Introduction
Dwarfism is one of the common diseases in
pediatrics. Different measures are taken according to the causes,
such as increase of the physical activities, monitoring of the
height regularly and increase of nutritional supplementation.
Short-term treatment is very important in early treatment, with the
combination of medical treatment, scientific eating habits and
exercise habits, which can promote normal height growth (1). Growth retardation phenomenon has a
certain impact on people's self-confidence, work, living conditions
and marriage (2,3). Studies have shown (4) that people with dwarfism account for 3%
of the total population, and dwarfism relates to >400 genetic
and endocrine factors. Also it is related to malnutrition,
psychological factors, living environment and economic situation.
However, some children with dwarfism have sufficient hormone
secretion and no problems, such as malnutrition or mental illness.
This is the idiopathic short stature (ISS) (5). ISS is the most common type of dwarfism,
accounting for ~60–80% (6).
Currently, the pathogenesis of ISS is unclear. However, as research
continues, studies have confirmed the safety and effectiveness of
recombinant human growth hormone (rhGH) in the treatment of ISS
(7). Clinically, disease analysis
and growth hormone (GH) stimulation tests are used to distinguish
whether the dwarfism is caused by growth hormone deficiency (GHD).
Insulin-like growth factor-1 (IGF-1) is a mediator of GH that
promotes human growth. Its role is to act directly on the GH
receptor. The synthesis of IGF-1 is also affected by the regulation
of GH and nutritional status (8,9). The
main mechanism of IGF-1 production is to promote the secretion of
IGF-1 by generating a stimulation effect by exogenous GH, and to
stimulate the GH according to the reaction condition and make
corresponding evaluation (10,11). In
normal serum, the IGF-1 binding protein is mainly insulin-like
growth factor binding protein-3 (IGFBP-3), and the GH stimulates
IGF-1 and promotes the production of IGFBP-3. Both levels of serum
are very inconsistent, both circadian rhythm and non-pulsed
secretion can reflect the state of hormone secretion (12). In this study, serum GH and IGFBP-3
levels were measured by stimulation tests, and the GHD and ISS
groups were determined according to the GH peak in the experimental
group. IGF-1 levels of the serum in the experimental and control
group were detected by chemiluminescence immunoassay (CLIA) and the
correlation between IGF-1 and GH, IGF-1 and IGFBP-3 was
analyzed.
Patients and methods
General information
From April 2014 to June 2017, 122 children with
dwarfism were treated in the Affiliated Wuxi No. 2 People's
Hospital of Nanjing Medical University (Wuxi, China) and The First
Affiliated Hospital of Xinxiang Medical University (Xinxiang,
China). Among them, 122 children with dwarfism were selected as the
experimental group and 51 normal children as the control group.
According to the peak of GH in the experimental group, there were
65 cases of GHD (GHD group) and 57 cases of idiopathic shortness
(ISS group). In the GHD group, there were 31 males and 34 females,
aged 6–11 years, with an average age of 9.23 years. In the ISS
group, there were 29 males and 28 females, aged 5–12 years, with an
average age of 8.85 years. In the control group, there were 27
males and 24 females, aged 5–11 years, with an average age of 9.01
years. All patients underwent thyroid function and urine routine
examination before the study was carried out. Children with kidney
disease, hypothyroidism or other kidney-related diseases were
excluded. The study was approved by the Ethics Committees of The
Affiliated Wuxi No. 2 People's Hospital of Nanjing Medical
University and The First Affiliated Hospital of Xinxiang Medical
University. The parents of the child patients who participated in
this research were informed of the details of the study before the
detection and signed an informed consent. Patients had complete
clinical data. There were no significant differences in terms of
sex, age, height, weight, birth weight, paternal weight, maternal
weight and bone age between the groups (P>0.05), which suggested
that the groups were comparable.
Specimen collection
A total of 4 ml of venous blood were taken in the
morning, on an empty stomach and in a quiet state, and the test was
performed in time to separate the serum. The serum GH and IGFBP-3
levels were measured by arginine and insulin hypoglycemia
stimulation tests, respectively.
