Introduction
Intrahepatic cholangiocarcinoma (ICC) is one of the
most common hepatic malignancies worldwide (1), the incidence and mortality of which has
increased in recent years (2).
Previous studies have suggested that multiple signaling pathways
are involved in the progression of ICC (3,4);
however, the specific mechanisms underlying ICC etiology remain
unknown. As cystoscopy is invasive and expensive, there is a need
to identify potential diagnostic biomarkers for ICC in order to
improve the early detection of ICC (5,6).
MicroRNAs (miRs) are small non-coding RNAs ~22
nucleotides in length that are associated with multiple biologic
processes including cell proliferation, differentiation and
apoptosis (7). Abnormal expression
of miRNAs has been widely identified in different diseases
(8,9). For instance, miR-590-3p inhibits
epithelial-mesenchymal transition in ICC via suppressing Smad
interacting protein 1 expression (9). Additionally, several differentially
expressed miRNAs have been reported as potential diagnostic
biomarkers for patients with ICC; for instance, high miR-146a
expression in the plasma and tumor tissues is reportedly associated
with prolonged overall survival in surgical patients with ICC
(7).
Abnormal miR-142-5p expression has been widely
reported in different tumors (10–12).
miR-142-5p acts as a tumor suppressor via targeting
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
in non-small cell lung cancer (10).
High miR-142-5p expression is also associated with the biological
aggressiveness of colorectal cancer (11) and has been reported as a potential
predictive biomarker for recurrence risk in patients with gastric
cancer (12). The focus of the
present study was miR-142-5p and its specific role in the
progression of ICC. The aim was to evaluate the expression of
miR-142-5p in ICC tissues and elucidate the potential underlying
mechanism.
Materials and methods
Cell culture
Two hundred and ninety-three cells were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% heat-inactivated fetal calf
serum (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
streptomycin in 25-cm2 culture flasks at 37°C in a
humidified atmosphere containing 5% CO2.
Patients and specimens
Human clinical samples were obtained from 100
patients with ICC between December 2016 and November 2017 at The
First People's Hospital of Tongxiang (Tongxiang, China).
Corresponding adjacent, non-neoplastic tissues from the macroscopic
tumor margin were isolated and used as controls. ICC diagnosis was
based on criteria outlined by the World Health Organization
(13) and tumor differentiation was
based on the classification proposed by Edmondson and Steiner
(13). The clinical classification
of tumors was performed according to the 7th edition of the
tumor-node-metastasis classification system of the International
Union Against Cancer (14). Patient
characteristics are presented in Table
I.
 | Table I.Clinicopathological features of
patients with ICC and healthy controls. |
Table I.
Clinicopathological features of
patients with ICC and healthy controls.
| Variables | Patients with
ICC | Healthy controls |
|---|
| Sex ratio
(male/female) | 75/25 | 39/11 |
| Age |
|
|
| ≥60
years | 64 | 35 |
| <60
years | 36 | 15 |
| Stage |
|
|
| Ta | 33 | – |
| T1 | 27 | – |
| T2 | 17 | – |
| T3 | 13 | – |
| T4 | 10 | – |
| Grade |
|
|
| 1 | 32 | – |
| 2 | 38 | – |
| 3 | 30 | – |
Patients were excluded from the current study if
they exhibited: Failure of important organs, including the heart,
lungs, kidneys and brain, intolerance to surgery, distant organ
metastasis, lymph node involvement beyond the hepatoduodenal
ligament, hilar or caval lymph nodes, preoperative chemotherapy or
radiotherapy and preoperative liver treatment (arterial
chemoembolization, radiofrequency ablation or percutaneous ethanol
injection). All samples were immediately snap-frozen in liquid
nitrogen and stored at −80°C. Whole blood samples were
prospectively collected from patients with ICC and healthy controls
without urologic malignancies. Whole blood (5–8 ml) was collected
in EDTA tubes and samples were centrifuged twice at 3,000 × g at
4°C for 15 min. The plasma was then stored at −80°C. All research
protocols were approved by The First People's Hospital of Tongxiang
and written informed consent was obtained from all
participants.
Plasma RNA isolation
Total RNA was isolated from whole blood samples
using RNAVzol LS or tissue samples using or RNAVzol (Vigorous
Biotechnology Beijing Co., Ltd., Beijing, China) according to the
manufacturer's protocol. The quality, quantity and integrity of RNA
were monitored using a NanoDrop spectrophotometer (ND-1000;
Nanodrop; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A total of 1 µg RNA was reverse transcribed using
Moloney Murine Leukemia Virus reverse transcription enzyme (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with specific primers.
The temperature protocol used for RT was as follows: 72°C for 10
min; 42°C for 60 min, 72°C for 5 min and 95°C for 2 min. qPCR was
performed using SYBR Green Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) in an iCycler iQ real-time PCR detection system.
