Biosciences, San Jose, CA, USA) was used to determine the
caspase-3 activity in brain tissues according to the manufacturer's
protocol. The results were evaluated using a microplate reader
(Bio-Rad Laboratories, Inc.) at a wavelength of 405 nm.
Measurement of mitochondrial
superoxide generation
Mitochondrial superoxide generation was measured by
superoxide flashes in single mitochondria in living brain cells
extracted from rats in each group as previously described (21). At least 20 brain cells/culture were
evaluated in each group.
Determination of the oxidative damage
parameters
Brain tissues isolated from rats were homogenized in
normal saline and centrifuged at 3,000 × g at 4°C for 10 min.
Supernatants were collected and the levels of malondialdehyde (MDA;
cat. no. A003-1), superoxide dismutase (SOD; cat. no. A001-1-1) and
glutathione peroxidase (GSH-Px; cat. no. A005) were detected
according to the manufacturer's protocol of the corresponding
detection kits (Nanjing Jiancheng Bioengineering Institute) using
the UV/Vis Spectrophotometer (Beijing Lab Tech Instrument Co.,
Beijing, China). The absorbance was measured at a wavelength of
532, 420 and 340 nm for MDA, SOD and GSH-Px, respectively.
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance followed by Bonferroni's post hoc test
was used to analyze the differences between groups using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA OR SPSS).
P<0.05 was considered to indicate a statistically significant
difference.
Results
β-PAE alleviates cerebral I/R
injury
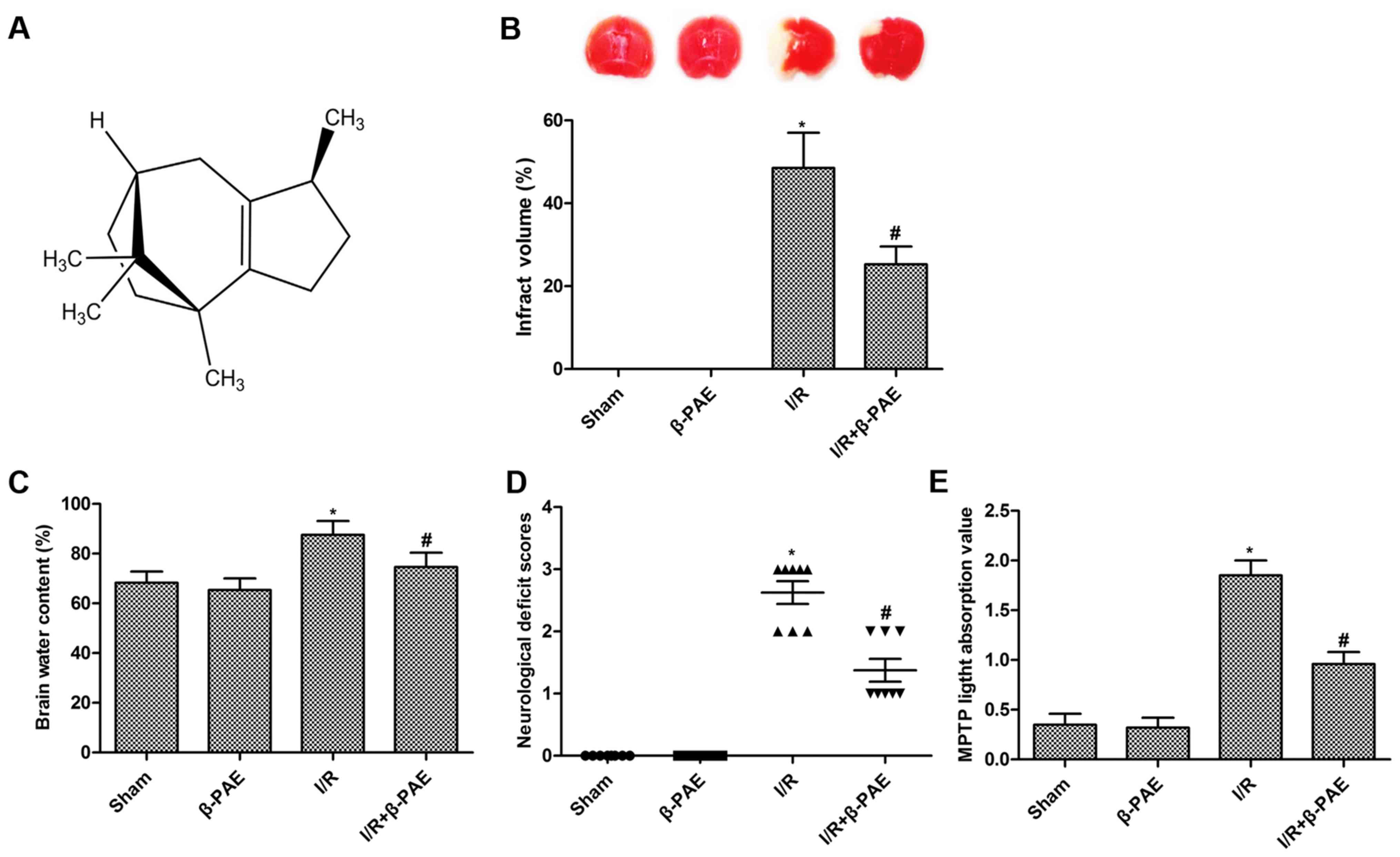
The structure of β-PAE is presented in Fig. 1A. To investigate the effect of β-PAE
on cerebral I/R injury, rats with cerebral I/R were treated with
β-PAE. The results indicated that treatment with β-PAE had no
impact on the infarction volume (Fig.
