Introduction
Although the incidence and mortality rates of
gastric cancer (GC) are slowly declining due to the reduction of
Helicobacter pylori infection rate and improvements in
socioeconomic conditions (1), GC
still poses a threat to public health, with >50% of cases
occurring in Asia (2). Surgery
remains the primary curative therapy for early gastric cancer;
however, chemotherapy can improve the outcome of resectable GC
(3). 5-fluorouracil (5-FU) is widely
used for the treatment of GC as it exerts an anticancer effect by
inducing apoptosis (4,5). Cisplatin (CDDP) is one of the most
effective broad-spectrum anticancer drugs for GC, which functions
by forming cross-links that activate apoptotic pathways causing
cell death (6). However, frequent
recurrences following surgical and medical therapies has resulted
in the unsuccessful prognosis of patients with advanced GC
(7). Furthermore, there are no
effective biomarkers of GC that predict prognosis or treatment
outcome, which may be a reason for the low survival rate or
occurrence of unsuccessful treatment. Therefore, it is of great
importance to assess novel molecular targeting agents for the
improvement of patient prognosis, particularly in combination with
cytotoxic agents.
Pituitary homeobox paired homeodomain transcription
(PITX1) was originally considered to be a bicoid-associated
transcription factor that was involved in the transcription of the
proopiomelanocortin gene in the adult pituitary. However, PITX1 may
serve a role in pituitary cell differentiation and pituitary
formation (8). In addition, PITX1 is
essential for limb development. The misexpression of PITX1 in the
forelimb results in the transformation and translocation of
specific muscles, tendons and bones such that they acquire a
hindlimb-like morphology (9).
Therefore, PITX1 is a key component for the identification and
structure of the hindlimb (10,11).
PITX1 has been revealed to be a tumor suppressor
that functions by downregulating the Ras pathway (12). Various studies have also indicated
that PITX1 expression is downregulated in certain types of
malignant cancer, including oral squamous cell carcinoma (13), esophageal (14), gastric (15,16),
lung (17), breast (18), hepatic (19), colorectal (20), pancreatic (21) and prostatic cancer (22), as well as malignant melanoma
(16,23). The strong expression of PITX1 is
associated with a favorable outcome in human osteosarcoma (24). PITX1 expression also serves as a
novel biomarker for the prediction of patient prognosis in oral
epithelial dysplasia (25) and may
be a predictive biomarker of human head and neck squamous cell
carcinoma chemosensitivity (26).
However, the association between PITX1 expression and GC patient
survival remains unclear. Additionally, whether the expression of
PITX1 is associated with the sensitivity of GC cells to
chemotherapy is poorly understood.
The present study assessed the effect of PITX1 on
the sensitivity of GC cells to 5-FU and CDDP. The results revealed
that increased PITX1 enhanced the sensitivity of GC cells (AGS and
BGC-823) to 5-FU and CDDP. The knockdown of PITX1 weakened the
sensitivity of GC cells (MCG-803 and SGC-7901) to 5-FU and CDDP.
These results may provide a potential therapeutic target for GC in
future studies.
Materials and methods
GC cell lines
GC cell lines (AGS, BGC-823, MCG-803 and SGC-7901)
and a gastric epithelial cell line (GES-1) were purchased from the
Institute of Biochemistry and Cell Biology of the Chinese Academy
of Sciences (Shanghai, China). All cell lines were maintained in
RPMI-1640 medium (Wisent, Inc., St Bruno, QC, Canada) supplemented
with 10% fetal bovine serum (Wisent, Inc.), 100 U/ml penicillin and
100 mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in a humidified incubator with 5% CO2
at 37°C.
Plasmid construction and PITX1 small
interfering (si)RNA
PITX1 cDNA was isolated via reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
then cloned into the HindIII/EcoRI sites of pcDNA3.1. The primer
sequences were as follows: PITX1-pcDNA3.1-HindIII,
5′-CCCCAAGCTTATGGACGCCTTCAAGGGGG-3′ and PITX1-pcDNA3.1-EcoRI,
5′-GGTGGAATTCGGCGGTCAGCTGTTGTACTGG-3′.
PITX1 siRNA and a scrambled/non-targeting siRNA were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
sequences were as follows: PITX1 siRNA1 forward,
5′-AUCGCCGUGUGGACCAACCUCACCGAGCC-3′ and reverse,
5′-GGCUCGGUGAGGUUGGUCCACACGGCGAU-3′; PITX1 siRNA2 forward,
5′-CACUUCACAAGCCAGCAGUUGCAAGAGCU-3′ and reverse,
5′-AGCUCUUGCAACUGCUGGCUUGUGAAGUG-3′; Scrambled/nontargeting siRNA
forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′.
Cell transfection
AGS and BGC-823 cells were transiently transfected
with 2 µg of the control pcDNA3.1 (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) or PITX1-pcDNA3.1 using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. PITX1 siRNA and negative control siRNA
were synthesized and purified by Shanghai GenePharma Co., Ltd.
(Shanghai, China) as aforementioned and transfected into cells at a
final concentration of 50 nM using Lipofectamine 2000 according to
the manufacturer's protocol. After the gastric cancer cells were
transfected in 6-well plates for 48 h, transfection efficiency was
evaluated and subsequent experiments were performed.
RT-qPCR
Total RNA from the gastric cancer cell lines GES-1,
AGS, BGC-823, MCG-803 and SGC-7901 was isolated using the TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). First strand
cDNA was synthesized from 1 µg of total RNA using PrimeScript™ RT
reagent Kit with gDNA Eraser (Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. qPCR was
performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.)
with the StepOne Plus system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Each
PCR was performed in triplicate and β-actin was used as the
internal control. The thermocycling conditions were as follows:
Pre-denaturation at 95°C for 5 min, denaturation at 98°C for 10
sec, annealing at 59°C for 5 sec, extension at 72°C for 2 min and
35 cycles. The relative expression of target RNA was evaluated via
the comparative 2−ΔΔCq method (27). The primer sequences of each gene were
as follows: PITX1 forward, 5′-TCCACCAAGAGCTTCACCTT-3′ and reverse,
5′-CGGTGAGGTTGTTGATGTTG-3′; β-actin forward,
5′-AAAGACCTGTACGCCAACAC-3 and reverse,
5′-GTCATACTCCTGCTTGCTGAT-3.
