Stroke is a partial or complete brain dysfunction
syndrome accompanied by acute cerebral circulatory disorders
(1). Stroke globally affects
millions of individuals each year (2). Of note, its incidence rate exceeds that
of heart disease. In particular, stroke has major life-long
consequences. Ischemic stroke, accounting for 70–80% of all
strokes, is caused by disturbances in cerebral circulation, leading
to cerebral ischemia, hypoxia, neuronal apoptosis and necrosis,
thereby leading to dysfunctions. Reconstruction or enhancement of
blood flow in the ischemic region is the current key treatment
strategy for stroke-associated ischemic brain injury. Recent
developments in thrombolytic therapy have led to increases in free
radical production, with consequent damage to the self-regulation
mechanisms of brain tissues (3). The
latter may determine secondary damage to cerebral vessels following
cerebral ischemia/reperfusion. Of note, excessive perfusion may
lead to the failure of the self-regulation mechanisms of brain
tissues, thereby increasing the area of brain edema and aggravating
cerebral ischemia. At present, ischemia/reperfusion injury is
receiving an increased amount of attention; however,
neuroprotective drugs currently used for treating neuronal injury
caused by cerebral ischemia/reperfusion are unable to repair and
perfuse injured neurons (4,5). Of note, clinicians are currently unable
to obtain good results owing to the time constraints associated
with and several side effects of thrombolytic and traditional
medical therapies. The development of a novel drug capable of
dealing with the multiple mechanisms involved in
ischemia/reperfusion injury is warranted (6). In recent years, leptin has been linked
to the occurrence and development of cerebrovascular diseases. The
metabolism and mechanism of action of leptin is similar in animals
and humans; however, the effective dose of leptin used in animals
is not feasible for use in humans due to drug pharmacokinetics
(7,8). Leptin receptor binding reportedly
regulates energy metabolism, respiration, neuroendocrine function,
immune inflammation, and afferent nerve and neuron regeneration. An
obesity-associated gene encoding leptin (OB-Rs), to which leptin
binds, are widely distributed in humans and rodents. In particular,
OB-Rs are distributed in the arcuate nucleus of the hypothalamus
and the lateral hypothalamus as well as in the ventromedial,
paraventricular, supraoptic and ventral perimammary nuclei
(9). Of note, leptin receptors were
determined to be distributed in the cortex, hippocampus and
striatum in a murine model of ischemia/reperfusion with leptin
pre-conditioning (Fig 1) (10,11).
Leptin receptors in locations including the striatum have
protective roles (12). Studies have
demonstrated considerable reductions in excitatory amino acids
(EAAs) (13,14), oxygen free radicals, inflammatory
factors (15–17), mitochondrial damage and apoptosis in
leptin-preconditioned groups compared with those in untreated
groups (Fig. 2). Leptin has an
important role in decreasing excitatory neurotransmitter levels,
protecting the mitochondria, decreasing superoxide and free radical
formation, increasing anti-inflammatory factor and -apoptotic
protein levels, decreasing apoptotic protein levels, and avoiding
the traditional role of protecting the brain from single and
potential side effects. In the present review, the protective
mechanisms of leptin on the brain are summarized.
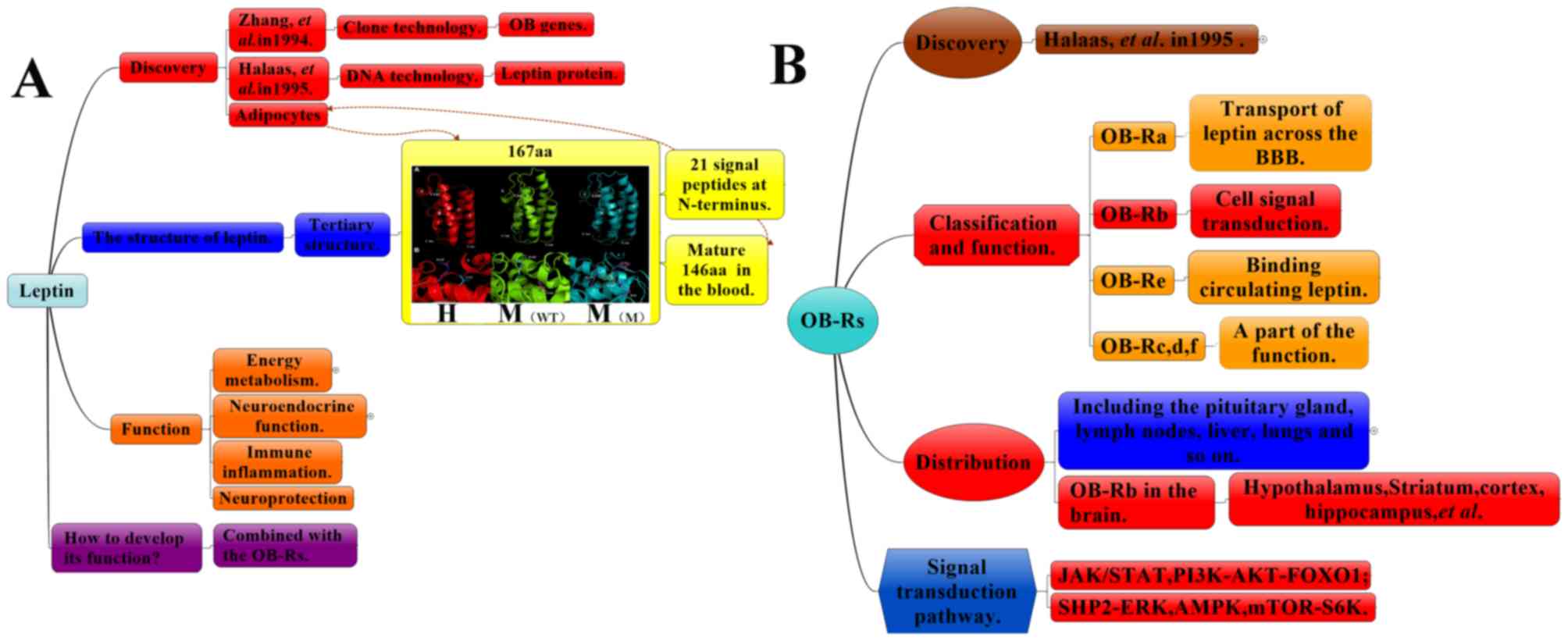
The leptin gene (OB gene) was originally produced by
two mutant mouse strains (ob/ob and db/db; leptin-deficient and
leptin-receptor-deficient, respectively) developed by the Jackson
Laboratory (Bar Harbor, ME, USA). These diabetic mice (db mice) had
obesity, polyphagia and polyuria as comorbidities (18). Obese mice (ob mice) encode an
appetite suppressor present in the blood, which was dectected by
conjoined symbiotic experiments. db mice lack the corresponding
receptor was discovered by conjoined symbiosis experiments
(19). In 1994, Zhang et al
(20) located the human OB gene
encoding the peptide hormone leptin to chromosome 7 (7q31.3) via
positional cloning. Subsequently, in 1995, Halaas et al
(21) reported on the synthesis of
the leptin protein. The metabolism of leptin may be summarized as
follows (22): Leptin is a 16-kDa
non-glycosylated protein encoded by the OB gene, which is located
on the human chromosome 7 and the mouse chromosome 6. The precursor
of leptin is a protein with 167 amino acids. During its secretion
into the blood, the signal peptide, composed of 21 hydrophilic
amino acid residues at the amino end, is hydrolyzed to form a
polypeptide chain composed of 146 amino acids (Fig. 1A). It is primarily synthesized by the
white adipose tissue, and leptin transport in the brain is
identical in humans and mice. Due to its large size, leptin cannot
passively cross the blood-brain barrier (BBB). Therefore, it is
transported across via a regulated saturable transport system
(23,24). Although the molecular identity of
this transporter system remains elusive, it appears to act
independently from leptin receptors. Studies (8,25,26) have
reported three mechanisms that aid the transport of leptin across
the BBB: Unidirectional Saturable (Leptin transport to
cerebrospinal fluid is saturated but not unlimited) transport of
leptin across the BBB; direct access of the leptin receptor neurons
to the circulation via projections close to fenestrated capillaries
(perivascular space) in circumventricular organs (e.g., median
eminence, area postrema and organum vasculum); and transport of
leptin by tanycytes into the cerebrospinal fluid within the
ventricular space. Leptin has strong hydrophilicity and is mostly
excreted by the kidneys. These mechanisms serve as the basis for
the administration of leptin through the lateral ventricle to
enhance its neuroprotective effects.