1B), brain water content (Fig.
1C), neurological function (Fig.
1D) and MMP (Fig. 1E) of rats
without I/R injury. However, the elevated infarct volume, brain
water content, mNSS and MMP induced by I/R injury were
significantly suppressed by treatment with β-PAE (Fig. 1). These results indicated that β-PAE
may markedly attenuate the cerebral I/R injury.
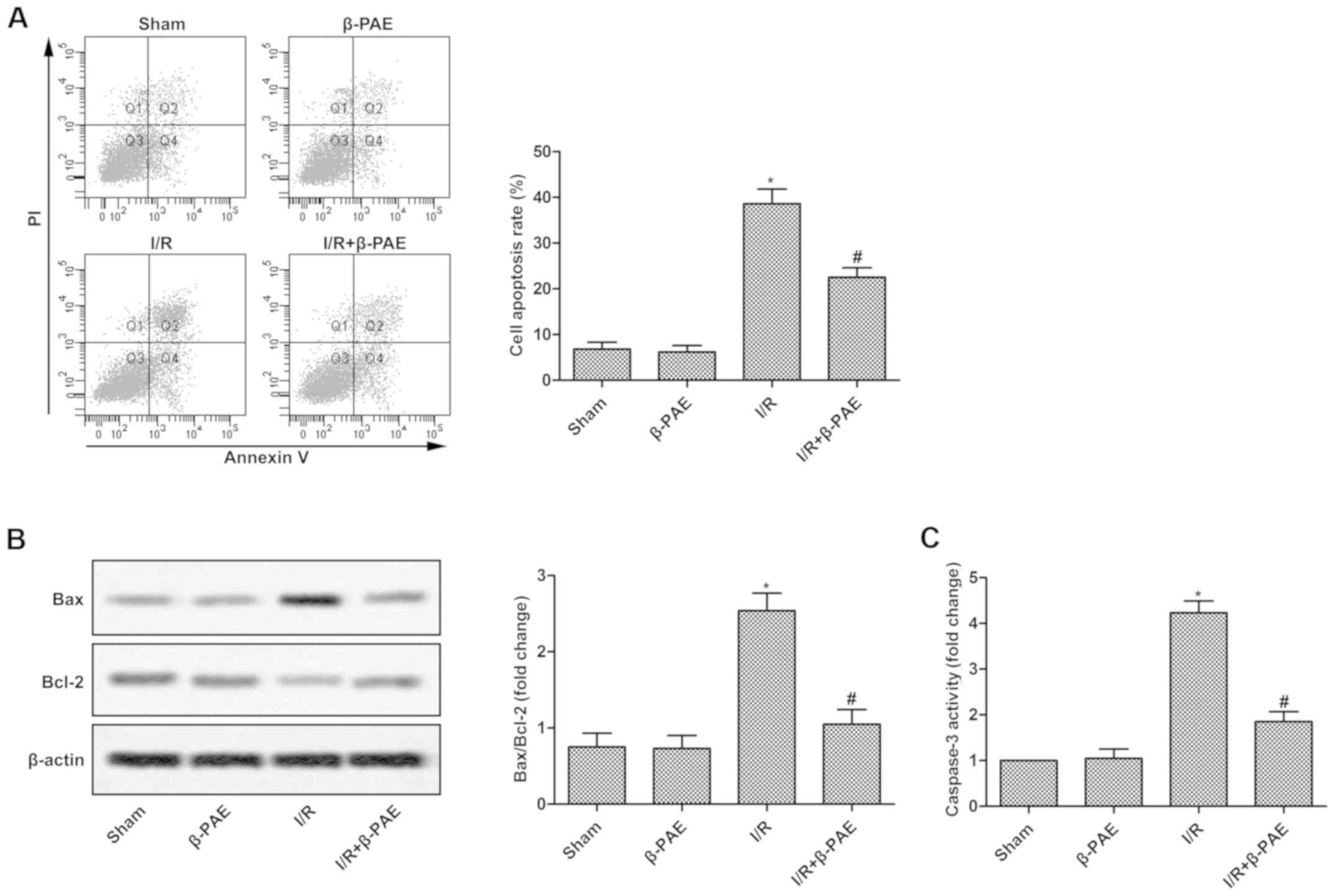
β-PAE decreases the I/R-induced cell
apoptosis
To measure the effect of β-PAE on the apoptosis of
brain cells, cell apoptosis rate and the expression levels of
associated proteins were measured. Cell apoptosis rate (Fig. 2A), Bax/Bcl-2 ratio (Fig. 2B) and casapase-3 activity (Fig. 2C) were not altered in the β-PAE group
compared with the sham group. However, cell apoptosis, Bax/Bcl-2
ratio and casapase-3 activity were significantly decreased in the
I/R + β-PAE group compared with the I/R group (Fig. 2). These results indicate that β-PAE
may markedly decrease brain cell apoptosis in rats with cerebral
I/R injury.