Method
IGF-1 was detected by the method of
immunochemiluminescence, using DPC Immulite 2000 chemiluminescence
instrument (Siemens AG, Munich, Germany).
GH excitation test
GH stimulation tests were performed using the
arginine stimulation test and the insulin hypoglycemia stimulation
test. The serum GH levels were examined at 0, 30, 60, and 90 min,
respectively. A peak of GH <10 ng/ml denoted GHD, while a peak
of GH >10 ng/ml denoted ISS.
Statistical analysis
SPSS 17.0 software (Beijing Strong-Vinda Information
Technology Co., Ltd., Beijing, China) was used for statistical
analysis. The enumeration data were expressed as n (%). The
comparison among groups was tested by F test, and the correlation
analysis was conducted by partial correlation analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
General information
There was no significant difference between the
experimental and control group in terms of sex, age, height,
weight, birth weight, paternal weight, maternal weight or bone age
(P>0.05) (Table I).
 | Table I.General clinical baseline data [n
(%)]. |
Table I.
General clinical baseline data [n
(%)].
|
| Experimental
group |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | GHD group (n=65) | ISS group (n=57) | Control group
(n=51) | F value | P-value |
|---|
| Sex |
|
|
| 0.327 | 0.849 |
| Male | 31 (47.69) | 29 (50.88) | 27 (52.94) |
|
|
|
Female | 34 (52.31) | 28 (49.12) | 24 (47.06) |
|
|
| Age (years) |
|
|
| 0.020 | 0.990 |
|
<6 | 30 (46.15) | 27 (47.37) | 24 (47.06) |
|
|
| ≥6 | 35 (53.85) | 30 (52.63) | 27 (52.94) |
|
|
| Height (cm) |
|
|
| 2.116 | 0.347 |
|
<100 | 45 (69.23) | 42 (73.68) | 31 (60.78) |
|
|
| ≥100 | 20 (30.77) | 15 (26.32) | 20 (39.22) |
|
|
| Weight (kg) |
|
|
| 1.631 | 0.442 |
|
<20 | 36 (55.38) | 26 (45.61) | 23 (45.10) |
|
|
| ≥20 | 29 (44.62) | 31 (54.39) | 28 (54.90) |
|
|
| Birth weight
(kg) |
|
|
| 0.858 | 0.651 |
|
<2 | 23 (35.38) | 19 (33.33) | 14 (27.45) |
|
|
| ≥2 | 42 (64.62) | 38 (66.67) | 37(72.55) |
|
|
| Paternal height
(cm) |
|
|
| 1.171 | 0.557 |
|
<100 | 33 (50.77) | 25 (43.86) | 21 (41.18) |
|
|
| ≥100 | 32 (49.23) | 32 (56.14) | 30 (58.82) |
|
|
| Maternal height
(cm) |
|
|
| 0.282 | 0.869 |
|
<100 | 31 (47.69) | 27 (47.37) | 22 (43.14) |
|
|
|
≥100 | 34 (52.31) | 30 (52.63) | 29 (56.86) |
|
|
| Bone age
(years) |
|
|
| 3.670 | 0.160 |
|
<7 | 35 (53.85) | 31 (54.39) | 23 (45.10) |
|
|
| ≥7 | 20 (30.77) | 26 (45.61) | 28 (54.90) |
|
|
Comparison of serum IGF-1 expression