The PCR amplifications were performed in a 10 µl reaction system
containing 5 µl SYBR Green Supermix, 0.4 µl forward primer, 0.4 µl
reverse primer, 2.2 µl double distilled H2O and 2 µl
template cDNA. Thermocycling conditions were as follows: 95°C for
10 min followed by 50 cycles of 95°C for 10 sec, 55°C for 10 sec,
72°C for 5 sec, 99°C for 1 sec, 59°C for 15 sec and 95°C for 1 sec,
followed by cooling to 40°C. Relative mRNA expression was
normalized to U6 using the 2−∆∆Cq method (15). Primer sequences were as follows:
miR-142-5p-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGTAG-3′; U6-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATG-3′;
miR-142-5p, forward 5′-CATAAAGTAGAAAGCACTACT-3′; U6, forward
5′-GCGCGTCGTGAAGCGTTC-3′; and universal reverse primer,
5′-GTGCAGGGTCCGAGGT-3′. The criteria for dividing low and high
expression of miR-142-5p were determined as follows: Relative mRNA
expression was normalized to U6 using the 2−∆∆Cq method
(15). Patients with below average
2−∆∆Cq were classed as low miR-142-5p expression, while
those with above average 2−∆∆Cq were classed as high
miR-142-5p expression.
Transient transfection
A total of 6×105 293 cells were seeded in
6-well plates with 2 ml DMEM supplemented with serum and
antibiotics as above. miR-142-5p mimics, inhibitors, or miR
negative controls (miR-NC; Shanghai GenePharma Co., Ltd., Shanghai,
China) were mixed with HiperFect transfection reagent (Qiagen GmbH,
Hilden, Germany) and incubated at room temperature for 10 min. This
mixture was then added to cultured 293 cells for 48 h. The interval
between transfection and subsequent experimentation was 48 h.
MiRNA target prediction and
dual-luciferase reporter assay
TargetScan (https://www.targetscan.org) was used to predict
potential target genes of miR-142-5p. The 3′-untranslated region
(3′UTR) of phosphate and tensin homolog (PTEN) was cloned into the