β-PAE reduces the inflammatory
response and oxidative stress
To determine whether β-PAE was associated with the
inflammatory response and oxidative stress induced by I/R injury in
brain tissues, the relevant inflammatory factors and oxidative
damage parameters were detected. Results suggested that there were
no marked differences in the expression levels of inflammatory
factors, including TNF-α, IL-1β and IL-6, and oxidative damage
parameters, including superoxide generation, MDA, GSH-Px and SOD,
between the sham and β-PAE groups. However, the TNF-α, IL-1β and
IL-6 expression, superoxide generation and MDA levels were reduced
while the levels of GSH-Px and SOD were increased in the I/R +
β-PAE group compared with the I/R group (Fig. 3). These results revealed that the
inflammatory response and oxidative stress were markedly suppressed
following treatment with β-PAE.
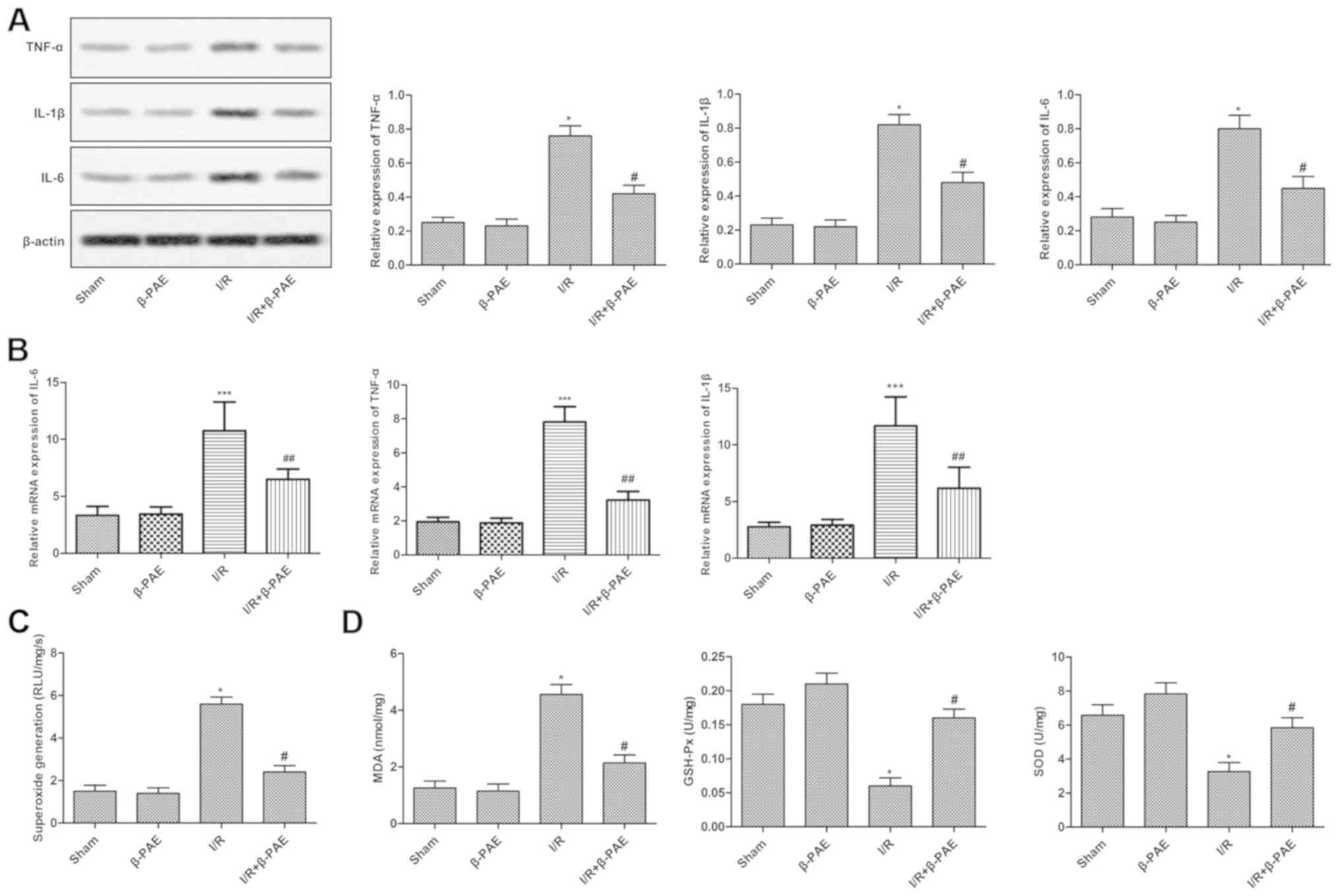 | Figure 3.β-PAE reduces the inflammatory
response and oxidative stress of rats with the I/R injury. (A)
Western blotting was used to analyze the expression levels of
TNF-α, IL-1β and IL-6 in the brain tissue. (B) The mRNA levels of
TNF-α, IL-1β and IL-6 were measured using reverse
transcription-quantitative polymerase chain reaction. (C)
Superoxide flashes were measured to evaluate mitochondrial
superoxide generation in brain tissues. (D) Oxidative damage
parameters of brain cells, including MDA, GSH-Px and SOD levels
were measured using the corresponding detection kits. Experiments
were repeated at least three times, and data are presented as the
mean ± standard deviation. *P<0.05, ***P<0.001 vs. sham