levels between the experimental and control group
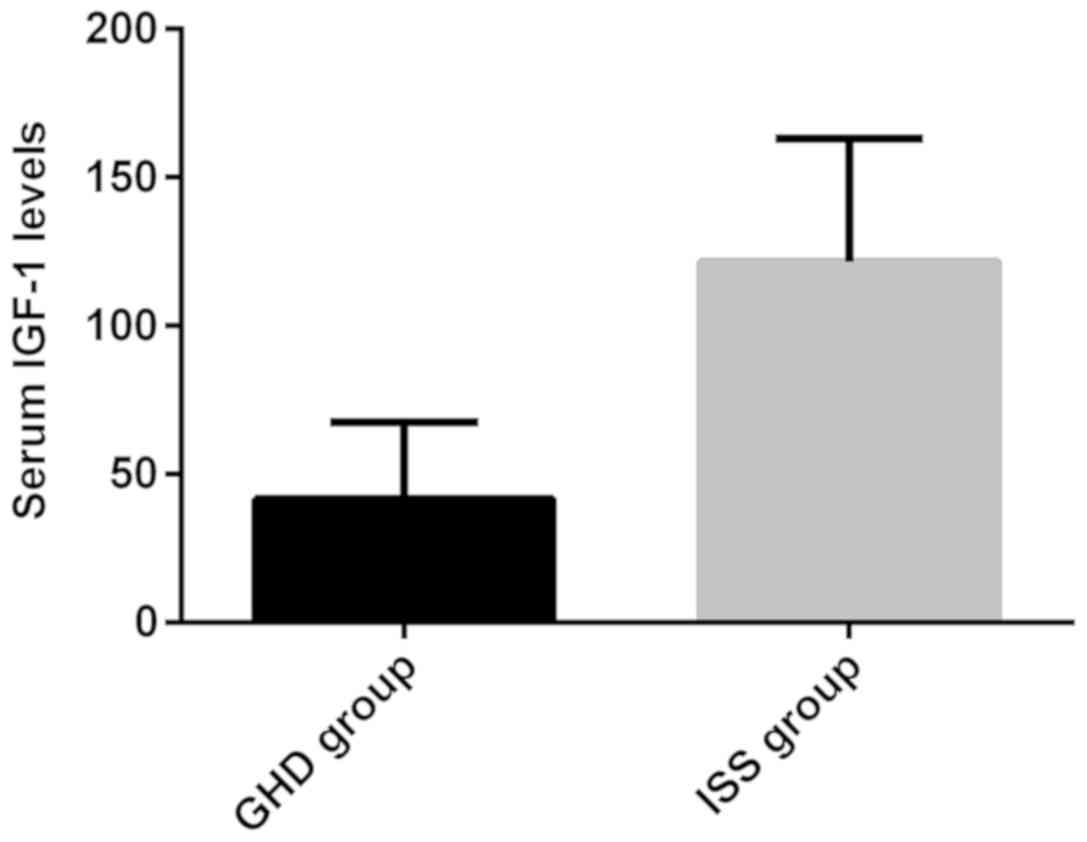
The levels of serum IGF-1 in GHD and ISS group were
41.75±25.75 and 121.53±41.51, respectively. The expression of serum
IGF-1 in GHD group was significantly lower than that in the ISS
group. There was significant difference between the groups
(t=12.92, P<0.05). The expression levels of serum IGF-1 in GHD
and control group were 41.75±25.75 and 154.54±59.27, respectively.
The expression level of serum IGF-1 in GHD group was significantly
lower than that in the control group, and the difference between
the groups was statistically significant (t=13.79, P<0.05). The
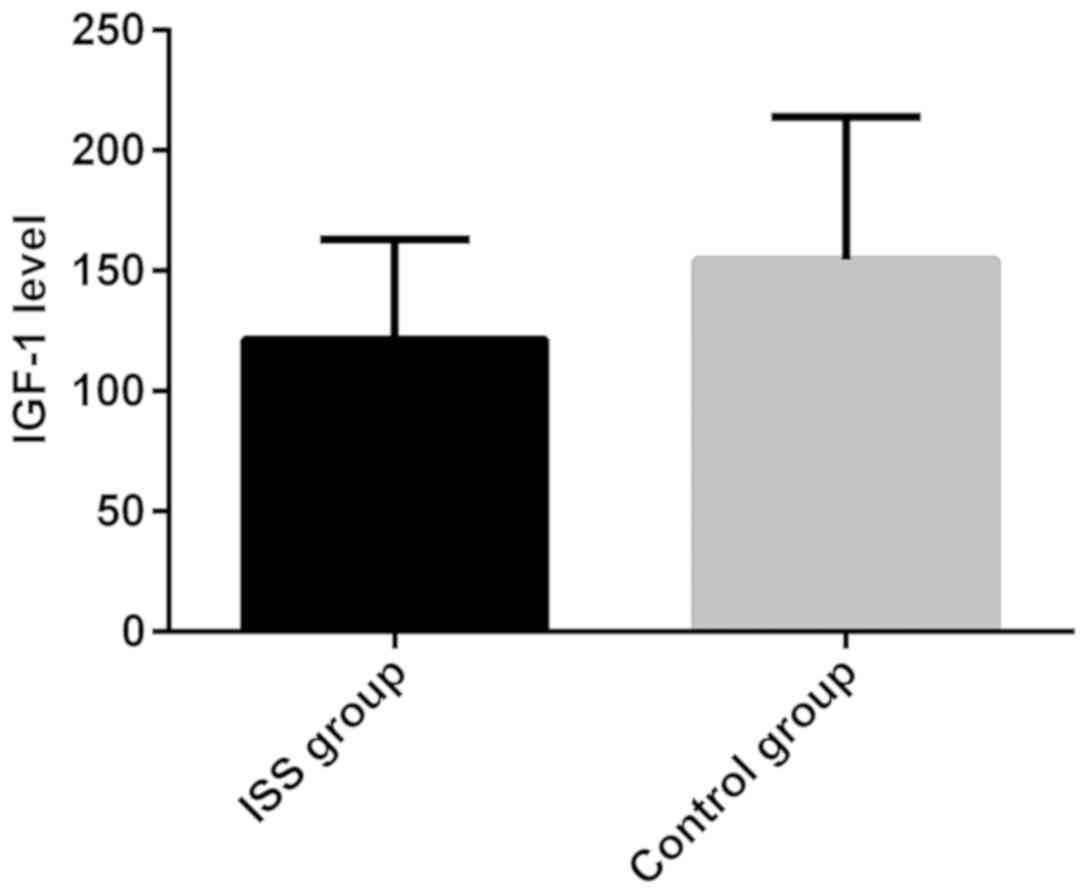
expression level of serum IGF-1 in the ISS and control group were
121.53±41.51 and 154.54±59.27, respectively. The expression level
of serum IGF-1 in ISS group was lower than that in the control
group, and the difference was statistically significant (t=3.38,
P<0.05) (Figs. 1–3).
Correlation between IGF-1 and GH in
dwarfism
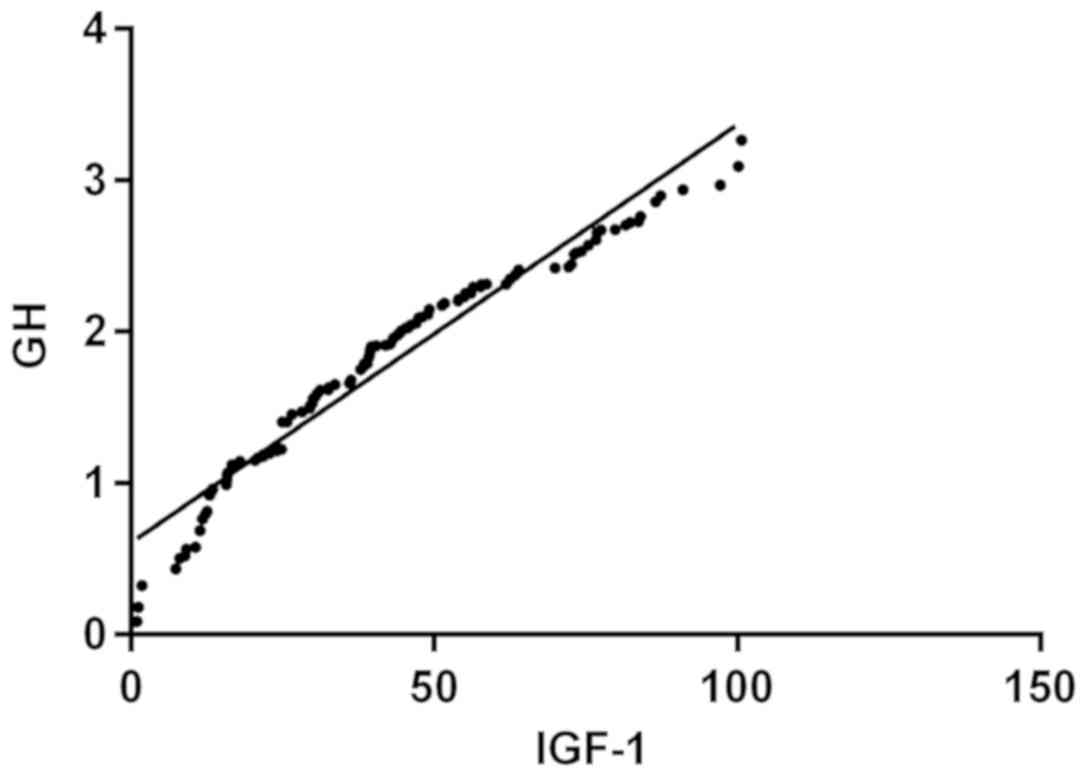
Partial correlation analysis showed that the
expression levels of GH and IGF-1 were positively correlated in the
serum of patients with dwarfism (r=0.974, P<0.001) (Fig. 4).