pmirGLO plasmid. After 293 cells were cultured for 24 h at 37°C in
DMEM medium, miR-142-5p or scramble were cotransfected with blank
pmirGLO or pmirGLO-PTEN-3′UTR using Vigofect (Vigorous
Biotechnology Beijing Co., Ltd.) according to the manufacturer's
protocol. Luciferase activity was analyzed using a Dual-Luciferase
Reporter Assay System (E1910; Promega Corp., Madison, WI, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Two-tailed unpaired Student's t-tests were used to
compare two groups. Multiple group comparisons were made using
one-way analysis of variance followed by Tukey's multiple
comparison test. Receiver operating characteristic curve (ROC)
analysis was used to assess the efficacy of miR-142-5p as a
biomarker. Kaplan-Meier survival analysis was also performed and
survival differences were assessed using a log-rank test. Cox
regression assay was performed to evaluate the prognostic value of
miR-142-5p in patients with ICC. SPSS (version 20.0, SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Plasma and tissue miR-142-5p levels
are upregulated in patients with ICC
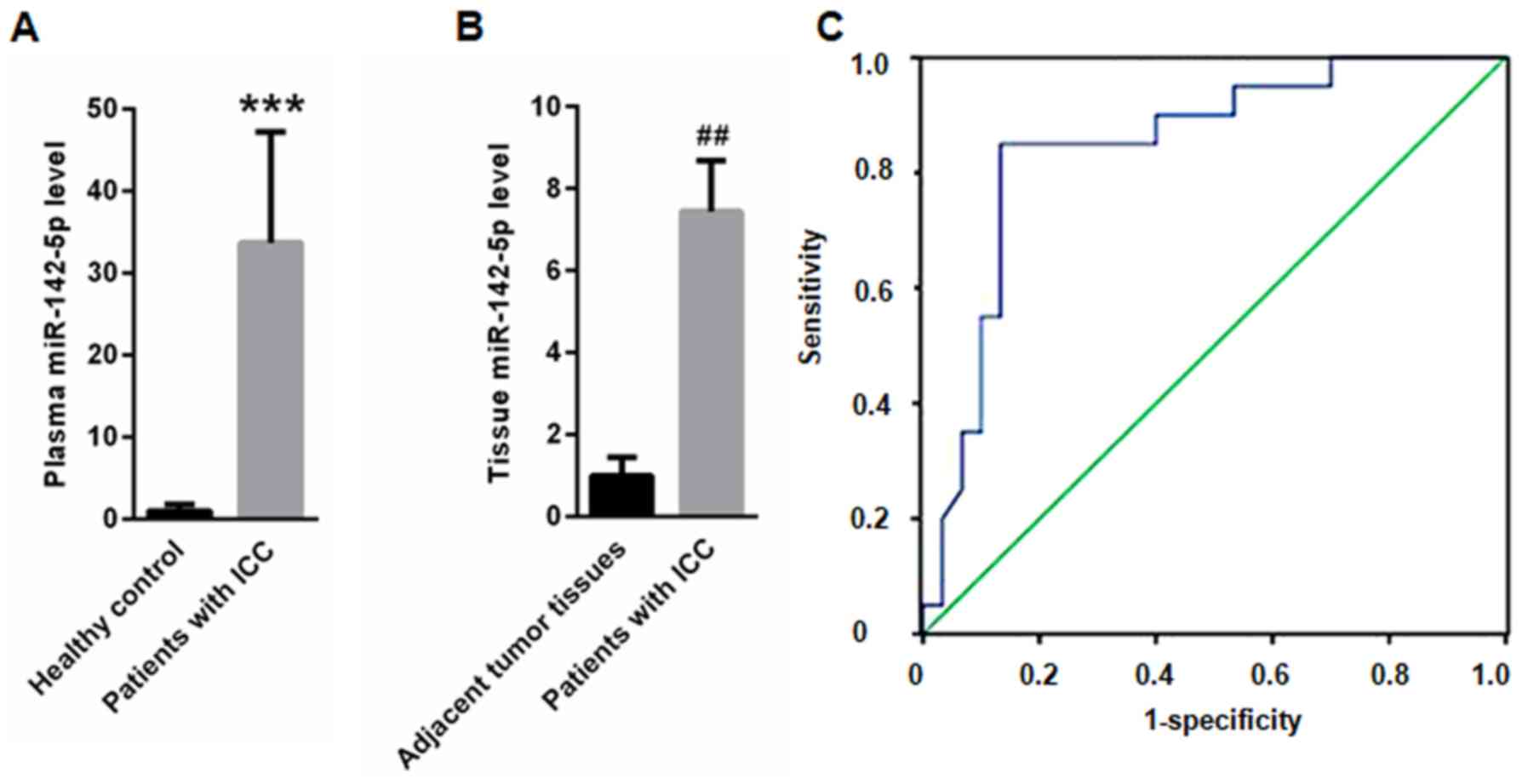
Compared with healthy controls (1±0.87), plasma
miR-142-5p was significantly upregulated in patients with ICC
(33.7±13.5; Fig. 1A). Furthermore,
miR-142-5p expression was significantly higher in tumor tissues
(7.45±1.23) compared with adjacent non-neoplastic tissues (1±0.45;
Fig. 1B). ROC analysis indicated
that plasma miR-142-5p could be used to screen patients with ICC
from healthy controls, with an area under the curve of 0.844, (95%
confidence interval: 0.730–0.959; Fig.
1C). Furthermore, the prognostic value of miR-142-5p was
assessed using Cox analysis. The results revealed that miR-142-5p
overexpression was an independent prognostic factor for patients
with ICC (hazard ratio 3.508, 95% confidence interval 1.783–6.968;