Leptin receptors (OB-Rs) are expressed in several
organs and tissue types, including the hypothalamus, pituitary
gland, lymph nodes, liver, lungs, uterus, adipose tissues, kidneys,
pancreas, stomach and gonads. Astrocytes are known to express
various isoforms of the leptin receptor (18–20).
OB-R splicing produces six isomers of the leptin receptor, named as
OB-Ra-f (Fig. 1B). Of those six,
OB-Rb is the longest receptor, and OB-Ra and OB-Rc are widely
distributed and are predominant in the brain's choroid plexus and
microvasculature. The distribution of leptin receptors is central
to the regulation of leptin transport, protein binding and the free
leptin in the blood through the BBB. The distribution of leptin
receptors regulate the amount of leptin passing through the BBB
(27). OB-Re exclusively contains
extracellular domains, circulating as a soluble receptor. By
contrast, other OB-Rs have identical N-terminals as well as
intracellular membranes and extracellular domains. OB-Rd and OB-Rf,
which are types of single transmembrane receptors, only have
partial functions. Among other OB-Rs, they are widely expressed in
the testicles, brain, liver, heart and lung tissues. OB-Rb
(Fig. 3), a leukocyte type-6
receptor encompassing an intracellular region, is considered the
only functional receptor involved in cellular signal transduction
(comprising intracellular, membrane and extracellular regions). The
extracellular region is a single transmembrane signal transduction
protein comprising two cytokines, combining the characteristic
sequences (cytokine receptor homology domains separated by an
immunoglobulin-like domain) and a fibronectin III region. The
membrane and intracellular regions are characterized by the same 29
amino acids of a highly conserved Box1 motif rich in proline, two
conservative Box2 motifs and three conservative tyrosine residues
(Tyr985, Tyr1077 and Tyr1138) (28).
Similarly, OB-Rb expresses various isoforms of the leptin receptor
and is prevalent in numerous areas of the mammalian brain,
including the cortex, hippocampus and striatum within neurons and
astrocytes, and in Schwann cells (29). Leptin receptors undergo mutagenesis
in the hypothalamus, hippocampus and prefrontal cortex of db/db
mice. Such an occurrence may be responsible for the decreased
neuroprotective functions of leptin following ischemia/reperfusion
injury. While leptin receptors do not possess intrinsic enzyme
activity, they may bind to tyrosine kinase in the cytoplasm.
Furthermore, (19,30) in the brain, exogenous leptin combined
with the OB-Rb has protective roles in the BBB. However, the
functions of short-form leptin receptors remain to be fully
elucidated.
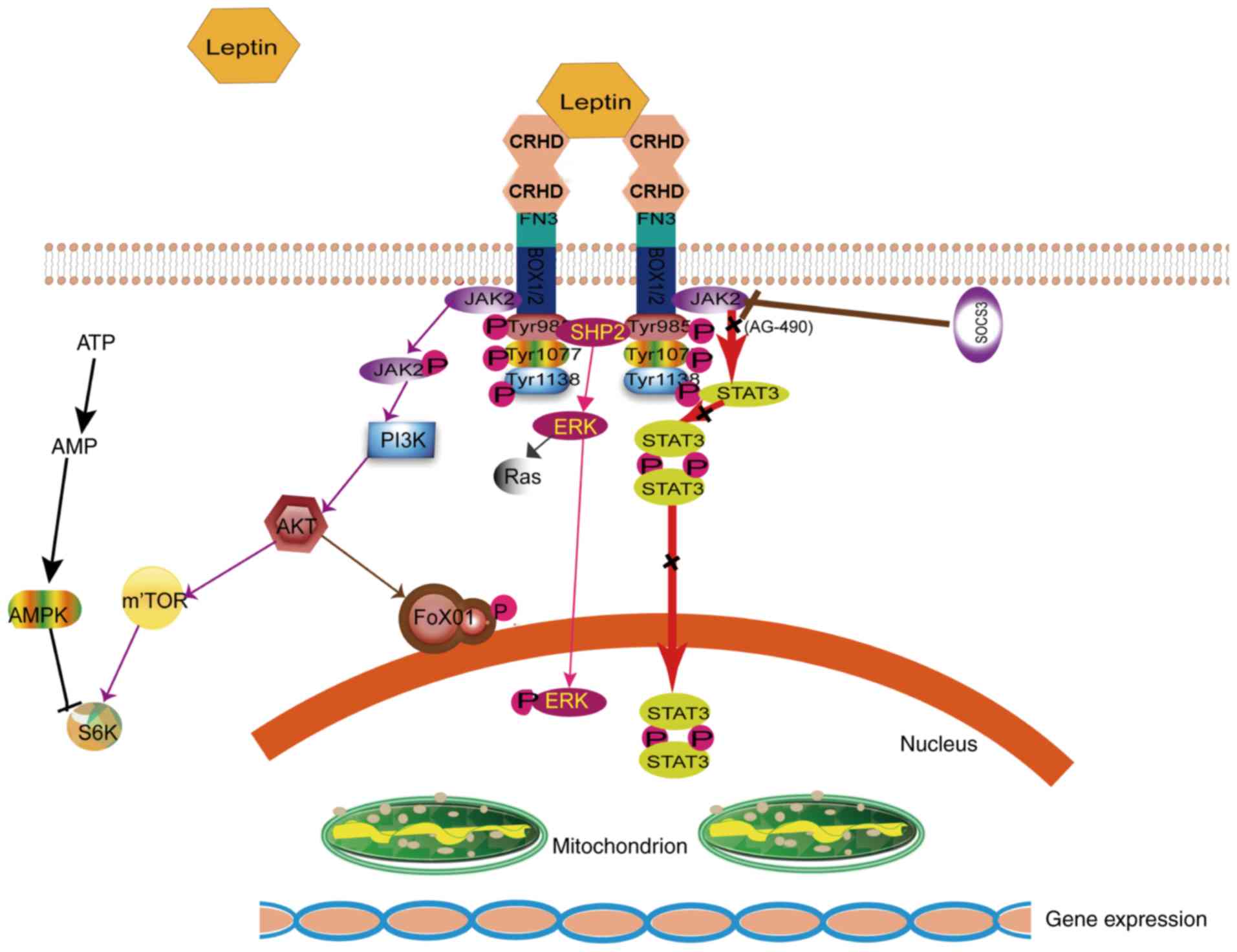
The binding of leptin to its receptors, OB-Rs, in
the central and peripheral tissues results in a wide range of
biological effects (31). The signal
transduction pathways are mediated at the membrane surface by long
functional receptors, OB-Rb (Figs. 1
and 3); these include Janus kinase
(JAK)/signal transducer and activator of transcription (STAT)
(32), Ras/extracellular
signal-regulated kinase (ERK)1/2 (33), phosphoinositide-3 kinase
(PI3K)/Akt/forkhead box O1 (34),
SH2 domain of protein tyrosine phosphatase 2/ERK, adenosine
monophosphate kinase (AMPK) and mammalian target of rapamycin/
ribosomal protein S6 kinase B1 (11,35).
