Introduction
Pain is a major public health problem and an
economic burden. In the United States alone, recent estimates
indicate that 100 million adults suffer from pain-associated
complaints (1). In Europe, severe
and frequent pain is associated with reduced quality of life in the
five largest European Union countries (2). In addition, the treatment of pain
places a large burden on the economy and health services. Indeed,
it is estimated that the annual cost of pain in the United States
alone ranges between $560 and $635 billion (including the direct
healthcare costs and health-associated loss of productivity); this
figure is greater than the annual costs of heart disease, cancer
and diabetes management combined (3).
Pain is classified into either acute or chronic
types depending on its duration. One-fifth of the world's
population is thought to suffer from chronic pain (4). Opioids and non-steroidal
anti-inflammatory drugs (NSAIDs) are among the most commonly
prescribed medications for the management of chronic pain (5). Despite their well-documented analgesic
effects, opioids and NSAIDs have a number of disadvantages if used
over a long period. For example, the chronic use of opioids
increases the risk of serious side effects, such as tolerance,
constipation and physical dependence (6). By contrast, the chronic use of NSAIDs
(including ibuprofen) is associated with an increased risk of
gastrointestinal bleeding, ulcers and thrombotic events (6).
Given the limitations associated with the use of
NSAIDs and opioids for the management of chronic pain, alternative
strategies have been proposed, such as combining the above
analgesics with non-pharmacological interventions (7,8). The use
of such a multimodal approach is expected to have added benefits
over the sole use of pharmacological agents. For instance,
combining analgesics with non-pharmacological interventions may
achieve the same therapeutic effect of NSAIDS or opioids at lower
doses. This dose reduction may subsequently decrease the risk of
adverse drug side effects and, in turn, reduce treatment costs. Of
note, the lowest effective dose of NSAID for the shortest time
period is recommended where possible (9). A number of non-pharmacological
interventions have been proposed for the management of chronic pain
(10); with exercise as the most
recommended intervention. The World Health Organization recognizes
insufficient physical activity as a leading risk factor for the
increase in chronic pain worldwide (11). In addition, a review of the
literature indicated that exercise is effective in reducing chronic
pain, whilst improving functional and psychological status
(12). A limitation of the studies
that have investigated the benefits of exercise in the management
of chronic pain is the use of subjective outcome measures, such as
questionnaires, as a primary method of assessment. These studies,
although useful in predicting or confirming associations, fail to
establish causal relationships and are inherently not well
controlled. Accordingly, the use of a suitable animal model would
be a supplemental approach to study the effect of exercise on
chronic pain using more objective outcome measures.
Complete Freund's Adjuvant (CFA) is a solution that
is widely used to study nociception in animals (13). CFA consists of heat-killed
Mycobacteria in suspension, which induces a local
inflammatory reaction at the site of injection. These injections
induce peripheral tissue injury accompanied by an increased
sensitivity to thermal and mechanical stimuli. The authors of the
current study hypothesized that swimming exercise may produce an
additive effect on NSAID-induced antinociception. To test this
hypothesis, intraplantar injections of CFA were used to induce
nociception in experimental rats. The effect of swimming exercise,
alone or in combination with ibuprofen, on the threshold of
nociception was then examined. To the best of the authors'
knowledge, this is the first study to evaluate the effects of
combined swimming exercise and NSAIDs in a rat model of chronic
inflammatory pain.
Materials and methods
Animals
A total of 78 adult (8 weeks old) male Sprague
Dawley rats (weight, 180–250 g at the time of study commencement)
were used in this study. All rats were obtained from the animal
housing facility at Jordan University of Science and Technology
(JUST; Irbid, Jordan). Rats were housed in stainless steel wire
cages, with 3 rats/cage. The temperature was controlled at 22±2°C
and the rats were exposed to 12 h light/dark cycles with lights on
between 6:00 a.m. and 6:00 p.m. Tap water and standard chow were
provided ad libitum. All experimental procedures were
conducted between 9:00 a.m. and 3:00 p.m. All rats were kept for 14
days for habituation prior to the initiation of any intervention.
The study protocol was approved by the National Committee of Animal
Care and Use at JUST.
Induction of inflammation
CFA was used to elicit an immune response at the
site of injection. A volume of 100 µl CFA (containing 1 mg/ml
Mycobacterium tuberculosis; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to induce the inflammation. On the day
of injection, the rats received light anesthesia using an
isoflurane vaporizer (Ugo Basile S.R.L, Gemonio, VA, Italy).
Intraplantar CFA or its vehicle (saline) was administered to the
left hind paw (CFA paw), while the right hind paw (control paw)
received saline.
Mechanical allodynia
Mechanical allodynia was assessed by measuring the
paw withdrawal threshold. Following the acclimation period and
prior to any injection, rats were habituated to a plastic apparatus
placed over a wire mesh bottom that facilitates poking of the paw
from below for 30 min on two consecutive days. Threshold readings
were measured for three consecutive days to serve as a baseline
prior to any CFA injections. Assessment was conducted using
Aesthesio® von Frey filaments (Bioseb, Vitrolles,
France) in a simple up-down method, as suggested previously
(14). The filament sizes ranged
between 0.4 and 15.0 g. The results were recorded as binary values
(i.e., response or no response).
Swimming protocol
The swimming protocol described by Ozbek et
al (15) and Wang et al
(16) was used in the present study.
In this protocol, rats were randomly assigned to one of six
experimental groups (n=8 in each group): i) No CFA + ibuprofen +
sham swimming; ii) 100 µl CFA + ibuprofen + sham swimming; iii) no
CFA + ibuprofen + swimming treatment; iv) no CFA + ibuprofen +
prophylactic swimming; v) 100 µl CFA + ibuprofen + swimming
treatment; and vi) 100 µl CFA + ibuprofen + prophylactic
swimming.
Rats were placed in one of two identical metal tanks
measuring 60×100×60 cm (width, length and depth, respectively). The
tanks were filled with tap water at 32±1°C, and a drop of soap was
added to minimize the surface tension and floating behavior. On the
first day, the rats were allowed to swim for a 5-min duration
session. Subsequently, the duration of the swimming exercise was
gradually increased by 5 min/day until the rats were able to swim
for 30 min continuously. All swimming sessions were supervised to
ensure that every rat received the required exercise. In rare
instances when signs of floating appeared, gentle stirring of the
water was performed by the observer to create a water current and
stimulate swimming. Rats receiving sham swimming (control) were
introduced to shallow water (5 cm), for 30 min/day for 5 days/week.
Rats in the swimming experiments were divided to two subgroups:
Prophylactic and treatment. In the prophylactic swimming group,
rats received the swimming exercise for 30 min continuously each
day, 5 days/week for 5 weeks (2 weeks prior to CFA injection and 3
weeks after CFA injection). Rats receiving swimming treatment swam
for 30 min continuously each day, 5 days/week for three weeks after
CFA injection only. Following swimming, the rats were removed from
the tank and gently dried with a cloth before they were returned to
their cages.
