Introduction
Prediabetes mellitus is a pathological state that
represents an elevation of plasma glucose above the normal range
but below that of clinical diabetes (1–5).
Prediabetes mellitus is characterized by impaired fasting glucose
(IFG) and/or impaired glucose tolerance (IGT) (4,5). IFG and
IGT are risk factors for type 2 diabetes, and the risk is even
greater when IFG and IGT occur together (1,2). The
annual risk of prediabetes developing into diabetes is 5–10%, with
a similar proportion converting back to normoglycaemia (2,3).
The intact vascular endothelium prevents injury to
blood vessels under physiological conditions and in diabetes
mellitus hyperglycemia is associated with the development of
endothelial dysfunction (4–6). Several studies have suggested that
endothelial dysfunction contributes to macro- and micro-vascular
complications in diabetes mellitus (7–9).
Beneficial effects of exercise training on remedying diabetic
endothelial dysfunction by inhibiting oxidative stress and
improving nitric oxide (NO) bioavailability in the vascular wall
have been reported (10,11). Xie et al (12) also demonstrated that exercise
protects endothelium continuity through increasing NO production in
collateral-dependent porcine coronary arterioles. Furthermore, NO
synthesis depends on physical stimuli that modulate the activity of
NO synthase (NOS) (13–15).
At present, three isoforms of NOS are recognized,
including NOS, brain (nNOS), macrophage or NOS, inducible (iNOS)
and NOS, endothelial (eNOS) (13).
Studies have revealed that exercise-induced relaxation of the
collateral coronary arteries is associated with the increased
expression of eNOS mRNA and protein in healthy dogs and miniature
swine (16,17). Miyauchi et al (18) demonstrated that, following exercise,
eNOS mRNA expression was remarkably increased and the protein level
decreased, while iNOS was not significantly affected in the lungs
of animals. Tatchum-Talom et al (19) identified that swimming enhanced
hindquarter acetylcholine-induced vasodilatation with an increase
in nNOS activation in skeletal muscle, and eNOS in the lung, atria
and aorta. Pellegrin et al (20,21)
observed that swimming raised eNOS protein expression in mice with
hypercholesterolemia and atherosclerosis, and had no effect on eNOS
protein levels in normal mice. The results of these studies
investigating exercise training suggest that the NOS/NO signaling
pathway serves an important role in the regulation of vascular
function. However, the contribution of eNOS/NO activity to the
mitigation of vascular endothelium-dependent dysfunction by aerobic
exercise in prediabetes mellitus requires further
investigation.
In the present study, the effects of low-moderate
exercise training on vascular pathological changes in prediabetic
rats were examined, as well as the potential molecular mechanisms
underlying these effects.
Materials and methods
Animals
The present study used 54 2-month-old male Wistar
rats (230–235 g) that were obtained from Wushi Experimental Animal
Supply Co., Ltd. (Fuzhou, China). All the rats were housed under
optimal hygiene conditions at 23–25°C and with 50–60% humidity and
a 12 h light/dark cycle. The animals were given a standard rat
pellet diet and water ad libitum. The experimental protocol
was approved in accordance with the Guide for the Care and Use of
Laboratory Animals prepared by the Institutional Animal Care and
Use Committee of Fujian Normal University (Fuzhou, China).
Experimental design
Rats were randomly divided into the control group
(n=24) and the prediabetes group (n=30). In the control group,
animals were fed a standard chow diet, while the prediabetes group
received an additional high-energy diet emulsion, as previously
described (22). For 1 month, the
rats were given the high-energy diet daily. In the first 5 days,
animals were intragastrically administered with the emulsion once a
day; 1 ml was added to the volume of the emulsion administered each
time until day 5. Thereafter, they were administered 5 ml of
emulsion. Water was intragastrically administered to the control
group at the same volume.
Blood was collected at the end of the tail using a
pin-prick technique. Blood glucose levels were assayed using the
Accu-Chek test strip (Roche Diabetes Care, Burgess Hill, UK)
according to the manufacturer's protocol. The glucose tolerance of
the rats was detected at 3 months of age, as described previously
(22). The animals underwent fasting
for 14–18 h prior to testing. An intraperitoneal (IP) injection
with 6 ml 30% glucose (w/v)/kg of body weight was administered to
each animal. Glucose levels were measured at 30, 60, 90 and 120 min
after glucose loading. The area-under-the-curve (AUC) after 120 min
of glucose level testing was calculated using the trapezoid
rule.
Following a comprehensive analysis of glucose levels
and the AUC of the glucose tolerance test for the prediabetes and
control groups, 28 rats comprised the prediabetes group and 24 rats
in the control group. Two rats in the prediabetes group succumbed.
The animals in each group were randomly divided into two subgroups:
The control and exercise intervention groups.
A total of 24 h after the last bout of aerobic
exercise training the rats were anesthetized with an IP injection
of pentobarbital solution (40 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and sacrificed by cervical dislocation. The
thoracic aorta was rapidly removed and loosely adherent tissues
were also removed. The aortic tissue was divided into two parts,
one part was frozen with liquid nitrogen and stored at −80°C for
biochemical analysis, and the other part was immersed in 4%
paraformldehyde at room temperature for 24–48 h for
histopathological evaluation.
Exercise training
Aerobic exercise training was performed according to
the method used by Braga et al (23), but with modifications. All rats were
acclimated to treadmill running for 10 min periods for 1 week. On
the first day, an electric shock of 1 mA was applied to make the
rats start running, following which they would run spontaneously.
The maximal aerobic velocity (MAV) was evaluated with an
incremental test to exhaustion using a protocol with an initial
velocity of 5 m/min being intensified every 5 min with an increase
in speed of 5 m/min, until the animal was unable or unwilling to
continue. Aerobic exercise training was performed on a treadmill at
a low-moderate intensity (50–60% MAV), 1 h/day, 5 days/week for 8
weeks. The experiment was performed in a quiet, well-ventilated
room with 30–40% humidity and at ~18±2°C. Sedentary rats in the
control group were handled in the same way as the exercise group;
however, they did not engage in the regular running.
RNA extraction and reverse
transcriptase-polymerase chain reaction (RT-PCR) analysis of NOS
mRNA expression
Total RNA was extracted from the thoracic aorta
samples using TRIzol solution (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), quantified by ultraviolet spectrometric
detection using BioPhotometer Plus (Eppendorf, Hamburg, Germany)
according to the manufacturer's protocol (24) and reverse transcribed into
complementary DNA using a PrimeScript RT Reagent kit (Takara
Biotechnology, Co., Ltd., Dalian, China), according to the
manufacturer's protocol. qPCR analysis was performed to analyze the
mRNA expression of eNOS, iNOS and nNOS using the SYBR Premix Ex Taq
II kit (Takara Biotechnology, Co., Ltd.) with a program of 30 sec
at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C.