group; #P<0.05, ##P<0.01 vs. the I/R group. β-PAE,
β-patchoulene; I/R, ischemia-reperfusion; TNF, tumor necrosis
factor; IL, interleukin; MDA, malondialdehyde; GSH-Px, glutathione
peroxidase; SOD, superoxide dismutase. |
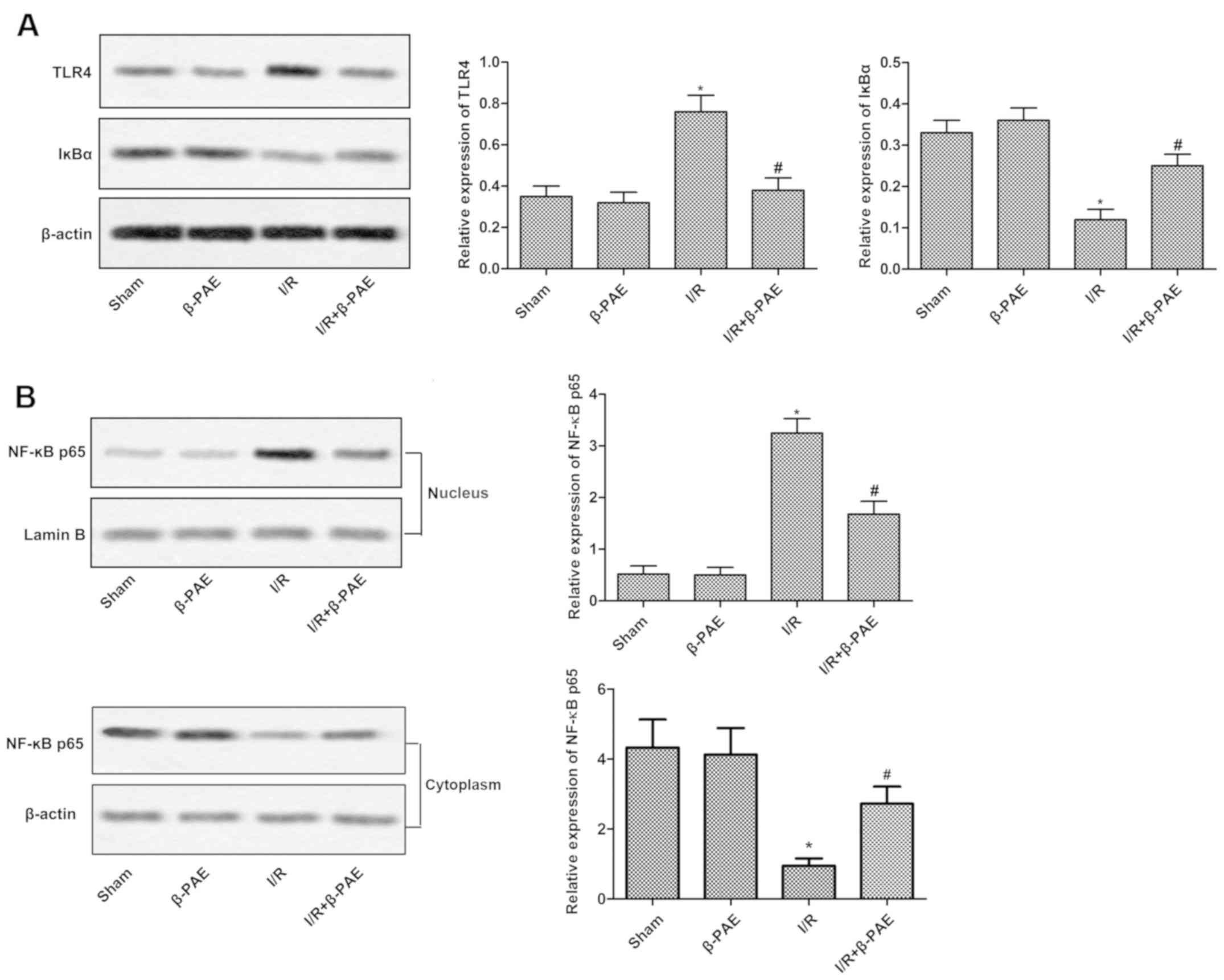
β-PAE inactivates the TLR4/NF-κB
signaling pathway
To further investigate the underlying mechanisms of
the effect of β-PAE on cerebral I/R injury, the activity of the
TLR4/NF-κB signaling pathway was measured using western blotting.
There was no difference in the TLR4 and IκBα expression in the
cytoplasm and nucleus. No difference of the NF-κB p65 expression in
the nucleus was observed between the β-PAE group and I/R + β-PAE
group (Fig. 4). However, the total
protein expression of TLR4 and nuclear expression of NF-κB p65 were
suppressed while the total protein expression of IκBα and
cytoplasmic protein expression of NF-κB p65 were increased in the
I/R + β-PAE group compared with the I/R group. These results
suggested that the TLR4/NF-κB pathway may be inactivated following
treatment with β-PAE.
Discussion
Stroke imposes a significant socio-economic burden
globally (22). At present,
restoration of the blood supply is the only effective therapy
available for patients with ischemic stroke (3). However, brain cell damage induced by
cerebral ischemia may be further aggravated when the blood supply
is recovered in the brain tissue, resulting in cerebral I/R injury
(23). Uncontrolled cerebral I/R
injury eventually exacerbates cerebral infarction, cerebral edema
and nerve injury (24). Therefore,