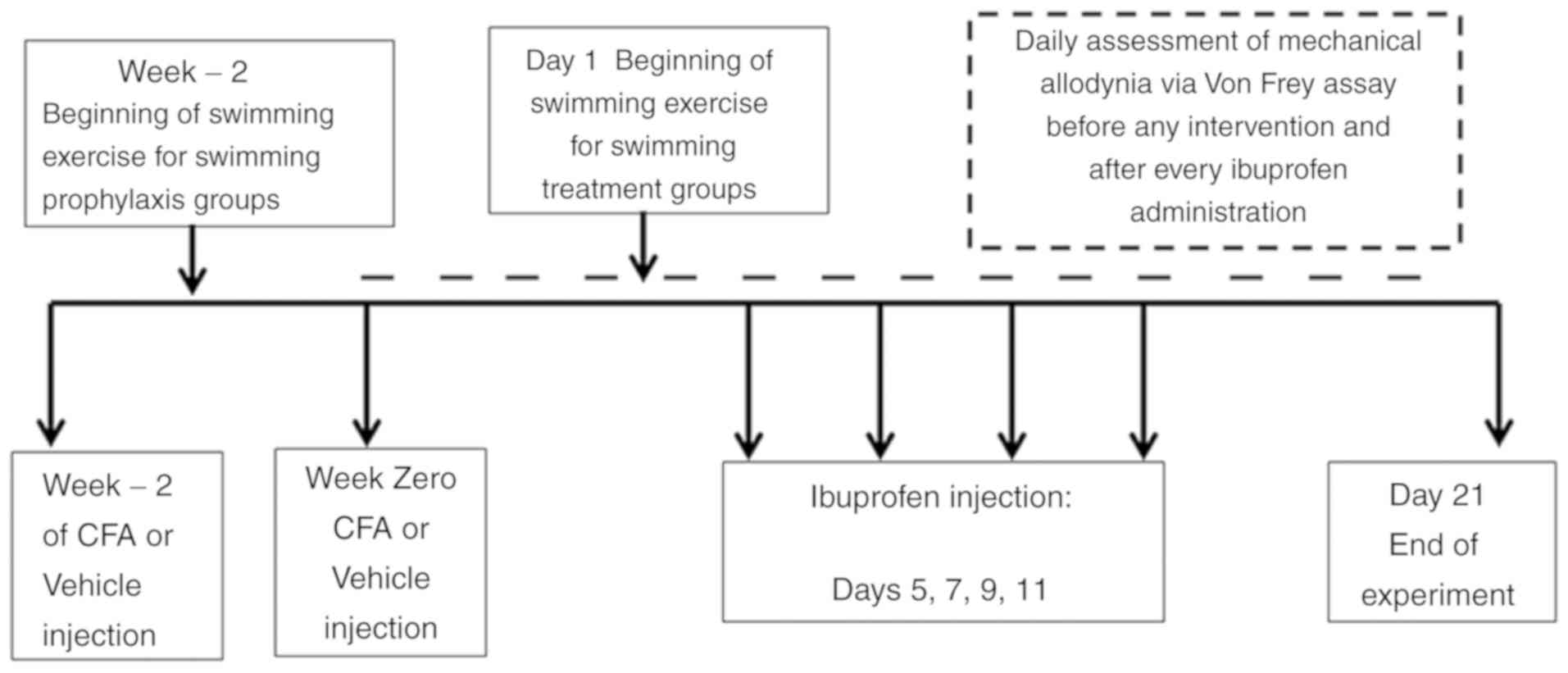
Assessment of mechanical allodynia was performed
daily directly prior to the swimming sessions. A summary of the
general workflow is presented in Fig.
1.
Drugs
Ibuprofen was administered at four doses (vehicle,
3.2, 10 and 32 mg/kg; based on a pilot study conducted in the
laboratory), and each dose was administered after at least 48 h had
elapsed since the previous dose to eliminate any leftover effect.
Thus, ibuprofen doses were administered in a randomized
Latin-square design on days 5, 7, 9, and 11 post-CFA injection.
Ibuprofen powder was obtained from the Jordanian Pharmaceutical
Manufacturing Company Co. PLC (Naour, Jordan) and dissolved in a
mixture of 20% ethanol, 10% cremophor oil and 70% water to prepare
the required concentrations. The assessor was blinded to the
ibuprofen doses throughout the experiment. On the ibuprofen test
day, the von Frey readings were performed twice, immediately prior
to drug administration and 60 min following ibuprofen
administration.
Statistical analysis
The primary dependent measure to assess mechanical
allodynia was the paw withdrawal threshold. Results are expressed
as the mean ± standard error of the mean. Analysis was performed
using repeated measures two-way analysis of variance followed by
Bonferroni's post hoc test. All statistical analyses and tests were
performed using GraphPad Prism software version 6.07 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
The percentage change in antinociception was
calculated using the following equation: Change (%) = [(threshold
after ibuprofen-threshold before ibuprofen)/(baseline
threshold-threshold before ibuprofen)] ×100.