Cell viability and proliferation
assays
The 30% inhibitory concentration (IC30) is a measure
of the potency of a substance in inhibiting a specific biological
or biochemical function and it represents the concentration of a
drug that is required for 30% inhibition in vitro. To
calculate this value for 5-FU and CDDP, AGS, BGC-823, MCG-803 and
SGC-7901 cells were seeded into 96-well plates at a density of
1,000 cells/well, treated with 5-FU (0, 25, 50, 100, 200, 400 or
800 µM) and CDDP (0, 1, 2.5, 5, 7.5, 10, 15 or 20 µM and cultured
for 48 h to determine the appropriate concentration for subsequent
experiments. Subsequently, 10 µl CCK-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) solution was added to each
well of the plate and incubated at 37°C for 3 h. AGS or BGC-823
cells (1,000 cells per well) were plated and transiently
transfected with the pPITX1 construct. MCG-803 and SGC-7901 cells
(1,000 cells/well) were transiently transfected with PITX1 siRNA in
96-well plates. Cell proliferation was determined using a Cell
Counting Kit-8 (CCK-8) assay for 4 days (Dojindo Molecular
technologies, Inc.). Optical density was measured at a wavelength
of 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and each experiment was performed in triplicate.
Cells (3.5×103 per well) were plated in 96-well plates
in triplicate at 37°C for 24 h. The cells were then incubated with
5-FU (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; AGS in 25 µM,
BGC-823 in 25 µM, MCG-803 in 12.5 µM and SGC7901 in 6.25 µM) or
CDDP (Sigma-Aldrich; Merck KGaA; AGS in 20 µM, BGC-823 in 5 µM,
MCG-803 in 2.5 µM and SGC-7901 in 2.5 µM) at 37°C for 24 h.
Following treatment at the indicated times, CCK-8 was added to the
culture for 3 h prior to the measurement of absorbance at 450 nm.
Each experiment was performed in triplicate.
Bioinformatics analysis of RNA
sequencing data from The Cancer Genome Atlas
The Cancer Genome Atlas (TCGA) dataset (http://ualcan.path.uab.edu/analysis.html), a large
cancer dataset with high-throughput sequencing data for protein
coding genes, was used to assess the expression of PITX1 mRNA in
stomach adenocarcinoma based on race. The Bioconductor package
edgeR (https://www.bioconductor.org/packages/release/bioc/html/edgeR.html)
was used to compute the P-values and fold changes of the
RNA-sequence read count data in the R platform (version 3.2.3;
http://www.r-project.org/). PITX1
expression was extracted from HTSeq-kilobase per million mapped
reads (FPKM) data (https://portal.gdc.cancer.gov/), excluding those
patients lacking complete clinical information or with low-quality
data. A box plot was used to present the differential expression of
PITX1 (PITX1 mRNA levels with an FPKM value ≤17.1 were considered
as low-expression) between 87 Asian GC tissues and 34 normal
gastric tissues. The 60-month survival time was assessed from the
TCGA data.
Bioinformatics analysis obtained from
Kyoto Encyclopedia of Genes and Genomes (KEGG)
The correlation between the expression level of
PITX1 and each protein coding gene (PCG) was calculated using
two-sided Pearson correlation coefficients and the z-test. PCGs
positively or negatively correlated with PITX1 were considered as
PITX1-associated PCGs (r≥0.4 and P<0.01). KEGG pathway analysis
(28) of PITX1-related PCGs was
analyzed using the clusterProfiler R package (https://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html),
with P-value <0.05 and adjusted P-value <0.05.
Statistical analysis
The proliferation of the single reagent and combined
therapy group was compared using a paired Student's t-test. An
independent Student's t-test was used to compare the results
(expressed as the mean ± standard deviation) between any two
preselected groups. One-way analysis of variance, followed by a
Dunnett's test, were performed to compare data between three or
more groups. P<0.05 was considered to indicate a statistically
significant difference. Patients were divided into low or high
PITX1 expression groups according to the PITX1 median value.
P-values for survival curves were determined from the Kaplan-Meier
survival curves by use of the log-rank test. The correlation
between the expression of PITX1 and each protein-coding gene was
assessed by performing a two-sided Pearson correlation coefficient
test and a z-test. If the protein-coding gene positively or
negatively correlated with PITX1, it was considered to be a
PITX1-associated protein-coding gene.
Results
Expression analysis of PITX1 mRNA in
GC cell lines
To select the appropriate cell model for each
experiment, levels of PITX1 were determined in several GC cell
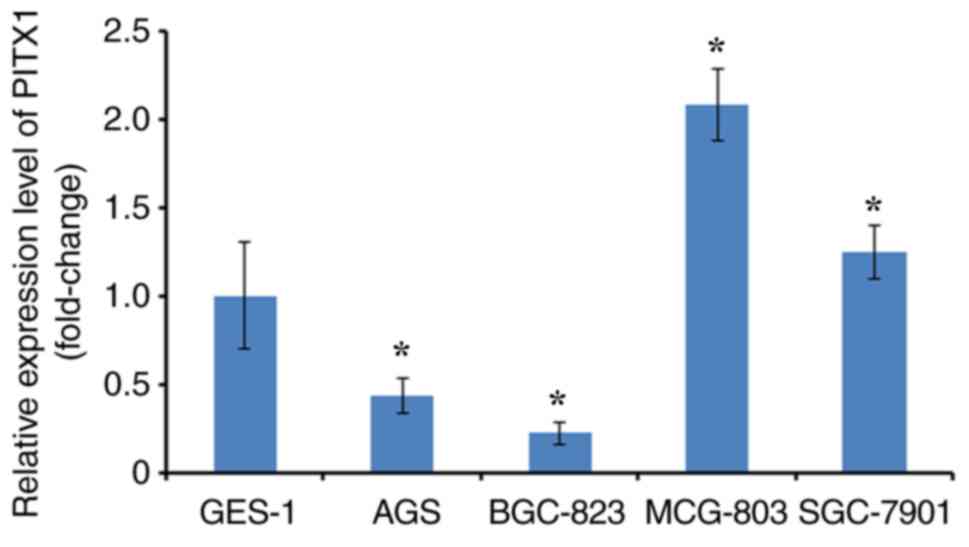
lines. RT-qPCR was performed to analyze PITX1 expression
(differentiated fold) between four GC cell lines and one normal
gastric mucosa cell line (GES-1). The expression of PITX1 in AGS
and BGC-823 was significantly lower compared with GES-1.
