Introduction
The oxidized compound 3-nitrotyrosine (3-NT) is
employed as an oxidative stress marker. The tyrosine present in
most proteins is a target for reactive nitrogen species (RNS), such
as peroxynitrite (ONOO−), which is produced by the
reaction of superoxide (O2−) with nitric
oxide (NO). The ONOO− reacts extremely quickly with the
aromatic amino acid tyrosine to form 3-NT (1,2). The
main problem of the occurrence of protein nitration is the
alteration or inhibition of their function. The protein oxidation
damage has been largely associated with the progression of some
degenerative diseases such as non-alcoholic fat liver, colitis,
lateral sclerosis, Alzheimer's disease and type 2 diabetes mellitus
(3–5).
The non-alcoholic fatty liver disease (NAFLD) begins
with hepatic steatosis, which is defined as the accumulation of
triglycerides (TG) at the level of the cytoplasm, in histology is
evidenced by the presence of droplets in cytoplasm of TG in more
than 5% of the hepatocytes, steatosis may progress to a state of
inflammation and cellular damage that may end in NAFLD and hepatic
cirrhosis (6). In the pathogenesis
of NAFLD it has been shown that the increase of free fatty acids
leads to a deregulation of the ways of assimilation and release of
free fatty acid in the liver, thus modifying the regulation of
transcription of the genes of lipogenesis and their transport out
of the liver (7,8). The excess of TG overloads the carrier
VLDL molecules, so VLDL-TG of greater diameter that exceed the
sinusoidal pores of epithelium accumulate in the liver, and may
generate stress in the endoplasmic reticulum (ER) and the
activation of the pathway NF-κB, which increase the transcription
of genes encoding for pro-inflammatory cytokines (8,9).
In addition, the excess of fatty acids modifies the
mitochondrial function, because when the fatty acids are processed
by β-oxidation, this increases the substrates of the respiratory
chain and the flow of electrons, which conduce to a higher
production of oxygen and reactive nitrogen species (ROS and RNS)
and may exceed the antioxidant response, and lead to oxidation of
biomolecules.
In the case of ulcerative colitis (UC), the
deficiencies of the intestine immune system and the epithelial
barrier that prevents the entry of bacteria or antigens to the
circulation, lead to inflammation, which causes a continuous
deterioration of the epithelium and the exposure to microorganisms.
In the lamina propia of the intestinal mucosa, the number of mature
dendritic cells of the immune system also increases, including a
large number of Toll-like (TLR) receptors, specifically TLR2 and
TLR4, which are activated and lead to the transcription of genes
that encode for pro-inflammatory cytokines. There is also an
atypical response of T helper cells (Th) in patients with
ulcerative colitis, specifically Th2, which exerts a cytotoxic
response against epithelial cells (10–12). In
addition, the recruitment of leukocytes is affected, because UC
increases the release of the chemo attractant molecule CXCL8, this
activates the leukocytes recruiting from the systemic circulation
to the intestinal mucosa, its entrance triggers an inflammatory
response with a high production of ROS, which is a defense response
to phagocytosis of bacteria, also the granular material or soluble
irritating compounds increase ROS levels (13).
In intestinal and hepatic diseases, the common
factor is an inflammatory condition, that begins with TLR4
receptors, and activates transcriptional factors such as NF-κB,
which in turn regulates the genetic expression of different
pro-inflammatory cytokines, for example in fatty liver disease,
there is an increase of TNF-α, IL-8, IL-1 α and IL-1 β (14). The cascade of TLR4 can induce
alterations of the mitochondrial function and thus the increase of
ROS production. Also, during the consumption of fat-rich diets, it
has been shown there is an overexpression of TLR4 gene
(15) and production of ROS
(16).
In addition, a relationship of incidence of hepatic
damage in patients with inflammatory diseases such as ulcerative
colitis (17) has been found
recently, which may be due to the passage of bacteria or endotoxins
from the intestine to liver, that produces an inflammation of the
liver and hepatobiliary damage, this condition is specifically
termed cholangitis (18,19). In patients with NAFLD the damage
derived from UC can be a factor of susceptibility for progress to
non-alcoholic steatohepatitis (NASH). As a result of the bacterial
translocation, the endotoxin of the bacterial cover (LPS) is fixed
to the type receptors of the cell surface and this promotes the
production of the pro-inflammatory cytokines and production of ROS
in the liver tissue. The establishment of incidence and correlation
between different diseases is also explored in other diseases to
prevent their worsening. For example, measurements of
echocardiography of healthy and NAFLD patients were compared and a
significant relationship was found, with an increase in the mass of
the left ventricle of the myocardium in patients with NAFLD, the
conclusion was that the echocardiography is a good indicator of
decreased cardiac function and may be key to modify feeding habits
in patients with NAFLD to improve cardiac function. In the case of
UC, its evaluation and monitoring could be used to prevent liver
damage.
The cells overcome the oxidative stress condition
mainly by neutralization systems such as superoxide dismutase,
catalase and oxide-reductase enzymes that react with ROS and in
consequence diminish ROS and RNS (13). The cellular mechanisms to repair
oxidative damage are scarce or null, such as the case of protein
3-NT elimination, which has been proposed as irreversible, and
although an enzymatic like activity of denitrase for histone 3 to
detoxify 3-NT has been demonstrated in humans (20), there are no data that clearly
demonstrate the presence of denitrase enzyme in higher eukaryotic
organisms. However, in microorganisms proteins have been found that
degrade 3-NT, these are denitrases enzymes or flavoprotein
monooxygenases that act directly on nitrated aromatic compounds to
remove the nitro group, originally described in bacteria as
oxygenase that in presence of an aromatic compound such as ortho or
para nitrophenol, require a reduced substrate such as NADPH or NADH
and oxygen to release nitrite (21–23). In
the yeast Debaryomyces hansenii (D. hansenii), we found
genes that encode for NADPH-dependent monooxygenase enzymes, and in
total protein extracts of the yeast, we measured the specific
activity of the denitrase with commercial free 3-nitrotyrosine and
determined that D. hansenii can degrade to a concentration
of 10 mM 3-NT. It was also found that the yeast can assimilate 3-NT
as the sole source of nitrogen (24).