Results
Effect of ibuprofen on CFA-induced
mechanical allodynia
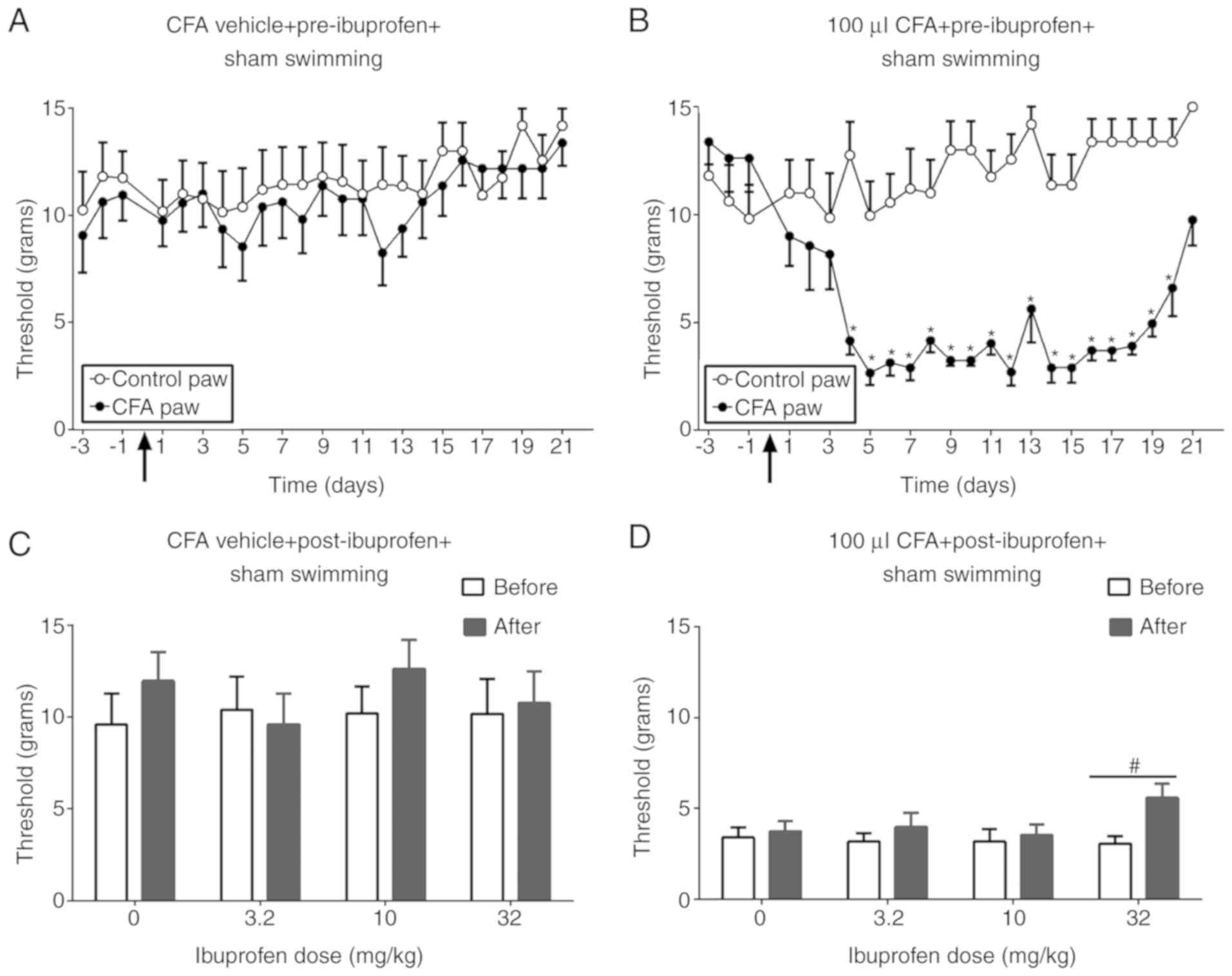
Fig. 2 illustrates
the effect of ibuprofen on CFA-induced mechanical allodynia. The
CFA vehicle did not produce any significant difference in
nociception threshold between the two paws [F (1, 14)=0.2628;
P=0.6162; Fig. 2A]. In the CFA
group, the threshold was significantly lower in the CFA paw
compared with the control paw from day 4 until 20 days following
CFA injection [F (1, 14)=54.56; P<0.0001; Fig. 2B]. However, ibuprofen (32 mg/kg)
partially reversed CFA-induced allodynia [F (1, 28)=21.74;
P<0.0001], without producing any significant effect in the CFA
vehicle group [F (1, 28)=2.853; P=0.1023; Fig. 2C and D].
Effect of swimming exercise and
ibuprofen on the CFA vehicle groups
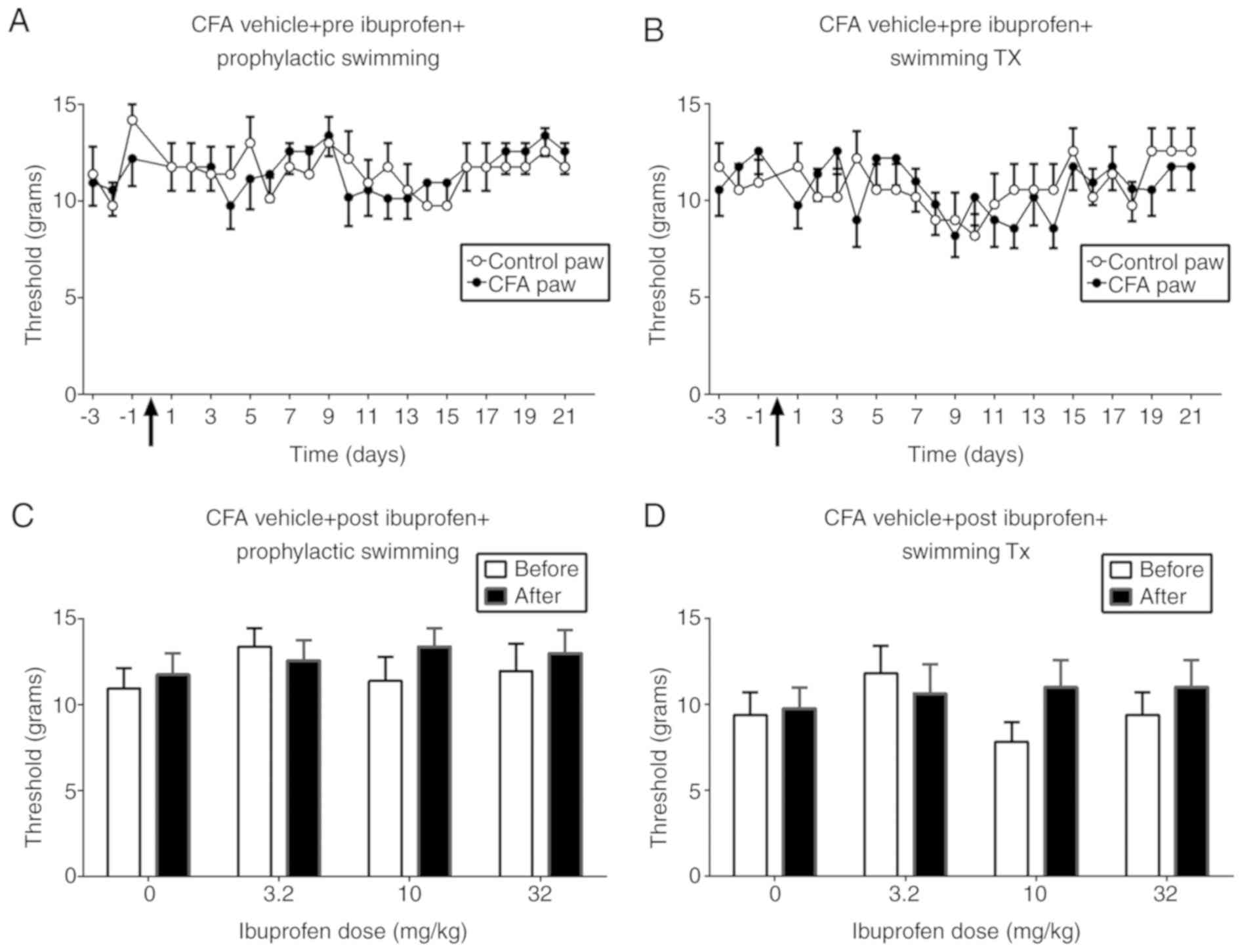
Fig. 3 illustrates
the effect of prophylactic swimming and swimming treatment on the
paw withdrawal threshold in the absence and presence of ibuprofen.