JAK/STAT is the most important signaling pathway and has a major
role in cerebral protection (36).
Suppressor of cytokine signaling reportedly inhibits the JAK/STAT
signaling pathway (37,38). Phosphorylation of tyrosine residues
(Tyr1138) was triggered and JAK2 was activated by OB-R, thereby
combining OB-Rb in cells; the phosphorylated site of OB-Rb serves
as a docking site and recruits the STAT3 monomer (39). STATs are a group of cytoplasmic
proteins that act as signal transducers and transcription factors
and participate in normal cell - cytokine interaction and cell
growth (40). STAT3, a potential
transcription factor belonging to the STAT family, reaches the
receptor and binds to the 705th tyrosine site via
phosphorylation. In particular, STAT3 is phosphorylated by
phosphorylated (p)-JAK2 (41,42).
p-STAT3 behaves homotypic or heterodimeric, depending on the action
of the SH2 region, and is then transferred to the cell nucleus.
p-STAT3 regulates downstream target genes by interacting with DNA
elements, other transcription factors or adjunct proteins. The
neuroprotective mechanism of leptin and the JAK2/STAT3 signal
transduction pathway are closely associated.
Studies have reported on the pleiotropic effects of
leptin on various biological functions of the central and
peripheral nervous systems. The presence of leptin receptors in
various tissues suggests its pleiotropic effects on numerous
biological functions (43–45). Certain arcuate nucleus (ARC) leptin
receptor (OB-Rs) neurons express pro-opiomelanocortin (POMC). Other
ARC OB-Rs neurons express agouti-related protein (AGRP) along with
the inhibitory neuropeptide Y (NPY) and the inhibitory
neurotransmitter γ-aminobutyric acid (GABA). POMC-expressing
neurons have low energy requirements, thereby promoting energy
expenditure, whereas those expressing AGRP, NPY and GABA
demonstrate opposite behaviors (32,33).
Blood leptin levels are typically proportional to body fat levels.
In particular, they reflect the organism's energy storage, with no
effects on obesity, thereby revealing the pathological state of
leptin resistance (46). Body weight
and the hypothalamus-pituitary-gonad axis, which is closely
associated with growth and weight, may be regulated by leptin. The
levels of circulating leptin predict the development of heart
failure in elderly individuals by modulating the influence of
obesity on the increasing risk of heart failure. Indeed, the
influence of leptin on the central nervous system (CNS) increases
the sympathetic activity of the nerves sub-serving various tissues,
including the cardiovascular organs and kidneys (33). In mice, blocking PI3K with either
LY294002 or wortmannin significantly attenuated the leptin-induced
increase in renal sympathetic activity. Of note, leptin promotes
the switch toward type 1 T-helper (Th1) cell immune responses by
increasing interferon-γ secretion, facilitating Th17 responses
(47), and stimulating the release
of inflammatory cytokines, including interleukin (IL)-1beta, IL-6
and IL-8, and the chemokine monocyte chemotactic protein-1.
Although most animal studies have indicated that
leptin protects the brain, human studies suggest certain
contradictions. What is known for certain is that the increased
circulating leptin levels may also contribute to low brain
natriuretic peptide concentrations observed in heart failure
patients with a high body mass index and may promote the obesity
paradox of heart failure (48). In
particular, the administration of leptin (14.1 ng/ml) to humans
would establish a risk model in coronary artery disease.
Furthermore, a prospective study involving 4,571 healthy African
Americans did not identify any association between the leptin
levels in the body and the risk of ischemic stroke in either men or
women (49–51). A few small-scale studies evaluated
leptin levels in the body during the acute phase of ischemic stroke
and reported an increase in the levels (52). The patients received escalating doses
of r-met-Hu-Leptin until a dose of 0.12 mg/kg/day was reached.
After 18 months of r-Met-Hu-Leptin therapy, a considerable
improvement in glucose homeostasis was achieved, as evidenced by
the normalization of fasting blood glucose levels, lowered glycated
hemoglobin and improved tolerance to an oral glucose load (7,52,53). In
recent years, significant efforts have been made to clarify the
neuroprotective mechanisms of leptin.