Additionally, PITX1 was significantly increased in the MCG-803 cell
line and markedly higher in the SGC-7901 cell line compared with
the GES-1 cell line (Fig. 1).
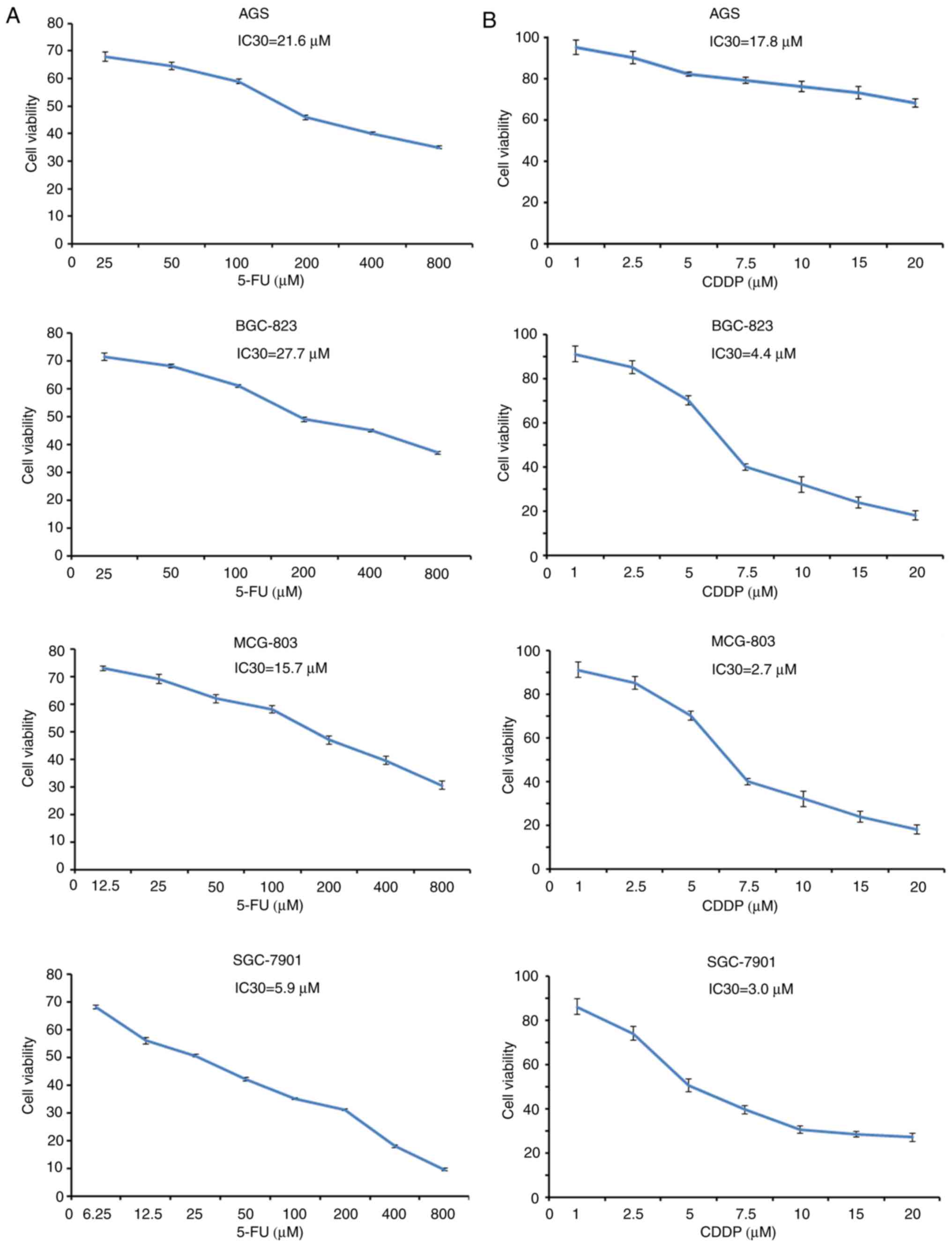
Establishment of the IC30 of each cell
line treated with 5-FU and CDDP
To determine the cytotoxic activity of 5-FU and CDDP
as single agents, the cell viability of four GC cell lines treated
with 5-FU and CDDP was determined (Fig.
2A and B, respectively). The cells were treated with single
5-FU and CDDP treatments at different concentrations. The results
revealed that the cell viability of each experimental group was
markedly inhibited by 5-FU and CDDP in a dose-dependant manner. In
addition, the IC30 of 5-FU in AGS, BGC823, MCG-803 and SGC-7901 was
21.6, 27.7, 15.7 and 5.9 µM, respectively. Therefore, the
concentrations of 5-FU at 25 µM in AGS, 25 µM in BGC-823, 12.5 µM
in MCG-803 and 6.25 µM in SGC-7901 were selected to perform the
following experiments. Similarly, the IC30 of CDDP in AGS, BGC823,
MCG-803 and SGC-7901 was 17.8, 4.4, 2.7 and 3.0 µM, respectively.
The selected concentrations of CDDP were 20 µM in AGS, 5 µM in
BGC-823, 2.5 µM in MCG-803 and 2.5 µM in SGC-7901.