The primers were synthesized by Takara Biotechnology, Co., Ltd.,
including eNOS primers [forward (F): 5′-GGCAGAGGAGTCCAGCGAAC-3′,
reverse (R): 5′-TGTGGAACAGACCCCATAGTGC-3′], iNOS primers (F:
5′-GGACCACCTCTATCAGGAA-3′, R: 5′-CCTCATGATAACGTTTCTGGC-3′), nNOS
primers (F: 5′-GGCAAACATGACTTCCGAGTGT-3′, R:
5′-CCCCAAGGTAGAGCCATCTG-3′) and GADPH primers (F:
5′-CGACCCCTTCATTGACCTCAAC-3′, R: 5′-AAGACGCCAGTAGACTCCACGAC-3′).
The amount of target gene mRNA relative to the internal control
gene, GADPH, mRNA was calculated in accordance with the
2−ΔΔCq method (25).
Relative mRNA levels are reported as 2−ΔΔCq values. The
results of three independent experiments were used for statistical
analysis.
Immunohistochemistry for NOS
Following fixation, aortic segments were embedded in
paraffin and then sectioned into 5-µm-thick sections. Certain
sections were stained using hematoxylin for 3 min and eosin for 30
sec at room temperature for pathological evaluation.
Immunohistochemical localization of eNOS, iNOS and nNOS was
performed using mouse anti-eNOS (1:1,000; cat. no. ab76198), goat
anti-nNOS (1:2,000; cat. no. ab1376) and rabbit anti-iNOS (1:100;
cat. no. ab15323) antibodies (Abcam, Cambridge, MA, USA). The
sections were incubated at room temperature overnight with the
primary antibodies. The immunoreactivity of the specific protein
was visualized using the Elite ABC kit (BioGenex Laboratories, San
Ramon, CA, USA), according to the manufacturer's protocol. Then,
the sections were counter-stained with hematoxylin for 2 min at
room temperature, and mounted with coverslips to identify the
structure and types of cells in the rat aorta. The negative control
was treated with concentrated goat serum (1:10 dilution; Boster
Biological Technology, Ltd., Wuhan, China) instead of primary
antibodies. The slides were examined under an optical microscope at
a magnification of ×100 or ×400.
Western blotting for examining eNOS
protein expression
The western blot procedure was performed as
previously described (26). Aorta
tissue was homogenized and left for 30 min in ice-cold RIPA buffer
[50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate and 0.1% SDS; all Beyotime Institute of Biotechnology,
Jiangsu, China] containing 2 mM phenylmethylsulfonyl fluoride
(Beyotime Institute of Biotechnology). Thereafter, protein was
extracted by centrifuging the cells at 4°C for 20 min at 13,000 ×
g, the protein concentration was calibrated using the BCA method
and the samples were boiled in SDS-PAGE sample loading buffer
(Beyotime Institute of Biotechnology). Sample were resolved by
SDS-PAGE (6% acrylamide gel, 35 µg protein per lane) and
transferred onto a polyvinylidene difluoride membrane. The
transferred blots were blocking with 5% non-fat milk at room
temperature for 1 h and then incubated at 4°C overnight with rabbit
polyclonal anti-eNOS antibodies (1:500; cat. no. sc-654; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Mouse monoclonal
anti-β-actin antibodies (1:1,000 dilution; cat. no. AF0003;
Beyotime Institute of Biotechnology, Jiangsu, China) were used as
the internal loading control for overnight at 4°C. Following
washing with PSB, the blots were incubated with goat anti-rabbit
(cat. no. A0208) and goat anti-rat (cat. no. A0192) horseradish
peroxidase-conjugated immunoglobulin G secondary antibodies
(1:1,000 dilution; Beyotime Institute of Biotechnology) at room
temperature for 2 h. Protein bands were detected with BeyoECL Star
Western Blotting Detection reagent (Beyotime Institute of
Biotechnology). The relative intensity of eNOS compared with
β-actin bands was quantified using the AlphaView® Q
software (version 3.0; Proteinsimple; Bio-Techne, Minneapolis, MN,
USA).
Determination of NOS activity and NO
content
According to the method for NOS activity established
by Wu et al (24), the total
(t)NOS [constitutive (c)NOS and iNOS] and iNOS activities were
assayed in accordance with the NOS typed assay kit (cat. no.
A014-1; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). As cNOS is eNOS in the rat aorta, the eNOS activity was
calculated as tNOS minus iNOS. The enzyme activities were expressed
as units/mg of protein. The results of six independent experiments
were used for statistical analysis. NO content was examined with
the nitrate reductase method using the NO assay kit (cat. no. A012;
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocol.
Statistical analysis
Data are presented as mean ± standard error of the
mean. The significant differences in mean values within and between
multiple groups were evaluated using one-way analysis of the
variance, followed by a Tukey's multiple range test. The tests were
performed using SPSS software (version 19.0, IBM Corp., Armonk, NY,
USA). P<0.05 indicated that the difference between groups was
statistically significant.
Results
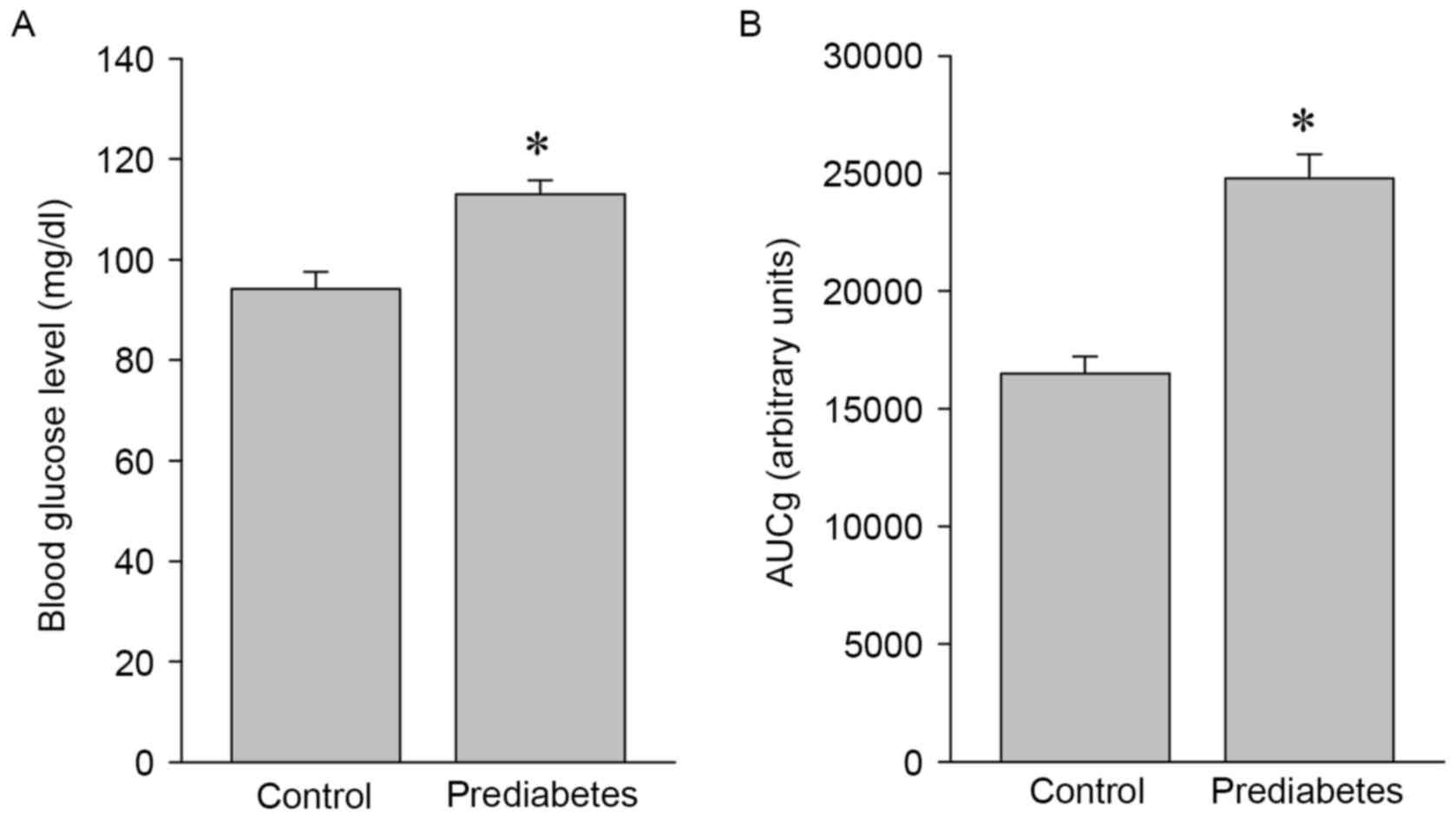
Blood glucose levels and glucose
intolerance are increased in prediabetic rats
To investigate the effects of aerobic exercise on
the expression and activity of NOS, a prediabetic rat model was
developed and used in the present study. Significant increases in
blood glucose levels (P<0.05; Fig.