it is necessary to develop effective therapeutic methods to
ameliorate cerebral I/R injury.
A number of studies indicated that numerous extracts
from Chinese herbs serve positive roles in the treatment of I/R
injury, including salvianolic acid B (25), trans-polydatin (26), vitexin (27) and triptolide (28). β-PAE, an active ingredient of the
Pogostemon cablin, exhibits a broad spectrum of
pharmacological functions, including anti-inflammatory and
antioxidative activities (4,13). Previous studies indicated that oxygen
supply and neuroinflammation are the major causes of cell death and
cerebral edema during the development of cerebral I/R injury
(29). Therefore, β-PAE may be
useful in the treatment of cerebral I/R injury. In the current
study, pretreatment with β-PAE effectively reduced the infarct
volume, decreased the brain water content, mitigated the
neurological impairment and recovered the MMP.
Apoptosis serves an important role in the
progression of cerebral I/R injury (30). A previous study indicated that the
expression level of caspase-3, Bax/Bcl-2 ratio and rate of cellular
apoptosis were elevated during cerebral I/R (31). Furthermore, increased caspase-3
levels and Bax/Bcl-2 ratio resulted in elevated chromatin
condensation, mitochondrial membrane permeabilization, DNA
fragmentation and cell apoptosis (32). Therefore, preventing the apoptosis of
nerve cells is considered to be a suitable therapeutic target for
limiting the volume of the cerebral infarct after stroke (33). In the current study, pretreatment
with β-PAE markedly reduced cell apoptosis, Bax/Bcl-2 ratio and
caspase-3 activity.