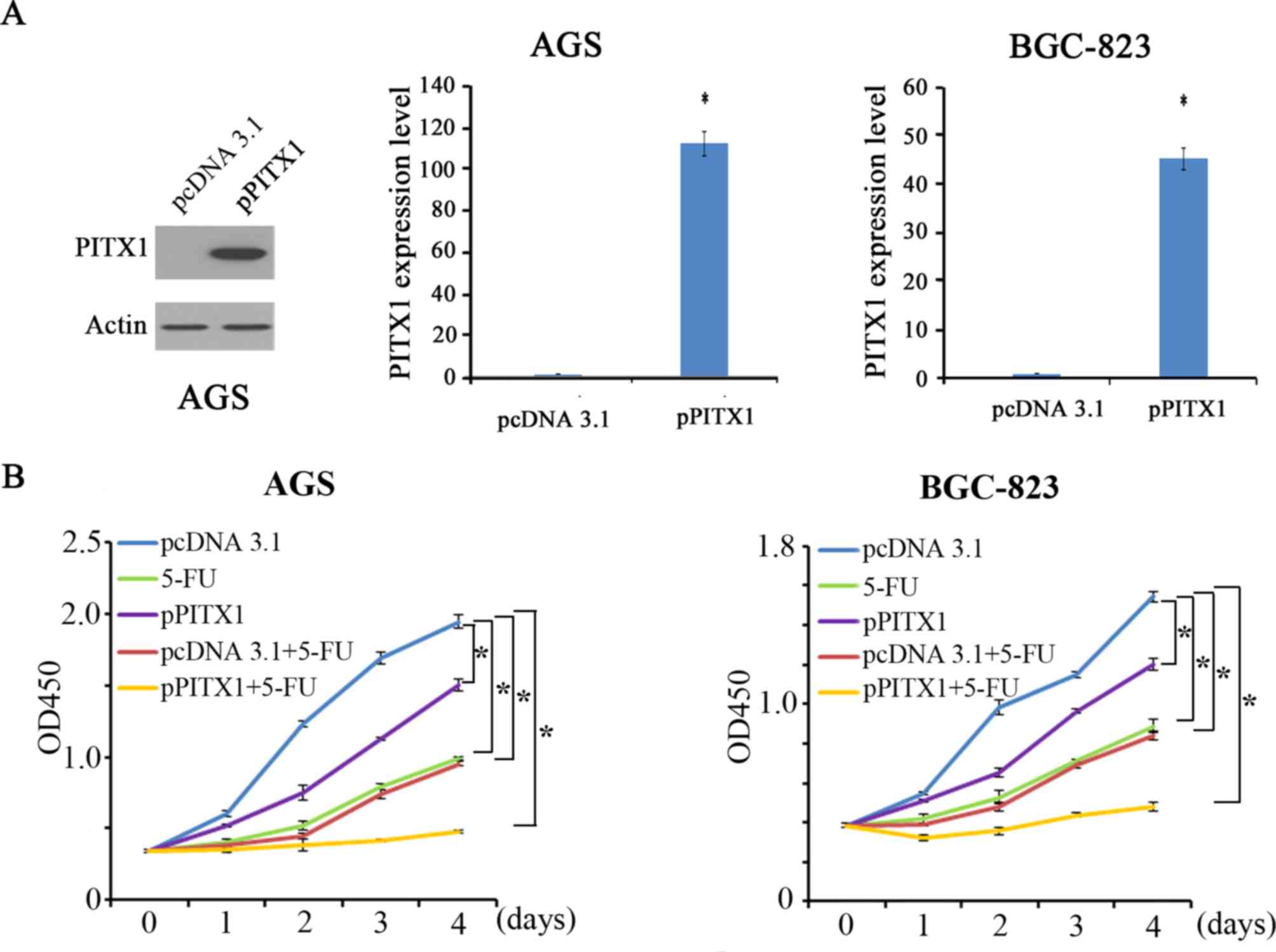
Overexpression of PITX1 enhances the
sensitivity of AGS and BGC-823 to 5-FU
To assess the effect of PITX1 on the
chemosensitivity of GC cells, PITX1 was overexpressed in AGS and
BGC-823. The expression of PITX1 was significantly increased in AGS
and BGC-823 cells transfected with pcDNA-PITX1 compared with that
of pcDNA3.1-transfected cells (Fig.
3A). Cells were then treated with 5-FU, which is clinically
used in the treatment of gastrointestinal cancer. In AGS and
BGC-823 cells, proliferation was measured via a CCK-8 assay in 5
groups: The control group (pcDNA 3.1), the 5-FU treatment group
(5-FU), the overexpression of PITX1 group (pPITX1), the group
treated with empty pcDNA3.1 vector+5-FU (pcDNA 3.1+5-FU) and 5-FU
treatment combined with the overexpression pPITX1 group
(pPITX1+5-FU). In AGS and BGC-823 cells, it was demonstrated that
cell proliferation was inhibited in the 5-FU, pPITX1, pcDNA3.1+5-FU
and pPITX1+5-FU groups compared with the pcDNA3.1 group (Fig. 3B). Furthermore, compared with 5-FU
treatment alone, pPITX1+5-FU treatment inhibited proliferation
significantly. These results indicate that the overexpression of
PITX1 enhances GC cell line sensitivity to 5-FU treatment.
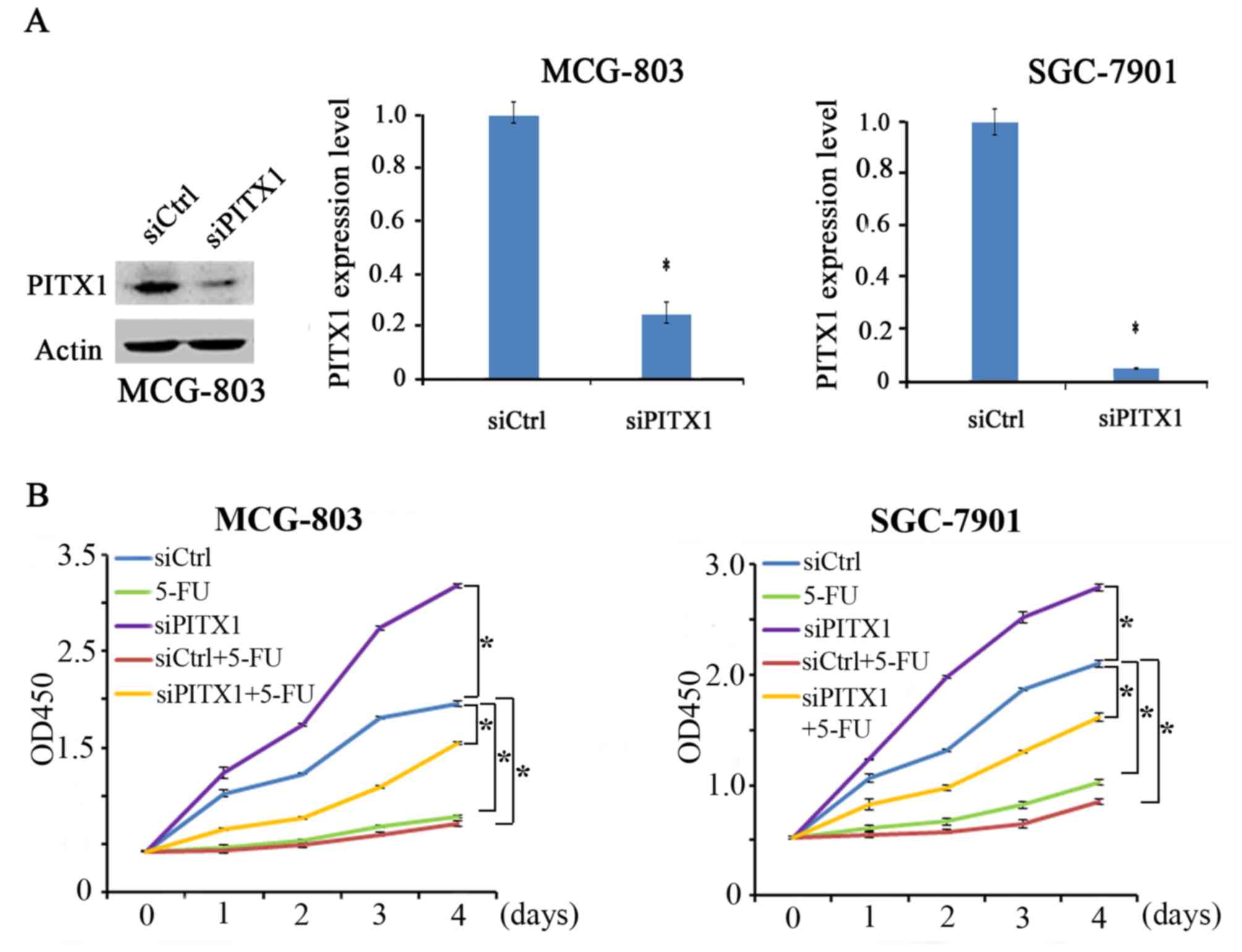
Silenced PITX1 impairs the sensitivity
of MCG-803 and SGC-7901 cells to 5-FU
PITX1 expression was determined via the transient