1A) and glucose intolerance (P<0.05; Fig. 1B) were identified in the prediabetes
group compared with the control group, indicating that these
animals were in a prediabetic state and suitable for the following
exercise intervention experiments.
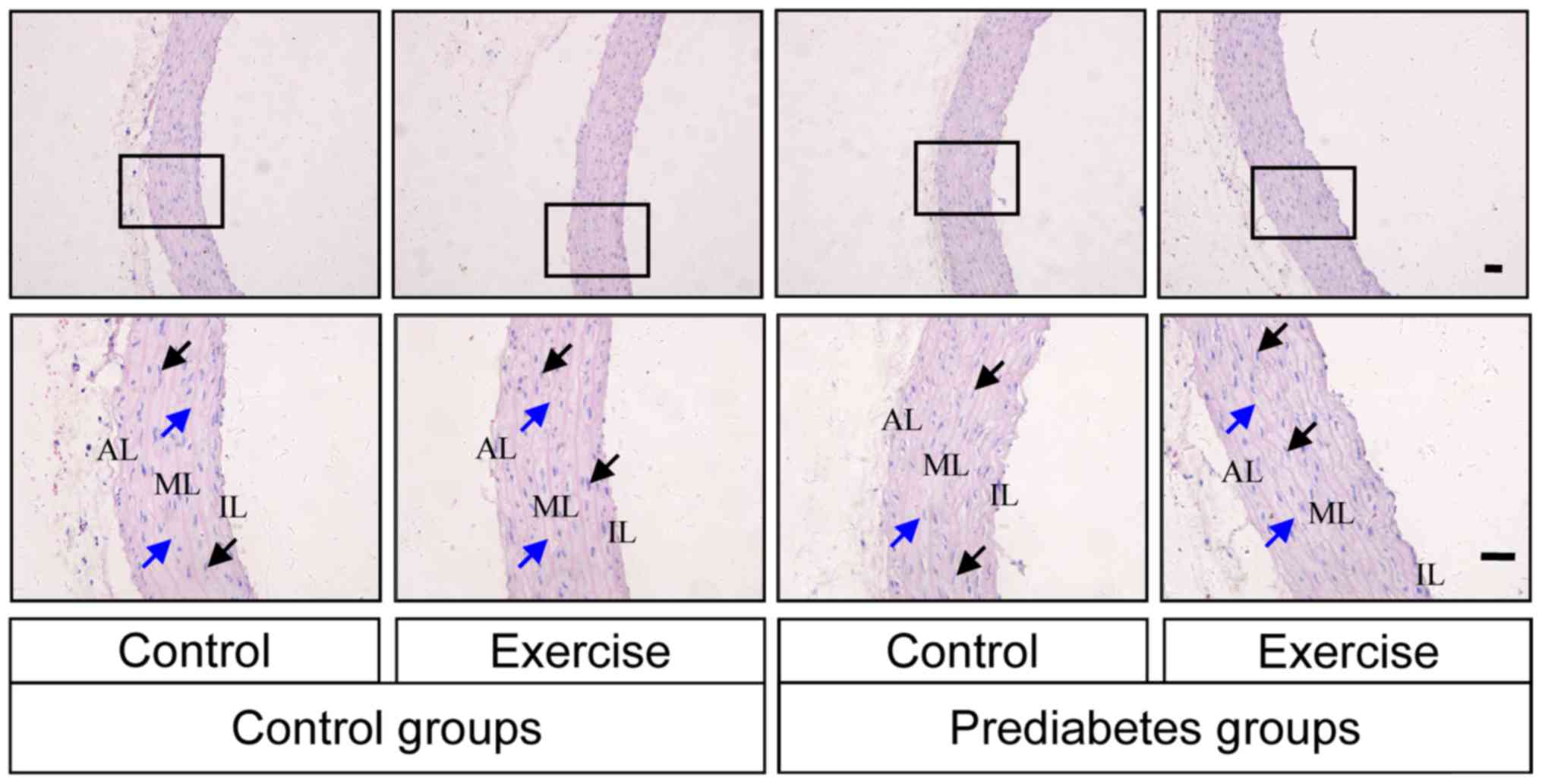
Aerobic exercise improves prediabetes
mellitus-induced aortic damage
The histological morphology of the thoracic aorta
was examined by HE staining in each group of rats. Intact vascular
endothelial layers and smooth muscle layers were identified in the
control groups, while, in the prediabetes groups, the vascular
tunica intima was damaged (Fig. 2).
Vascular endothelium staining was shallow and the internal elastic
fibers were partially broken in the prediabetic rats, while the
severity of histopathological alterations in the prediabetic rats
subjected to aerobic exercise intervention was lower compared with
those without exercise intervention. These observations indicating
that aerobic exercise improved the damage caused to the thoracic
aorta by prediabetes mellitus.