The extremophile organisms are an excellent source
to search for genes to detoxify oxidized molecules, such as the
extreme halotolerant yeast D. hansenii, that during salt
stress support a high ROS production (25,26). In
response, the yeast activates the transcription of genes to
ameliorate oxidative stress. In a previous work, we reported that
the increased expression of the RNA messenger of DhARO4 gene
in D. hansenii is a strategy to survive to salt stress,
because after the transcription of DhARO4 there is more
enzyme to produce tyrosine, that is used as antioxidant compound to
avoid the protein oxidation, because the free tyrosine can react
with ONOO− that, in turn, reduces the ONOO−
levels that can nitrate proteins. Taking into account this model,
we supported our results, because after the overexpression of the
DhARO4 gen, there is a high specific activity of the DhAro4p
without an increase of tyrosine because we observed a maximum
concentration of the 3-NT compound (27). In fact, after grown D.
hansenii in a medium with high NaCl concentration, there is
also oxidative stress and we proposed that the synthesis of
tyrosine occurred immediately, this is oxidized to 3-NT as a
mechanism to decrease the cell damage of high ROS or RNS production
(26).
As we mentioned, recently we found that D.
hansenii can assimilate 3-NT as unique nitrogen source, and
determined that the cell extract of this yeast has denitrase
specific activity over residual free 3-NT (24). However, it is necessary determine
whether the cell extracts of D. hansenii can revert the 3-NT
oxidation when it is bound to proteins of higher eukaryotic
organisms.
In this work, we evaluated the denitrase activity of
D. hansenii in liver proteins from mice with and without
colitis measuring the ROS production and the 3-NT oxidative marker,
and evaluating the nitrite concentration to corroborate the removal
of NO2 from 3NT.
Materials and methods
Mice
Female BALB/c mice were purchased from Envigo México
(Envigo RMS S.A., Coyoacán, Mexico). Six to eight weeks old mice
were used in this study. All mice were housed under specific
pathogen-free conditions according to Faculty Animal Care and Use
Committe and government guidelines (Official Mexican regulation
NOM-062-ZOO-1999).
DSS-induced colitis
The model of colitis in mice was implemented
according to Ledesma-Soto (28).
Briefly, BALB/c strain mice were assigned to the control or colitis
group. The control group received standard food with free access to
water, whereas the colitis group received in their drinking water
4% dextran sodium sulphate (DSS) (MW: 40000; Alfa Aesar) for 7 to
10 days to cause colitis. The mice were weighted daily to evaluate
the body weight percent. At the end of the treatament, the mice
were euthanized in a CO2 chamber and liver samples with
or without colitis were placed in 2 ml polypropylene conical tubes,
immediately frozen with liquid nitrogen and stored at −70°C for
later use.
Histology
Ethanol-fixed colon tissues were embedded in a
paraffin block. Distal parts of colons obtained from colitis mice
at day 9 were cut into 5 µm and used for hematoxylin and eosin
(H&E) staining. The histological severity of colitis was
examined under a microscope (Axio Vert A1, Carl Zeiss), tissue
samples stained with H&E and were based on the extent of edema,
ulceration, crypt loss and infiltration of immune cells as
previously described (29).
Mice liver crude extracts
The crude extracts were done according to the
modified assay of Song (30). Then,
200 mg of each sample was taken and to remove the excess of blood,
samples were washed with 1X phosphate buffer (PBS). The tissue was
transferred to a 2 ml propylene conical tube and added 500 µl of 1X
PBS. Cellular lysis was done with PTFE micro pestle and 5 µl of 100
mM phenylmethylsulfonyl fluoride (PMSF) (SIGMA-ALDRICH). The
cellular macerate was centrifuged at 13,000 × g for 10 min at 4°C.
The aqueous phase was transferred to a new tube and kept on ice.
The protein concentration was determined using the UV light
spectrophotometry method at 280 nm and a standard curve of bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany).
ROS production
ROS levels were determined in the liver extracts of
M. musculus by the reaction of ROS with the 2′7′-
dichlorofluorescein diacetate (DCFA-DA) (Sigma-Aldrich; Merck
KGaA), according to the modified method of Hempel (31). With this method, the increase of
fluorescence is used as index of the production of ROS in the
sample. From each cell extract, 50 µl were placed in triplicate in
a 96-well plate and 195 µl of 1X PBS and 5 µl of 500 µM DCFA-DA
were added. The plate was placed in a fluorometer with 96-well
plate reader (BioTek Instruments, Inc., Winooski, VT, USA); the
fluorescence was recorded at an excitation wavelength of 485 nm and
emission of 520 nm for 60 min. The fluorescence value was reported
as the relative fluorescence unit per mg of total protein in the
crude extract.