Correlation between IGF-1 and IGFBP-3
in dwarfism
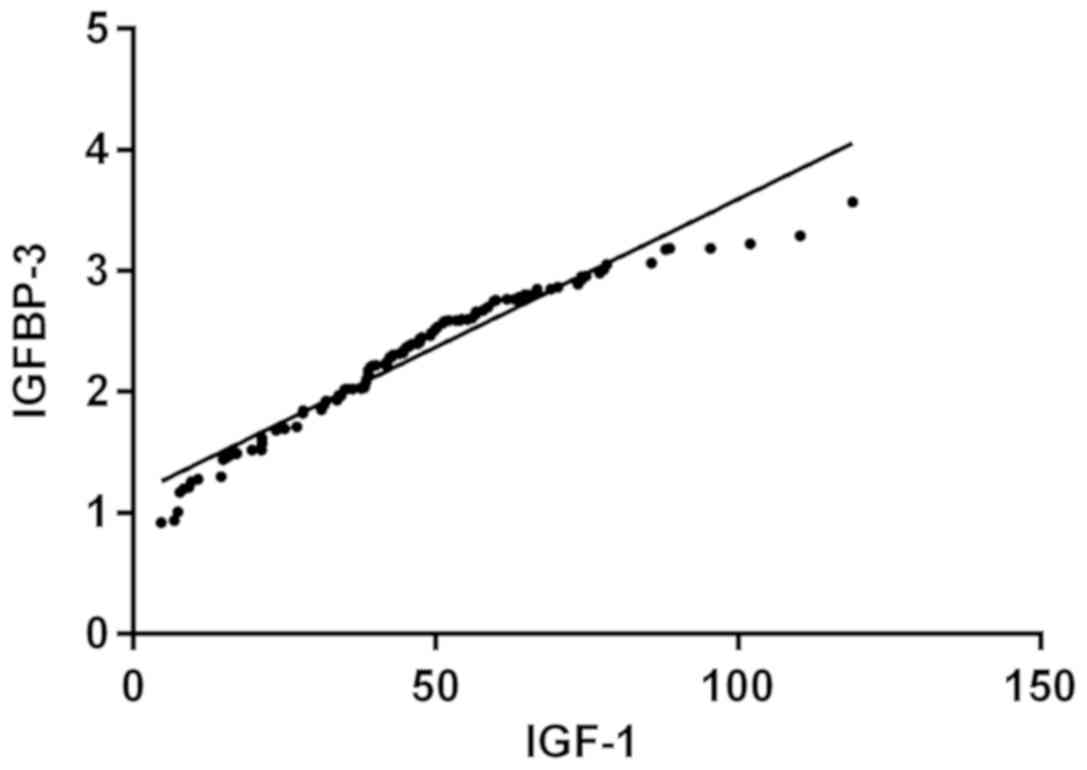
Partial correlation analysis showed that the
expression levels of IGFBP-3 and IGF-1 were positively correlated
in the serum of patients with dwarfism (r=0.970, P<0.001)
(Fig. 5).