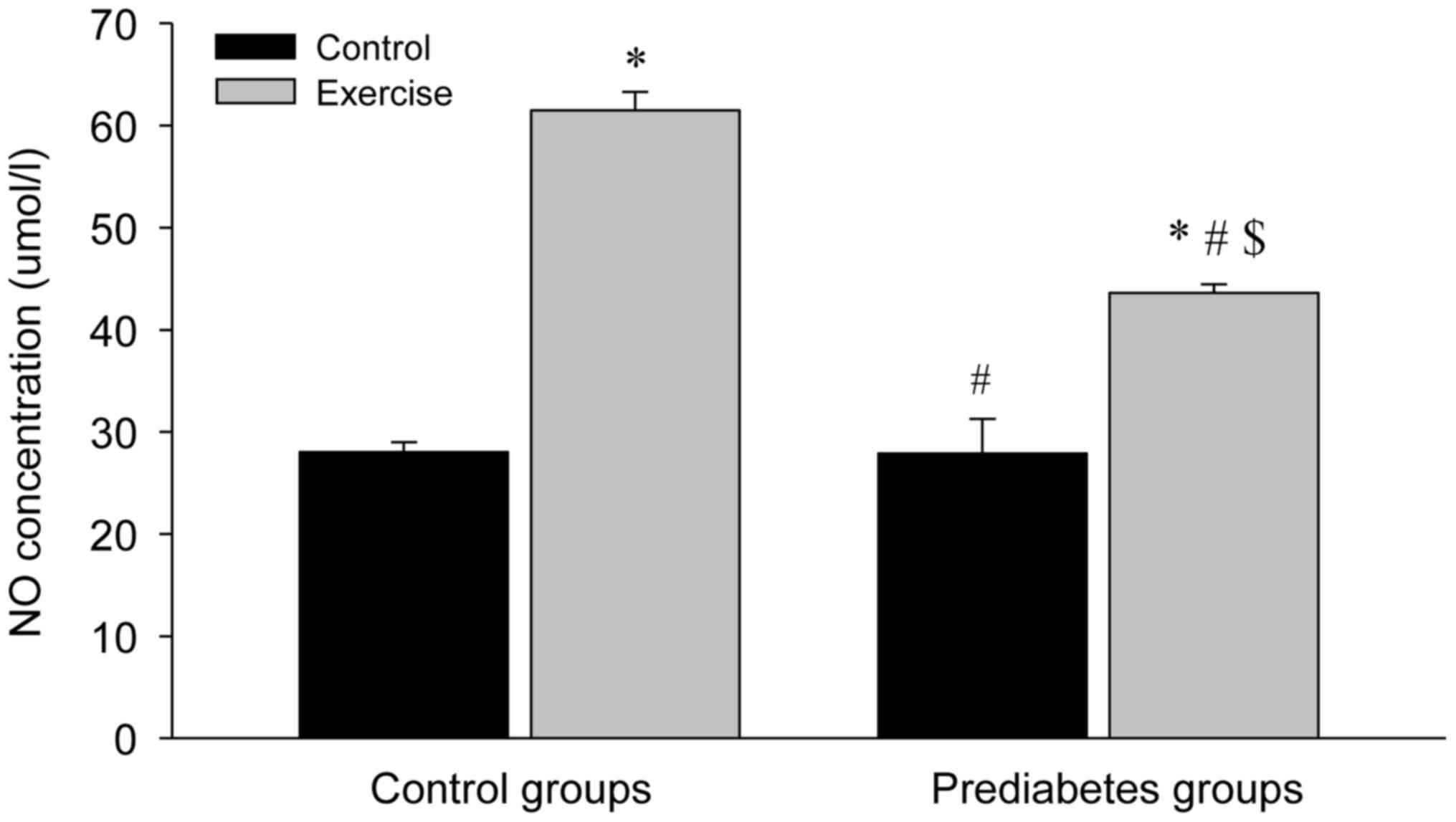
Aerobic exercise increases NO levels
in the aorta
The NO levels in the aortas of the different groups
were significantly increased following exercise intervention
compared with the control groups (P<0.05; Fig. 3). However, the NO concentration in
the control group with exercise intervention increased more
compared with that in the prediabetes group with exercise
intervention (P<0.05). Notably, NO concentrations between the
two groups without exercise intervention were similar. These
results indicated that increased NO production is associated with
the repair of the vascular endothelial injury in prediabetic rats
following exercise intervention.
Aerobic exercise increases eNOS and
decreases iNOS mRNA expression in the aorta of prediabetic
rats
Given the regulatory role of NOS in NO production,
the mRNA expression of NOS was detected in the aortas of rats in
each group (Fig. 4). This revealed a
significant decrease in eNOS mRNA expression (P<0.05; Fig. 4A), a significant increase in iNOS
mRNA expression (P<0.05; Fig. 4B)
and no obvious change in nNOS mRNA expression (Fig. 4C) in the prediatetes rats compared
with the control rats, suggesting a possible mechanism for why
there was no significant change in NO levels between these two
groups. Following exercise intervention, eNOS mRNA expression
increased and iNOS mRNA expression decreased significantly,
indicating that eNOS serves an important role in the reversal of
endothelial injury following aerobic exercise intervention.
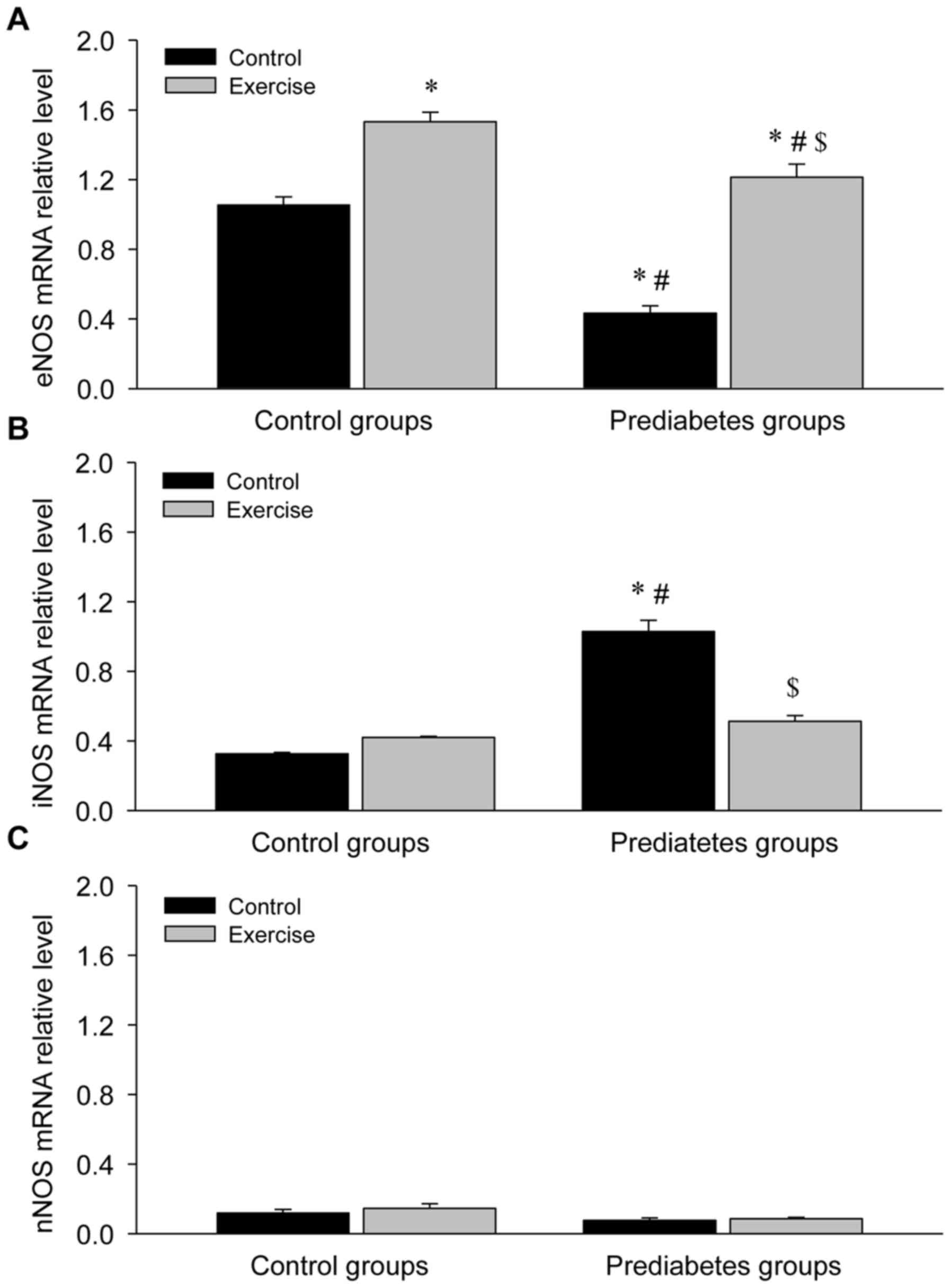 | Figure 4.Aerobic exercise increases eNOS and
decreases iNOS mRNA expression in the aortas of prediabetic rats.