D. hansenii growth conditions
The yeast D. hansenii strain Y7426 (CBS 767)
was obtained from the Department of Agriculture of Peoria (Peoria,
IL, USA). The strain was maintained in YPD medium (yeast extract
1%, peptone 1% and glucose 2%) (Sigma-Aldrich; Merck KGaA) and Agar
1.5% (Sigma-Aldrich; Merck KGaA). In order to grow D.
hansenii in salt stress, first a pre-culture of the yeast was
made in YPD medium for 24 h at 28°C with constant stirring (250
rpm). Then aliquots were taken from the preculture to inoculate
three Erlenmeyer flasks with YPD medium and/or 1 and 2 M NaCl (JT
Baker, Phillipsburg, NJ, USA). Cultures were started with an
optical density (OD) of 0.01 to 0.2. Cells were collected by
centrifugation when the cultures reached OD value of 0.8 to 1 at
600 nm. D. hansenii crude extracts were obtained (26). The protein concentration was
determined using the UV light spectrophotometric method at 280 nm
and a standard curve of BSA.
Incubation assay of crude extracts of
mice liver and yeast
From each liver crude extract (with and without
colitis) and yeast (YPD, with 1 and 2 M NaCl), 100 µg ml of
proteins were added to each eppendorf tube. The capacity of
denitrase activity was evaluated according to the modified assay of
Zeyer and Kocher (21). To each
extract mixture 4 mM NADPH (Sigma-Aldrich; Merck KGaA) and 4 mM
MgSO4 (Sigma-Aldrich; Merck KGaA) were added and the
volume was adjusted to 100 µl with 1X PBS, each sample was kept at
room temperature for 30 min, then frozen at −70°C.
3-NT concentration in crude
extracts
3-NT-modified proteins in the mixture of extracts
were quantified by a 3-NT primary antibody (enzyme-linked
immunosorbent assay) assay (Ab116691; Abcam, Cambridge UK)
following the instructions of the supplier. Quantification was done
against a standard curve of nitrated BSA (Ab116691; Abcam). From
each sample an aliquot was taken which was added to each well of
the plate and incubated for two h at room temperature. Subsequently
the secondary antibody was added which recognizes the 3-NT antibody
and changes color when the HRP substrate is added. The plate was
read on a UV–VIS spectrophotometer (BioTek Instruments, Inc.) at a
wavelength of 600 nm.
Nitrite production
The concentration of nitrites from 3-NT degradation
was determined in all extracts with the Griess reaction of the
commercial nitrite and nitrate system (no. 780001; Cayman Chemical
Company, Ann Arbor, MI, USA). The added Griess reagents