No significant difference in the nociception threshold between the
two paws in the groups receiving prophylactic swimming [F (1,
14)=0.00001421; P=0.9970] or swimming treatment [F (1,
14)=0.003911; P=0.9510; Fig. 3A and
B] was observed. In addition, ibuprofen did not produce a
significant change in threshold in either group, regardless of
ibuprofen dose (Fig. 3C and D).
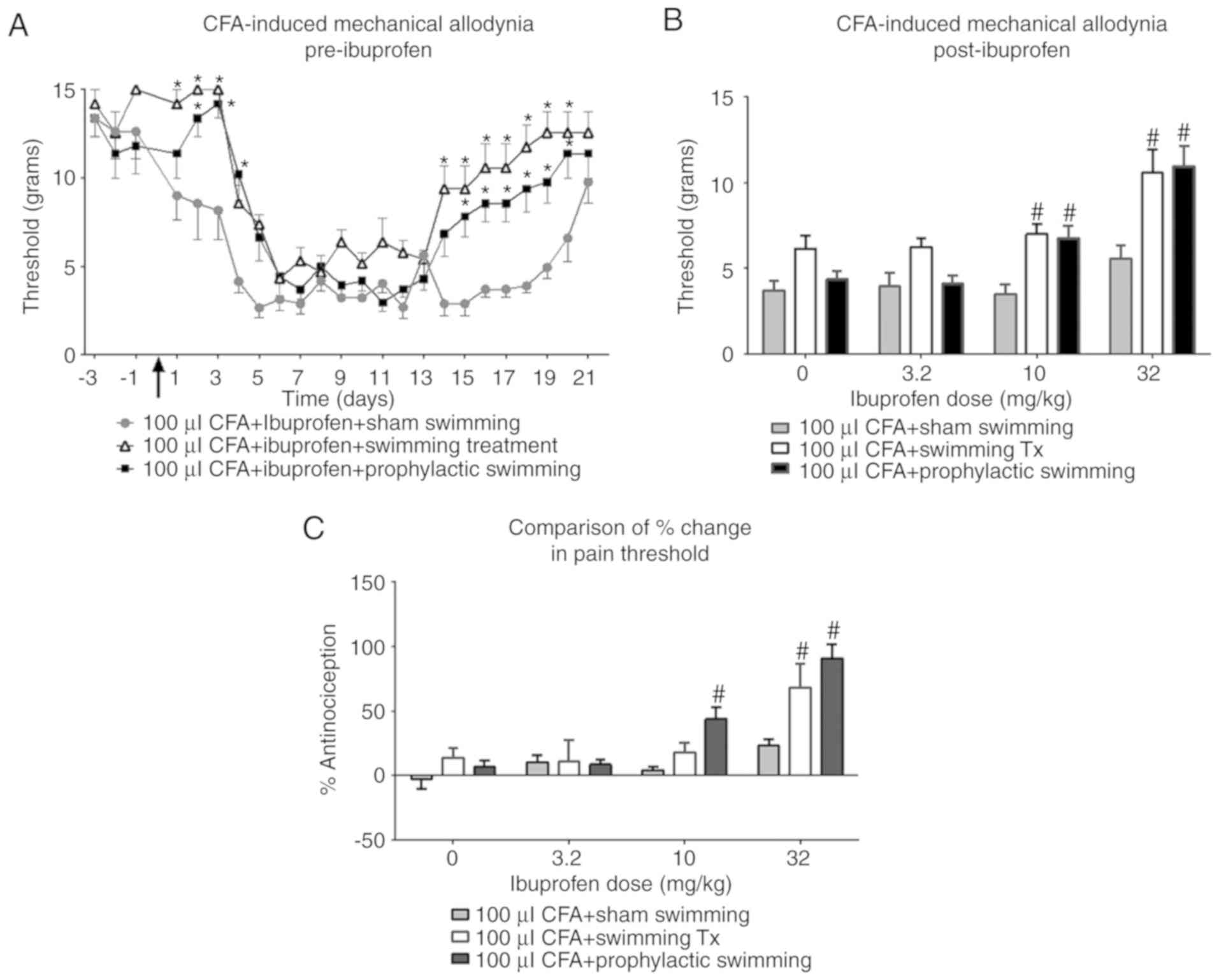
Effect of swimming exercise and
ibuprofen on CFA-induced mechanical allodynia
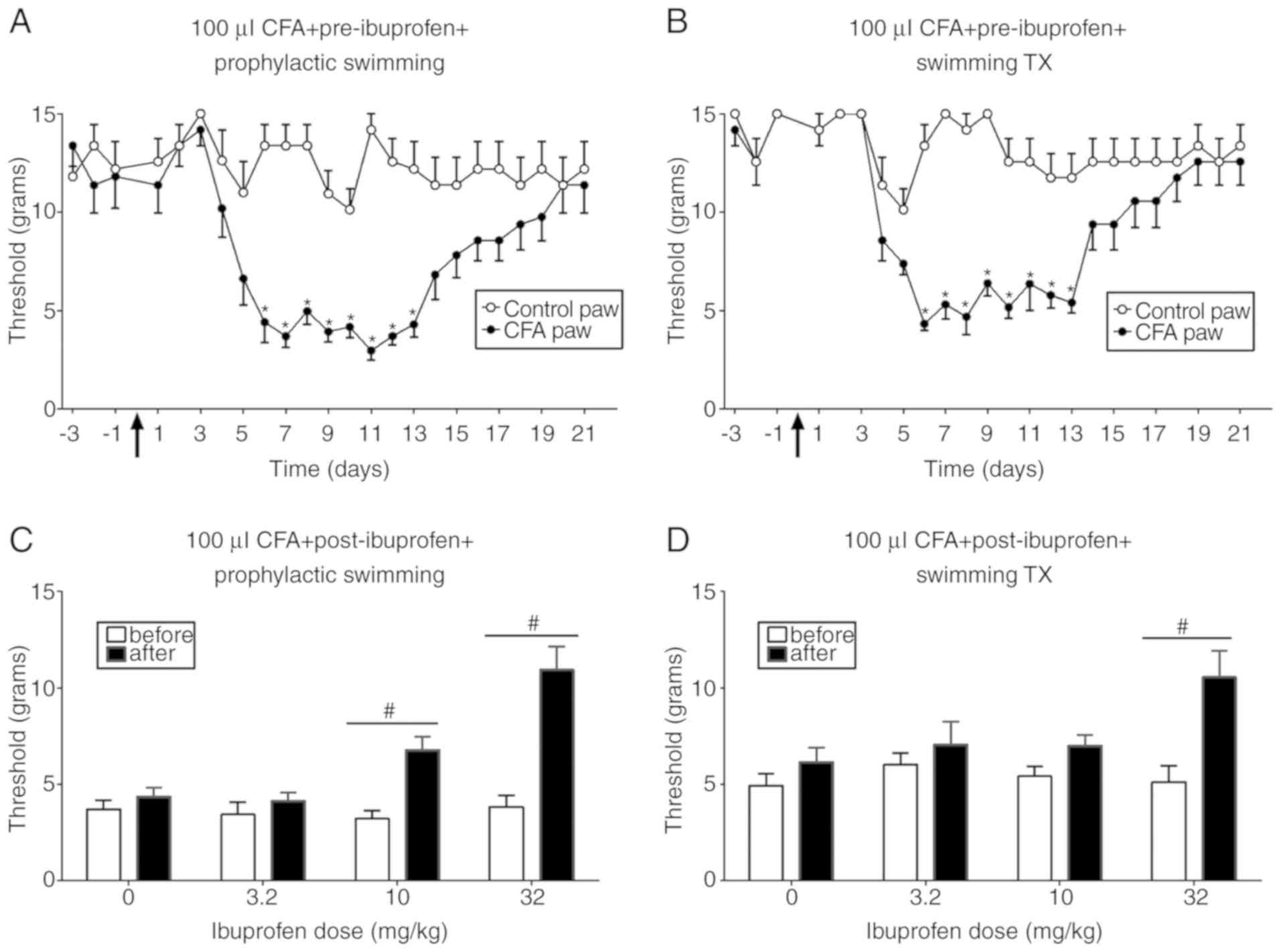
Fig. 4 illustrates
the effects of ibuprofen in combination with prophylactic swimming
or swimming treatment on CFA-induced mechanical allodynia. In the
prophylactic swimming group, ibuprofen administration produced a
dose-dependent increase in nociception threshold, which was
significant at 10 and 32 mg/kg (from 3.22 to 6.7 g and from 3.83 to
11 g, respectively) compared with pre-ibuprofen administration [F
(1, 56)=42.16; P<0.0001]. In addition, ibuprofen produced a
significant increase in the nociception threshold at 32 mg/kg in
the swimming treatment group [F (1, 56)=14.90; P=0.0003].
Effect of swimming exercise and
ibuprofen on CFA-induced mechanical allodynia
Fig. 5A illustrates
the effect of prophylactic swimming and swimming treatment on paw
withdrawal threshold in the presence of ibuprofen. Prophylactic
swimming produced a significantly higher threshold when compared
with the sham swimming group on days 2–4 and 15–20 following CFA
injection. Likewise, swimming treatment produced a significant
increase in threshold compared with sham swimming, which was
significant on days 1–3 and 14–20 following CFA injection.
Statistical analysis revealed a significant effect of swimming
exercise on mechanical allodynia in prophylactic swimming and
swimming treatment groups when compared with the sham swimming
group [F (2, 21)=14.54; P=0.0001]. Fig.
5 also demonstrates the effect of different swimming exercise
protocols on CFA-induced mechanical allodynia in the CFA paw
following the administration of ibuprofen. The nociception
threshold following administration of 10 mg/kg ibuprofen was 3.53 g
in the sham swimming group, 7 g in the swimming treatment group,
and 6.77 g in the prophylactic swimming group. The thresholds in
the latter two groups were significantly higher compared with that
in the sham swimming group (F=29.33; P<0.0001; Fig. 5B). Similarly, the thresholds in the
swimming treatment and prophylactic swimming groups were
significantly higher compared with the threshold in the sham
swimming group following the administration of 32 mg/kg of
ibuprofen (F=12.37; P<0.0001; Fig.
5B); these were 5.58 g in the sham swimming group, 10.57 g in
the swimming treatment group, and 11 g in the prophylactic swimming
group.
When the change in threshold was converted into a
percentage change in antinociception, 32 mg/kg ibuprofen only
