Introduction
As one of the most common types of cancer, gastric
carcinoma is one of leading causes of cancer-associated mortality
worldwide (1). Gastric carcinoma
primarily affects people residing in Central and Eastern Europe,
East Asia and South Africa (2). In
China, there are approximately 380,000 new cases of gastric cancer
each year, which accounts for >40% of the total worldwide number
of cancer diagnoses each year (3).
Despite efforts to improve treatment outcomes for patients with
gastric carcinoma, postoperative survival is extremely poor, and
the overall 5-year survival rate of patients with gastric carcinoma
is <10% (4). Early, accurate
diagnosis and reliable prognosis prediction are key factors for
improving the survival of patients with gastric carcinoma.
Therefore, identifying effective diagnostic and prognostic
biomarkers of gastric cancer is required.
It is widely accepted that the occurrence of gastric
carcinoma is closely associated with several factors including
mutations in tumor suppressor genes or oncogenes, bacterial and
viral infections and diet (5–7). The
Wnt/β-catenin signaling pathway serves a role in the pathogenesis
of several types of cancer by promoting tumor growth or metastasis
(8,9). In many cases, Wnt/β-catenin achieves
its biological function by interacting with long non-coding RNAs
(lncRNAs) (10); a subgroup of
non-coding RNAs with roles in multiple human diseases (11). Growth arrest associated lncRNA 1
(GASL1) is a newly discovered tumor suppressor lncRNA with a
functional role in osteosarcoma (12); however, its role in other
malignancies remains unknown. Preliminary microarray data has
demonstrated that lncRNA GASL1 expression is downregulated in
patients with gastric carcinoma (data not shown). The current study
demonstrated that lncRNA GASL1 may inhibit tumor growth in patients
with gastric carcinoma by inactivating the Wnt/β-catenin
signalingpathway.
Materials and methods
Clinical samples
Gastric cancer and adjacent normal tissue samples
were collected from 88 patients (male, n=52; female, n=36; age
range, 26–70 years; mean age, 47.7±6.3 years) who were diagnosed
and treated at the Union Hospital, Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China) from March 2011
to January 2013. In addition, serum samples were also obtained from
each patient. Inclusion criteria for the study included: i)
patients diagnosed with gastric carcinoma for the first time, as
confirmed by pathological assessment, and that had not previously
undergone treatment; ii) patients with complete clinical data; iii)
patients that had completed a 5-year follow-up following discharge;
iv) patients and/or their familes willing to participate. Exclusion
criteria for the study included: i) patients that had received any
treatment prior to admission; ii) patients that had failed to
complete treatment or the 5-year follow-up procedure; iii) patients
with other malignancies and/or gastric diseases; iv) patients that
had succumbed to other diseases or accidents during follow-up. In
addition, serum samples from 72 healthy control patients (male,
n=44; female, n=28; age range, 25–68 years; mean age, 47.1±6.1
years) were also included as a control group. Healthy patients
received routine physiological examinations at the Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology from March 2011 to January 2013. No significant
differences in age and gender were found between the two groups.
The current study was approved by the Ethics Committee at the Union
Hospital, Tongji Medical College, Huazhong University of Science
and Technology (Wuhan, China), and all patients and/or their
families provided written informed consent.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cancer tissues,
adjacent healthy tissues, serum and in vitro cultivated
cells using TRIrizol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Total RNA concentation was
measured using a NanoDrop™ 2000 Spectrophotometer (Thermo Fisher
Scientific, Inc.), and RNA samples with a A260/A280 ratio of
1.8–2.0 were reverse transcribed into cDNA. qPCR was subsequently
performed using the SYBR® Green PCR Master Mix (Thermo
Fisher Scientific, Inc.). The following primer pairs were used in
the qPCR reactions: GASL1 forward, 5′-CATGTTCCAATATGATTCCACC-3′,
and reverse, 5′-GATGGGATTTCCATTGATGAC-3′; β-actin forward, 5′-
GACCTCTATGCCAACACAGT-3′, and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
All PCR reactions were performed using an ABI PRISM 7500 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used for the
qPCR reactions: Initial denaturation at 95°C for 45 sec; 40 cycles
of 95°C for 12 sec and 60°C for 45 sec. GASL1 mRNA levels were
quantified using the 2−ΔΔCq method (13) and normalized to the internal
reference gene β-actin.
Cell culture and transfection
Human gatric carcinoma cell lines SNU-16
(ATCC® CRL-5974™) and NCI-N87 (ATCC®
CRL-5822™) were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). Cells were cultured in ATCC-formulated
RPMI-1640 medium (ATCC® 30-2001™) supplemented with 10%
fetal bovine serum (ATCC® 30-2020™). GASL1 siRNA
(CCUGAGGCUAGAGGGUCUAAGAGAA) and the siRNA Universal Negative
Control were provided by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The full-length GASL1 cDNA clone surrounded by EcoRI
restriction sites was obtained by PCR amplification and cloned into
the linearized pIRSE2-EGFP vector (Clontech Laboratories, Inc.,
Mountainview, CA, USA) to generate a GASL1 expression vector. Prior
to transfection, cells were cultured to reach 80–90% confluence.