Table II).
 | Table II.Multivariate Cox regression analysis
for miR-142-5p in patients with intrahepatic
cholangiocarcinoma. |
Table II.
Multivariate Cox regression analysis
for miR-142-5p in patients with intrahepatic
cholangiocarcinoma.
| Parameter | Hazard ratio | 95% confidence
interval | P-value |
|---|
| miR-142-5p | 3.508 | 1.783–6.968 | <0.001 |
| Sex | 0.843 | 0.532–1.467 |
0.486 |
| Age | 1.513 | 0.879–1.987 |
0.114 |
| Tumor size | 1.021 | 0.621–1.831 |
0.876 |
| Lymph node
metastasis | 1.032 | 0.653–1.902 |
0.821 |
| Clinical stage | 0.926 | 0.602–1.623 |
0.821 |
| Histological
type | 1.024 | 0.597–1.821 |
0.921 |
|
Differentiation | 1.067 | 0.672–1.906 |
0.798 |
miR-142-5p expression is positively
correlated with ICC metastasis and invasion
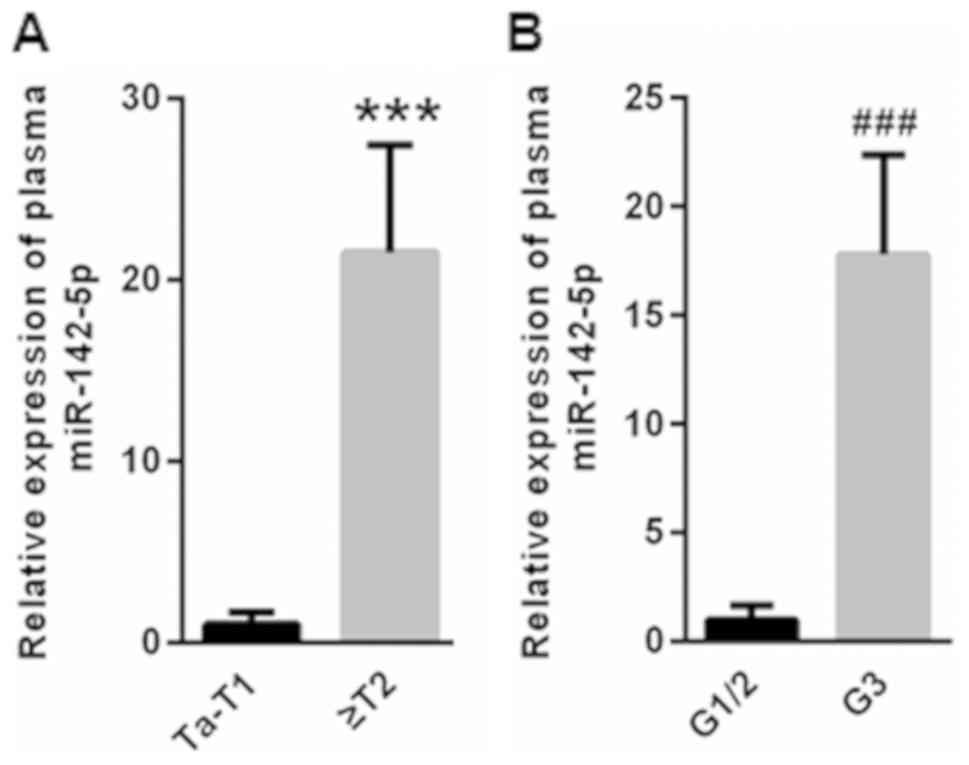
Compared with patients with Ta-T1 stage ICC,
(1±0.68), plasma miR-142-5p was significantly elevated in patients
with ICC with ≥T2 staging (21.5±5.93; Fig. 2A). Furthermore, patients with ICC at
G3 stage had significantly higher plasma miR-142-5p levels
(17.8±4.56) compared with those at G1/2 stage (1±0.67; Fig. 2B).
Plasma miR-142-5p is negatively
correlated with survival in ICC cancer patients
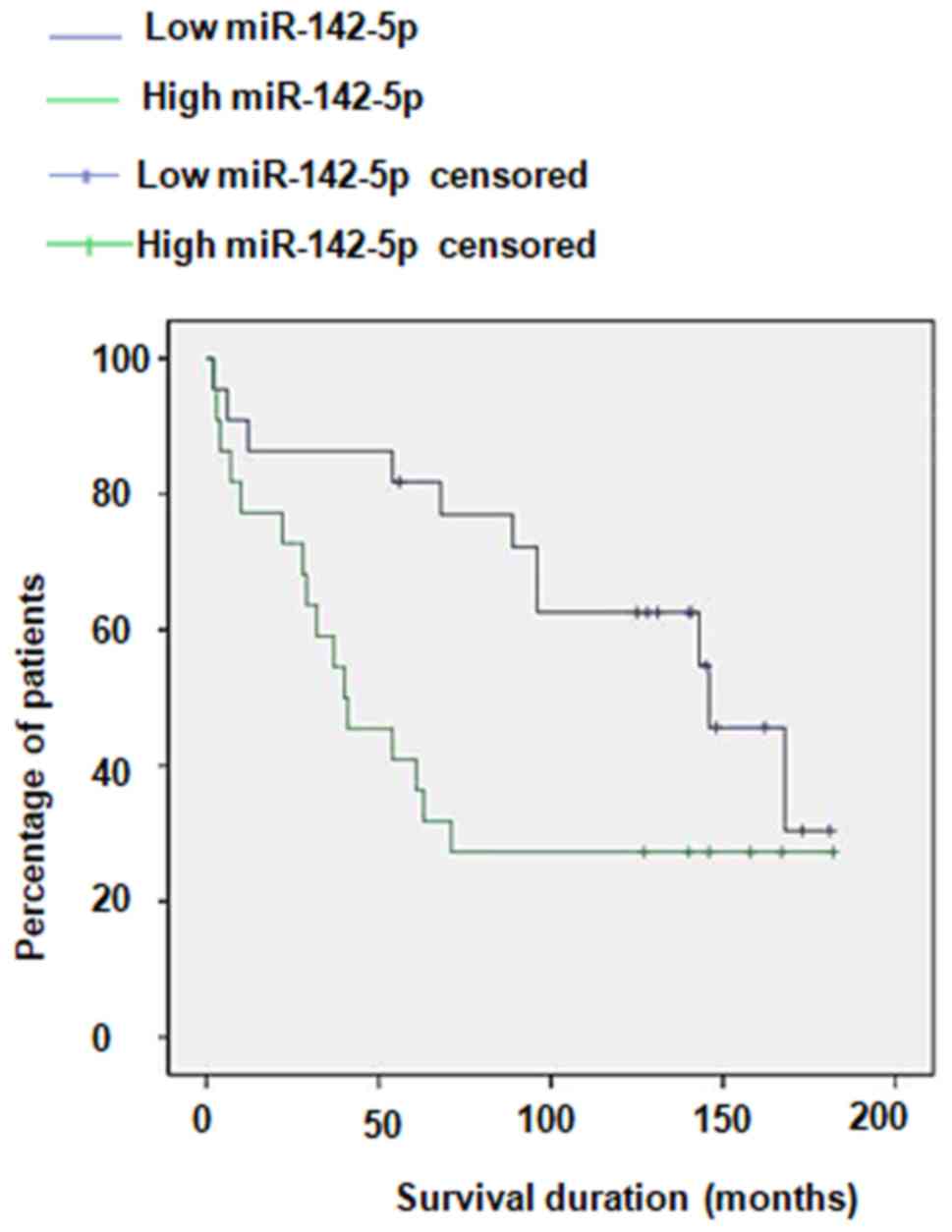
The results of Kaplan-Meier analysis revealed that
patients with high plasma miR-142-5p had a poorer survival rate
compared with those with low plasma miR-142-5p, with 5-year overall
survival rates of 27.86 and 51.46%, respectively (Fig. 3). In addition, to analyze whether
clinical factors, including sex, age, tumor diameter and tumor
differentiation affect ICC prognosis, Kaplan-Meier survival curves
were plotted and compared using a log-rank test (Table III). Log-rank analysis demonstrated
that increased miR-142-5p was significantly correlated with tumor
differentiation and malignancy (Table
III).