sulfanilamide and n-(1-naphthyl) ethylendiamine react with the
nitrite to form a violet diazonium salt choromophore, whose
concentration is determined by measuring absorbance at 545 nm. The
samples were read on a 96-well plate reader spectrometer (BioTek
Instruments, Inc.) at a wavelength of 545 nm.
Statistical analysis
In order to establish whether the differences in the
values of the parameters of experimental and control mice were
statistically significant, a Student's t-test was applied when the
sample number of mice were five to six (data of change of weight
and ROS concentration). While in the determinations with a number
of mice of three, as the case of 3-NT and NO2
concentrations, the data were analyzed with a Kruskal-Wallis to
establish if there were differences among experimental and control
groups; next, a Mood's median test was applied as a pos hoc
test for comparisons between pairs of groups using Bonferroni
correction. P<0.05 was considered to indicate a statistically
significant difference.
Results
Induction of colitis in a mouse model
by Dextran sodium sulphate (DSS)
Taking into account that in the model of murine
colitis severe intestine damage has been reported, we wanted to
evaluate whether there was also liver damage and we established
first the model of colitis according to Ledesma-Soto (28). We then evaluated whether the
intestine damage can affect the liver. We corroborated that 4% DSS
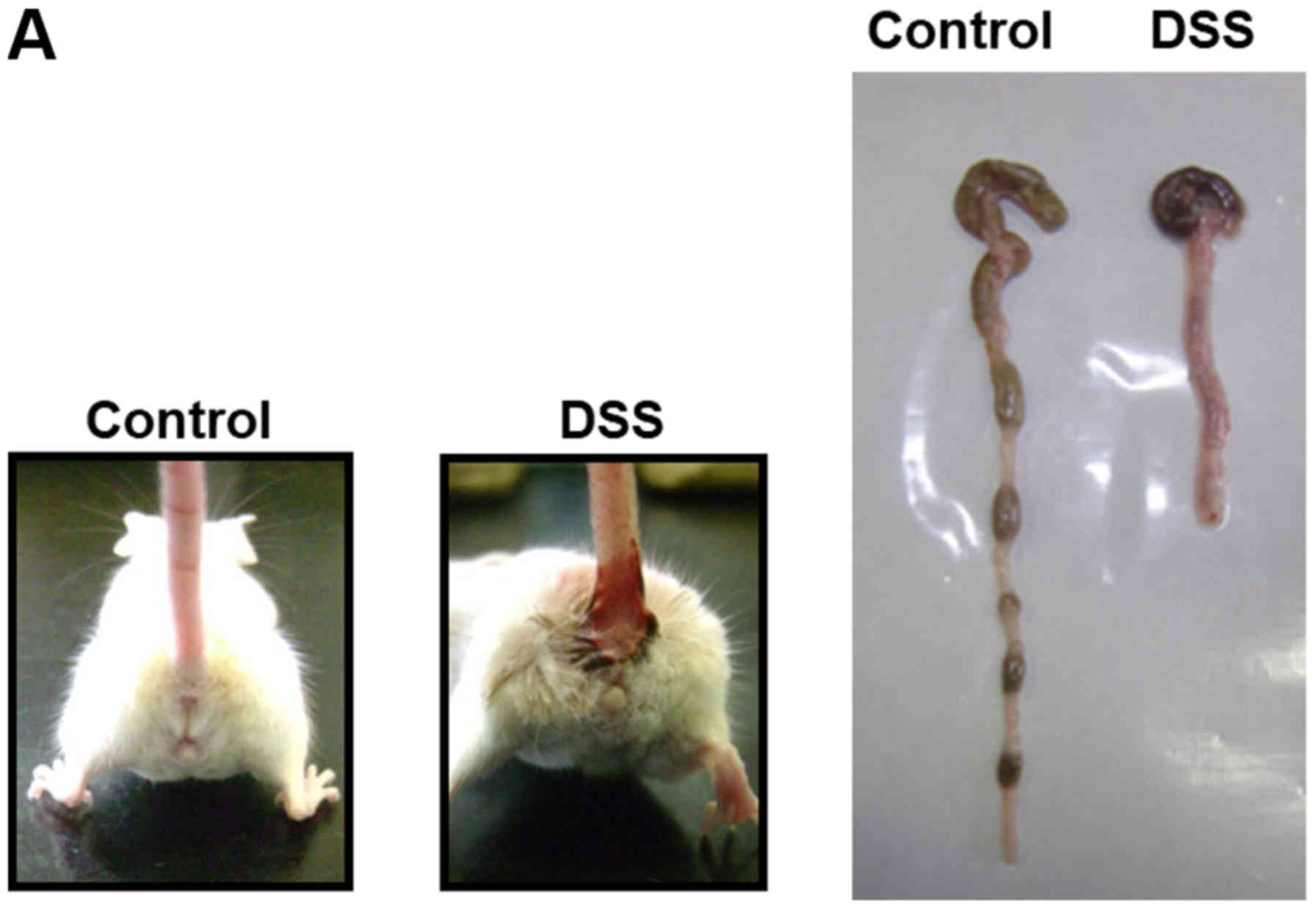
induces colitis when it is administered orally for 9 days (Fig. 1A). During the course of the
experiment, the treated mice exhibited profound body weight loss
(Fig. 1B) and bloody diarrhea,
whereas the control group exhibited no symptoms. Histological
analysis indicated that DSS generates signs of the histological
damage such as abnormal crypts, crypts loss, and inflammatory cell
infiltration (Fig. 1C).
Colitis produces an increase of ROS
and protein damage in liver
To evaluate liver damage, we measured the reactive
oxygen species (ROS) in liver samples of mice with and without
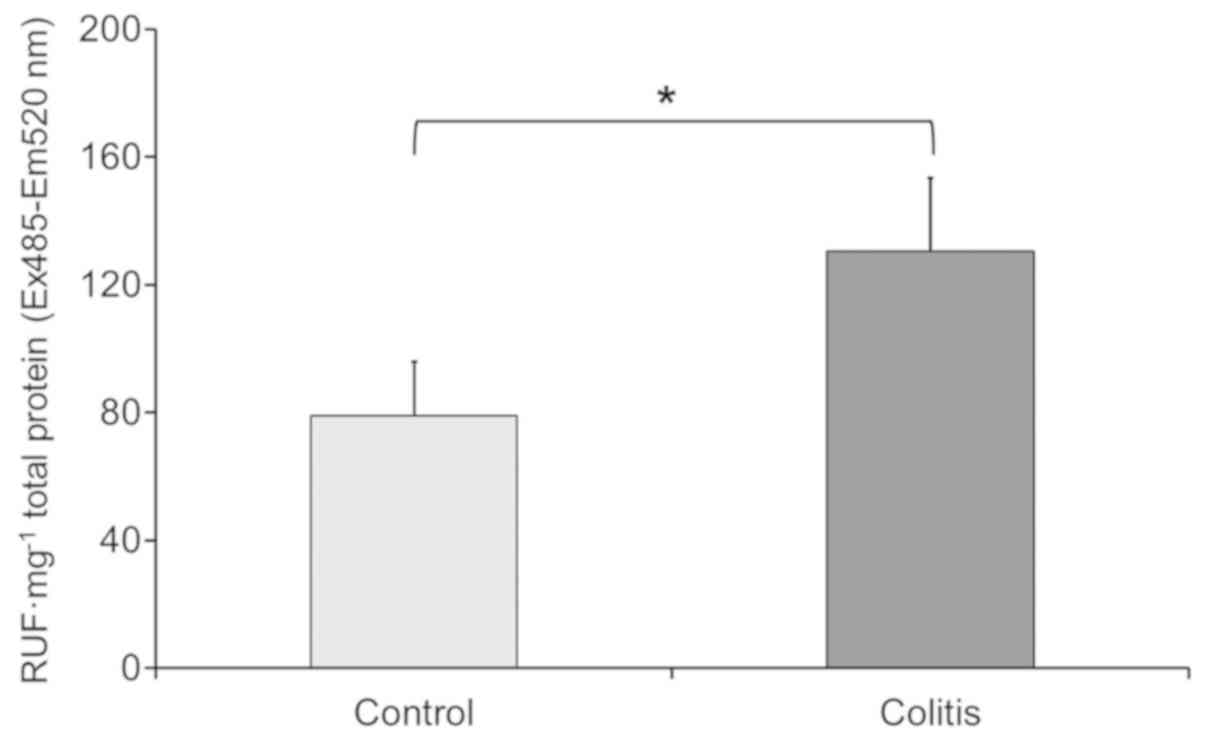
colitis. The crude extracts of mice liver without colitis had a ROS
level of 79 relative units of fluorescence (RUF) total protein •
mg−1. In contrast the crude extracts of mice liver with
colitis presented higher ROS level 130.6 RUF total protein •
mg−1, (Fig. 2), these
results indicate that effectively, ROS levels increase in the liver
samples from mice with colitis.