Cells (5×105 cells/well of a 6-well plate) were
transfected with siRNAs (50 nM) and vectors (10 nM) using
Lipofectamine® 2000 reagent (cat. no. 11668-019,
Invitrogen; Thermo Fisher Scientific, Inc.). GASL1 downregulation
and overexpression was confirmed by RT-qPCR before subsequent
experimentation. Cells were harvested at 24 h following
transfection for subsequent experiments. Untransfected cells were
control cells, and cells transfected with empty vectors were
negative control cells.
Cell proliferation assay
Cell proliferation was analyzed using the Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Brifely, SNU-16 and NCI-N87 cells in the
logarithmic growth phase were harvested and single-cell suspensions
with a density of 4×104 cells/ml were prepared.
Subsequently, cells were seeded in 96-well plates at a density of
4×103 cells/well and incubated at 37°C in a 5%
CO2-humidified incubator for 24, 48, 72 and 96 h.
Following incubation, 10 µl CCK-8 reagent was added to each well
and cells were incubated for an additional 5 h. Cell proliferation
was determined by measuring the optical density OD at a wavelength
of 450 nm using a microplate reader.
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.). Total protein was quantified using a bicinchoninic acid
assay and 20 µg protein/lane was separated by 10% SDS-PAGE. The
separated proteins were transferred onto polyvinylidene difluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
blocked with 5% skimmed milk at room temperature for 2 h. Following
washing with PBS solution, membranes were incubated with primary
antibodies against β-catenin (rabbit anti-human; cat. no. ab16051;
dilution, 1:1,200) and GAPDH (rabbit anti-human; cat. no. ab9485;
dilution, 1:1,000; both Abcam, Cambridge, UK) overnight at 4°C.
Following washing, membranes were incubated with horseradish
peroxidase-labeled anti-rabbit IgG secondary antibody (cat. no.
MBS435036; dilution, 1:1,000; MyBioSource, Inc., San Diego, CA,
USA) at room temperature for 2 h. Protein bands were visualized
using the Amersham™ ECL™ western blotting reagent (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). β-catenin protein expression was
quantified using Image J v.1.47 software (National Institutes of
Health, Bethesda, MD, USA) and normalized to the GAPDH loading
control.
Statistical analysis
Data are presented as the mean ± standard deviation.
All statistical analyses were performed using GraphPad Prism
software (version 6.0; GraphPad Software, La Jolla, CA, USA).GASL1
and β-catenin expression data were analyzed using an unpaired
t-test, whilst the statistical significance between GASL1
expression levels in tumor and adjacent healthy tissue samples were
analyzed using a paired Student's t-test. One-way analysis of
variance followed by the least significant difference test was used
to analyze differences among multiple groups. The overall survival
of patients with gastric cancer was evaluated using the
Kaplan-Meier method and the log-rank test was used to compare the
survival distribution between the two groups. The correlation
between serum levels of GASL1 and clinicopathological data from
patients with gastric carcinoma were analyzed using a Chi-square
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
GASL1 expression in gastric cancer
tissue and adjacent normal tissue samples from 88 patients with
gastric carcinoma
The differential expression of a gene in cancer
tissues and adjacent normal tissues typically indicates the
involvement of this gene in a malignancy. Therefore, the expression
level of GASL1 in cancer and normal adjacent tissue samples from
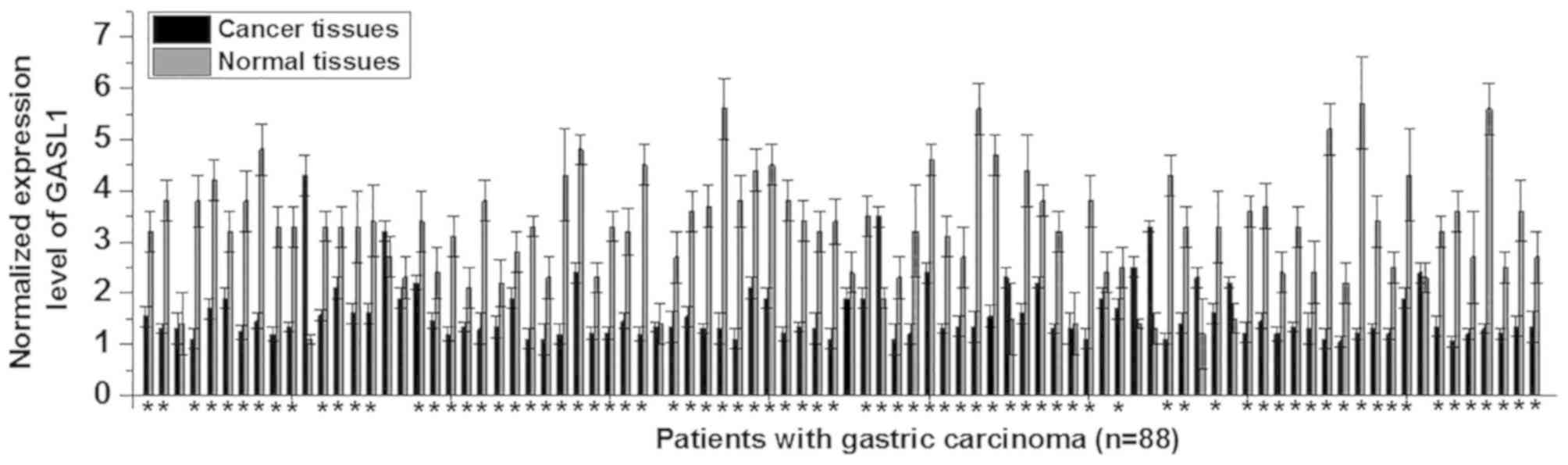
all 88 patients with gastric carcinoma was examined. The expression
level of GASL1 was significantly decreased in gastric cancer
tissues when compared with normal adjacent tissues in 76/88
patients with gastric carcinoma (Fig.