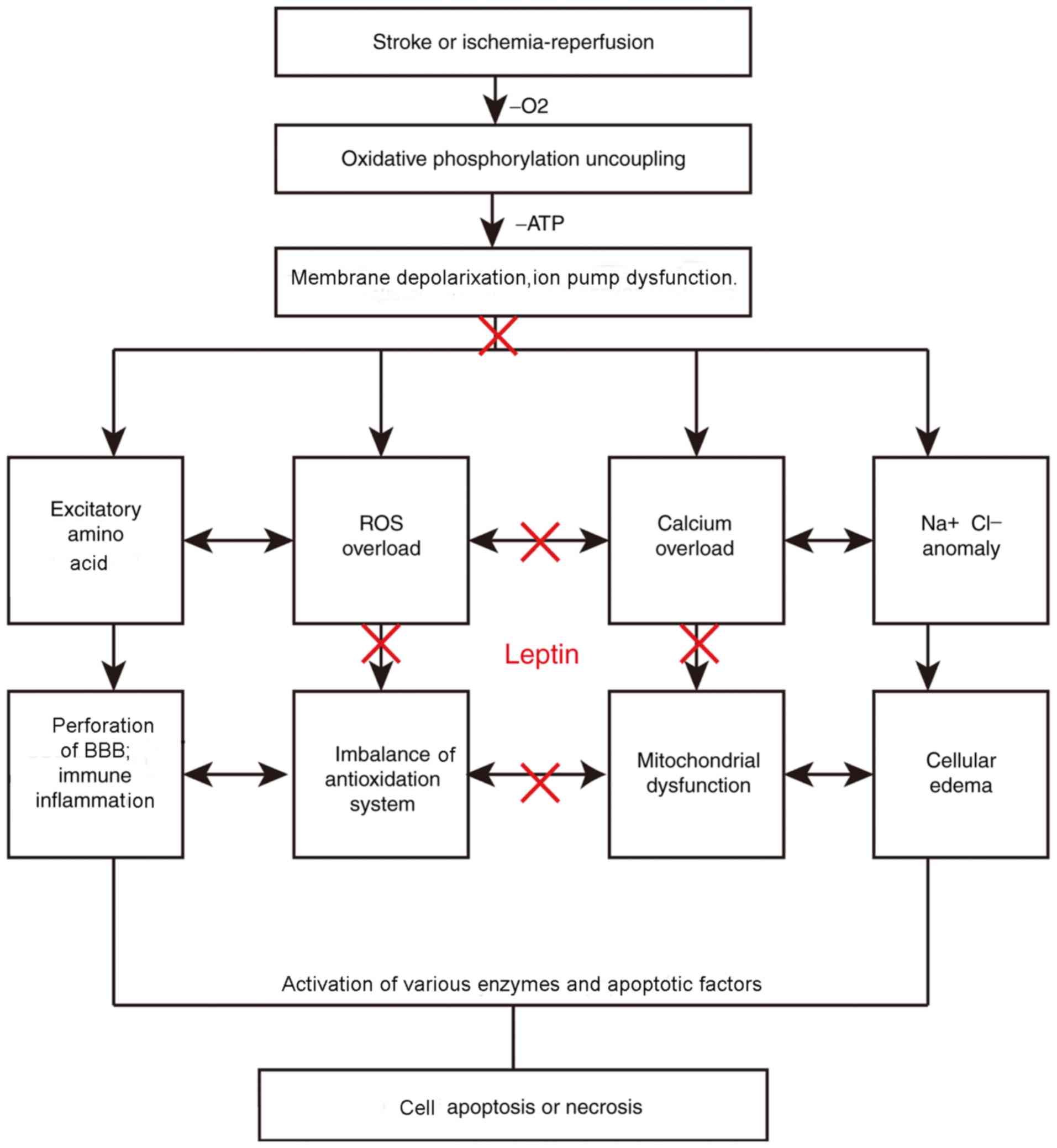
The pathogenesis of cerebral ischemia/reperfusion
injury has been linked to increases in the toxicity of EAAs
(54), mitochondrial damage
(55), free oxygen radical
production, inflammatory factors and apoptosis, as well as the
interaction among those factors (Fig.
2). EAAs, including glutamic and aspartic acids, are present in
the mammalian CNS and most abundantly in the cerebral cortex and
hippocampus, where they deliver excitatory nerve impulses (56). N-Methyl-D-aspartic acid receptors are
expressed in oligodendrocytes. Of note, 40% of glutamic acid is
released from synapses that are activated during ischemia (57). In general, EAAs present in the
synaptic vesicles of nerve endings are released into the synaptic
cleft acting on the corresponding receptor, transmitting nerve
impulses once the nerve cell membrane is depolarized. Excessive
excitatory transmitters are either hydrolyzed by proteases or
re-absorbed by neurons (57).
Calcium, sodium and potassium channels are activated following
cerebral ischemia. Excitatory neurotransmitters are simultaneously
released in the synaptic space and cannot be absorbed or used
effectively. The accumulation of sodium and chloride ions causes
cellular edema, thereby resulting in the accumulation of excitatory
amino acids in interstitial cells, causing cellular toxicity.
Dopaminergic neurons of the striatum and prefrontal cortex
originate from the ventral tegmental area (VTA) of the
mesencephalon.
Leptin treatment provided following transient
ischemia markedly reduces neurologic deficits, cerebral infarct
volumes and brain edema (58,59).
Leptin treatment increased the ATP, leptin and p-Akt levels and
decreased the lactate dehydrogenase levels and lactic acid/pyruvate
ratio; it also alleviated histopathologic injuries, all of which
were entirely reversed following treatment with the PI3K inhibitor
LY294002. Leptin inhibits the release of pre-synaptic glutamate
into the VTA via JAK2 and PI3K activation. Furthermore, leptin
reportedly suppresses the release of glutamate and regulates the
dopamine pathway in the hippocampus to alleviate the depressive
symptoms of cerebral ischemia. AG-490, a JAK2 signal transduction
protein and transcriptional inhibitor activator, inhibits the
effects of leptin on JAK2/PI3K signaling. (60,61) The
JAK2/PI3K/Akt pathway is critical for the mediation of
leptin-induced neuroprotection. These results support the use of
leptin in the treatment of ischemic stroke. The effects of leptin
in increasing angiogenesis and neurogenesis following brain
ischemia may be partially attributed to an increase in OB-Rs, AMPK
activation, transient receptor potential cation channel subfamily V
member 1 expression and biosynthesis of pro-angiogenic factors
(e.g., vascular endothelial growth factor). By contrast, studies
assessing microsphere infusion under maximal vasodilation have
reported that that leptin does not have a central role in cerebral
perfusion enhancement (59,62). Systemic leptin administration for 1
week following an occlusion surgery increased the cerebral
hemodynamic reserve in a similar fashion to that caused by
granulocyte-macrophage colony-stimulating factor. Leptin enhances
cell growth and differentiation via the mitogen-activated protein
kinase/ERK signaling pathway. Leptin infusion increases Akt and
ERK1/2 phosphorylation in brain tissues. These effects were
associated with upregulation of ERK1/2 phosphorylation and nitric
oxide (NO) release, which promote the repair of damaged mucosa
(63,64). Mitochondria are the powerhouse of
cells. Ischemic or hypoxic injury to the brain terminates
mitochondrial oxidative phosphorylation (65), thereby leading to the loss of
mitochondrial membrane potential and decreasing the membrane
permeability of ATP (66,67). Ischemia/reperfusion leads to further
mitochondrial damage; in particular, it causes secondary damage
with the release of cellular oxygen free radicals due to a series
of chain reactions involving cell membrane damage, lysosome rupture
and cell lysis. Mitochondrial biogenesis is promoted via the
activation of JAK/STAT by leptin, via downstream proteins, such as
proliferator-activated receptor γ a co-activator-1α and
mitochondrial transcription factor A. Such activation has been
indicated to be involved in neuronal cell protection via the
enhancement of anti-oxidant enzyme activities (15,47). It
has been demonstrated that leptin increases superoxide dismutase
(SOD) levels, stabilizes the mitochondrial membrane and alleviates
endoplasmic reticulum pressure (17); this in turn inhibits the production
of free radicals and ischemic injury in mice by binding to OB-Rb in
the cortex, hippocampus and striatum. Inflammatory responses of
leukocyte infiltration have an important role in cerebral ischemia
and reperfusion. The reduction in oxidative damage to brain tissue
caused by reactive oxygen represents a therapeutic approach for
cerebral ischemia/reperfusion injury (1). Oxygen free radicals activate
inflammatory cytokines and amplify inflammatory signals (68,69).