 | Table III.Clinicopathological features of
patients with intrahepatic cholangiocarcinoma and healthy control
subjects. |
Table III.
Clinicopathological features of
patients with intrahepatic cholangiocarcinoma and healthy control
subjects.
|
|
| miR-142-5p | Overall survival,
months |
|---|
|
|
|
|
|
|---|
| Variables | N | Low | High | P-value | Mean | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
Male | 75 | 37 | 38 | 0.985 | 44.67 | 39.05–51.94 | 0.435 |
|
Female | 25 | 13 | 12 |
| 38.25 | 32.56–43.15 |
|
| Age |
|
|
|
|
|
|
|
|
≥60 | 64 | 34 | 30 | 0.936 | 40.87 | 36.87–47.35 | 0.098 |
|
<60 | 36 | 18 | 18 |
| 42.86 | 31.45–50.87 |
|
|
Stage |
|
|
|
|
|
|
|
| Ta | 33 | 15 | 18 | 0.016 | 52.56 | 46.87–57.67 | 0.012 |
| T1 | 27 | 13 | 14 |
| 46.53 | 39.75–53.12 |
|
| T2 | 17 | 9 | 8 |
| 36.54 | 30.57–43.22 |
|
| T3 | 13 | 6 | 7 |
| 29.35 | 27.01–34.52 |
|
| T4 | 10 | 4 | 6 |
| 20.91 | 5.78–32.87 |
|
| Grade |
|
|
|
|
|
|
|
| 1 | 32 | 17 | 15 | 0.032 | 46.08 | 40.35–50.76 | 0.009 |
| 2 | 38 | 19 | 19 |
| 36.45 | 30.23–43.12 |
|
| 3 | 30 | 16 | 14 |
| 21.34 | 6.56–35.96 |
|
PTEN is a target gene of
miR-142-5p
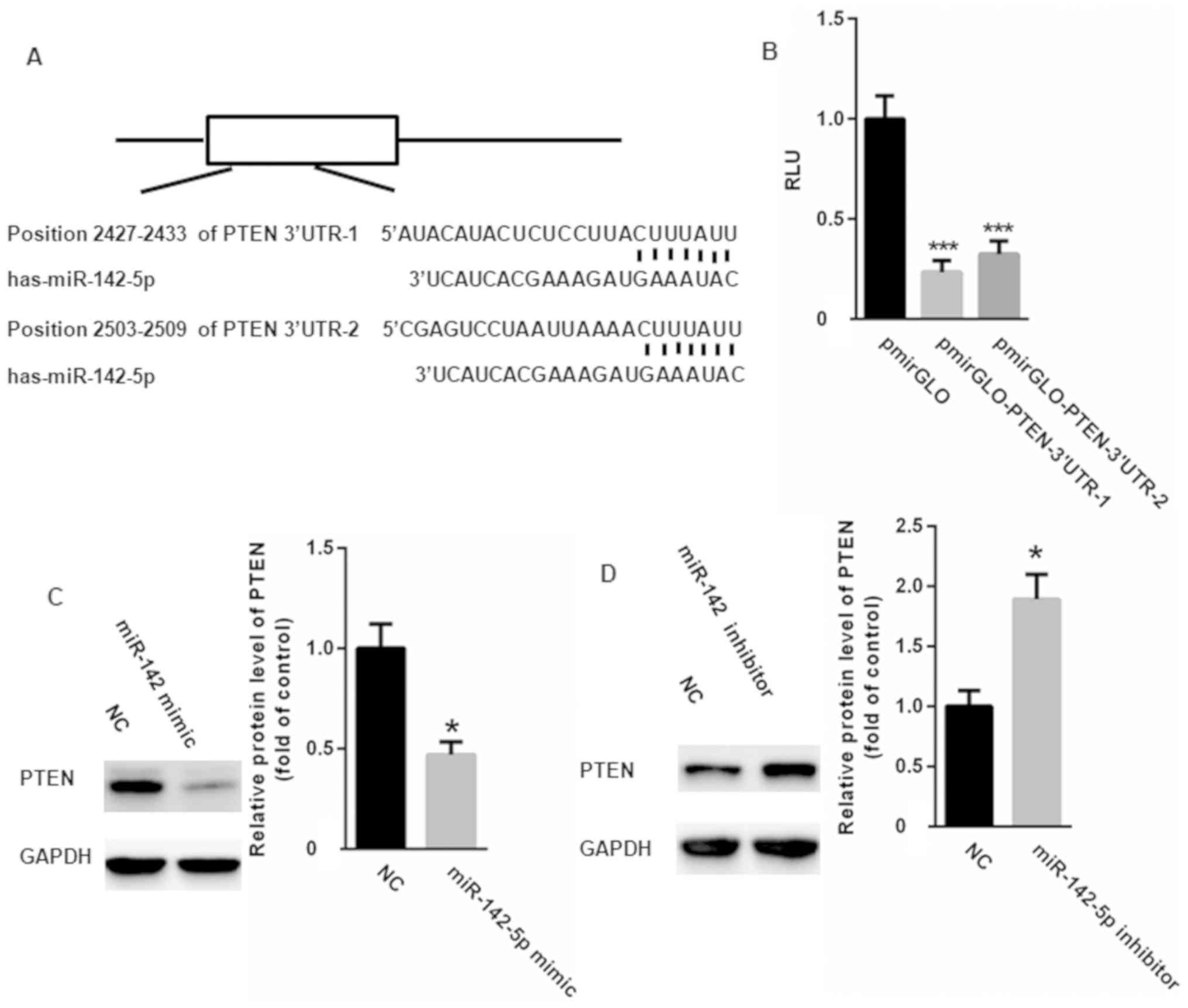
Based on the results of TargetScan analysis, a
conserved binding site of miR-142-5p in the 3′UTR of PTEN was
identified (Fig. 4A). Dual
luciferase reporter assay results indicated that miR-142-5p
significantly suppressed the relative luciferase activity of
pmirGLO-PTEN-3′UTR compared with the control (Fig. 4B). Western blotting also revealed
that miR-142-5p overexpression downregulated PTEN (Fig. 4C), while miR-142-5p knockdown
resulted in PTEN upregulation (Fig.
4D). These results confirm PTEN as a target gene of
miR-142-5p.
Discussion
ICC is the second most common intrahepatic primary
tumor after hepatocellular carcinoma and ICC is highly invasive by
nature and frequently metastasizes (16). It has been reported that chromosomal
anomalies, genetic polymorphisms and genetic or epigenetic
alterations may contribute to the tumorigenesis and progression of
ICC (17,18). It is important to detect ICC early in