(A) eNOS, (B) iNOS and (C) nNOS mRNA expression levels. *P<0.05
vs. the control/control group, #P<0.05 vs. the
control/exercise group, $P<0.05 vs. the
prediabetes/control group. NOS, nitric oxide synthase; eNOS, NOS,
endothelial; iNOS, NOS, inducible; nNOS, NOS, brain. |
For further determination of the changes in NOS
expression, immunostaining was performed to examine NOS expression
in the thoracic aorta from each group. The expression and
localization of NOS was revealed by arterial cross sections
immunolabelled with antibodies directed against different NOS
isoforms (Fig. 5). The results
demonstrated that prior to exercise intervention, eNOS was
decreased in the endothelial and smooth muscle layers of thoracic
aorta in the prediabetes group compared with the control group.
Following exercise intervention, eNOS immunostaining was stronger
in these two groups. Nevertheless, iNOS expression decreased
following exercise intervention. Changes in nNOS immunostaining
were not evident. These immunostaining results were consistent with
the NOS mRNA expression results and further suggest that eNOS
serves an important role in the regulation of endothelial
dysfunction in prediabetic rats.
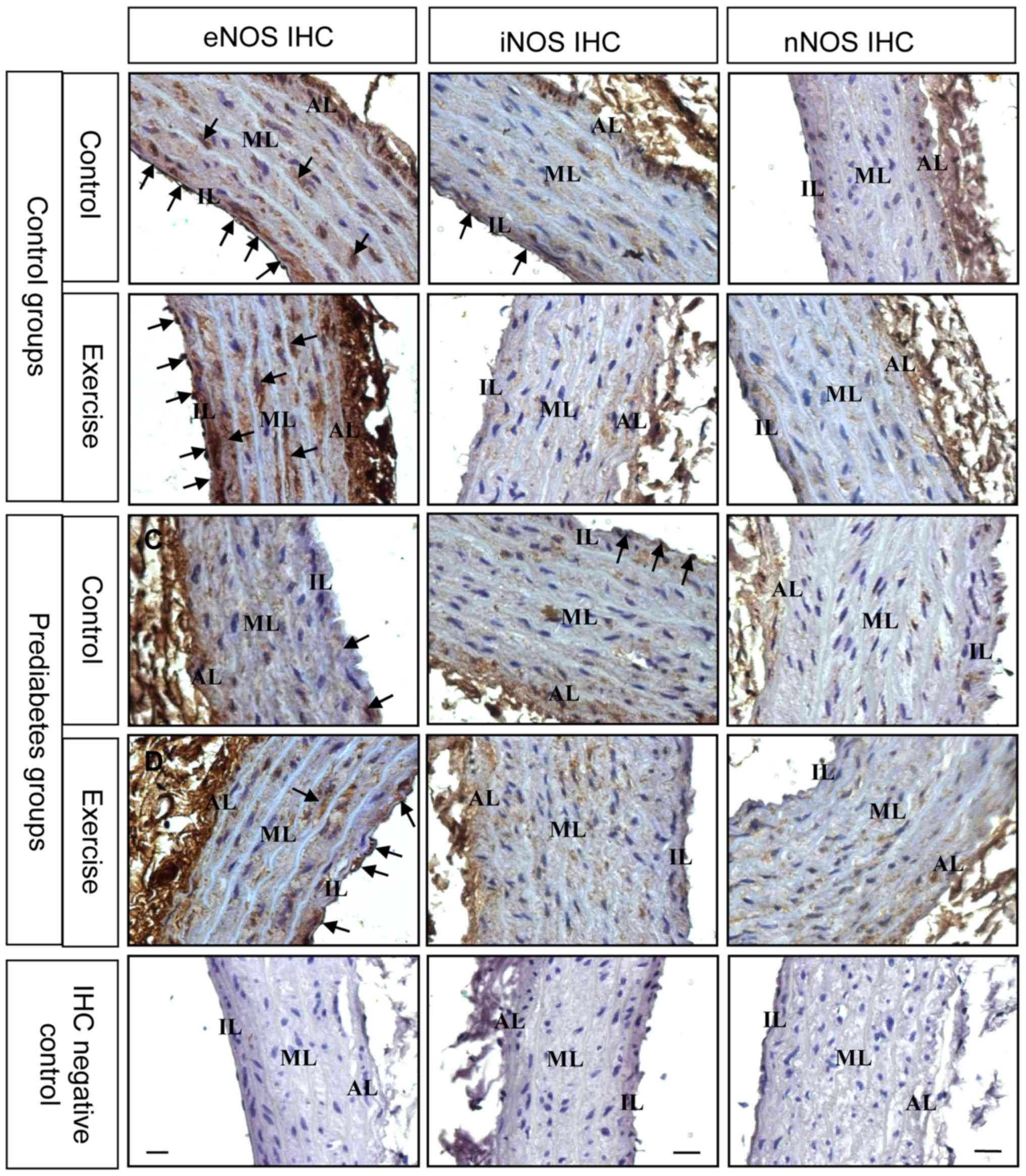 | Figure 5.Aerobic exercise increases eNOS and
decreases iNOS protein expression in the aortas of prediabetic