Considering the high ROS production observed in the
samples of liver from mice with colitis, we evaluated whether the
samples displayed oxidation of proteins such as the formation of
3-NT. Next, we measured the concentration of 3-NT. We observed that
samples of liver from mice without colitis showed an average
concentration of 3-NT of 36.8 ng • ml−1 (n=3) while the
samples of liver from mice with colitis displayed significant
higher 3-NT concentrations 78.1 ng • ml−1 (n=3) compared
to normal mice, these data suggest a higher protein oxidation
damage in the liver of mice with colitis.
Denitrases of Debabryomyces hansenii
decrease the levels of oxidation of proteins of mice liver with
colitis
In order to evaluate the denitrase capacity of D.
hansenii to reduce protein-bound 3-NT, we performed incubation
assays of crude extracts of liver samples (with and without
colitis) with crude extracts of D. hansenii. Also, we
considered that higher denitrase activity was reported in cell
extracts of the yeast grown with 1 and 2 M NaCl (24), thus we included these cell growth
conditions to obtain the cell extracts. We found that in the
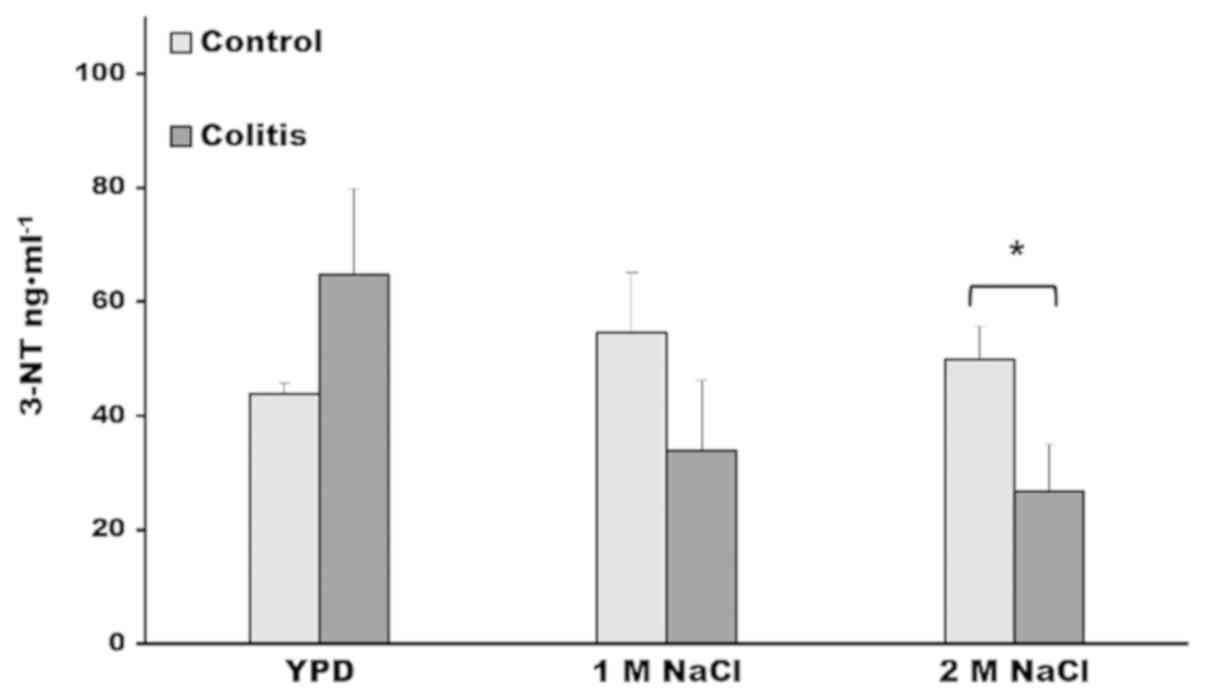
incubation assay, in which the samples of control and colitis liver
were incubated with cells extract of D. hansenii grown in
YPD medium, the higher value of 3-NT corresponded to the samples
with colitis, because, first, the samples of colitis, as we
described above, had a higher 3NT concentration, and second, the
YPD growth condition is not stressful for the yeast and in
consequence, there is only a basal denitrase activity (24). However, in the incubation assay in
which the cell extract of D. hansenii grown in 1 M NaCl, the
3-NT concentration of samples with colitis decreased and dropped
their values below that obtained in the control samples (Fig. 3). Moreover, in the incubation assay
of the samples with colitis and the cell extract of D.
hansenii grown with 2 M NaCl, the 3-NT levels were
significantly lower compared with control samples. The lack of
reduction of 3-NT concentration in the control and colitis samples
when the cell extracts of the yeast were recovered from YPD medium,
and the increase of degradation of 3-NT with cell extracts of D.
hansenii grown in 1 and 2 M NaCl, suggest de novo
synthesis of denitrase enzyme to overcome the oxidative damage
generated during salt stress.
Decreased levels of 3-nitrotyrosine in liver samples
with colitis are notorious, comparing the levels in the samples
incubated with the yeast cell extract grown in YPD medium against
those of the extract with 2 M NaCl (Fig.
3), it is observed that there is a 50% decrease in 3-NT levels,
this fact underscores the decrease of oxidized proteins of colitis
with the denitrase of the cellular extract of D.
hansenii.