transfection of PITX1 siRNA in MCG-803 and SGC-7901 cells (Fig. 4A). In the absence of 5-FU, PITX1
knockdown (siPITX1 group) significantly promoted the proliferation
of MGC-803 cells compared with the control. Similarly, PITX1
knockdown significantly increased the proliferation of SGC-7901
cells. The other 3 groups (5-FU, siCtrl+5-FU and siPITX1+5-FU)
exhibited a lower proliferation in MCG-803 and SGC-7901 cell lines
compared with the control (Fig. 4B).
Furthermore, the proliferation of MCG-803 and SGC-7901 cells
treated with siPITX1+5-FU were not significantly inhibited compared
with the 5-FU group. The results revealed that the silencing of
PITX1 in GC cells attenuated the sensitivity of GC cells to 5-FU
treatment.
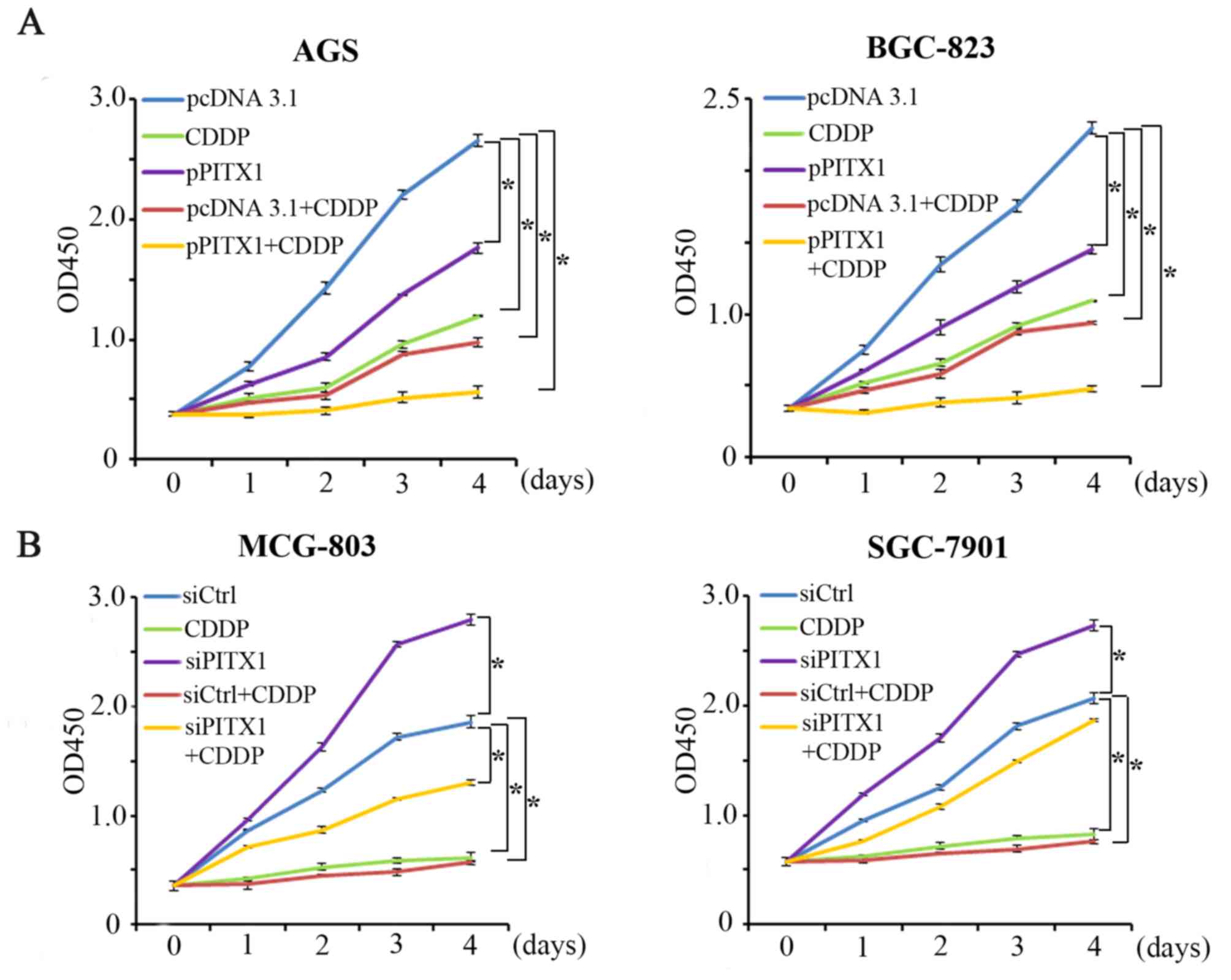
Ectopic PITX1 expression increases the
sensitivity of AGS and BGC-823 cells to CDDP
CDDP is also commonly used for the clinical
treatment of GC. Cell proliferation was assessed via a CCK-8 assay
following transfection with a PITX1 construct. The impact of PITX1
on CDDP treatment was assessed by dividing the cells into five
groups. The proliferation of AGS and BGC-823 cells was markedly
reduced in the pPITX1, CDDP, pcDNA3.1+CDDP and the pPITX1+CDDP
group (Fig. 5A) compared with the
pcDNA3.1 group. Furthermore, pPITX1+CDDP treated cells induced a
lower reduction of AGS and BGC-823 cell proliferation compared with
the CDDP group.