Upregulation of adhesion molecule expression on the surface of
neutrophils, reactive leukocytes and vascular endothelial cells was
observed; furthermore, there was an increase in neutrophil adhesion
to vascular endothelial cells (70,71).
In cerebral ischemia/reperfusion injury,
inflammation caused by leukocyte infiltration and fluid has an
important role; a therapeutic target is represented by oxidative
damage of the brain tissue determined by reactive oxygen species
(ROS) (72). In particular, the
latter activates inflammatory cytokines and amplifies inflammatory
signals (71,73). Hence, upregulation of adhesion
molecule expression on the neutrophil surface, reactive leukocytes
and vascular endothelial cells may be observed. In addition,
neutrophil adhesion to vascular endothelial cells inhibited the
production of NO, as determined by the aggravation of cell edema
(74). Leptin has an
anti-inflammatory role (75,76); it activates the nuclear factor-κB
pathway, inhibits the expression of pro-inflammatory factors [tumor
necrosis factor (TNF)-α, IL-6, IL-1β] within 7 h and increases the
expression of IL-10 (74,77) Within 100 min, the human body is able
to recover from hypothermia and hypotension caused by
ischemia/reperfusion injury (78).
Within 90–240 min, TNF-α is inhibited. This is not accompanied by
changes in the levels of other cytokines. In 1981, the concept of
ischemic penumbra, which refers to the reversible damage caused by
the brain outside the central area of focal ischemic necrosis, was
introduced (79). While the energy
supply ceases, the ionic pump in the cell remains accessible. The
ischemic penumbra, an area unaffected by the stroke for a few hours
from its occurrence, represents an important therapeutic target
(3,80). During cerebral ischemia/reperfusion,
oxidative stress leads to the breakdown of the redox balance; this
is due to the accumulation of ROS and the dysfunction of important
redox-sensitive protein kinases, membrane receptors and ion
channels (81). ROS may damage DNA
to trigger apoptosis, peroxidising the phospholipid membrane and
releasing cytochrome C from the mitochondria, thereby leading to
activation of pro-apoptotic caspases and apoptosis initiation
(13). Leptin treatment causes the
production of SOD, upregulation of the anti-apoptotic protein
B-cell lymphoma-2 (Bcl-2) and downregulation of caspase-3 and
Bcl-2-associated X protein expression. This stabilizes the
mitochondrial membrane potential, and reduces oxidative stress and
induction of apoptosis (82).
Stroke determines high morbidity, disability and
mortality following cancer, and precedes cardiovascular disease
(83). Leptin has an important role
in decreasing excitatory neurotransmitter levels, protecting
mitochondria, decreasing superoxide and free radical formation,
increasing anti-inflammatory factor and -apoptotic protein levels,
decreasing apoptotic protein levels and avoiding thrombolysis and
the risk of cerebral hemorrhage (84). Leptin has drawn the attention of
numerous experts and scholars. Several studies have indicated that
exogenous leptin has a protective role in the brain (85), while also interfering with the body's
energy regulation. Most studies on human subjects are relevant and
provide contradictory points of view regarding leptin in this
field. Regarding the association of the serum levels of leptin with
stroke, the opinion of scholars is divided. By contrast, a
prospective study on 4,571 healthy African Americans did not
identify any association between leptin levels and ischemic stroke
risk in either men or women (8,22,51,86).
Only a few small studies evaluated leptin levels during the acute
phase of ischemic stroke and reported increased leptin levels
(7,26,50,52,87). It
is currently indicated that leptin administration after stroke may
be a promising treatment strategy. In particular with use of a gel,
the polymer (synthetic leptin protein) may be directed to the
damaged brain tissue to stimulate different functions, including
the regulation of nutrition and growth, and the differentiation of
neural stem cells. Based on these results, leptin may be
selectively administered to the damaged parts of the brain to
subsequently observe whether leptin stimulates the growth and
differentiation of neural stem cells (88–90).
However, further research is warranted in this regard. Leptin is a
novel brain protective drug, but its application has not yet been
successfully translated into the clinic. Additional research is
required to implement applications developed in animal experiments
as clinical treatments in humans.
Not applicable.
This study was supported by the Shandong Provincial
Natural Science Foundation, China (grant no. ZR2014HM026), the
National Natural Science Foundation of China (grant no. 81701301)
and the Science and Technology Plan of Universities in Shandong
Province (grant no. J14LL01).
Not applicable.
WZ, JC and DW conceived and designed the article. WZ
and YJ analyzed the relevant literature. WZ wrote the manuscript
and drew the figures. YJ, ZY, DW and XL made suggestions for
revision. WZ, DW and JC are responsible for text layout.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Chouchani ET, Pell VR, Gaude E,
Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord
ENJ, Smith AC, et al: Ischaemic accumulation of succinate controls
reperfusion injury through mitochondrial ROS. Nature. 515:431–435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu H, Tang C, Tai LW, Yao W, Guo P, Hong
J, Yang X, Li X, Jin Z, Ke J and Wang Y: Flurbiprofen axetil
attenuates cerebral ischemia reperfusion injury by reducing
inflammation in a rat model of transient global cerebral ischemia
reperfusion. Biosci Rep. 38:BSR201715622018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou H, Wang J, Jiang J, Stavrovskaya IG,
Li M, Li W, Wu Q, Zhang X, Luo C, Zhou S, et al: N-acetyl-serotonin
offers neuroprotection through inhibiting mitochondrial death
pathways and autophagic activation in experimental models of
ischemic injury. J Neurosci. 34:2967–2978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coutinho JM, Liebeskind DS, Slater LA,
Nogueira RG, Clark W, Dávalos A, Bonafé A, Jahan R, Fischer U,
Gralla J, et al: Combined intravenous thrombolysis and thrombectomy
vs thrombectomy alone for acute ischemic stroke: A pooled analysis
of the SWIFT and STAR studies. JAMA Neurol. 74:268–274. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Ghanem M, Al-Mufti F, Thulasi V, Singh
IP and Gandhi C: Expanding the treatment window for ischemic stroke
through the application of novel system-based technology. Neurosurg
Focus. 42:E72017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh HK, Choi YS, Yang YI, Kim JH, Leung PC
and Choi JH: Leptin receptor is induced in endometriosis and leptin
stimulates the growth of endometriotic epithelial cells through the
JAK2/STAT3 and ERK pathways. Mol Hum Reprod. 19:160–168. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gairolla J, Kler R, Modi M and Khurana D:
Leptin and adiponectin: pathophysiological role and possible
therapeutic target of inflammation in ischemic stroke. Rev
Neurosci. 28:295–306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nowzari Z, Masoumi M, Nazari-Robati M,
Akbari H, Shahrokhi N and Asadikaram G: Association of
polymorphisms of leptin, leptin receptor and apelin receptor genes
with susceptibility to coronary artery disease and hypertension.