Discussion
Dwarfism in children affects the growth and
development of children's height and has an impact on their study
and living abilities. Thus, it causes mental and physical burden.
Studies have shown that the incidence of dwarfism in children is
related to a certain genetic predisposition (13,14).
Some studies have reported (15)
that children's height is affected by congenital and acquired
environmental factors, such as physical exercise, eating habits and
quality of life. The causes of dwarfism in children are
complicated. They are generally thought to be associated with
decreased hormone sensitivity in children or decreased receptor
sensitivity, such as growth factors. Maintaining the expression
level of IGF-1 is important for promoting the growth and
development of children. Moreover, IGF-1 also has a certain effect
on the organ function and physiological regulation mechanism of
children (16,17). The presence of IGF-1 in human serum
is mostly synthesized by the liver, and the expression level is
also affected by various factors. The most important factor is GH.
After liver GH binds to GH, IGF-1 is secreted, and IGF-1 inhibits
GH secretion by negative feedback (18). The IGFBP-3 gene is located in the
7p12-p13 region and is highly conserved. Studies have shown
(19) that more than half of the
IGFBP-3 levels are genetically determined, but the specific
mechanism remains unclear. Polymorphisms in the promoter region of
IGFBP-3 gene can change the levels of IGFBP-3 and IGF-1, thereby
affecting the development of the disease (20).
Herfs et al (21) have reported that IGF-1 is usually
synthesized by the action of GH, and GH acts on bone through the
auxiliary action of IGF-1. IGFBP-3 is the most important
insulin-like growth factor, as well as the most powerful one. GH
stimulates the synthesis of IGFBP-3 while producing IGFBP-3. IGF-1
has significant negative effects on GH, and IGF-1 is the main
regulator of GH. In this study, the differences between the
experimental and control group in terms of sex, age, height,
weight, birth weight, paternal weight, maternal weight, and bone
age were not statistically significant. The expression level of
IGF-1 in GHD group was lower than that in ISS group, and there was
a significant difference between the groups (P<0.05). The
expression level of IGF-1 in the GHD group was significantly lower
than that in the control group (P<0.05); and the expression
level of serum IGF-1 in the ISS group was significantly lower than
that in the control group (P<0.05). This suggests that IGF-1 has
an important significance in the diagnosis of children with
dwarfism.
Related studies have shown that the levels of serum
IGF-1 in patients with GHD are significantly lower than in normal
children, and IGF-1 detection can be used as an effective indicator
for screening growth retardation caused by abnormal GH-IGF axis
(22), which is consistent with the
view of this study. However, this study has obvious advantages in
terms of number of subjects investigated and the results are more
convincing. We also analyzed the correlation between GH and IGF-1
in the serum of patients with dwarfism, and partial correlation
analysis showed that GH and IGF-1 are positively correlated
(P<0.001). Therefore, it is speculated that the expression
levels of GH and IGF-1 are closely related in dwarfism. Previous
studies (23) have shown that the
pathogenic mechanisms of ISS and GHD are different. The GHD is
caused by various factors leading to decreased GH levels, resulting
in limited growth and development. Currently, regarding the
treatment of rhGH, some scholars have compared the treatment of ISS
and GHF and found that the standard deviation of the mean height of
children with GHF has been significantly improved (24). However, another study has suggested
that when comparing the treatment of ISS and GHD, the rhGH
treatment can improve the height of children with ISS once they
enter adulthood (25).
Lanes et al (26) have confirmed that rhGH treatment of
ISS and GHD is a safe and effective treatment. These studies are
also consistent with our results. Finally, we analyzed the
correlation between IGF-1 and IGFBP-3 in the serum of patients with
dwarfism, and the results showed that the expression levels of
IGFBP-3 and IGF-1 are positively correlated (P<0.001).
Therefore, it is speculated that the expression levels of IGFBP-3
and IGF-1 are closely related in dwarfism. Rasat et al
(27) have shown that the detection
of both IGFBP-3 and IGF-1 are better than IGF-1 for the diagnosis
of dwarfism. This shows that the joint diagnosis can improve the
accuracy.