The knockdown of PITX1 weakens the
sensitivity of MCG-803 and SGC-7901 cells to CDDP
Following the successful transfection of siPITX1
into MCG-803 and SGC-7901 cells the effect of downregulated PITX1
on CDDP sensitivity in these cell lines were assessed (Fig. 5B). The cells were divided into 5
groups: The control group (siCtrl), the CDDP-treated group (CDDP),
the PITX1 knockdown group (siPITX1), the CDDP with
scrambled/non-targeting siRNA group (siCtrl+CDDP) and the CDDP with
PITX1 knockdown group (siPITX1+CDDP). Compared with the siCtrl
group, it was revealed that the CDDP, siCtrl+CDDP and siPITX1+CDDP
groups inhibited the proliferation of MCG-803 cells. However, the
siPITX1 group significantly promoted the proliferation of MCG-803
(Fig. 5B). Furthermore, compared
with the CDDP group, the inhibition of cell proliferation was
reduced in the siPITX1+CDDP group. Additionally, a similar result
was demonstrated in the SGC-7901 cell line (Fig. 5B). These data indicate that the
knockdown of PITX1 in GC cells weakens the sensitivity of GC cells
to CDDP treatment.
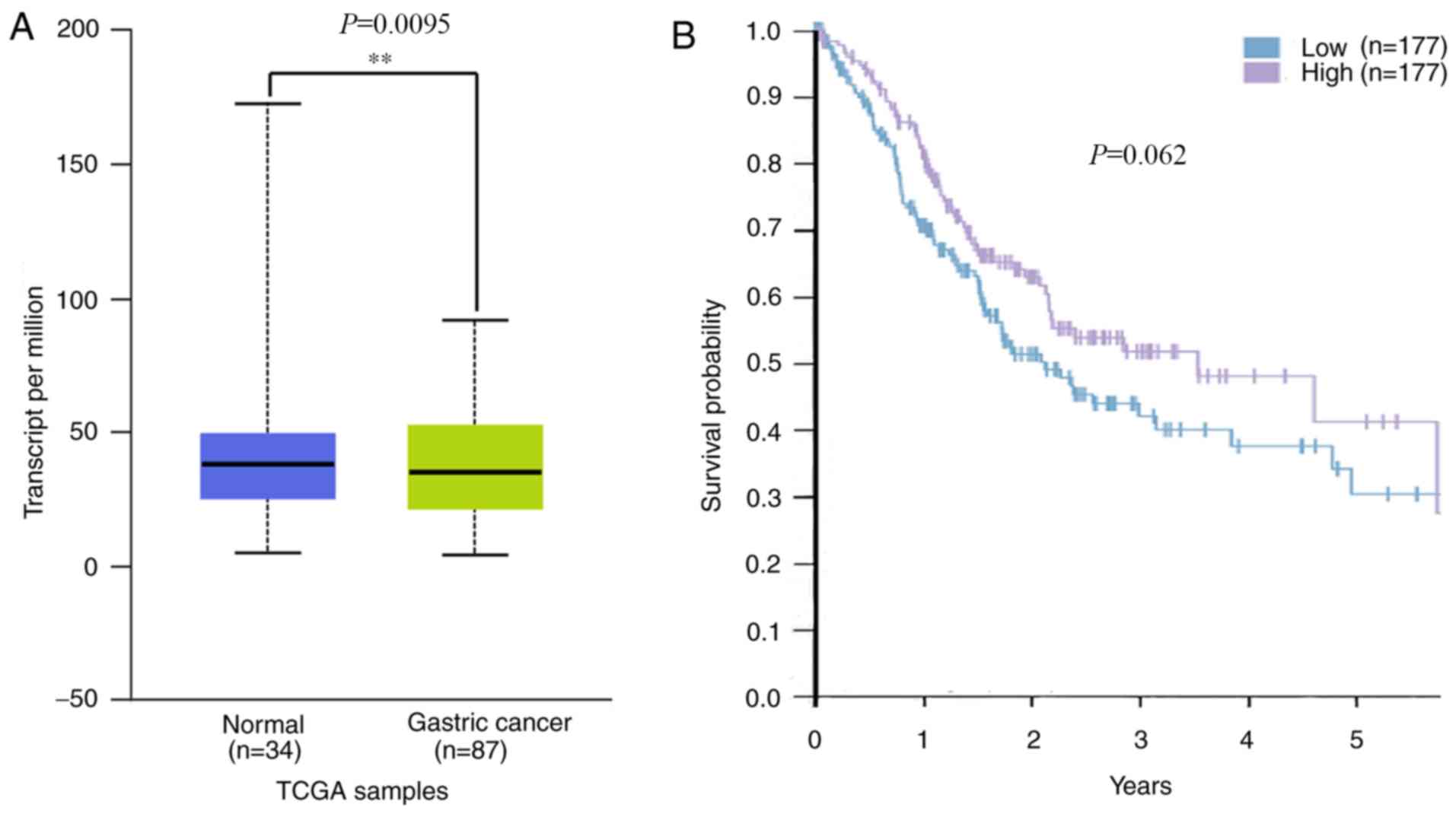
The decreased expression of PITX1
exhibits a negative outcome in human GC based on TCGA data
A previous study revealed that patients with GC that
exhibited lower PITX1 protein levels tended to have a poorer
prognosis than those with higher levels (29). To assess whether PITX1 serves a
prognostic role in GC of Asian populations, mRNA levels of PITX1 in
the TCGA database were analyzed. The results revealed that PITX1
was markedly decreased at the transcriptional level in the 87 Asian
GC tissues compared with 34 normal gastric tissues (Fig. 6A). Although there was no significant
difference in the survival rates of patients with GC between the
low and the high PITX1 mRNA expression groups (Fig. 6B), the data indicates that the
patient's survival was affected by the low expression of PITX1.