Life Sci. 207:166–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng ZH, Liao J, Zhang JY, Liang C, Song
CH, Han M, Wang LH, Xue H, Zhang K, Zabeau L, et al: Inhibition of
the connexin 43 elevation may be involved in the neuroprotective
activity of leptin against brain ischemic injury. Cell Mol
Neurobiol. 34:871–879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Porzionato A, Rucinski M, Macchi V, Stecco
C, Castagliuolo I, Malendowicz LK and De Caro R: Expression of
leptin and leptin receptor isoforms in the rat and human carotid
body. Brain Res. 1385:56–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y and Rui L: Leptin signaling and
leptin resistance. Front Med. 7:207–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang JY, Yan GT, Liao J, Deng ZH, Xue H,
Wang LH and Zhang K: Leptin attenuates cerebral
ischemia/reperfusion injury partially by CGRP expression. Eur J
Pharmacol. 671:61–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JY Jr, Si YL, Liao J, Yan GT, Deng
ZH, Xue H, Wang LH and Zhang K: Leptin administration alleviates
ischemic brain injury in mice by reducing oxidative stress and
subsequent neuronal apoptosis. J Trauma Acute Care Surg.
72:982–991. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuda K: Leptin and nitric oxide
production against ischemic neuronal injury. Stroke. 39:e3–e4.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Musman J, Pons S, Barau C, Caccia C, Leoni
V, Berdeaux A, Ghaleh B and Morin D: Regular treadmill exercise
inhibits mitochondrial accumulation of cholesterol and oxysterols
during myocardial ischemia-reperfusion in wild-type and ob/ob mice.
Free Radic Biol Med. 101:317–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grabacka MM, Wilk A, Antonczyk A, Banks P,
Walczyk-Tytko E, Dean M, Pierzchalska M and Reiss K: Fenofibrate
induces ketone body production in melanoma and glioblastoma cells.
Front Endocrinol (Lausanne). 7:52016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye R, Yang Q, Kong X, Li N, Zhang Y, Han
J, Xiong L, Liu X and Zhao G: Sevoflurane preconditioning improves
mitochondrial function and long-term neurologic sequelae after
transient cerebral ischemia: Role of mitochondrial permeability
transition. Crit Care Med. 40:2685–2693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz MW and Baskin DG: Leptin and the
brain: then and now. J Clin Investig. 123:2344–2345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maffei M, Fei H, Lee GH, Dani C, Leroy P,
Zhang Y, Proenca R, Negrel R, Ailhaud G and Friedman JM: Increased
expression in adipocytes of ob RNA in mice with lesions of the
hypothalamus and with mutations at the db locus. Proc Natl Acad Sci
USA. 92:6957–6960. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halaas JL, Gajiwala KS, Maffei M, Cohen
SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK and Friedman JM:
Weight reducing effects of the plasma protein Encoded by the obese
gene. Science. 269:543–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niklowitz P, Rothermel J, Lass N, Barth A
and Reinehr T: Bioactive leptin is stronger related to parameters
of fat mass and distribution than conventionally measured leptin:
Findings from a longitudinal study in obese children participating
in a lifestyle intervention. Clin Chim Acta. 480:225–229. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Munzberg H and Morrison CD: Structure,
production and signaling of leptin. Metabolism. 64:13–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perez-Perez A, Vilarino-Garcia T,
Fernandez-Riejos P, Martin-Gonzalez J, Segura-Egea JJ and
Sanchez-Margalet V: Role of leptin as a link between metabolism and
the immune system. Cytokine Growth Factor Rev. 35:71–84. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kastin AJ and Pan WH: Dynamic regulation
of leptin entry into brain by the blood-brain barrier. Regul Pept.
92:37–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Witte AV, Kobe T, Graunke A, Schuchardt
JP, Hahn A, Tesky VA, Pantel J and Flöel A: Impact of leptin on
memory function and hippocampal structure in mild cognitive
impairment. Hum Brain Mapp. 37:4539–4549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chowen JA, Argente-Arizon P,
Freire-Regatillo A, Frago LM, Horvath TL and Argente J: The role of
astrocytes in the hypothalamic response and adaptation to metabolic
signals. Prog Neurobiol. 144:68–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Escobar S, Rocha A, Felip A, Carrillo M,
Zanuy S, Kah O and Servili A: Leptin receptor gene in the European
sea bass (Dicentrarchus labrax): Cloning, phylogeny, tissue
distribution and neuroanatomical organization. Gen Comp Endocrinol.
229:100–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engel O, Kolodziej S, Dirnagl U and Prinz
V: Modeling stroke in mice - middle cerebral artery occlusion with
the filament model. J Vis Exp. 6:24232011.
|
|
30
|
Thomas SA, Preston JE, Wilson MR, Farrell
CL and Segal MB: Leptin transport at the blood–cerebrospinal fluid
barrier using the perfused sheep choroid plexus model. Brain Res.
895:283–290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwon O, Kim KW and Kim MS: Leptin
signalling pathways in hypothalamic neurons. Cell Mol Life Sci.
73:1457–1477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng ZH Jr, Yan GT, Wang LH, Zhang JY, Xue
H and Zhang K: Leptin relieves intestinal ischemia/reperfusion
injury by promoting ERK1/2 phosphorylation and the NO signaling
pathway. J Trauma Acute Care Surg. 72:143–149. 2012.PubMed/NCBI
|
|
33
|
Zhang J, Deng Z, Liao J, Song C, Liang C,
Xue H, Wang L, Zhang K and Yan G: Leptin attenuates cerebral
ischemia injury through the promotion of energy metabolism via the
PI3K/Akt pathway. J Cereb Blood Flow Metab. 33:567–574. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang F, Wang S, Signore AP and Chen J:
Neuroprotective effects of leptin against ischemic injury induced
by oxygen-glucose deprivation and transient cerebral ischemia.
Stroke. 38:2329–2336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amantea D, Tassorelli C, Russo R, Petrelli
F, Morrone LA, Bagetta G and Corasaniti MT: Neuroprotection by
leptin in a rat model of permanent cerebral ischemia: Effects on
STAT3 phosphorylation in discrete cells of the brain. Cell Death
Dis. 2:e2382011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Z, Gan L, Zhou Z, Jin W and Sun C:
SOCS3 promotes inflammation and apoptosis via inhibiting JAK2/STAT3
signaling pathway in 3T3-L1 adipocyte. Immunobiology. 220:947–953.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Lu R, Feng DY and Zhang H:
Sevoflurane inhibits glutamate-aspartate transporter and glial
fibrillary acidic protein expression in hippocampal astrocytes of
neonatal rats through the janus kinase/signal transducer and
activator of transcription (JAK/STAT) pathway. Anesth Analg.