Denitrase activity increases nitrite
production
It is known that after a denitrase reaction occurrs
there is an increase in the nitrite levels (22). Thus, next we quantified the nitrites
concentration after the incubation assay of crude extracts of
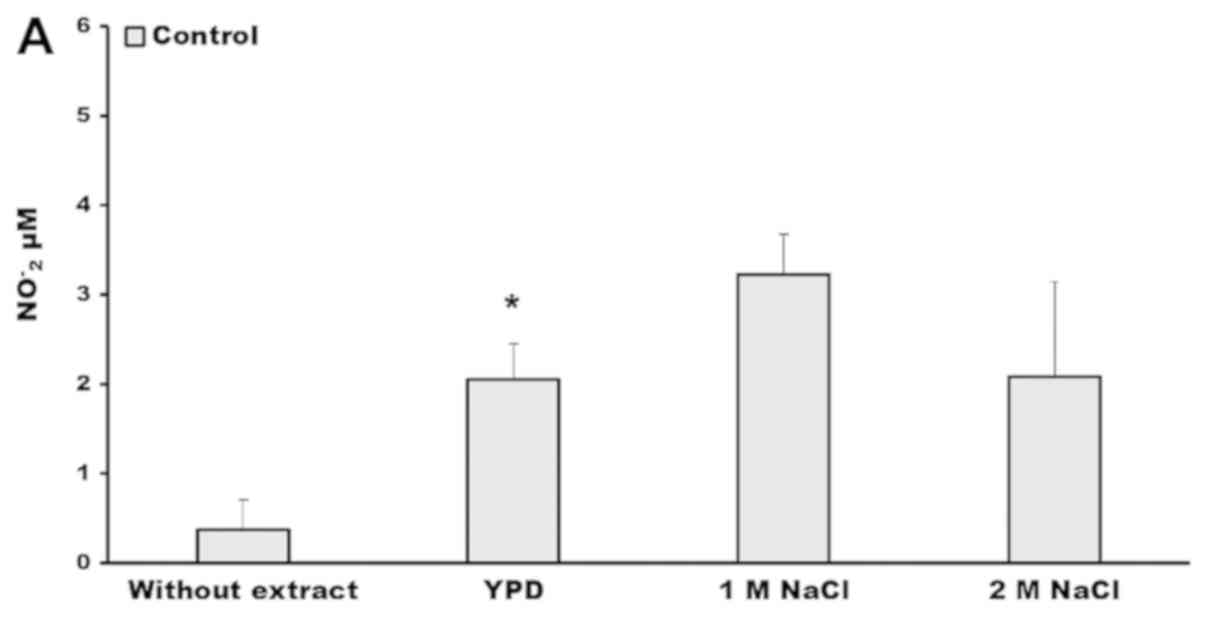
livers with the crude extract of the yeast. We found in the liver
samples without yeast extract that the concentration of nitrites
were 0.41 and 0.37 µM, respectively (Fig. 4). By contrast, after the incubation
with the yeast extract the nitrites reached the highest
concentration, and significant differences of nitrites were
observed in colitis samples incubated with the extract of yeast
grown in 1 M NaCl (Fig. 4B).
However, we observed almost the same value of nitrites
concentration in the incubation assay of control and colitis
samples with the crude extract of the yeast grown in 2 M NaCl
(Fig. 4).
Discussion
The oxidative-mediated damage of proteins is a key
event in many degenerative and inflammatory diseases (32,33). In
the present study, we provided evidence that colitis can affect
other organs such as liver, given that we observed an increase of
ROS production in the crude extracts of livers from mice with
colitis. In this respect, research is scarce, due to the complexity
of evaluating that a disease such as colitis or obesity in animal
models lead to oxidative stress condition in the liver (16,34).
The research about inflammatory disease such as
colitis is fundamental to establish strategies to decrease the
damage of liver and preserve its functions. In particular, it has
been demonstrated in colitis that the oxidative stress condition
leads to oxidation of biomolecules, which can be detrimental, if
the oxidation modifies or inhibits protein functions, for example,
when the irreversible oxidation of tyrosine occurs to form 3-NT
(35).
To date, a specific biological system has not been
reported in higher eukaryotes that reverts the oxidation of the
3-NT bound to proteins. In a previous work, we demonstrated that
D. hansenii can assimilate free 3-NT as a unique source of
nitrogen and that in vitro the crude extracts of this yeast
have denitrase activity (24), but
it is necessary to provide evidence on whether or not such
denitrase activity may function with 3-NT bound to proteins. To our
knowledge this is the first report demonstrating that cell extracts
from D. hansenii cultured in salt stress conditions have a
potent denitrase activity that is able to degrade 3-NT in protein
samples of liver from mice with acute colitis. Also, we
demonstrated that the crude extracts of D. hansenii grown in
1 and 2 M NaCl has the highest denitrase activity, and this fact is
in agreement with the postulate of the transcriptional response of
D. hansenii to overcome the effects of the oxidative stress
condition generated during salt stress (24,27).
Our findings are supported by an expected increase
in the nitrite concentration, because when the reaction of
denitrase occurs, the 3-NT disappears and the nitrite concentration
increases, as has been shown by Nishino (22). In particular, we found an elevated
increase of nitrites in the liver samples from mice with colitis,
this result can be taken as an additional demonstration that the
3-NT concentration was higher in the liver of mice with colitis
because there was an elevated ROS production. And, as we suspected,
in the assays where we used the cell extracts of D. hansenii
grown in salt stress, nitrite production increased due to the high
denitrase activity observed in 1 and 2 M NaCl conditions. However,
in the 2 M NaCl crude extract of the yeast there was no higher
increase of nitrites concentration, probably because the sodium
residual in the cell extracts reacts with nitrites to form
nitrates, as occurred in the reaction of nitrite oxidation in
presence of sodium (36,37).