order to improve treatment outcomes. Increasing evidence has
suggested that miRNAs may be used as potential diagnostic
biomarkers for ICC and may also serve as therapeutic targets
(19,20).
miRNAs are able to stably exist in bodily fluids,
including serum, plasma, saliva, urine and tears (20,21).
Furthermore, miRNAs can be easily detected in small amounts and are
resistant to degradation (20,22).
These characteristics make miRNAs attractive as potential
biomarkers (20). In the present
study it was determined that data showed that plasma miR-142-5p was
significantly increased in patients with ICC compared with healthy
controls. Furthermore, miR-142-5p levels were increased in ICC
tumor tissues compared with adjacent non-neoplastic tissues.
Further analysis revealed a positive correlation between miR-142-5p
and clinical outcome. Compared with patients with ICC at Ta-T1
stage, miR-142-5p was significantly upregulated in patients with
ICC at ≥T2 stage. Additionally, patients with ICC at G3 stage had
higher plasma miR-142-5p levels compared with those at G1/2 stage.
These data indicate that miR-142-5p expression is positively
correlated with therapy and outcome. ROC analysis indicated that
miR-142-5p could be used to differentiate patients with ICC from
healthy controls. Additionally, Kaplan-Meier analysis revealed that
plasma miR-142-5p is negatively correlated with survival in
patients with ICC. These data indicate that plasma miR-142-5p may
be useful for the early detection of cancer and individualized
therapies. The main focus of the present study was PTEN, which is
an important tumor suppressor in the development of ICC (23). Mutation and genomic loss of PTEN have
been widely reported in a number of cancers (24,25). It
has been also demonstrated that liver-specific deletion of PTEN in
a mouse model results in the development of ICC (26,27). As
an important tumor suppressor, PTEN mainly acts to dephosphorylate
phosphatidylinositol-3,4,5-trisphosphate [PtdIns(3,4,5)P3], which
potently activates 3-phosphoinositide-dependent kinase (PDK) and
protein kinase B (AKT) (28).