123:93–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin G, Zhang H, Sun F, Lu Z,
Reed-Maldonado A, Lee YC, Wang G, Banie L and Lue TF: Brain-derived
neurotrophic factor promotes nerve regeneration by activating the
JAK/STAT pathway in Schwann cells. Transl Androl Urol. 5:167–175.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv J, Wang X, Liu SY, Liang PF, Feng M,
Zhang LL and Xu AP: Protective effect of Fenofibrate in renal
ischemia reperfusion injury: Involved in suppressing kinase 2
(JAK2)/transcription 3 (STAT3)/p53 signaling activation. Pathol
Biol (Paris). 63:236–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garama DJ, White CL, Balic JJ and Gough
DJ: Mitochondrial STAT3: Powering up a potent factor. Cytokine.
87:20–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gurzov EN, Stanley WJ, Pappas EG, Thomas
HE and Gough DJ: The JAK/STAT pathway in obesity and diabetes. FEBS
J. 283:3002–3015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng HL, Li XX, Chen YT, Yu LJ, Zhang H,
Lao JM, Zhang X and Xu Y: Neuronal soluble fas ligand drives
M1-microglia polarization after cerebral ischemia. CNS Neurosci
Ther. 22:771–781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Friedman J: The long road to leptin. J
Clin Invest. 126:4727–4734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moon HS, Dalamaga M, Kim SY, Polyzos SA,
Hamnvik OP, Magkos F, Paruthi J and Mantzoros CS: Leptin's role in
lipodystrophic and nonlipodystrophic insulin-resistant and diabetic
individuals. Endocr Rev. 34:377–412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan WW and Myers MG Jr: Leptin and the
maintenance of elevated body weight. Nat Rev Neurosci. 19:95–105.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Davis C, Mudd J and Hawkins M:
Neuroprotective effects of leptin in the context of obesity and
metabolic disorders. Neurobiol Dis. 72:61–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nuñez-Figueredo Y, Pardo-Andreu GL,
Ramírez-Sánchez J, Delgado-Hernández R, Ochoa-Rodríguez E,
Verdecia-Reyes Y, Naal Z, Muller AP, Portela LV and Souza DO:
Antioxidant effects of JM-20 on rat brain mitochondria and
synaptosomes: Mitoprotection against Ca2+-induced mitochondrial
impairment. Brain Res Bull. 109:68–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hana V, Silha JV, Justova V, Lacinova Z,
Stepan JJ and Murphy LJ: The effects of GH replacement in adult
GH-deficient patients: Changes in body composition without
concomitant changes in the adipokines and insulin resistance. Clin
Endocrinol (Oxf). 60:442–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cundrle I Jr, Somers VK, Singh P, Johnson
BD, Scott CG and Olson LJ: The relationship between leptin and
ventilatory control in heart failure. J Card Fail. 19:756–761.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang H, Zhang Z, Li ZK, Lin J and Fang DZ:
Association of leptin receptor gene polymorphisms with genetic
susceptibility to ischemic stroke. J Stroke Cerebrovasc Dis.
24:2128–2133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Auer MK, Ebert T, Pietzner M, Defreyne J,
Fuss J, Stalla GK and T'Sjoen G: Effects of sex hormone treatment
on the metabolic syndrome in transgender individuals: Focus on
metabolic cytokines. J Clin Endocrinol Metab. 103:790–802. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Saber H, Himali JJ, Shoamanesh A, Beiser
A, Pikula A, Harris TB, Roubenoff R, Romero JR, Kase CS, Vasan RS
and Seshadri S: Serum leptin levels and the risk of stroke: The
framingham study. Stroke. 46:2881–2885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Diaz M, Chacon MR, Lopez-Bermejo A,
Maymó-Masip E, Salvador C, Vendrell J, de Zegher F and Ibáñez L:
Ethinyl estradiol-cyproterone acetate versus low-dose
pioglitazone-flutamide-metformin for adolescent girls with androgen
excess: Divergent effects on CD163, TWEAK receptor, ANGPTL4, and
LEPTIN expression in subcutaneous adipose tissue. J Clin Endocrinol
Metab. 97:3630–3638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Scott I, Webster BR, Chan CK, Okonkwo JU,
Han K and Sack MN: GCN5-like protein 1 (GCN5L1) controls
mitochondrial content through coordinated regulation of
mitochondrial biogenesis and mitophagy. J Biol Chem. 289:2864–2872.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Martinez-Abundis E, Rajapurohitam V,
Gertler A and Karmazyn M: Identification of functional leptin
receptors expressed in ventricular mitochondria. Mol Cell Biochem.
408:155–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Karadottir R, Cavelier P, Bergersen LH and
Attwell D: NMDA receptors are expressed in oligodendrocytes and
activated in ischaemia. Nature. 438:1162–1166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hamilton NB, Kolodziejczyk K,
Kougioumtzidou E and Attwell D: Proton-gated Ca(2+)-permeable TRP
channels damage myelin in conditions mimicking ischaemia. Nature.
529:523–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Greco SJ, Hamzelou A, Johnston JM, Smith
MA, Ashford JW and Tezapsidis N: Leptin boosts cellular metabolism
by activating AMPK and the sirtuins to reduce tau phosphorylation
and β-amyloid in neurons. Biochem Biophys Res Commun. 414:170–174.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Busch HJ, Schirmer SH, Jost M, van Stijn
S, Peters SL, Piek JJ, Bode C, Buschmann IR and Mies G: Leptin
augments cerebral hemodynamic reserve after three-vessel occlusion:
Distinct effects on cerebrovascular tone and proliferation in a
nonlethal model of hypoperfused rat brain. J Cereb Blood Flow
Metab. 31:1085–1092. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gleitz HF, Kramann R and Schneider RK:
Understanding deregulated cellular and molecular dynamics in the
haematopoietic stem cell niche to develop novel therapeutics for
bone marrow fibrosis. J Pathol. 245:138–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ray A and Cleary MP: The potential role of
leptin in tumor invasion and metastasis. Cytokine Growth Factor
Rev. 38:80–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ni H, Sun Q, Tian T, Feng X and Sun BL:
Long-term expression of metabolism-associated genes in the rat
hippocampus following recurrent neonatal seizures and its
regulation by melatonin. Mol Med Rep. 12:2727–2734. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang XG, Zhao L, Zhang Y, Li YY, Wang H,
Duan GL, Xiao L, Li XR and Chen HP: Extracellular
Cl(−)-free-induced cardioprotection against hypoxia/reoxygenation
is associated with attenuation of mitochondrial permeability
transition pore. Biomed Pharmacother. 86:637–644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Warne J, Pryce G, Hill JM, Shi X, Lennerås
F, Puentes F, Kip M, Hilditch L, Walker P, Simone MI, et al:
Selective inhibition of the mitochondrial permeability transition
pore protects against neurodegeneration in experimental multiple
sclerosis. J Biol Chem. 291:4356–4373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ham PB 3rd and Raju R: Mitochondrial
function in hypoxic ischemic injury and influence of aging. Prog
Neurobiol. 157:92–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Holmstrom MH, Tom RZ, Bjornholm M,
Garcia-Roves PM and Zierath JR: Effect of leptin treatment on
mitochondrial function in obese leptin-deficient ob/ob mice.