rats. The black arrows denote the positive signals. Original
magnification, ×400. NOS, nitric oxide synthases; eNOS, NOS,
endothelial; iNOS, NOS, inducible; nNOS, NOS, brain; AL, adventitia
layer; ML, medial layer; IL, intimal layer. |
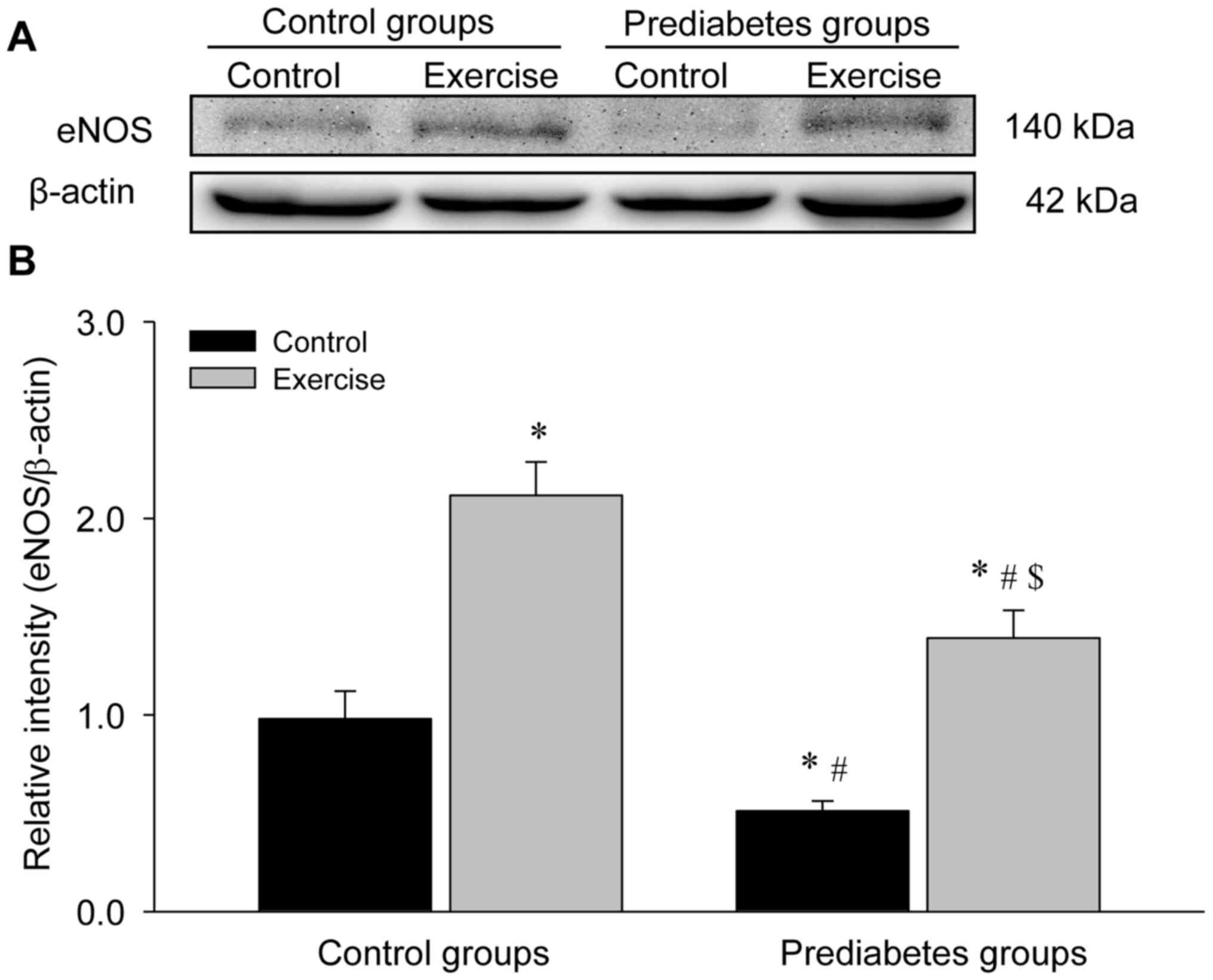
Aerobic exercise increases eNOS
protein expression in the aorta of prediabetic rats
The present study detected eNOS protein expression
levels through western blot analyses (Fig. 6). The results demonstrated a
significant decrease in eNOS protein levels in the sedentary
prediabetic rats compared with the sedentary control rats
(P<0.05), and exercise intervention significantly increased eNOS
protein expression in these two groups (both P<0.05 vs. their
respective control groups), which was consist with the identified
changes in eNOS mRNA expression.
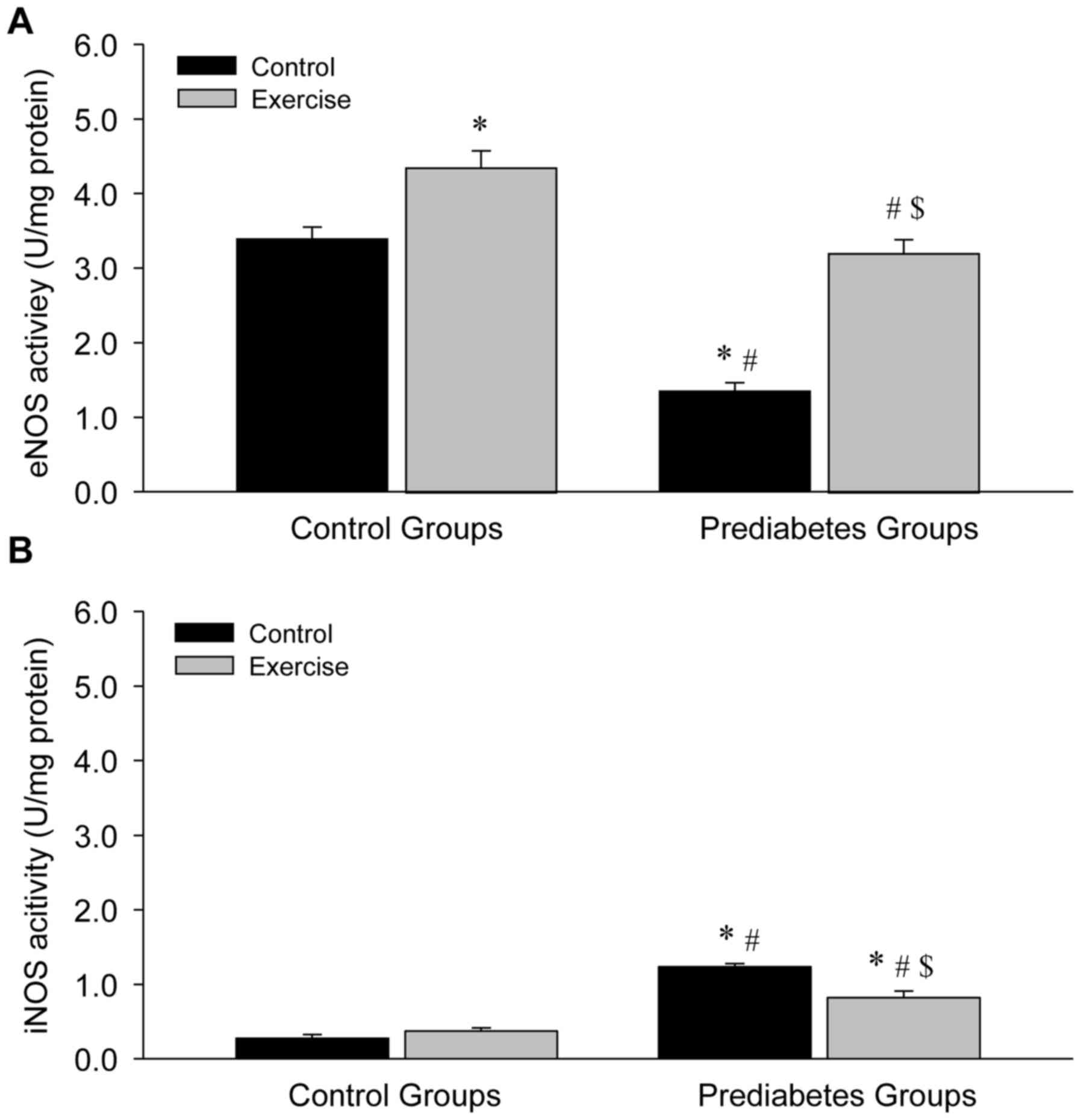
Aerobic exercise increases eNOS and
decreases iNOS activity in the aortas of prediabetic rats
NOS activity was examined in the present study in
order to further explore the association between exercise
intervention and NO production. Compared with the sedentary control
group, a significant decrease in eNOS activity (P<0.05; Fig. 7A) and a significant increase in iNOS
activity (P<0.05; Fig. 7B) in the
vessels were identified in the sedentary prediabetic group.