However, PTEN loss leads to excessive recruitment of
PtdIns(3,4,5)P3 at the plasma membrane, thereby activating a subset
of proteins, including the AKT family and PDK1 (28). AKT signaling induces cell survival,
cell proliferation, angiogenesis and cellular metabolism via
phosphorylating downstream signaling proteins (29,30). The
results of the present study revealed that PTEN was a target gene
of miR-142-5p. PTEN is widely acknowledged as a tumor suppressor
that is mutated in multiple tumors (31–33). In
the progression of ICC cancer, PTEN could negatively regulate the
AKT/PKB signaling pathway, thereby increasing cancer cell growth
and survival (34,35). The results of the present study
indicate that miR-142-5p may suppress PTEN expression, thereby
resulting in the malignant proliferation and increased viability of
cancer cells.
In summary, this study presents novel data
indicating that plasma miR-142-5p is significantly upregulated in
patients with ICC. Further analysis demonstrated that plasma
miR-142-5p could be used to screen patients with ICC from healthy
controls by targeting PTEN. However, only limited samples were
included in the current study. Thus, in further study, it may be
necessary to include more patients to validate the application of
miR-142-5p as a potential biomarker.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guided
Science And Technology Program Of Tongxiang City (grant nos.
81700765 and 81570789), the Zhejiang Provincial Natural Science
Foundation of China (grant no. LY14H030006), the Foundation for
Young Scientists of Zhejiang Province Traditional Chinese Medicine
(grant no. 2011ZQ008) and the Health and Family Planning Committee
of Zhejiang Province (grant no. 2012KYB143).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW performed the experiments and analyzed the data.
YY, XH and LJ performed RT-qPCR experiments. DJ designed all
experiments, analyzed the data and gave final approval of the
version of the manuscript to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The research protocols were approved by the First
People's Hospital of Tongxiang (Tongxiang, China) and written
informed consent was obtained from the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu J, Chen FY, Zhou KQ, Zhou C, Cao Y, Sun
HC, Fan J, Zhou J and Wang Z: Intrahepatic cholangiocarcinoma
patients without indications of lymph node metastasis not benefit
from lymph node dissection. Oncotarget. 8:113817–113827. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn DH and Bekaii-Saab T: Biliary cancer:
Intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma
vs. gallbladder cancers: Classification and therapeutic
implications. J Gastrointest Oncol. 8:293–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bagante F, Spolverato G, Weiss M,
Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C,
Bauer TW, Shen F, et al: Impact of morphological status on
long-term outcome among patients undergoing liver surgery for
intrahepatic cholangiocarcinoma. Ann Surg Oncol. 24:2491–2501.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhalla A, Mann SA, Chen S, Cummings OW and
Lin J: Histopathological evidence of neoplastic progression of von
meyenburg complex to intrahepatic cholangiocarcinoma. Hum Pathol.
67:217–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang ME, Lei HJ, Chen MH, Yeh YC, Li CP,
Hung YP, Hsia CY, Liu CA, Chau GY and Chao Y: Evaluation of
prognostic factors and implication of lymph node dissection in
intrahepatic cholangiocarcinoma: 10-year experience at a tertiary
referral center. J Chin Med Assoc. 80:140–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Liu D, Liu P, Chen Y, Yu H and
Zhang Q: Identification of biomarkers of intrahepatic
cholangiocarcinoma via integrated analysis of mRNA and miRNA
microarray data. Mol Med Rep. 15:1051–1056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang RX, Zheng Z, Li K, Wu XH and Zhu L:
Both plasma and tumor tissue miR-146a high expression correlates
with prolonged overall survival of surgical patients with
intrahepatic cholangiocarcinoma. Medicine (Baltimore).
96:e82672017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Xiao J, Chai Y, Du YY, Liu Z,
Huang K, Zhou X and Zhou W: LncRNA-CCAT1 promotes migration,
invasion, and EMT in intrahepatic cholangiocarcinoma through
suppressing miR-152. Dig Dis Sci. 62:3050–3058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zu C, Liu S, Cao W, Liu Z, Qiang H, Li Y,
Cheng C, Ji L and Li J and Li J: MiR-590-3p suppresses
epithelial-mesenchymal transition in intrahepatic
cholangiocarcinoma by inhibiting SIP1 expression. Oncotarget.
8:34698–34708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Liu Z, Fang X and Yang H:
MiR-142-5p suppresses tumorigenesis by targeting PIK3CA in
non-small cell lung cancer. Cell Physiol Biochem. 43:2505–2515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Islam F, Gopalan V, Vider J, Lu CT and Lam
AK: MiR-142-5p act as an oncogenic microRNA in colorectal cancer:
Clinicopathological and functional insights. Exp Mol Pathol.
104:98–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui
J, Liu Y, Gao Z, Li J, Shen L and Lu Y: Combination of hsa-miR-375
and hsa-miR-142-5p as a predictor for recurrence risk in gastric
cancer patients following surgical resection. Ann Oncol.