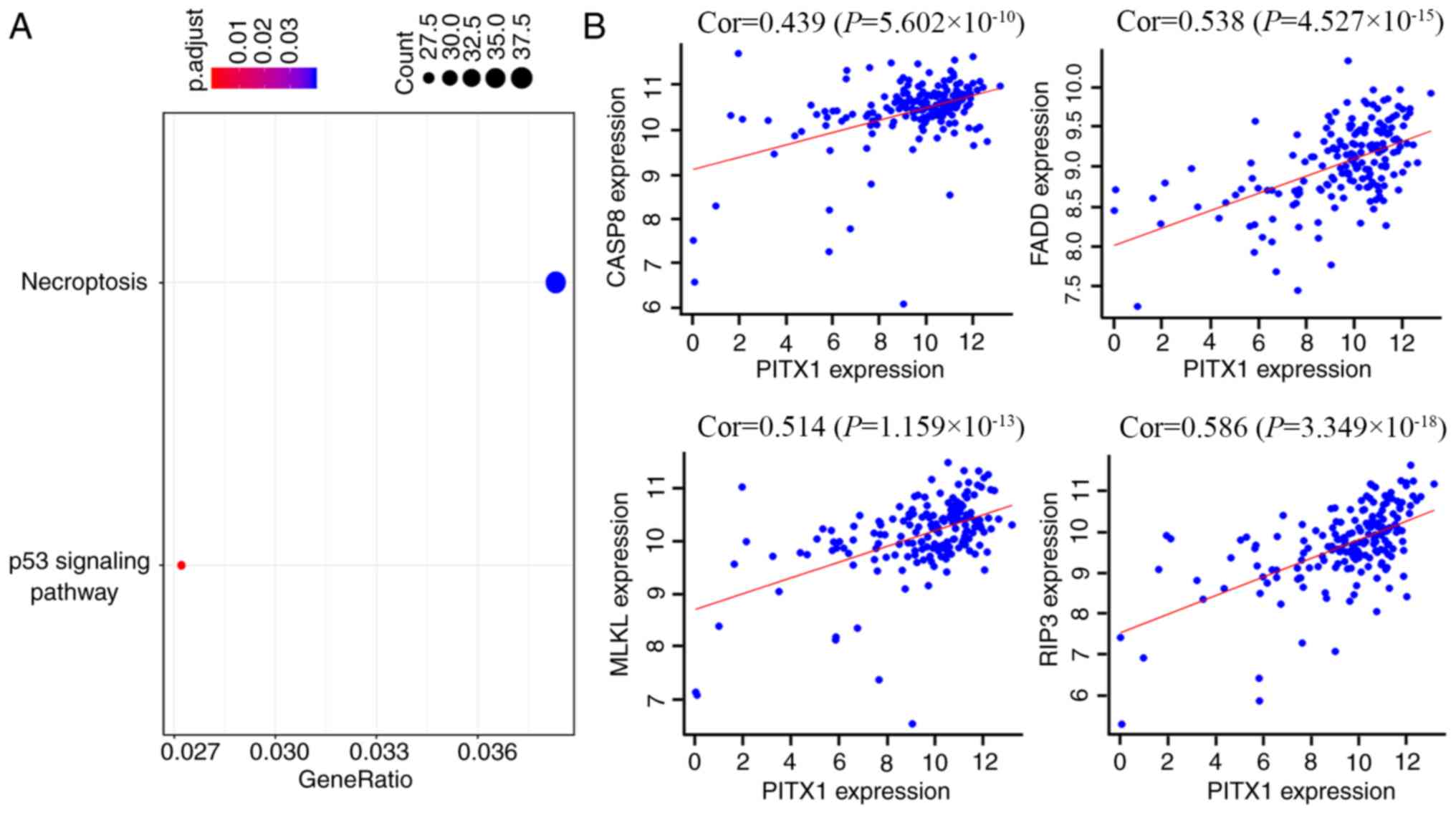
To assess the functional similarity of genes
co-expressed with PITX1, a Pearson correlation coefficient was
performed. Co-expressed genes were selected at a certain cutoff
threshold and then filtered to 1,620 genes by considering
correlation coefficients at r≥0.4 and significances at P<0.01. A
KEGG analysis was utilized to assess the potential function of
PITX1 in the chemotherapeutic resistance to 5-FU and CDDP in GC
cells. The majority of these co-expressed genes were revealed to
participate in the regulation of necroptosis and certain genes were
also associated with the p53 signaling pathway (Fig. 7A). Certain genes, which are widely
thought to be involved in the necroptosis pathway, including
caspase-8, Fas-associated protein with death domain (FADD),
receptor-interacting serine/threonine-protein kinase 3 (RIP3) and
mixed lineage kinase domain like pseudokinase (MLKL), were
positively correlated with PITX1 (Fig.
7B).
Discussion
Although PITX1 was first reported in a study on the
development of hindlimb morphology (30,31), an
increasing number of reports have determined that decreased PITX1
levels in various types of human cancer serve important roles in
carcinogenesis. The downregulation of PITX1 expression may
contribute to the progression of cutaneous malignant melanoma by
promoting cell proliferative activity (23). Furthermore, a decreased PITX1
expression has been revealed in bronchial (17,18),
hepatic (19), colorectal (20), pancreatic (21), prostatic (22) and gastric malignancies (15,16). It
has also been demonstrated that the level of PITX1 is correlated
with patient survival; patients with higher PITX1 levels exhibit a
longer median overall survival in human osteosarcoma (24). The reduced expression of PITX1 is
also independently correlated with shorter patient survival and may
serve as a prognostic marker in colorectal carcinoma (32). In a previous study (29), it was revealed that the expression of
PITX1 is decreased in GC tissues and that the increased expression
of PITX1 significantly suppresses the proliferation of GC cells and
tumorigenesis in vitro and in vivo. The present study
assessed the role of PITX1 expression in GC cell sensitivity to the
chemotherapeutic drugs 5-FU and CDDP, which are clinically used in
the treatment of gastrointestinal cancer.