However, there are certain limitations in our study,
as we did not evaluate the prognosis and survival rate of dwarfism,
and the clinical and pathological features of IGFBP-3 and IGF-1 in
patients with dwarfism were not studied in depth. In the future, we
aim to improve our research based on the patients' data in the
experimental group, follow-up patients regularly and evaluate the
results of this analysis.
In summary, the detection of IGF-1 and IGFBP-3 is
important for the early diagnosis and comprehensive evaluation of
children with dwarfism. IGF-1 can reflect the therapeutic effect of
dwarfism on rhGH, which is worthy of clinical application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and HZ conceived and designed the study and were
responsible for the statistical analysis. YW wrote the manuscript.
MC acquired the general data of the patients. LK and MC assisted
with the specimen collection. XG and YW performed the GH excitation
test. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committees of
The Affiliated Wuxi No. 2 People's Hospital of Nanjing Medical
University (Wuxi, China) and The Fisrt Affiliated Hospital of
Xinxiang Medical University (Xinxiang, China). The parents of the
child patients who participated in this research signed an informed
consent, and the patients had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Toumba M, Kokotsis V, Savva SC and Skordis
N: Expensive therapies in children: Benefit versus cost of combined
treatment of recombinant human growth hormone and
gonadotropin-releasing hormone analogue in girls with poor height
potential. J Pediatr Endocrinol Metab. 27:311–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandberg DE and Gardner M: Short stature:
Is it a psychosocial problem and does changing height matter?
Pediatr Clin North Am. 62:963–982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gohlke BC and Stanhope R: Final height in
psychosocial short stature: Is there complete catch-up? Acta
Paediatr. 91:961–965. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baten J, Pelger I and Twrdek L: The
anthropometric history of Argentina, Brazil and Peru during the
19th and early 20th century. Econ Hum Biol. 7:319–333. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quitmann JH, Bullinger M, Sommer R,
Rohenkohl AC and Bernardino Da Silva NM: Associations between
psychological problems and quality of life in pediatric short
stature from patients' and parents' perspectives. PLoS One.
11:e01539532016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen LE: Idiopathic short stature: A
clinical review. JAMA. 311:1787–1796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cuttler L: Safety and efficacy of growth
hormone treatment for idiopathic short stature. J Clin Endocrinol
Metab. 90:5502–5504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bereket A, Turan S, Omar A, Berber M, Ozen
A, Akbenlioglu C and Haklar G: Serum IGF-I and IGFBP-3 levels of
Turkish children during childhood and adolescence: Establishment of
reference ranges with emphasis on puberty. Horm Res. 65:96–105.
2006.PubMed/NCBI
|
|
9
|
Cappa M, Iughetti L, Loche S, Maghnie M
and Vottero A; GeNeSIS National Board on behalf of the GeNeSIS
Italian Investigators, : Efficacy and safety of growth hormone
treatment in children with short stature: The Italian cohort of the
GeNeSIS clinical study. J Endocrinol Invest. 39:667–677. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wit JM and Rekers-Mombarg LT; Dutch Growth
Hormone Advisory Group, : Final height gain by GH therapy in
children with idiopathic short stature is dose dependent. J Clin
Endocrinol Metab. 87:604–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kozuki N, Katz J, Lee AC, Vogel JP,
Silveira MF, Sania A, Stevens GA, Cousens S, Caulfield LE,
Christian P, et al Child Health Epidemiology Reference Group
Small-for-Gestational-Age/Preterm Birth Working Group, : Short
maternal stature increases risk of small-for-gestational-age and
preterm births in low- and middle-income countries: Individual
participant data meta-analysis and population attributable
fraction. J Nutr. 145:2542–2550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu S, Gu X, Pan H, Zhu H, Gong F, Li Y and
Xing Y: Reference ranges for serum IGF-1 and IGFBP-3 levels in
Chinese children during childhood and adolescence. Endocr J.