1). These results suggest that downregulation of GASL1 may be
involved in the pathogenesis of gastric carcinoma.
Comparison between GASL1 serum levels
in patients with gastric cancer and healthy controls and the
potential diagnostic value
LncRNAs are present in the circulatory system and
serve as molecular signals participating in several biological
processes (11). In the current
study, serum levels of GASL1 were detected in all patients with
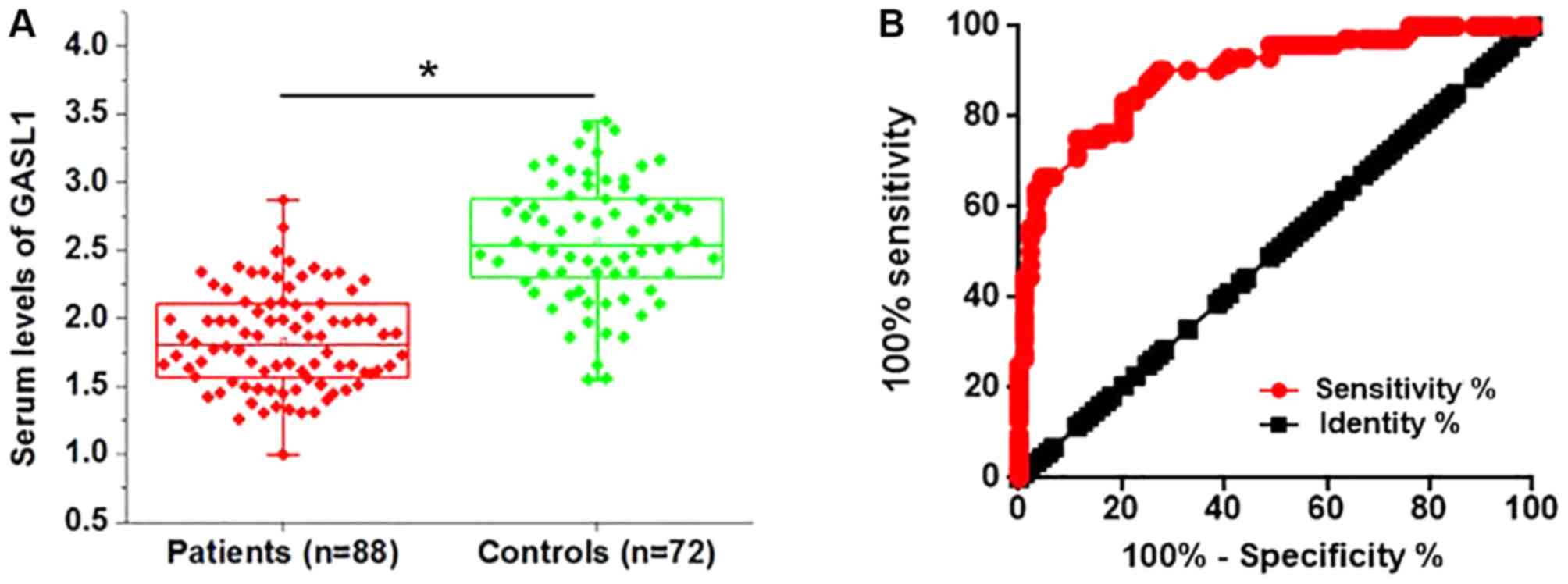
gastric carcinoma. The serum level of GASL1 was significantly
decreased in patients with gastric carcinoma compared with healthy
controls (Fig. 2A). Receiver
operating characteristic (ROC) curve analysis was used to evaluate
the diagnostic value of serum lncRNA GASL1 in discriminating
patients with gastric carcinoma from normal controls. The area
under the curve was 0.8945 (95% confidence interval, 0.8452–0.943;
standard error, 0.02313) (Fig. 2B).