Ischemic hypoxia results in an increased number of
free radicals during reperfusion, and such reperfusion stimulating
extensive oxidative damage is considered one of the major reasons
for brain cell damage and death in I/R injury (34). Tan et al (30) observed that oxidative stress was
induced by cerebral I/R injury as indicated by suppressed SOD and
GSH-Px levels, and increased MDA level (35). Numerous researchers have demonstrated
the anti-oxidative effect of β-PAE. According to Wu et al
(5), β-PAE inhibited MAD expression
in LPS-induced lung injury (8). A
previous study revealed that pretreatment with β-PAE at doses of
10, 20 and 40 mg/kg markedly enhanced the SOD, CAT and GSH
activity, and significantly suppressed the MDA level in
ethanol-induced gastric injury (13). In the current study, superoxide
generation and MDA levels increased while the GSH-Px and SOD
activities decreased in rats with cerebral I/R injury. However,
pretreatment with β-PAE reversed these alterations.
The TLR4 signaling pathway is associated with the
occurrence and development of the ischemic stroke (36). Interaction between TLR4 and MyD88
results in the activation of NF-κB (37). NF-κB, a ubiquitous transcription
factor participating in the inflammatory and immune responses, is
inhibited by IκBα proteins (38).
The activation of NF-κB leads to the synthesis of inflammatory
cytokines, including IL-1β, IL-6 and TNF-α. A previous study
indicated that proinflammatory responses exacerbate cerebral I/R
injury, especially at the early stage of this condition (39). β-PAE exhibits well-established
anti-inflammatory abilities and is widely used in the treatment of
numerous inflammatory diseases (7,8).
According to Chen et al (8),
increased secretion of TNF-α, IL-6 and IL-1β induced by LPS was
markedly suppressed by pretreatment with β-PAE, and this effect may
have occurred due to the inactivation of the NF-κB signaling
pathway in the bronchoalveolar lavage fluid. In addition, in the
carrageenan-induced paw edema, pretreatment with β-PAE inhibited
the release of TNF-α, IL-1β, IL-6, prostaglandin E2 and nitric
oxide in a dose-dependent manner (9). Furthermore, the overexpression of
TNF-α, IL-6 and IL-1β was significantly suppressed by β-PAE through
the inactivation of the NF-κB signaling pathway in ethanol-induced
gastric injury in rat model (13).
The current study revealed that the activity of the TLR4/NF-κB
signaling pathway was decreased following pretreatment with β-PAE
due to the inhibition of TLR4 expression, enhanced IκBα activity
and suppressed nuclear translocation of NF-κB p65 in brain tissues
with I/R injury. In addition, the expression levels of TNF-α, IL-1β
and IL-6 were suppressed following pretreatment with β-PAE.
In conclusion, to the best of our knowledge, the
current study is the first to reveal that β-PAE may induce
neuroprotective effects on cerebral I/R injury. The protective
function was demonstrated by the decreased infarct volume, reduced
brain water content, alleviated neurological dysfunction, restored
MMP and decreased cell apoptosis rates. The underlying mechanism
may include the inhibition of oxidative stress via suppressing
superoxide generation and MDA level, and increasing the levels of
GSH-Px and SOD. Furthermore, the release of proinflammatory
factors, including TNF-α, IL-1β and IL-6 was suppressed by β-PAE
through the inactivation of the TLR4/NF-κB signaling pathway. The
present results suggest that β-PAE may be further developed as a
potential therapeutic agent in the treatment of cerebral I/R
injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FBZ constructed the middle cerebral artery occlusion
model and performed western blotting; JPW and HXZ were involved in
the in evaluation of Modified Neurological Severity Scores, infarct
volume and statistical analysis. GMF conducted the mitochondrial
membrane potential assay. XC conducted reverse
transcription-quantitative polymerase chain reaction. XC was
responsible for the design of the present study and drafting the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Cangzhou Central Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GSH-Px
|
glutathione peroxidase
|
|
I/R
|
ischemia/reperfusion
|
|
IL
|
interleukin
|
|
MCAO
|
middle cerebral artery occlusion
|
|
MDA
|
malondialdehyde
|
|
MMP
|
mitochondrial membrane potential
|
|
NF-κB
|
nuclear factor-κB
|
|
SOD
|
superoxide dismutase
|
|
TLR4
|
Toll-like receptor 4
|
|
TNF-α
|
tumor necrosis factor-α
|
|
β-PAE
|
β-patchoulene
|
References
|
1
|
Zhao L, Liu X, Liang J, Han S, Wang Y, Yin
Y, Luo Y and Li J: Phosphorylation of p38 MAPK mediates hypoxic
preconditioning-induced neuroprotection against cerebral ischemic
injury via mitochondria translocation of Bcl-xL in mice. Brain Res.