57:221–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Şıklar Z, Kocaay P, Çamtosun E, İsakoca M,
Hacıhamdioğlu B, Savaş Erdeve Ş and Berberoğlu M: The effect of
recombinant growth hormone treatment in children with idiopathic
short stature and low insulin-like growth factor-1 levels. J Clin
Res Pediatr Endocrinol. 7:301–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grimberg A, DiVall SA, Polychronakos C,
Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C and Murad MH;
Drug and Therapeutics Committee and Ethics Committee of the
Pediatric Endocrine Society, : Guidelines for growth hormone and
insulin-like growth factor-I treatment in children and adolescents:
growth hormone deficiency, idiopathic short stature, and primary
insulin-like growth factor-I deficiency. Horm Res Paediatr.
86:361–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huchko MJ, Leslie H, Maloba M, Zakaras J,
Bukusi E and Cohen CR: Outcomes up to 12 months after treatment
with loop electrosurgical excision procedure for cervical
intraepithelial neoplasia among HIV-infected women. J Acquir Immune
Defic Syndr. 69:200–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cengiz P, Bas F, Atalar F, Ucar A,
Darendeliler F, Akan G, Tarhan T and Bundak R: Growth
hormone/insulin-like growth factor-1 axis as related to body mass
index in patients with idiopathic short stature. J Clin Res Pediatr
Endocrinol. 5:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawashima Y, Hakuno F, Okada S, Hotsubo T,
Kinoshita T, Fujimoto M, Nishimura R, Fukushima T, Hanaki K,
Takahashi S, et al: Familial short stature is associated with a
novel dominant- negative heterozygous insulin-like growth factor 1
receptor (IGF1R) mutation. Clin Endocrinol (Oxf). 81:312–314. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeshita H, Fujihara J, Soejima M, Koda
Y, Kimura-Kataoka K, Ono R, Yuasa I, Iida R, Ueki M, Nagao M, et
al: Confirmation that SNPs in the high mobility group-A2 gene
(HMGA2) are associated with adult height in the Japanese
population; wide-ranging population survey of height-related SNPs
in HMGA2. Electrophoresis. 32:1844–1851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teng RJ, Wu TJ and Hsieh FJ: Cord blood
level of insulin-like growth factor-1 and IGF binding protein-3 in
monochorionic twins. J Formos Med Assoc. 114:359–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaplan RC, Petersen AK, Chen MH, Teumer A,
Glazer NL, Döring A, Lam CS, Friedrich N, Newman A, Müller M, et
al: A genome-wide association study identifies novel loci
associated with circulating IGF-I and IGFBP-3. Hum Mol Genet.
20:1241–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herfs M, Somja J, Howitt BE,
Suarez-Carmona M, Kustermans G, Hubert P, Doyen J, Goffin F,
Kridelka F, Crum CP, et al: Unique recurrence patterns of cervical
intraepithelial neoplasia after excision of the squamocolumnar
junction. Int J Cancer. 136:1043–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bohé J, Joly MO, Arkouche W, Laville M and
Fouque D: Haemodialysis with the biocompatible high permeability
AN-69 membrane does not alter plasma insulin-like growth factor-I
and insulin-like growth factor binding protein-3. Nephrol Dial
Transplant. 16:590–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SA, Choe YR, Yang EM and Kim CJ:
Comparison of growth hormone treatment in patients with idiopathic
short stature and idiopathic growth hormone deficiency. Chonnam Med
J. 50:63–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoffman AR: Treatment of the adult growth
hormone deficiency syndrome: Directions for future research. Growth
Horm IGF Res. 15 (Suppl A):48–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sotos JF and Tokar NJ: Growth hormone
significantly increases the adult height of children with
idiopathic short stature: Comparison of subgroups and benefit. Int
J Pediatr Endocrinol. 2014:152014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lanes R, Gunczler P, Esaa S and Weisinger
JR: The effect of short- and long-term growth hormone treatment on
bone mineral density and bone metabolism of prepubertal children
with idiopathic short stature: A 3-year study. Clin Endocrinol
(Oxf). 57:725–730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasat R, Livesey JL, Espiner EA, Abbott GD
and Donald RA: IGF-1 and IGFBP-3 screening for disorders of growth
hormone secretion. N Z Med J. 109:156–159. 1996.PubMed/NCBI
|