22:2257–2266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wittekind C: Pitfalls in the
classification of liver tumors. Pathologe. 27:289–293. 2006.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB and Compton CC: The american joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartella I and Dufour JF: Clinical
diagnosis and staging of intrahepatic cholangiocarcinoma. J
Gastrointestin Liver Dis. 24:481–489. 2015.PubMed/NCBI
|
|
17
|
Rahnemai-Azar AA, Weisbrod A, Dillhoff M,
Schmidt C and Pawlik TM: Intrahepatic cholangiocarcinoma: Molecular
markers for diagnosis and prognosis. Surg Oncol. 26:125–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pawlik TM: Intrahepatic
cholangiocarcinoma: From diagnosis to treatment. Hepatobiliary Surg
Nutr. 6:12017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang S, Yin J, Li T, Yuan L, Wang D, He J,
Du X and Lu J: Upregulated circulating miR-150 is associated with
the risk of intrahepatic cholangiocarcinoma. Oncol Rep. 33:819–825.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Correa-Gallego C, Maddalo D, Doussot A,
Kemeny N, Kingham TP, Allen PJ, D'Angelica MI, DeMatteo RP, Betel
D, Klimstra D, et al: Circulating plasma levels of MicroRNA-21 and
MicroRNA-221 are potential diagnostic markers for primary
intrahepatic cholangiocarcinoma. PLoS One. 11:e01636992016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng Q, Feng F, Zhu L, Zheng Y, Luo X,
Liu C, Yi B and Jiang X: Circulating miR-106a is a novel prognostic
and lymph node metastasis indicator for cholangiocarcinoma. Sci
Rep. 5:161032015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan R, Wang G, Xu Z, Zhao H, Chen H, Han
Y, Wang B, Zhou J, Hu H, Guo Z, et al: Up-regulated circulating
miR-106a by DNA methylation promised a potential diagnostic and
prognostic marker for gastric cancer. Anticancer Agents Med Chem.
16:1093–1100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou GY, Pan CW, Jin LX, Zheng JJ and Yi
YX: Neoalbaconol inhibits cell growth of human cholangiocarcinoma
cells by up-regulating PTEN. Am J Transl Res. 8:496–505.
2016.PubMed/NCBI
|
|
24
|
Horie Y, Suzuki A, Kataoka E, Sasaki T,
Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, et
al: Hepatocyte-specific pten deficiency results in steatohepatitis
and hepatocellular carcinomas. J Clin Invest. 113:1774–1783. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trimboli AJ, Cantemir-Stone CZ, Li F,
Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H,
Chong JL, et al: Pten in stromal fibroblasts suppresses mammary
epithelial tumours. Nature. 461:1084–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu X, Kobayashi S, Qiao W, Li C, Xiao C,
Radaeva S, Stiles B, Wang RH, Ohara N, Yoshino T, et al: Induction
of intrahepatic cholangiocellular carcinoma by liver-specific
disruption of Smad4 and pten in mice. J Clin Invest. 116:1843–1852.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marsh V, Davies EJ, Williams GT and Clarke
AR: PTEN loss and KRAS activation cooperate in murine biliary tract
malignancies. J Pathol. 230:165–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chung JY, Hong SM, Choi BY, Cho H, Yu E
and Hewitt SM: The expression of phospho-AKT, phospho-mTOR, and
PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res.
15:660–667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YR, Chen M and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor: New modes
and prospects. Nat Rev Mol Cell Biol. 19:547–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rimawi MF, De Angelis C, Contreras A,
Pareja F, Geyer FC, Burke KA, Herrera S, Wang T, Mayer IA, Forero
A, et al: Low PTEN levels and PIK3CA mutations predict resistance
to neoadjuvant lapatinib and trastuzumab without chemotherapy in
patients with HER2 over-expressing breast cancer. Breast Cancer Res
Treat. 167:731–740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malaney P, Palumbo E, Semidey-Hurtado J,
Hardee J, Stanford K, Kathiriya JJ, Patel D, Tian Z, Allen-Gipson D
and Davé V: PTEN Physically interacts with and regulates
E2F1-mediated transcription in lung cancer. Cell Cycle. 17:947–962.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bian X, Gao J, Luo F, Rui C, Zheng T, Wang
D, Wang Y, Roberts TM, Liu P, Zhao JJ and Cheng H: PTEN deficiency
sensitizes endometrioid endometrial cancer to compound PARP-PI3K
inhibition but not PARP inhibition as monotherapy. Oncogene.
37:341–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikenoue T, Terakado Y, Nakagawa H, Hikiba
Y, Fujii T, Matsubara D, Noguchi R, Zhu C, Yamamoto K, Kudo Y, et
al: Corrigendum: A novel mouse model of intrahepatic
cholangiocarcinoma induced by liver-specific Kras activation and
Pten deletion. Sci Rep. 7:395672017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikenoue T, Terakado Y, Nakagawa H, Hikiba
Y, Fujii T, Matsubara D, Noguchi R, Zhu C, Yamamoto K, Kudo Y, et
al: A novel mouse model of intrahepatic cholangiocarcinoma induced
by liver-specific Kras activation and Pten deletion. Sci Rep.
6:238992016. View Article : Google Scholar : PubMed/NCBI
|