1503:78–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo
C, Peng J, Li J, Yung KK and Mo Z: Neuroprotective effects of
bilobalide on cerebral ischemia and reperfusion injury are
associated with inhibition of pro-inflammatory mediator production
and down-regulation of JNK1/2 and p38 MAPK activation. J
Neuroinflammation. 11:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chien A and Vinuela F: Analyzing Circle of
Willis blood flow in ischemic stroke patients through 3D stroke
arterial flow estimation. Interv Neuroradiol. 23:427–432. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao P, Zhou R, Zhu XY, Hao YJ, Li N, Wang
J, Niu Y, Sun T, Li YX and Yu JQ: Matrine attenuates focal cerebral
ischemic injury by improving antioxidant activity and inhibiting
apoptosis in mice. Int J Mol Med. 36:633–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu J, Chen Y, Yu S, Li L, Zhao X, Li Q,
Zhao J and Zhao Y: Neuroprotective effects of sulfiredoxin-1 during
cerebral ischemia/reperfusion oxidative stress injury in rats.
Brain Res Bull. 132:99–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou L, Bondy SC, Jian L, Wen P, Yang F,
Luo H, Li W and Zhou J: Tanshinone IIA attenuates the cerebral
ischemic injury-induced increase in levels of GFAP and of
caspases-3 and −8. Neuroscience. 288:105–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu JZ, Liu YH, Liang JL, Huang QH, Dou YX,
Nie J, Zhuo JY, Wu X, Chen JN, Su ZR and Wu QD: Protective role of
β-patchoulene from Pogostemon cablin against indomethacin-induced
gastric ulcer in rats: Involvement of anti-inflammation and
angiogenesis. Phytomedicine. 39:111–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen XY, Dou YX, Luo DD, Zhang ZB, Li CL,
Zeng HF, Su ZR, Xie JH, Lai XP and Li YC: β-Patchoulene from
patchouli oil protects against LPS-induced acute lung injury via
suppressing NF-κB and activating Nrf2 pathways. Int
Immunopharmacol. 50:270–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Chen X, Chen H, Wang L, Liang J,
Luo D, Liu Y, Yang H, Li Y, Xie J and Su Z: Anti-inflammatory
activity of β-patchoulene isolated from patchouli oil in mice. Eur
J Pharmacol. 781:229–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bi Y, Zhu Y, Zhang M, Zhang K, Hua X, Fang
Z, Zhou J, Dai W, Cui Y, Li J and You T: Effect of shikonin on
spinal cord injury in rats via regulation of HMGB1/TLR4/NF-kB
signaling pathway. Cell Physiol Biochem. 43:481–491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ntoufa S, Vilia MG, Stamatopoulos K, Ghia
P and Muzio M: Toll-like receptors signaling: A complex network for
NF-κB activation in B-cell lymphoid malignancies. Semin Cancer
Biol. 39:15–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Su L, Zhang X, Zhang C, Wang L, Li
Y, Zhang Y, He T, Zhu X and Cui L: Ulinastatin downregulates TLR4
and NF-kB expression and protects mouse brains against
ischemia/reperfusion injury. Neurol Res. 39:367–373. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Liang J, Wu J, Chen H, Zhang Z,
Yang H, Chen L, Chen H, Su Z and Li Y: Transformation of patchouli
alcohol to β-patchoulene by gastric juice: β-patchoulene is more
effective in preventing ethanol-induced gastric injury. Sci Rep.
7:55912017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Sanberg PR, Li Y, Wang L, Lu M,
Willing AE, Sanchez-Ramos J and Chopp M: Intravenous administration
of human umbilical cord blood reduces behavioral deficits after
stroke in rats. Stroke. 32:2682–2688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong D, Deng Y, Huang B, Yin C, Liu B,
Shi J and Gong Q: Icariin attenuates cerebral ischemia-reperfusion
injury through inhibition of inflammatory response mediated by
NF-κB, PPARα and PPARγ in rats. Int Immunopharmacol. 30:157–162.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Wang L, Ning FB, Wang T, Liang YC
and Liu YL: Erythropoietin reduces hippocampus injury in neonatal
rats with hypoxic ischemic brain damage via targeting matrix
metalloprotein-2. Eur Rev Med Pharmacol Sci. 21:4327–4333.
2017.PubMed/NCBI
|
|
18
|
Tao T, Li CL, Yang WC, Zeng XZ, Song CY,
Yue ZY, Dong H and Qian H: Protective effects of propofol against
whole cerebral ischemia/reperfusion injury in rats through the
inhibition of the apoptosis-inducing factor pathway. Brain Res.