These results suggest that low serum GASL1 may serve as a
diagnostic biomarker for gastric carcinoma.
Association between GASL1 serum levels
and clinicopathological characteristics of patients with gastric
cancer
Patients were divided into the high expression group
(n=44) and low expression group (n=44) according to the median
serum level of GASL1. The correlation between the serum levels of
GASL1 and clinicopathological data from patients with gastric
carcinoma were analyzed using a Chi-square test. As presented in
Table I, no significant correlation
between the serum level of GASL1 and age, gender, distant tumor
metastasis and the smoking and alcohol consumption of patients was
observed. However, serum levels of GASL1 were significantly
correlated with tumor size. Therefore, GASL1 may participate in the
regulation of tumor growth in gastric carcinoma.
 | Table I.Association between serum GASL1 and
clinicopathological characteristics of patients with gastric
cancer. |
Table I.
Association between serum GASL1 and
clinicopathological characteristics of patients with gastric
cancer.
|
|
| GASL1 expression
level |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristics | No. of cases | High (n=44) | Low (n=44) | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
>45 | 48 | 22 | 26 | 0.73 | 0.39 |
|
<45 | 40 | 22 | 18 |
|
|
| Gender |
|
|
|
|
|
| Male | 52 | 23 | 29 | 1.69 | 0.19 |
|
Female | 36 | 21 | 15 |
|
|
| Drinking |
|
|
|
|
|
| Yes | 47 | 22 | 25 | 0.41 | 0.52 |
| No | 41 | 22 | 19 |
|
|
| Smoking |
|
|
|
|
|
| Yes | 42 | 19 | 23 | 0.73 | 0.39 |
| No | 46 | 25 | 21 |
|
|
| Tumor size (cm) |
|
|
|
|
|
|
>5 | 45 | 17 | 28 | 5.50 | 0.02 |
|
<5 | 43 | 27 | 16 |
|
|
| Tumor distant
metastasis |
|
|
|
|
|
| Yes | 41 | 18 | 23 | 1.14 | 0.29 |
| No | 47 | 26 | 21 |
|
|
Association between GASL1 serum levels
and the overall survival of patients with gastric cancer
No significant correlation between the serum levels
of GASL1 and distant tumor distant metastasis was observed. Distant
metastasis is a major cause of death in gastric carcinoma patients;
therefore, the 5-year follow-up data from patients with and without
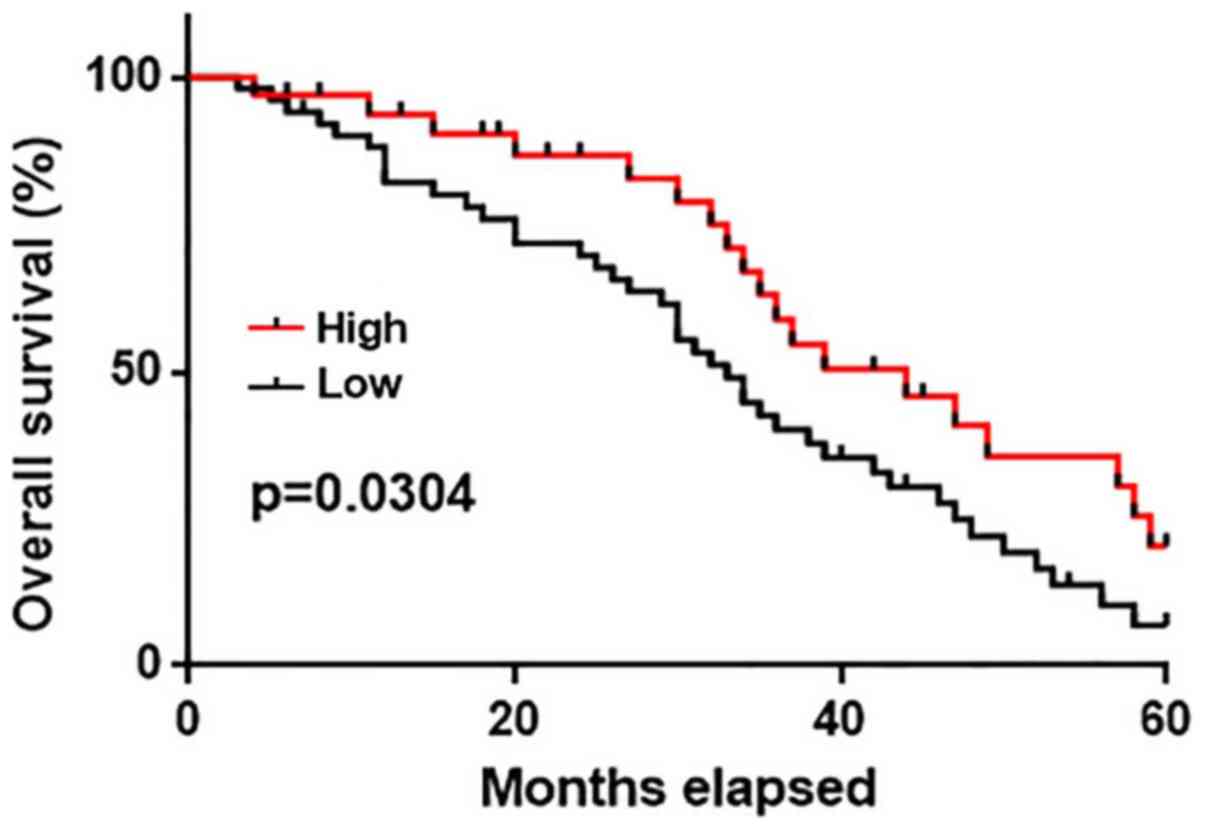
metastasis were analyzed. The survival curves of patients in the
high and low expression groups were plotted using the Kaplan-Meier
method and compared by log-rank test. The overall survival of
patients in the high expression group was significantly longer when
compared with patients in the low expression group (P<0.05;
Fig. 3). These results suggest that
serum GASL1 levels may also serve as a potential prognostic
biomarker for patients with gastric cancer.