produced only a 23.11% change in the sham swimming group. In
comparison with the sham swimming group, the percentage change was
significantly higher in the swimming treatment and prophylactic
swimming groups, such that it was increased to 68.12 and 91%,
respectively (F=9.189; P=0.0002; Fig.
5C). Only the prophylactic swimming group produced a
significantly greater change in percentage antinociception
following the administration of 10 mg/kg ibuprofen (43.9%) when
compared with the sham swimming group, which was only 4.30%
(F=20.22; P<0.0001; Fig. 5C).
Discussion
The present study examined the effect of swimming
treatment and prophylactic swimming in combination with ibuprofen
on CFA-induced nociception in rats. There were three principal
findings: First, swimming treatment and prophylactic swimming
shortened the duration of CFA-induced nociception when compared
with the non-swimming group. Second, the two types of swimming
enhanced the efficacy of ibuprofen-induced antinociception. Third,
prophylactic swimming increased the potency of ibuprofen to produce
antinociception. Collectively, these results indicated that
exercise may enhance the antinociceptive effect of ibuprofen in
inflammatory conditions
CFA is commonly used in rodents to induce
nociception for up to 4 weeks (17,18). The
results of the current study are consistent with the
well-established profile of CFA-induced inflammation reported in
previous literature (19). Ibuprofen
is widely used in the clinic for the management of different types
of inflammatory pain, including osteoarthritis and rheumatoid
arthritis (20). In the present
study, ibuprofen produced significant antinociception effects in
CFA-induced mechanical allodynia at a dose of 32 mg/kg without
shortening the duration of CFA-induced mechanical allodynia. This
result was consistent with previous studies that examined the
dose-associated effects of ibuprofen in CFA-induced mechanical
allodynia (20,21). An additional study reported that the
median effective dose of ibuprofen alone was 22 mg/kg, with no
significant antinociception observed below 17.8 mg/kg (22).
The present study demonstrated that swimming
exercise decreased the duration of CFA-induced mechanical
allodynia. The duration of mechanical allodynia produced by CFA was
15 days in the control group (between days 4 and 19), while in the
swimming groups the duration of mechanical allodynia was decreased
to only 10 days. Similar results were reported previously in a
post-ischemic inflammatory model (23,24).
Additional studies have reported that exercise facilitates the
recovery of brain and spinal cord injuries in a relatively short
time frame, with better physical and cognitive outcomes (25,26). The
current study adds to a growing body of evidence indicating that
swimming exercise shortens the recovery period of inflammatory
conditions, including osteoarthritis.