The present work is preliminary and in further
studies of in vivo application of the yeast extract in a
mouse model of colitis, we consider carrying out a liver function
test, by measuring hepatic enzymes in blood, such as γ-glutamyl
transferase (GGT), aspartate transaminase (AST), alanine
aminotransferase (ALT) or alkaline phosphatase, because these
enzymes are released from the liver in response to damage or
disease; a measurement and identification of nitrated proteins of
liver and blood by HPLC and mass spectrometry, will be useful to
stablish the kind and number of nitrated proteins before and after
application of the yeast extract, for example it has been
determined that both the glutamine synthase and apolipoprotein A1
are nitrated in inflammatory and hepatic diseases (2). Given that in this work we determine
only whether there were changes in the production of ROS in the
liver of mice with or without colitis, we consider using ROS
determination in specific cells employing flow cytometry. All of
these measurements are required to state the results of the present
study.
In conclusion, here we presented evidence that the
yeast D. hansenii possess a high denitrase activity to
detoxify oxidized 3-NT bound to proteins. This finding will benefit
experimental and therapeutic medicine, considering that oxidation
of proteins in degenerative and chronic diseases is a problem in
medicine today. Also the demand for repair mechanisms justify the
need for more effective therapy approaches. Thus the research that
put in practice strategies to revert the oxidation damage derived
from the results of this study will be able to make a breakthrough
in medicine, because the study will help uncover a critical area in
the reduction of the oxidized compound 3-NT that inhibits or
modifies the function of proteins and because until now there is
not a report giving evidence of a gene or enzyme that reduces this
compound in humans. Thus a new application to reduce oxidation in
inflammatory diseases and thereby ameliorate the symptoms and
perhaps stop the progress to more severe illness may found.
Acknowledgements
We are grateful to Dr Antonio Peña Díaz (Institute
of Cellular Physiology, National Autonomous University of Mexico)
for his kind support of this study.
Funding
This study was partially financed by grant no.
N226716 from National Autonomous University of Mexico,
DGAPA-PAPIIT.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CMC-T was responsible for study design. YL-S and
LS-C performed the experiments. Data interpretation and statistical
analysis was performed by CMC-T, LS-C and MM-R. LIT-V was involved
in drafting the manuscript and revising it critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal research protocol for this work were
reviewed and approved by the ethics committee
(CE/FESI/042017/1168), Faculty of Higher Studies Iztacala, National
Autonomous University of Mexico, Mexico.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
3-NT
|
3-nitrotyrosine
|
|
ROS
|
reactive oxygen species
|
|
RNS
|
reactive nitrogen species
|
References
|
1
|
Ischiropoulos H: Biological tyrosine
nitration: A pathophysiological function of nitric oxide and
reactive oxygen species. Arch Biochem Biophys. 356:1–11. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahsan H: 3-Nitrotyrosine: A biomarker of
nitrogen free radical species modified proteins in systemic
autoimmunogenic conditions. Hum Immunol. 74:1392–1399. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820. 2011.
View Article : Google Scholar
|
|
4
|
Ceriello A and Testa R: Antioxidant
anti-inflammatory treatment in type 2 diabetes. Diabetes Care. 32
(Suppl 2):S232–S236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Butterfield DA, Reed T and Sultana R:
Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain
proteins in the progression and pathogenesis of Alzheimer's
disease. Free Radical Res. 45:59–72. 2011. View Article : Google Scholar
|
|
6
|
Academia Nacional de Medicina de México, .
Enfermedad por hígado graso no alcohólico. Boletín De Información
Clínica Y Terapéutica. 24:7–8. 2015.
|
|
7
|
Di Rosa M and Malaguarnera L: Genetic
variants in candidate genes influencing NAFLD progression. J Mol
Med (Berl). 90:105–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Botteri G, Montori M, Gumà A, Pizarro J,
Cedó L, Escolà-Gil JC, Li D, Barroso E, Palomer X, Kohan AB and
Vázquez-Carrera M: VLDL and apolipoprotein CIII induce ER stress
and inflammation and attenuate insulin signaling via Toll-like
receptor 2 in mouse skeletal muscle cells. Diabetologia.
60:2262–2273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ipsen DH, Lykkesfeldt J and Tveden-Nyborg
P: Molecular mechanisms of hepatic lipid accumulation in
non-alcoholic fatty liver disease. Cell Mol Life Sci. 75:3313–3327.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Danese S and Fiocchi C: Ulcerative
colitis. N Engl J Med. 365:1713–1725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kmieć Z, Cyman M and Ślebioda TJ: Cells of
the innate and adaptive immunity and their interactions in
inflammatory bowel disease. Adv Med Sci. 62:1–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ungaro R, Mehandru S, Allen PB,
Peyrin-Biroulet L and Colombel JF: Ulcerative colitis. Lancet.
389:1756–1770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Sun H, Meng P, Wang M, Tian M,
Xiong Y, Zhang X and Huang P: Dose and time effect of CdTe quantum
dots on antioxidant capacities of the liver and kidneys in mice.
Int J Nanomedicine. 12:6425–6435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stojsavljević S, Gomerčić Palčić M,
Virović Jukić L, Smirčić Duvnjak L and Duvnjak M: Adipokines and
proinflammatory cytokines, the key mediators in the pathogenesis of
nonalcoholic fatty liver disease. World J Gastroenterol.