1644:9–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan J, Guo X, Liu Z, Zhao X, Feng Y, Song
S, Cui C and Jiang P: Vitamin D receptor activation influences the
ERK pathway and protects against neurological deficits and neuronal
death. Int J Mol Med. 41:364–372. 2018.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Fang H, Groom L, Cheng A, Zhang W,
Liu J, Wang X, Li K, Han P, Zheng M, et al: Superoxide flashes in
single mitochondria. Cell. 134:279–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: Systematic analysis of population health data.
Lancet. 367:1747–1757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gomis M and Dávalos A: Recanalization and
reperfusion therapies of acute ischemic stroke: What have we
learned, what are the major research questions and where are we
headed? Front Neurol. 5:2262014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M, Wang J, Liu Z, Guo X, Wang N, Jia
N, Zhang Y and Yuan J: Effects of intermedin on autophagy in
cerebral ischemia/reperfusion injury. Neuropeptides. 68:15–21.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiao Z and Xu Y: Salvianolic acid B
alleviating myocardium injury in ischemia reperfusion rats. Afr J
Tradit Complement Altern Med. 13:157–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ming D, Songyan L, Yawen C, Na Z, Jing M,
Zhaowen X, Ye L, Wa D and Jie L: Trans-Polydatin protects the mouse
heart against ischemia/reperfusion injury via inhibition of the
renin-angiotensin system (RAS) and Rho kinase (ROCK) activity. Food
Funct. 8:2309–2321. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Che X, Wang X, Zhang J, Peng C, Zhen Y,
Shao X, Zhang G and Dong L: Vitexin exerts cardioprotective effect
on chronic myocardial ischemia/reperfusion injury in rats via
inhibiting myocardial apoptosis and lipid peroxidation. Am J Transl
Res. 8:3319–3328. 2016.PubMed/NCBI
|
|
28
|
Zhang B, Song C, Feng B and Fan W:
Neuroprotection by triptolide against cerebral ischemia/reperfusion
injury through the inhibition of NF-κB/PUMA signal in rats. Ther
Clin Risk Manag. 12:817–824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miao M, Yan X, Guo L and Shao S: Effects
of the Rabdosia rubescens total flavonoids on focal cerebral
ischemia reperfusion model in rats. Saudi Pharm J. 25:607–614.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan H, Chen L and Ma J: Penehyclidine
hydrochloride post-conditioning reduces
ischemia/reperfusion-induced cardiomyocyte apoptosis in rats. Exp
Ther Med. 14:4272–4278. 2017.PubMed/NCBI
|
|
31
|
Shabanzadeh AP, D'Onofrio PM, Monnier PP
and Koeberle PD: Targeting caspase-6 and caspase-8 to promote
neuronal survival following ischemic stroke. Cell Death Dis.
6:e19672015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren Z, Zhang R, Li Y, Li Y, Yang Z and
Yang H: Ferulic acid exerts neuroprotective effects against
cerebral ischemia/reperfusion-induced injury via antioxidant and
anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med.
40:1444–1456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Zhang L and Liang J: Activation of
the Nrf2 defense pathway contributes to neuroprotective effects of
phloretin on oxidative stress injury after cerebral
ischemia/reperfusion in rats. J Neurol Sci. 351:88–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoshino K, Takeuchi O, Kawai T, Sanjo H,
Ogawa T, Takeda Y, Takeda K and Akira S: Pillars Article: Cutting
edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive
to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J
Immunol. 162:3749–3752. 1999.PubMed/NCBI
|
|
37
|
Wang D, Wang X, Tong W, Cui Y, Li X and
Sun H: Umbelliferone alleviates lipopolysaccharide-induced
inflammatory responses in acute lung injury by down-regulating
TLR4/MyD88/NF-κB signaling. Inflammation. Jan 15–2019.(Epub ahead
of print). View Article : Google Scholar
|
|
38
|
Wan F and Lenardo MJ: The nuclear
signaling of NF-kappaB: Current knowledge, new insights, and future
perspectives. Cell Res. 20:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Fu B, Zhang X, Chen L, Zhang L,
Zhao X, Bai X, Zhu C, Cui L and Wang L: Neuroprotective effect of
bicyclol in rat ischemic stroke: Down-regulates TLR4, TLR9, TRAF6,
NF-κB, MMP-9 and up-regulates claudin-5 expression. Brain Res.
1528:80–88. 2013. View Article : Google Scholar : PubMed/NCBI
|