Effect of GASL1 overexpression and
knockdown on β-catenin expression
The results presented in Table I indicate that GASL1 may be involved
in the regulation of gastric tumor growth. The Wnt/β-catenin
signaling pathway promotes cancer cell proliferation in several
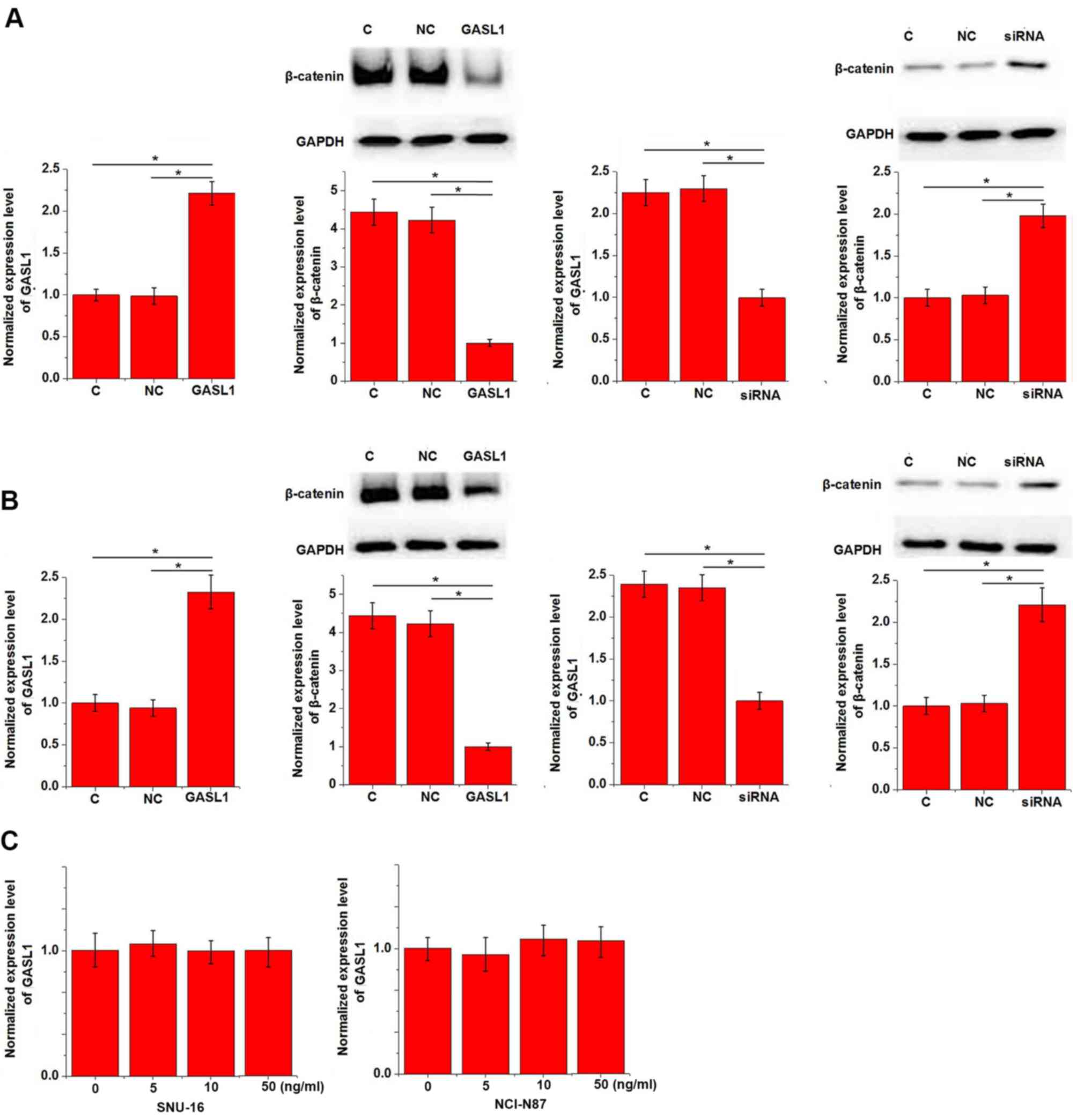
types of cancer, including gastric cancer (8). To investigate the potential mechanism
underlying the function of GASL1, the effect of GASL1 expression on
the Wnt/β-catenin signaling pathway was examined in vitro
using gastric carcinoma cell lines, SNU-16 and NCI-N87. GASL1
overexpression inhibited and GASL1 knockdown promoted the
expression of β-catenin in both SNU-16 (Fig. 4A) and NCI-N87 (Fig. 4B) gastric cell lines. In addition,
treatment with a Wnt agonist, a potent and selective activator of
Wnt signaling, demonstrated no significant effect on GASL1
expression at doses of 5, 10 and 50 ng/ml (Fig. 4C).