In the present study, a non-weight bearing exercise
(NWB) (i.e., swimming) was selected, rather than weight-bearing
(WB) exercise (e.g., walking and running). WB exercises generate
force activity that exerts load on skeletal regions. On the other
hand, NWB exercises are performed while the bodyweight of the
individual is supported artificially (i.e., without the person
supporting his/her own weight), and thus have the advantage of
being performed without any added load from the bodyweight itself
(27). The choice of either type of
exercise depends on the specific considerations of the patient. For
example, engaging individuals with joint pain in WB exercises has
the potential to exacerbate disease symptoms. This is attributed to
the excessive loading of the joint, which can increase swelling,
inflammation and pain (28). Age and
risk of falling are also significant considerations that should be
considered when selecting the type of exercise. Falling is the
primary cause of fatal and nonfatal injuries in individuals aged
>65 years (29) and 54% of falls
occur during exercise, with 15% while walking (30). Water-based exercise increases energy
expenditure with decreased impact loading on the joints or injured
tissues. The resistance characteristics of water supports
bodyweight during exercise, and thus positive outcomes will be
achieved while avoiding the joint load and increased falling risk
associated with WB exercise (31).
In one prospective randomized study, water gymnastics was effective
in reducing the intensity of back pain during pregnancy (32).
For the aforementioned reasons, swimming, as an NWB
exercise, was selected to examine its effect on nociception in
combination with ibuprofen in the present study. Swimming exercise
leads to a significant reduction in acetic acid-induced nociception
in mice (33). In addition, repeated
sessions of swimming exercise was observed to significantly reduce
mechanical allodynia in a mouse model of chronic neuropathic pain
(34). The swimming protocol used in
these previous studies is consistent with the swimming protocol
employed in the current study. This high-intensity extended
swimming exercise protocol is clinically relevant, as it is the
protocol recommended by the American Heart Association and the
American College of Sports Medicine (35). The association recommended that all
adults between 18 and 65 years of age engage in 30 min of
moderately intense physical activity each day for at least 5
days/week. These recommendations were the result of cumulative
evidence that revealed the health benefits of regular exercise and
an active lifestyle (25).
Prophylactic swimming, in particular, was reported to ameliorate
diabetes-induced muscular atrophy (36), prevent bone mass loss (37), and prevent diabetes and
hypertension-associated inflammatory consequences (38,39). In
the present study, prophylactic swimming exercise was efficacious
in preventing inflammatory pain.
The two swimming protocols employed in the present
study, increased the efficacy of the antinociceptive effect of
ibuprofen. Ibuprofen produced a significant increase in the
nociception threshold at 10 and 32 mg/kg in the swimming treatment
and prophylactic swimming groups when compared with the control
group. A recent study demonstrated that combined indomethacin
(another NSAID) and exercise training produced more rapid recovery
from the inflammatory process associated with ischemic infarcts
compared with exercise training alone (24). Although swimming exercise may produce
fatigue (40), the authors of the
present study considered that the observed increase in nociception
threshold from combined ibuprofen and swimming treatment was
primarily due to direct antinociceptive effects of ibuprofen rather
than a fatigue-induced effect for three reasons: First, the
percentage change in antinociception did not reach (or exceed) 100%
in any group, although the equation used would allow for the
detection of such an effect; second, daily swimming sessions were
conducted following the administration of ibuprofen and von Frey
testing to eliminate any fatigue or swimming-induced stress;
finally, no change in swimming behavior was noted by the assessor
in any of the treatment groups. These results are therefore
consistent with the primary target of pain control, which is to
relieve pain while restoring normal function and the ability to
perform daily activities (41).
The results of the present study demonstrate that
prophylactic swimming produces improved outcomes with respect to
ibuprofen-induced antinociception when compared with swimming
treatment plus ibuprofen treatment. For example, the percentage
change in antinociception of 32 mg/kg ibuprofen was higher in the
prophylactic swimming group when compared with the swimming
treatment group. In addition, prophylactic swimming increased the
potency of ibuprofen to produce antinociception, such that the
lower doses (10 mg/kg) produced a significant increase in the
nociception threshold. The present results thus support the
recommendation of continuous physical activity as a strategy to
decrease pain in patients with chronic arthritis (42). Growing evidence has recently changed
the common misconception that exercise may be harmful to patients
suffering from pain (43). It has
also been reported that people with an active lifestyle have a
decreased risk of developing inflammatory diseases that are
associated with chronic pain (12).
Furthermore, it is recommended that individuals with no active
injury should be encouraged to exercise regularly as a type of
prophylactic pain management strategy (44). Finally, the results of the current
study are consistent with a previous study demonstrating that
physically active subjects reported a 1.5–4-fold lower prevalence
of daily analgesic use upon developing chronic pain conditions
(45).
The mechanism by which exercise enhances the
antinociceptive effect of ibuprofen was not investigated in the
present study. However, earlier studies have demonstrated that
repeated exercise in rats increases the cerebrospinal fluid and
plasma concentrations of endogenous opioids with long-lasting
antinociception (46). Endorphins
are produced from the pituitary gland and the hypothalamus
following pain, excitement and exercise; they lead to analgesia and
a sense of well-being by activating µ-opioid receptors (47–49).
Increased production of endogenous opioids results in
antinociceptive effects in animals and humans (50). These results were further confirmed
when exercise-induced antinociception was reversed by naltrexone;
an opioid receptor antagonist (51).
Furthermore, combining opioids and NSAIDs produced synergistic
antinociceptive effects in many preclinical nociception models,
such as a postoperative rat pain model (52) and neuropathic pain model (53), as well as in clinical studies
(54).
One limitation of the present study was the use of a
subjective nociception assessment method (von Frey). To overcome
this subjectivity, the assessor was blinded to the administered
ibuprofen doses. The administration of CFA was not able to be
blinded due to the obvious signs of inflammation (i.e., redness and
swelling). A second limitation is the lack of data regarding the
molecular assessment of inflammatory biomarkers. The assessment of
inflammatory biomarkers is important, as they may determine the
mechanism by which exercise is able to enhance ibuprofen-induced
antinociception. The biomarkers usually assessed in similar studies
include interleukin (IL)-6, IL-1β, tumor necrosis factor-α,
endogenous endorphins, C-reactive protein and numerous others
(55). Given the positive effects of
swimming exercise on nociception demonstrated in the current study,
it is hypothesized that swimming may also produce a decrease in the
concentration of inflammatory biomarkers. As such, future studies
that will measure inflammatory biomarker levels in an animal model
of inflammatory pain with and without exercise is warranted.
Further studies may include the evaluation of NSAIDs other than
ibuprofen, as well as assessing the effects of swimming sessions of
different durations, to examine the possibility of constructing a
dose-response relationship for swimming exercise intervention.
In conclusion, the results of the present study
demonstrated that combined ibuprofen and swimming exercise may be
effective in controlling nociception in a rat pain model. The
ability of exercise to enhance ibuprofen-induced antinociception
suggests that this combination may be recommended as an effective
intervention to help control chronic pain. In addition,
prophylactic swimming may be combined with ibuprofen administration
to enhance its potency, and thus decrease its side effects.