Metabolism. 62:1258–1267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hayakawa K, Esposito E, Wang X, Terasaki
Y, Liu Y, Xing C, Ji X and Lo EH: Transfer of mitochondria from
astrocytes to neurons after stroke. Nature. 535:551–555. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Antico Arciuch VG, Elguero ME, Poderoso JJ
and Carreras MC: Mitochondrial regulation of cell cycle and
proliferation. Antioxid Redox Signal. 16:1150–1180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Valerio A, Bertolotti P, Delbarba A,
Perego C, Dossena M, Ragni M, Spano P, Carruba MO, De Simoni MG and
Nisoli E: Glycogen synthase kinase-3 inhibition reduces ischemic
cerebral damage, restores impaired mitochondrial biogenesis and
prevents ROS production. J Neurochem. 116:1148–1159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Madathil RJ, Hira RS, Stoeckl M, Sterz F,
Elrod JB and Nichol G: Ischemia reperfusion injury as a modifiable
therapeutic target for cardioprotection or neuroprotection in
patients undergoing cardiopulmonary resuscitation. Resuscitation.
105:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vermeij JD, Westendorp WF, Dippel DW, van
de Beek D and Nederkoorn PJ: Antibiotic therapy for preventing
infections in people with acute stroke. Cochrane Database Syst Rev.
1:CD0085302018.PubMed/NCBI
|
|
72
|
Chung HK, Kim YK, Park JH, Ryu MJ, Chang
JY, Hwang JH, Lee CH, Kim SH, Kim HJ, Kweon GR, et al: The indole
derivative NecroX-7 improves nonalcoholic steatohepatitis in ob/ob
mice through suppression of mitochondrial ROS/RNS and inflammation.
Liver Int. 35:1341–1353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rustenhoven J, Aalderink M, Scotter EL,
Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, Curtis MA,
Park TI and Dragunow M: TGF-beta1 regulates human brain pericyte
inflammatory processes involved in neurovasculature function. J
Neuroinflammation. 13:372016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Agrawal S, Gollapudi S, Su H and Gupta S:
Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10
via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin
Immunol. 31:472–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xu H, Qin W, Hu X, Mu S, Zhu J, Lu W and
Luo Y: Lentivirus-mediated overexpression of OTULIN ameliorates
microglia activation and neuroinflammation by depressing the
activation of the NF-κB signaling pathway in cerebral
ischemia/reperfusion rats. J Neuroinflammation. 15:832018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rummel C: Inflammatory transcription
factors as activation markers and functional readouts in
immune-to-brain communication. Brain Behav Immun. 54:1–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lopez-Rodriguez AB, Mela V, Acaz-Fonseca
E, Garcia-Segura LM and Viveros MP: CB2 cannabinoid receptor is
involved in the anti-inflammatory effects of leptin in a model of
traumatic brain injury. Exp Neurol. 279:274–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Flatow EA, Komegae EN, Fonseca MT, Brito
CF, Musteata FM, Antunes-Rodrigues J and Steiner AA: Elucidating
the role of leptin in systemic inflammation: A study targeting
physiological leptin levels in rats and their macrophages. Am J
Physiol Regul Integr Comp Physiol. 313:R572–R582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Astrup J, Symon L and Siesjo BK:
Thresholds in cerebral ischemia-the ischemic penumbra. Stroke.
12:723–725. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stetler RA, Leak RK, Yin W, Zhang L, Wang
S, Gao Y and Chen J: Mitochondrial biogenesis contributes to
ischemic neuroprotection afforded by LPS pre-conditioning. J
Neurochem. 123 (Suppl 2):125–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tan DX, Manchester LC, Qin L and Reiter
RJ: Melatonin: A mitochondrial targeting molecule involving
mitochondrial protection and dynamics. Int J Mol Sci. 17:E21242016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Geng HX, Li RP, Li YG, Wang XQ, Zhang L,
Deng JB, Wang L and Deng JX: 14,15-EET suppresses neuronal
apoptosis in ischemia-reperfusion through the mitochondrial
pathway. Neurochem Res. 42:2841–2849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Owens B: Stroke. Nature.
26:510(7506)–S12014
|
|
84
|
Grummisch JA, Jadavji NM and Smith PD: tPA
promotes cortical neuron survival via mTOR-dependent mechanisms.
Mol Cell Neurosci. 74:25–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Arita S, Kinoshita Y, Ushida K, Enomoto A
and Inagaki-Ohara K: High-fat diet feeding promotes stemness and
precancerous changes in murine gastric mucosa mediated by leptin
receptor signaling pathway. Arch Biochem Biophys. 610:16–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Carboni L, Marchetti L, Lauria M, Gass P,
Vollmayr B, Redfern A, Jones L, Razzoli M, Malki K, Begni V, et al:
Cross-species evidence from human and rat brain transcriptome for
growth factor signaling pathway dysregulation in major depression.
Neuropsychopharmacology. 43:234–2145. 2018. View Article : Google Scholar
|
|
87
|
Yang H, Guo W, Li J, Cao S, Zhang J, Pan
J, Wang Z, Wen P, Shi X and Zhang S: Leptin concentration and risk
of coronary heart disease and stroke: A systematic review and
meta-analysis. PLoS One. 12:e01663602017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Brüstle O, Choudhary K, Karram K, Hüttner
A, Murray K, Dubois-Dalcq M and McKay RD: Chimeric brains generated
by intraventricular transplantation of fetal human brain cells into
embryonic rats. Nat Biotechnol. 16:1040–1044. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nakatomi H, Kuriu T, Okabe S, Yamamoto S,
Hatano O, Kawahara N, Tamura A, Kirino T and Nakafuku M:
Regeneration of hippocampal pyramidal neurons after ischemic brain
injury by recruitment of endogenous neural progenitors. Cell.
110:429–441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Carlen M, Meletis K, Goritz C, Darsalia V,
Evergren E, Tanigaki K, Amendola M, Barnabé-Heider F, Yeung MS,
Naldini L, et al: Forebrain ependymal cells are Notch-dependent and
generate neuroblasts and astrocytes after stroke. Nat Neurosc.
12:259–267. 2009. View Article : Google Scholar
|