Effects of GASL1 overexpression and
knockdown on cell proliferation
A CCK-8 assay was performed to investigate the
effects of GASL1 overexpression and knockdown on the proliferation
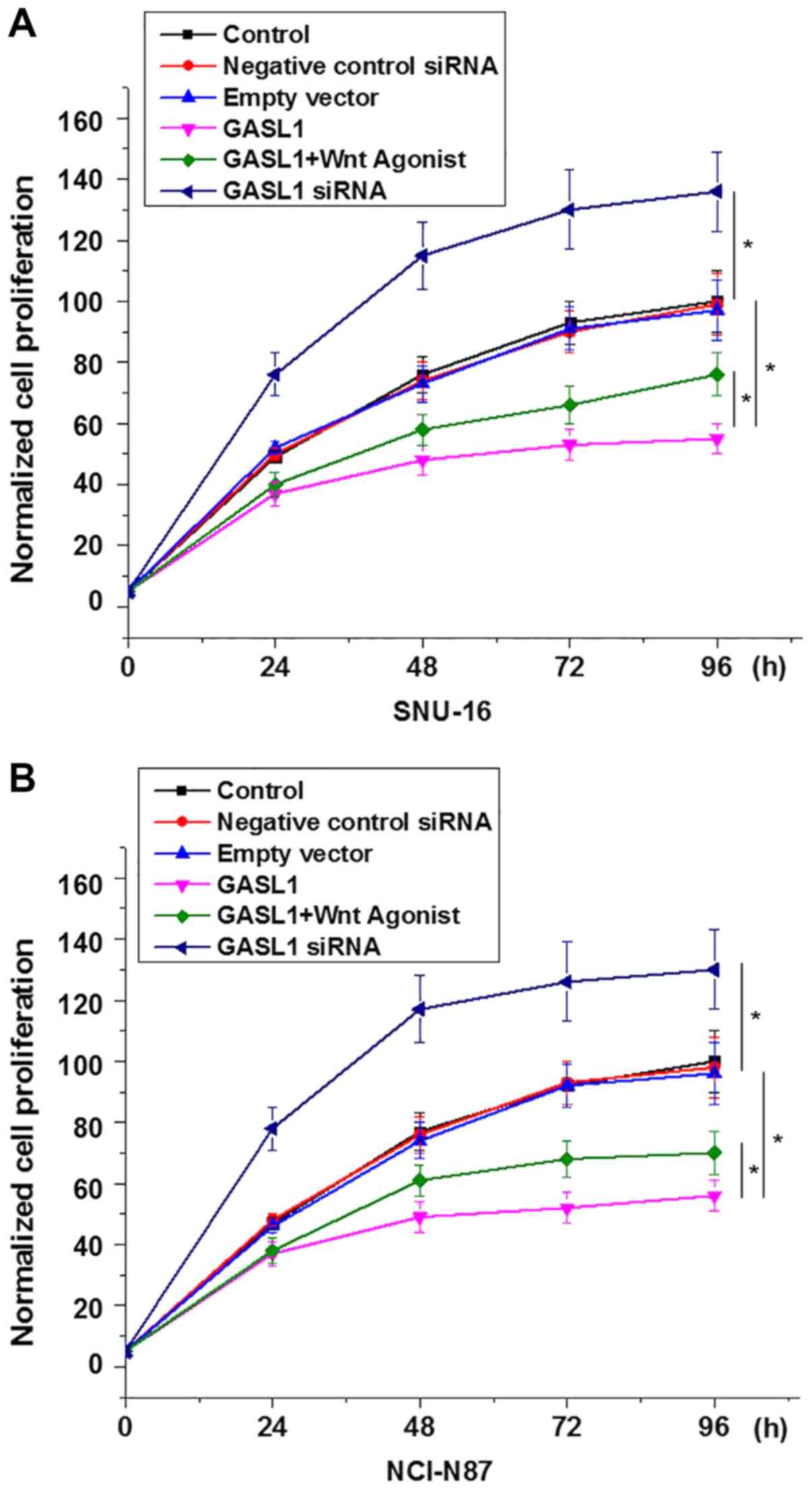
of gastric carcinoma cell lines, SNU-16 and NCI-N87. GASL1
overexpression inhibited and GASL1 knockdown promoted the
proliferation of SNU-16 (Fig. 5A)
and NCI-N87 (Fig. 5B) cells. In
addition, treatment with a Wnt agonist (10 ng/ml) significantly
reduced the inhibitory effect of GASL1 overexpression on the
proliferation of both cell lines (Fig.