Acknowledgements
The authors would like to thank Professor Karem
Alzoubi (Faculty of Pharmacy, Jordan University of Science and
Technology) for providing the swimming tanks for the swimming
exercise. They would also like to thank Ms. Khawla Al-Mhedat and
Ms. Nama'a Amawi (both Department of Pharmacology, Jordan
University of Science and Technology) for their technical
support.
Funding
The present study was funded by a research grant
from Jordan University of Science and Technology (grant no.
130/2016). The funding was exclusively used for data
collection.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. However, detailed datasets and
individual data points are available from the corresponding author
on reasonable request.
Authors' contributions
AA was responsible for designing the study and
writing the manuscript. ZK was responsible for conducting the
experiments and writing. SK was involved in the conception of the
study and data interpretation. MaA performed the statistical
analysis and writing. MoA was responsible for designing the study
and writing the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the National
Committee of Animal Care and Use at Jordan University of Science
and Technology and complied with the National Research Council
Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lapane KL, Quilliam BJ, Benson C, Chow W
and Kim MS: Impact of noncancer pain on healthrelated quality of
life. Pain Pract. 15:333–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Langley P, Müller-Schwefe G, Nicolaou A,
Liedgens H, Pergolizzi J and Varrassi G: The societal impact of
pain in the European Union: Health-related quality of life and
healthcare resource utilization. J Med Econ. 13:571–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaskin DJ, Spencer CS, Richard P, Anderson
G, Powe NR and LaVeist TA: Do minority patients use lower quality
hospitals? Inquiry. 48:209–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hague M and Shenker N: How to investigate:
Chronic pain. Best Pract Res Clin Rheumatol. 28:860–874. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Labianca R, Sarzi-Puttini P, Zuccaro SM,
Cherubino P, Vellucci R and Fornasari D: Adverse effects associated
with non-opioid and opioid treatment in patients with chronic pain.
Clin Drug Investig. 32 (Suppl 1):S53–S63. 2012. View Article : Google Scholar
|
|
6
|
Carter GT, Duong V, Ho S, Ngo KC, Greer CL
and Weeks DL: Side effects of commonly prescribed analgesic
medications. Phys Med Rehabil Clin N Am. 25:457–470. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kroll HR: Exercise therapy for chronic
pain. Phys Med Rehabil Clin N Am. 26:263–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brandt KD: The importance of
nonpharmacologic approaches in management of osteoarthritis. Am J
Med. 105:S39–S44. 1998. View Article : Google Scholar
|
|
9
|
Airaksinen O, Brox J, Cedraschi C,
Hildebrandt J, Klaber-Moffett J, Kovacs F, Mannion A, Reis S, Staal
J, Ursin H, et al: Chapter 4 European guidelines for the management
of chronic nonspecific low back pain. Eur Spine J. 15 (Suppl
2):S192–S300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedersen BK and Saltin B: Evidence for
prescribing exercise as therapy in chronic disease. Scand J Med Sci
Sports1. 6 (Suppl 1):S3–S63. 2006. View Article : Google Scholar
|
|
11
|
Vonkeman HE and van de Laar MA:
Nonsteroidal anti-inflammatory drugs: Adverse effects and their
prevention. Semin Arthritis Rheum. 39:294–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geneen LJ, Moore RA, Clarke C, Martin D,
Colvin LA and Smith BH: Physical activity and exercise for chronic
pain in adults: An overview of Cochrane reviews. Cochrane Database
Syst Rev. 4:CD0112792017.PubMed/NCBI
|
|
13
|
McCarson KE: Models of inflammation:
Carrageenan- or Complete Freund's Adjuvant (CFA)-induced edema and
hypersensitivity in the rat. Curr Protoc Pharmacol. 70:5.4.1–9.
2015. View Article : Google Scholar
|
|
14
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozbek E, Tasci AI, Ilbey YO, Simsek A,
Somay A and Metin G: The effect of regular exercise on penile
nitric oxide synthase expression in rats. Int J Androl. 33:623–628.
2010.PubMed/NCBI
|
|
16
|
Wang J, Wang L, Yang H, You Y, Xu H, Gong
L, Yin X, Wang W, Gao S, Cheng L, et al: Prevention of
atherosclerosis by Yindan Xinnaotong capsule combined with swimming
in rats. BMC Complement Altern Med. 15:1092015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagakura Y, Okada M, Kohara A, Kiso T,
Toya T, Iwai A, Wanibuchi F and Yamaguchi T: Allodynia and
hyperalgesia in adjuvant-induced arthritic rats: Time course of
progression and efficacy of analgesics. J Pharmacol Exp Ther.
306:490–497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stein C, Millan M and Herz A: Unilateral
inflammation of the hindpaw in rats as a model of prolonged noxious
stimulation: Alterations in behavior and nociceptive thresholds.
Pharmacol Biochem Behav. 31:445–451. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilson AW, Medhurst SJ, Dixon CI, Bontoft
NC, Winyard LA, Brackenborough KT, De Alba J, Clarke CJ, Gunthorpe
MJ, Hicks GA, et al: An animal model of chronic inflammatory pain:
Pharmacological and temporal differentiation from acute models. Eur
J Pain. 10:537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andrews N, Harper S, Issop Y and Rice AS:
Novel, nonreflex tests detect analgesic action in rodents at
clinically relevant concentrations. Ann N Y Acad Sci. 1245:11–13.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rutten K, Schiene K, Robens A, Leipelt A,
Pasqualon T, Read S and Christoph T: Burrowing as a nonreflex
behavioural readout for analgesic action in a rat model of
subchronic knee joint inflammation. Eur J Pain. 18:204–212. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López JR, Domínguez-Ramírez AM, Cook HJ,
Bravo G, Díaz-Reval MI, Déciga-Campos M and López-Muñoz FJ:
Enhancement of antinociception by co-administration of ibuprofen
and caffeine in arthritic rats. Eur J Pharmacol. 544:31–38. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Acheson A, Conover JC, Fandl JP, DeChiara
TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD and Lindsay
RM: A BDNF autocrine loop in adult sensory neurons prevents cell
death. Nature. 374:450–453. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liebigt S, Schlegel N, Oberland J, Witte
OW, Redecker C and Keiner S: Effects of rehabilitative training and
anti-inflammatory treatment on functional recovery and cellular
reorganization following stroke. Exp Neurol. 233:776–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Devine JM and Zafonte RD: Physical
exercise and cognitive recovery in acquired brain injury: A review
of the literature. PM R. 1:560–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ying Z, Roy RR, Edgerton VR and
Gómez-Pinilla F: Exercise restores levels of neurotrophins and
synaptic plasticity following spinal cord injury. Exp Neurol.