However, following exercise intervention, eNOS activity
significantly increased in the exercise and control groups (both
P<0.05; Fig. 7A), while iNOS
activity significantly decreased in the prediabetes group
(P<0.05; Fig. 7B). These changes
were similar to the observed changes in NOS expression, indicating
that NOS activity depended upon NOS expression.
Discussion
The present study demonstrated that aerobic exercise
intervention helped to ameliorate vascular endothelium-dependent
dysfunction through the NOS/NO signaling pathway, primarily
regulated by NOS expression and activity in prediabetes mellitus.
These findings provide important insight that can be used in
further investigations of the underlying molecular mechanism of
exercise intervention and prevention of diabetes mellitus.
Prediabetes mellitus is a pathological state between
normoglycemia and diabetes mellitus, which is characterized by IGT
and mild hyperglycemia (1–3). In the present study, a prediabetic rat
model was established based on the method of Rato et al
(22), and was used to examine blood
glucose levels and glucose intolerance. An intact vascular
endothelium inhibits atherosclerosis under physiological conditions
and vascular compliance is a direct indicator of the functional
status of the arteries (27). In the
present study, an incomplete vascular endothelium and broken
internal elastic fibers were observed in the prediabetic rats,
indicating vascular endothelium-dependent dysfunction. These rats
were used in the following exercise intervention experiments.
Aerobic exercise intervention is widely considered
to be an important non-pharmacological tool for the improvement of
vascular endothelial function (10).
Previous studies by our group have indicated that regular aerobic
exercise intervention promotes the maintenance of vasomotor
functions (1–5,9). Xie
et al (12) demonstrated that
exercise protects the endothelium through increasing NO production,
which was consistent with the results of the present study. The
present study further demonstrated that aerobic exercise
intervention alleviated vascular histopathological alterations
through improving the vascular endothelium and elevated NO
production through inducing eNOS expression in prediabetic
rats.
NO is a key signaling molecule in vascular
homeostasis (28), which was
originally identified as an endothelium-derived relaxation factor
(12). The present study identified
that under normal conditions without exercise intervention, marked
changes in NO levels were not identified in the prediabetic rats.
Notably, the expression of eNOS mRNA and protein decreased
significantly following exercise in the prediabetic group. Given
the regulatory role of NOS in the process of NO biosynthesis, the
expression of different NOS isoforms was detected in order to
further understand the molecular mechanism of aerobic exercise
intervention in the regulation of vascular endothelium-dependent
dysfunction in prediabetic rats. The significant decrease in eNOS
mRNA and protein expression identified in prediabetic rats was
reversed by exercise intervention in the prediabetic rats, which is
consistent with previous reports (16–21).
Several studies have indicated that exercise-induced relaxation of
the collateral coronary arteries is associated with the increased
expression of eNOS mRNA and protein in healthy animals (16,17), and
that swimming increases eNOS expression at the protein level in
mice prone to hypercholesterolemia and atherosclerosis (20,21). The
present study demonstrated that the expression of iNOS mRNA was in
contrast to eNOS mRNA expression in the prediabetic group, which
led to the homeostasis of NO production. Gielen et al
(29) identified that physical
exercise decreased the expression of iNOS at the mRNA and protein
levels in blood vessels, and Wu et al (24) revealed that high glucose incubation
led to a significant decrease in eNOS expression and NO
concentration, with increased iNOS mRNA and protein levels in rat
thoracic aortic rings. The activity of eNOS and iNOS was also
examined in the present study, which revealed increased eNOS
activity in prediabetic rats following exercise. These results
suggest that the eNOS/NO signaling pathway serves an important role
in the regulation of vascular dysfunction in prediabetes
mellitus.
In conclusion, the present study demonstrated that
aerobic exercise intervention attenuated vascular injury of the
thoracic aorta through activating the eNOS/NO signaling pathway in
prediabetic rats. However, the specific mechanism of eNOS induction
requires further investigation. In addition, the exercise threshold
may be important for the regulation of NO production, but the
precise identification of this threshold requires further study.
The results of the current study demonstrated that aerobic exercise
intervention improved endothelial function and reduced aortic
histopathological injury, due to activating the eNOS/NO signaling
pathway, in prediabetes mellitus, which highlights a mechanism that
could be targeted to prevent diabetes mellitus in clinical
practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31271255), the
Fujian Provincial Natural Science Foundation (grant nos. 2016J01145
and 2016J01150) and the Education Department of Fujian Province
Science and Technology Project (grant no. JAT160118).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW, YL and ZW designed the study. SW, JL, CZ and GX
performed the experiments. SW, ZT, ZZ, YL and ZW analyzed the data.
SW, YL and ZW interpreted the data and discussed the results. SW
wrote the manuscript and ZW revised it with YL. All authors read
and approved the final version of the manuscript for
publication.