5). Furthermore, GASL1 overexpression inhibited and GASL1
knockdown demonstrated no significant effect on gastric carcinoma
cell migration and invasion (data not shown), which suggests the
specific involvement of GASL1 in gastric carcinoma growth but not
metastasis.
Discussion
In the current study, the expression of GASL1 in
patients with gastric carcinoma and healthy controls was detected
and the funtional role of GASL1 in gastric carcinoma was
investigated. The present study demonstrated that GASL1, a known
tumor suppressor lncRNA in osteosarcoma (12), inhibits gastric carcinoma cell
proliferation. In addition, the GASL1 lncRNA may exert its tumor
suppressor function in gastric carcinoma by inactivating the
Wnt/β-catenin signaling pathway.
The occurrence and development of gastic carcinoma
is associated with alterations in the expression pattern of a large
number of lncRNAs (14), indicating
the involvement of lncRNAs in gastric carcinoma. LncRNA expression
profiles in tumor tissue and adajcent normal tissues serve
important roles by inhibiting or promoting cancer progression
(11). It has been reported that the
expression of lncRNA-H19 is significantly upregulated in gastric
carcinoma tissues compared with adjacent normal tissues, and
overexpression of lncRNA-H19 promotes distant tumor metastasis
(15). By contrast, the expression
of the maternally expressed gene 3 lncRNA is downregulated in
patients with gastric carcinoma and was reported to be an indicator
of poor prognosis (16). GASL1
lncRNA expression is downregulated in osteosarcoma (12). In the current study, the expression
level of GASL1 was significantly decreased in gastric cancer
tissues when compared with adjacent normal tissues in the majority
of patients, indicating that downregulation of GASL1 may be
involved in the pathogenesis of gastric carcinoma.
The survival of patients with gastric carcinoma
remains low, and this is in part due to the low rate of early
diagnosis (17). The gold standard
for the diagnosis of gastric carcinoma is pathological examination,
however application is often limited due to the invasive nature of
the procedure (18). The development
of human disease is often associated with alterations in certain
substances (such as circulating proteins, hormones and RNAs) in the
circulatory system, and monitoring changes in these substances may
facilitate the diagnosis of disease (19). In the current study, serum levels of
GASL1 were significantly decreased in patients with gastric
carcinoma when compared with healthy controls. ROC curve analysis
demonstrated that low levels of serum GASL1 effectively
distinguished patients with gastric carcinoma from healthy
controls. In addtion, survival analysis revealed that low serum
GASL1 levels were closely correlated with poor survival. GASL1
expression in other diseases remains unkown. Therefore, multiple
biomarkers should be combined to improve the diagnostic and
prognostic accuracy of serum GASL1 levels as a potential diagnostic
marker for gastric carcinoma. In addition, serum levels of GASL1 do
not always reflect the expression levels of GASL1 in tumor tissues.
In the current study, 4 patients in the high GASL1 serum level
group also demonstrated relatively high expression levels of GASL1
in tumor tissues.
The survival rate of patients with gastric carcinoma
in the early stages of disease is significnatly higher than those
with distant tumor metastasis (20).
In the current study, the serum levels of GASL1 demonstrated no
significant correlation with distant tumor metastases, indicating
that GASL1 may not be involved in the invasion and migration of
gastric cancer cells. Therefore, the 5-year follow-up records
included data from patients with and without distant metastases.
Taken together, these results suggest that serum GASL1 may serve as
a potential prognostic biomarker for gastric carcinoma.
Correlation analysis demonstrated a significant
correlation between the serum levels of GASL1 and tumor size. In
addition, in vitro cell proliferation assays revealed that
GASL1 inhibits cancer cell proliferation in gastric carcinoma. The
Wnt/β-catenin signaling pathway promotes cancer cell proliferation
in several types of cancer, including gastric cancer (8). The current study confirmed that GASL1
inhibits the Wnt/β-catenin signaling pathway. By contrast,
activation of the Wnt/β-catenin signaling pathway did not affect
GASL1 expression, suggesting that GASL1 may be an upstream
inhibitor of the Wnt/β-catenin signaling pathway in gastric
carcinoma. Activation of the Wnt/β-catenin signaling pathway
promotes tumor growth in gastric cancer (21), and rescue experiments in the current
study revealed that activation of Wnt/β-catenin can reduce the
inhibitory effects of GASL1 overexpression on gastric cancer cell
proliferation. Therefore, GASL1 may inhibit gastric cancer cell
proliferation by inactivating the Wnt/β-catenin signaling
pathway.