20:18070–18091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manček-Keber M, Frank-Bertoncelj M,
Hafner-Bratkovič I, Smole A, Zorko M, Pirher N, Hayer S,
Kralj-Iglič V, Rozman B, Ilc N, et al: Toll-like receptor 4 senses
oxidative stress mediated by the oxidation of phospholipids in
extracellular vesicles. Sci Signal. 8:ra602015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ortiz-Reyes AE and Calderón-Torres CM:
Incremento de la expresión de TLR4 y efecto antioxidante del ácido
acetilsalicílico en conejos con dieta alta en grasas. Revista De
Salud Pública Y Nutrición. 16:1–10. 2017.
|
|
17
|
Solís-Herruzo JA and Solís-Muñoz P:
Manifestaciones hepatobiliares en la enfermedad inflamatoria
Intestinal. Rev Esp De Enferm Dig. 99:525–542. 2007. View Article : Google Scholar
|
|
18
|
Erkan G: Inflammatory bowel disease and
primary sclerosing cholangitis. Ulcerative colitis epidemiology,
pathogenesis and complications. O'Connor Dr Mortimer: ISBN:
978-953-307-880-889. 2011, View
Article : Google Scholar
|
|
19
|
Dohan A, Faraoun SA, Barral M, Guerrache
Y, Boudiaf M, Dray X, Hoeffel C, Allez M, Farges O, Beaugerie L, et
al: Extra-intestinal malignancies in inflammatory bowel diseases:
An update with emphasis on MDCT and MR imaging features. Diagn
Interv Imaging. 96:871–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smallwood HS, Lourette NM, Boschek CB,
Bigelow DJ, Smith RD, Pasa-Tolić L and Squier TC: Identification of
a denitrase activity against calmodulin in activated macrophages
using high-field liquid chromatography-FTICR mass spectrometry.
Biochemistry. 46:10498–10505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeyer J and Kocher HP: Purification and
characterization of a bacterial nitrophenol oxygenase which
converts ortho-nitrophenol to catechol and nitrite. J Bacteriol.
170:1789–1794. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishino SF and Spain JC: Biodegradation of
3-nitrotyrosine by Burkholderia sp. strain JS165 and Variovorax
paradoxus JS171. Appl Environ Microbiol. 72:1040–1044. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arora PK, Srivastava A and Singh VP:
Application of monooxygenases in dehalogenation, desulphurization,
denitrification and hydroxylation of aromatic compounds. J Bioremed
Biodegrad. 1:1122010. View Article : Google Scholar
|
|
24
|
Castro DE, Murguía-Romero M, Thomé PE,
Peña A and Calderón-Torres M: Putative 3-nitrotyrosine detoxifying
genes identified in the yeast Debaryomyces hansenii: In silico
search of regulatory sequences responsive to salt and nitrogen
stress. Electron J Biotechn. 29:1–6. 2017. View Article : Google Scholar
|
|
25
|
Chao HF, Yen YF and Ku MS:
Characterization of a salt-induced DhAHP, a gene coding for alkyl
hydroperoxide reductase, from the extremely halophilic yeast
Debaryomyces hansenii. BMC Microbiol. 9:1822009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calderón-Torres M, Castro DE, Montero P
and Peña A: DhARO4 induction and tyrosine nitration in response to
reactive radicals generated by salt stress in Debaryomyces
hansenii. Yeast. 28:733–746. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calderón-Torres M, Peña A and Thomé PE:
DhARO4, an amino acid biosynthetic gene, is stimulated by high
salinity in Debaryomyces hansenii. Yeast. 23:725–734. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ledesma-Soto Y, Callejas BE, Terrazas CA,
Reyes JL, Espinoza-Jiménez A, González MI, León-Cabrera S, Morales
R, Olguín JE, Saavedra R, et al: Extraintestinal helminth infection
limits pathology and proinflammatory cytokine expression during
DSS-induced ulcerative colitis: A role for alternatively activated
macrophages and prostaglandins. Biomed Res Int. 2015:5634252015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang YC, Ching YH, Chiu CC, Liu JY, Hung
SW, Huang WC, Huang YT and Chuang HL: TLR2 and interleukin-10 are
involved in Bacteroides fragilis-mediated prevention of DSS-induced
colitis in gnotobiotic mice. PLoS One. 12:e01800252017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song J, Ke SF, Zhou CC, Zhang SL, Guan YF,
Xu TY, Sheng CQ, Wang P and Miao CY: Nicotinamide
phosphoribosyltransferase is required for the calorie
restriction-mediated improvements in oxidative stress,
mitochondrial biogenesis, and metabolic adaptation. J Gerontol A
Biol Sci Med Sci. 69:44–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hempel SL, Buettner GR, O'Malley YQ,
Wessels DA and Flaherty DM: Dihydrofluorescein diacetate is
superior for detecting intracellular oxidants: comparison with
2′,7′-dichlorodihydrofluorescein diacetate, 5(and
6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and
dihydrorhodamine 123. Free Radic Biol Med. 27:146–159. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gadjeva VS, Goycheva P, Nikolova G and
Zheleva A: Influence of glycemic control on some real-time
biomarkers of free radical formation in type 2 diabetic patients:
An EPR study. Adv Clin Exp Med. 26:1237–1243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zuwała-Jagiełło J, Pazgan-Simon M, Simon K
and Warwas M: Elevated advanced oxidation protein products levels
in patients with liver cirrhosis. Acta Biochim Pol. 56:679–685.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bronsart L, Nguyen L, Habtezion A and
Contag C: Reactive oxygen species imaging in a mouse model of
inflammatory bowel disease. Mol Imaging Biol. 18:473–478. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Souza JM, Choi I, Chen Q, Weisse M,
Daikhin E, Yudkoff M, Obin M, Ara J, Horwitz J and Ischiropoulos H:
Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem
Biophys. 380:360–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Winkler T, Goschinick J and Ache HJ:
Reactions of nitrogen oxides with NaCl as model of sea salt
aerosol. J Aerosol Sci. 22 (Suppl 1):S605–S608. 1991. View Article : Google Scholar
|
|
37
|
Sun CC and Chou TC: Kinetic of anodic
oxidation of nitrite ion using in situ electrogenerated HCIO in a
NaCl aqueous solution. Ind Eng Chem Res. 38:4545–4551. 1999.
View Article : Google Scholar
|