Ethics approval and consent to
participate
The experimental protocol was approved in accordance
with the Guide for the Care and Use of Laboratory Animals prepared
by the Institutional Animal Care and Use Committee of Fujian Normal
University (Fuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tabák AG, Herder C, Rathmann W, Brunner EJ
and Kivimäki M: Prediabetes: A high-risk state for diabetes
development. Lancet. 379:2279–2290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nathan DM, Davidson MB, DeFronzo RA, Heine
RJ, Henry RR, Pratley R, Zinman B and American Diabetes
Association: Impaired fasting glucose and impaired glucose
tolerance: Implications for care. Diabetes Care. 30:753–759. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta A, Al-Aubaidy HA and Mohammed BI:
Glucose dependent insulinotropic polypeptide and dipeptidyl
peptidase inhibitors: Their roles in management of type 2 diabetes
mellitus. Diabetes Metab Syndr 10 (2 Suppl 1). S170–S175. 2016.
View Article : Google Scholar
|
|
4
|
Liu Y, Li J, Zhang Z, Tang Y, Chen Z and
Wang Z: Endocrinological analysis of endothelium-dependent
vasodilation in middle-aged patients with impaired glucose
tolerance during prediabetes mellitus. Exp Ther Med. 7:697–702.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Li J, Zhang Z, Tang Y, Chen Z and
Wang Z: Effects of exercise intervention on vascular endothelium
functions of patients with impaired glucose tolerance during
prediabetes mellitus. Exp Ther Med. 5:1559–1565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fiorentino TV, Prioletta A, Zuo P and
Folli F: Hyperglycemia-induced oxidative stress and its role in
diabetes mellitus related cardiovascular diseases. Curr Pharm Des.
19:5695–5703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sena CM, Pereira AM and Seiça R:
Endothelial dysfunction-a major mediator of diabetic vascular
disease. Biochim Biophys Acta. 1832:2216–2231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fowler MJ and Michael J: Microvascular and
macrovascular complications of diabetes. Clin Diabetes. 26:77–82.
2008. View Article : Google Scholar
|
|
9
|
De Vriese AS, Verbeuren TJ, Van de Voorde
J, Lameire NH and Vanhoutte PM: Endothelial dysfunction in
diabetes. Br J Pharmacol. 130:963–974. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chakraphan D, Sridulyakul P, Thipakorn B,
Bunnag S, Huxley VH and Patumraj S: Attenuation of endothelial
dysfunction by exercise training in STZ-induced diabetic rats. Clin
Hemorheol Microcirc. 32:217–226. 2005.PubMed/NCBI
|
|
11
|
Chis IC, Coseriu A, Simedrea R, Oros A,
Nagy AL and Clichici S: In vivo effects of quercetin in association
with moderate exercise training in improving streptozotocin-induced
aortic tissue injuries. Molecules. 20:21770–21786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie W, Parker JL and Heaps CL: Effect of
exercise training on nitric oxide and
superoxide/H2O2 signaling pathways in
collateral-dependent porcine coronary arterioles. J Appl Physiol
(1985). 112:1546–1555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Wei QW, Wang ZC, Ding W, Wang W
and Shi FX: Cell-specific expression and immunolocalization of
nitric oxide synthase isoforms and the related nitric oxide/cyclic
GMP signaling pathway in the ovaries of neonatal and immature rats.
J Zhejiang Univ Sci B. 12:55–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng K, Sulieman FJ, Li J, Wei Q, Xu M
and Shi F: Nitric oxide and thyroid hormone receptor alpha 1
contribute to ovarian follicular development in immature hyper- and
hypo-thyroid rats. Reprod Biol. 15:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu M, Wei Q, Zheng K, Mao D, Zheng Y, Li Y
and Shi F: Protective effects of Big-leaf mulberry and
physiological roles of nitric oxide synthases in the testis of mice
following water immersion and restraint stress. Acta Histochem.
116:1323–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laughlin MH, Pollock JS, Amann JF, Hollis
ML, Woodman CR and Price EM: Training induces nonuniform increases
in eNOS content along the coronary arterial tree. J Appl Physiol
(1985). 90:501–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sessa WC, Pritchard K, Seyedi N, Wang J
and Hintze TH: Chronic exercise in dogs increases coronary vascular
nitric oxide production and endothelial cell nitric oxide synthase
gene expression. Circ Res. 74:349–353. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyauchi T, Maeda S, Iemitsu M, Kobayashi
T, Kumagai Y, Yamaguchi I and Matsuda M: Exercise causes a
tissue-specific change of NO production in the kidney and lung. J
Appl Physiol (1985). 94:60–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tatchum-Talom R, Schulz R, McNeill JR and
Khadour FH: Upregulation of neuronal nitric oxide synthase in
skeletal muscle by swim training. Am J Physiol Heart Circ Physiol.
279:H1757–H1766. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pellegrin M, Berthelot A, Houdayer C,
Gaume V, Deckert V and Laurant P: New insights into the vascular
mechanisms underlying the beneficial effect of swimming training on
the endothelial vasodilator function in apolipoprotein E-deficient
mice. Atherosclerosis. 190:35–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pellegrin M, Miguet-Alfonsi C, Berthelot
A, Mazzolai L and Laurant P: Long-term swimming exercise does not
modulate the Akt-dependent endothelial nitric oxide synthase
phosphorylation in healthy mice. Can J Physiol Pharmacol. 89:72–76.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rato L, Duarte AI, Tomás GD, Santos MS,
Moreira PI, Socorro S, Cavaco JE, Alves MG and Oliveira PF:
Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating
mitochondrial bioenergetics and oxidative stress. Biochim Biophys
Acta. 1837:335–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braga VA, Couto GK, Lazzarin MC, Rossoni
LV and Medeiros A: Aerobic exercise training prevents the onset of
endothelial dysfunction via increased nitric oxide bioavailability
and reduced reactive oxygen species in an experimental model of
menopause. PLoS One. 10:e01253882015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Xue L, Du W, Huang B, Tang C, Liu C,
Qiu H and Jiang Q: Polydatin restores endothelium-dependent
relaxation in rat aorta rings impaired by high glucose: A novel
insight into the PPARβ-NO signaling pathway. PLoS One.
10:e01262492015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang SB, Xing BS, Yi L, Wang W and Xu YX:
Expression of Frizzled 2 in the mouse ovary during oestrous cycle.
J Anim Physiol Anim Nutr (Berl). 94:437–445. 2010.PubMed/NCBI
|
|
27
|
Westerterp KR: Perception, passive
overfeeding and energy metabolism. Physiol Behav. 89:62–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ignarro LJ: Nitric oxide as a unique
signaling molecule in the vascular system: A historical overview. J
Physiol Pharmacol. 53:503–514. 2002.PubMed/NCBI
|
|
29
|
Gielen S, Adams V, Linke A, Erbs S,
Möbius-Winkler S, Schubert A, Schuler G and Hambrecht R: Exercise
training in chronic heart failure: Correlation between reduced
local inflammation and improved oxidative capacity in the skeletal
muscle. Eur J Cardiovasc Prev Rehabil. 12:393–400. 2005. View Article : Google Scholar : PubMed/NCBI
|