Further studies are required to investigate the
potential in vivo association between GASL1 and the
Wnt/β-catenin signaling pathway, which include analyzing the
correlation between GASL1 and Wnt/β-catenin expression levels in
tumor tissues. In addition, the number of clinicopathologic factors
evaluated in the current study were relatively low and therefore
more factors should be included in future studies.
In conclusion, the GASL1 lncRNA is significantly
downregulated in gastric carcinoma. Downregulation of serum GASL1
distinguishes patients with gastric carcinoma from healthy
controls, and low expression also indicated poor survival. GASL1
may function as an inhibitor of tumor growth in gastric carcinoma
through inactivation of the Wnt/β-catenin signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CP, XL, YY and JC were responsible for the
conception and design of the study. CP, XL and YY performed the
experiments. CP and XL analyzed the data. CP and YY prepared the
manuscript. CP and JC revised the final draft of the
manuscript.
Ethics approval and consent to
participate
The protocol used in the present study was approved
by the Ethics Review Committee of Union Hospital, Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China), and all patients and/or their families provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hohenberger P and Gretschel S: Gastic
cancer. Lancet. 362:305–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu X and Li J: Gastric carcinoma in
China: Current status and future perspectives. Oncol Lett.
1:407–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rugge M, Fassan M and Graham DY:
Epidemiology of gastric cancer. Gastric Cancer. Springer; Cham: pp.
23–34. 2015, View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canedo P, Durães C, Pereira F, Regalo G,
Lunet N, Barros H, Carneiro F, Seruca R, Rocha J and Machado JC:
Tumor necrosis factor alpha extended haplotypes and risk of gastric
carcinoma. Cancer Epidemiol Biomarkers Prev. 17:2416–2420. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song HJ and Kim KM: Pathology of
epstein-barr virus-associated gastric carcinoma and its
relationship to prognosis. Gut Liver. 5:143–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sjödahl K, Lu Y, Nilsen TI, Ye W, Hveem K,
Vatten L and Lagergren J: Smoking and alcohol drinking in relation
to risk of gastric cancer: A population-based, prospective cohort
study. Int J Cancer. 120:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akaboshi S, Watanabe S, Hino Y, Sekita Y,
Xi Y, Araki K, Yamamura K, Oshima M, Ito T, Baba H and Nakao M:
HMGA1 is induced by Wnt/beta-catenin signaling pathway and
maintains cell proliferation in gastric cancer. Am J Pathol.
175:1675–1685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang GB, Kim JY, Cho SD, Park KS, Jung JY,
Lee HY, Hong IS and Nam JS: Blockade of Wnt/β-catenin signaling
suppresses breast cancer metastasis by inhibiting CSC-like
phenotype. Sci Rep. 5:124652015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R,
Cao Z, Singh B, Franklin JL, Wang J, Hu H, et al: lncRNA
MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance
via Wnt/β-catenin signaling. Nat Med. 23:1331–1341. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lalevée S and Feil R: Long noncoding RNAs
in human disease: Emerging mechanisms and therapeutic strategies.
Epigenomics. 7:877–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gasri-Plotnitsky L, Ovadia A, Shamalov K,
Nizri-Megnaji T, Meir S, Zurer I, Cohen CJ and Ginsberg D: A novel
lncRNA, GASL1, inhibits cell proliferation and restricts E2F1
activity. Oncotarget. 8:23775–23786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang K, Shi H, Xi H, Wu X, Cui J, Gao Y,
Liang W, Hu C, Liu Y, Li J, et al: Genome-wide lncRNA microarray
profiling identifies novel circulating lncRNAs for detection of
gastric cancer. Theranostics. 7:213–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun M, Xia R, Jin F, Xu T, Liu Z, De W and
Liu X: Downregulated long noncoding RNA MEG3 is associated with
poor prognosis and promotes cell proliferation in gastric cancer.
Tumuor Biol. 35:1065–1073. 2014. View Article : Google Scholar
|
|
17
|
Jin Z, Jiang W and Wang L: Biomarkers for
gastric cancer: Progression in early diagnosis and prognosis. Oncol
Lett. 9:1502–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahn S, Van Vrancken M, Lee M, Ha SY, Lee
H, Min BH, Lee JH, Kim JJ, Choi S, Jung SH, et al: Ideal number of
biopsy tumor fragments for predicting HER2 status in gastric
carcinoma resection specimens. Oncotarget. 6:38372–38380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawata K, Liu CY, Merkel SF, Ramirez SH,
Tierney RT and Langford D: Blood biomarkers for brain injury: What
are we measuring? Neurosci Biobehav Rev. 68:460–473. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ono H, Kondo H, Gotoda T, Shirao K,
Yamaguchi H, Saito D, Hosokawa K, Shimoda T and Yoshida S:
Endoscopic mucosal resection for treatment of early gastric cancer.
Gut. 48:225–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5:e10392014. View Article : Google Scholar : PubMed/NCBI
|