193:411–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahmann AE: Exercise for people with hip
or knee osteoarthritis: A comparison of land-based and aquatic
interventions. Open Access J Sports Med. 1:123–125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin DH, Lin CHJ, Lin YF and Jan MH:
Efficacy of 2 non-weight-bearing interventions, proprioception
training versus strength training, for patients with knee
osteoarthritis: A randomized clinical trial. J Orthop Sports Phys
Ther. 39:450–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burns ER, Stevens JA and Lee R: The direct
costs of fatal and non-fatal falls among older adults-United
States. J Safety Res. 58:99–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mertz KJ, Lee DC, Sui X, Powell KE and
Blair SN: Falls among adults: The association of cardiorespiratory
fitness and physical activity with walking-related falls. Am J Prev
Med. 39:15–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu KS, Eng JJ, Dawson AS, Harris JE,
Ozkaplan A and Gylfadóttir S: Water-based exercise for
cardiovascular fitness in people with chronic stroke: A randomized
controlled trial. Arch Phys Med Rehabil. 85:870–874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kihlstrand M, Stenman B, Nilsson S and
Axelsson O: Water-gymnastics reduced the intensity of back/low back
pain in pregnant women. Acta Obstet Gynecol Scand. 78:180–185.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mazzardo-Martins L, Martins DF, Marcon R,
Dos Santos UD, Speckhann B, Gadotti VM, Sigwalt AR, Guglielmo LGA
and Santos ARS: High-intensity extended swimming exercise reduces
pain-related behavior in mice: Involvement of endogenous opioids
and the serotonergic system. J Pain. 11:1384–1393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martins D, Mazzardo-Martins L, Soldi F,
Stramosk J, Piovezan A and Santos A: High-intensity swimming
exercise reduces neuropathic pain in an animal model of complex
regional pain syndrome type I: Evidence for a role of the
adenosinergic system. Neuroscience. 234:69–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haskell WL, Lee IM, Pate RR, Powell KE,
Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD and Bauman
A: Physical activity and public health: Updated recommendation for
adults from the American College of Sports Medicine and the
American Heart Association. Circulation. 116:1081–1093. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee Y, Kim JH and Hong Y, Lee SR, Chang KT
and Hong Y: Prophylactic effects of swimming exercise on
autophagy-induced muscle atrophy in diabetic rats. Lab Anim Res.
28:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsukahara N, Toda A, Goto J and Ezawa I:
Cross-sectional and longitudinal studies on the effect of water
exercise in controlling bone loss in Japanese postmenopausal women.
J Nutr Sci Vitaminol (Tokyo). 40:37–47. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghiasi R, Ghadiri Soufi F, Mohaddes G,
Alihemmati A, Somi MH, Ebrahimi H, Mirzaie Bavil F and Alipour MR:
Influance of regular swimming on serum levels of CRP, IL-6, TNF-α
in high-fat diet-induced type 2 diabetic rats. Gen Physiol Biophys.
35:469–476. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cardoso AM, Abdalla FH, Bagatini MD,
Martins CC, Fiorin Fda S, Baldissarelli J, Costa P, Mello FF,
Fiorenza AM, Serres JD, et al: Swimming training prevents
alterations in acetylcholinesterase and butyrylcholinesterase
activities in hypertensive rats. Am J Hypertens. 27:522–529. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Z, Wu F and Shao H: Does the fragrance
of essential oils alleviate the fatigue induced by exercise? A
biochemical indicator test in rats. Evid Based Complement Alternat
Med 2017. 50273722017.
|
|
41
|
Bijlsma J and Knahr K: Strategies for the
prevention and management of osteoarthritis of the hip and knee.
Best Pract Res Clin Rheumatol. 21:59–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Valderrabano V and Steiger C: Treatment
and prevention of osteoarthritis through exercise and sports. J
Aging Res 2011. 3746532010.
|
|
43
|
Hunter DJ and Eckstein F: Exercise and
osteoarthritis. J Anat. 214:197–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
LaStayo PC, Ewy GA, Pierotti DD, Johns RK
and Lindstedt S: The positive effects of negative work: Increased
muscle strength and decreased fall risk in a frail elderly
population. J Gerontol A Biol Sci Med Sci. 58:M419–M424. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dale O, Borchgrevink PC, Fredheim OMS,
Mahic M, Romundstad P and Skurtveit S: Prevalence of use of
non-prescription analgesics in the Norwegian HUNT3 population:
Impact of gender, age, exercise and prescription of opioids. BMC
Public Health. 15:4612015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoffmann P, Terenius L and Thorén P:
Cerebrospinal fluid immunoreactive β-endorphin concentration is
increased by voluntary exercise in the spontaneously hypertensive
rat. Regul Pept. 28:233–239. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dishman RK and O'Connor PJ: Lessons in
exercise neurobiology: The case of endorphins. Mental Health
Physical Activity. 2:4–9. 2009. View Article : Google Scholar
|
|
48
|
Boecker H, Sprenger T, Spilker ME,
Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A and
Tolle TR: The runner's high: Opioidergic mechanisms in the human
brain. Cerebral Cortex. 18:2523–2531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fichna J, Janecka A, Costentin J and Do
Rego JC: The endomorphin system and its evolving neurophysiological
role. Pharmacol Rev. 59:88–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Koltyn KF: Analgesia following exercise: A
review. Sports Med. 29:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stagg NJ, Mata HP, Ibrahim MM, Henriksen
EJ, Porreca F, Vanderah TW and Philip Malan T Jr: Regular exercise
reverses sensory hypersensitivity in a rat neuropathic pain model:
Role of endogenous opioids. Anesthesiology. 114:940–948. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Merlos M, Portillo-Salido E, Brenchat A,
Aubel B, Buxens J, Fisas A, Codony X, Romero L, Zamanillo D and
Vela JM: Administration of a co-crystal of tramadol and celecoxib
in a 1:1 molecular ratio produces synergistic antinociceptive
effects in a postoperative pain model in rats. Eur J Pharmacol.
833:370–378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shinozaki T, Yamada T, Nonaka T and
Yamamoto T: Acetaminophen and non-steroidal anti-inflammatory drugs
interact with morphine and tramadol analgesia for the treatment of
neuropathic pain in rats. J Anesth. 29:386–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oh E, Ahn HJ, Sim WS and Lee JY:
Synergistic effect of intravenous ibuprofen and hydromorphone for
postoperative pain: Prospective randomized controlled trial. Pain
Physician. 19:341–348. 2016.PubMed/NCBI
|
|
55
|
Chennaoui M, Gomez-Merino D, Drogou C,
Geoffroy H, Dispersyn G, Langrume C, Ciret S, Gallopin T and Sauvet
F: Effects of exercise on brain and peripheral inflammatory
biomarkers induced by total sleep deprivation in rats. J Inflamm
(Lond). 12:562015. View Article : Google Scholar : PubMed/NCBI
|