The current study first assessed the connection
between PITX1 expression and the sensitivity of GC cells to
chemotherapy and the prognosis of patients with GC. To determine
whether PITX1 expression was correlated with GC cell sensitivity to
chemotherapeutic drugs, 5-FU and CDDP were utilized. 5-FU inhibits
DNA synthesis and is used to treat colorectal, breast and head and
neck cancer (33). CDDP is an
inorganic compound that exerts cytotoxicity by inducing apoptosis
(34) and is commonly administered
in ovarian (35), testicular
(36) and esophageal cancer
(37). The present study transiently
transfected a PITX1 construct into the GC cell lines, AGS and
BGC-823. The results revealed that 5-FU/CDDP and PITX1
significantly suppressed GC cell proliferation. Compared with the
5-FU/CDDP treatment, pPITX1+5-FU/CDDP treatment significantly
inhibited cell proliferation. These results indicated that the
overexpression of PITX1 in the GC cell lines, AGS and BGC-823,
enhanced the efficacy of 5-FU/CDDP treatment. Furthermore, compared
with the 5-FU/CDDP group, the inhibition of cell proliferation in
the siPITX1+5-FU/CDDP group was reduced, indicating that the
knockdown of PITX1 in the GC cell lines, MCG-803 and SGC-7901,
weakens the sensitivity of GC cells to CDDP and 5-FU treatment.
To determine the correlation of PITX1 with GC
prognosis, the TCGA dataset, which consists of high-throughput
sequencing data for protein-coding gene expression, was considered
in the further analysis. The current study demonstrated that PITX1
mRNA expression was significantly lower in 87 Asian GC tissues than
in 34 normal gastric mucous tissues. A Kaplan-Meier survival curve
of patients with GC classified into 2 groups depending on the high
and low expression of PITX1 from the TCGA database. The results
revealed that a high and low expression of PITX1 influenced patient
survival. Those with a high PITX1 mRNA expression had a lower
survival than those with a low PITX1 mRNA expression. Combining the
results of a previous study (29),
the results indicate that patients with higher PITX1 levels have a
longer survival time than those with a lower PITX1 level. The
expression of PITX1 may therefore be a reliable biomarker for the
prediction of GC patient prognosis. To further assess the mechanism
by which PITX1 contributes to chemotherapy insensitivity, all known
co-expressed genes were categorized using a KEGG analysis. A total
of ~1620 target genes were screened, the biological processes of
which were primarily implicated in necroptosis. The kinase RIP3,
the adaptor protein FADD and the proximal initiator caspase-8, have
been identified as fundamental regulators of the necroptotic cell
death pathway (38–40). In addition, MLKL, a key component
downstream of RIP3, is suggested to be a terminal executor of
necroptosis (41). Previous studies
also revealed that the four aforementioned genes were positively
correlated with necroptosis (42,43). The
present study hypothesized that PITX1 enhances the cytotoxicity of
5-FU and CDDP in GC cells partially by inducing necroptosis;
however, the mechanism requires further exploration.
In summary, a high PITX1 expression increases the
sensitivity of GC cell lines to 5-FU and CDDP. However, the precise
molecular mechanism by which this occurs requires further
study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472548 and
81672414).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS conceived the study and was a major contributor
in writing the manuscript. GY, SY, YM and YZ contributed to cell
culture and CCK-8 assay. PG was responsible for bioinformatics
analysis. YL, FQ and ZZ were involved in drafting the manuscript
and revising it critically for important intellectual content. HF
designed the research protocols and gave final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinou JC, Desagher S and Antonsson B:
Cytochrome c release from mitochondria: All or nothing. Nat Cell
Biol. 2:E41–E43. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Tang XM, Half E, Kuo MT and
Sinicrope FA: Cyclooxygenase-2 overexpression reduces apoptotic
susceptibility by inhibiting the cytochrome c-dependent apoptotic
pathway in human colon cancer cells. Cancer Res. 62:6323–6328.
2002.PubMed/NCBI
|
|
6
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wagner AD, Syn NL, Moehler M, Grothe W,
Yong WP, Tai BC, Ho J and Unverzagt S: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev.
8:CD0040642017.PubMed/NCBI
|
|
8
|
Lamonerie T, Tremblay JJ, Lanctôt C,
Therrien M, Gauthier Y and Drouin J: Ptx1, a bicoid-related homeo
box transcription factor involved in transcription of the
pro-opiomelanocortin gene. Genes Dev. 10:1284–1295. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeLaurier A, Schweitzer R and Logan M:
Pitx1 determines the morphology of muscle, tendon, and bones of the
hindlimb. Dev Biol. 299:22–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang J, Li X, Ring HZ, Clayton DA and
Francke U: Backfoot, a novel homeobox gene, maps to human
chromosome 5 (BFT) and mouse chromosome 13 (Bft). Genomics.
40:108–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shang J, Luo Y and Clayton DA: Backfoot is
a novel homeobox gene expressed in the mesenchyme of developing
hind limb. Dev Dyn. 209:242–253. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kolfschoten IG, van Leeuwen B, Berns K,
Mullenders J, Beijersbergen RL, Bernards R, Voorhoeve PM and Agami
R: A genetic screen identifies PITX1 as a suppressor of RAS
activity and tumorigenicity. Cell. 121:849–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Libório TN, Acquafreda T,
Matizonkas-Antonio LF, Silva-Valenzuela MG, Ferraz AR and Nunes FD:
In situ hybridization detection of homeobox genes reveals distinct
expression patterns in oral squamous cell carcinomas.
Histopathology. 58:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lord RV, Brabender J, Wickramasinghe K,
DeMeester SR, Holscher A, Schneider PM, Danenberg PV and DeMeester
TR: Increased CDX2 and decreased PITX1 homeobox gene expression in
Barrett's esophagus and Barrett's-associated adenocarcinoma.
Surgery. 138:924–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YN, Chen H, Xu Y, Zhang X and Luo Y:
Expression of pituitary homeobox 1 gene in human gastric
carcinogenesis and its clinicopathological significance. World J
Gastroenterol. 14:292–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi DL, Ohhira T, Fujisaki C, Inoue T, Ohta
T, Osaki M, Ohshiro E, Seko T, Aoki S, Oshimura M and Kugoh H:
Identification of PITX1 as a TERT suppressor gene located on human
chromosome 5. Mol Cell Biol. 31:1624–1636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Knösel T, Ye F, Pacyna-Gengelbach
M, Deutschmann N and Petersen I: Decreased PITX1 homeobox gene
expression in human lung cancer. Lung Cancer. 55:287–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stender JD, Stossi F, Funk CC, Charn TH,
Barnett DH and Katzenellenbogen BS: The estrogen-regulated
transcription factor PITX1 coordinates gene-specific regulation by
estrogen receptor-alpha in breast cancer cells. Mol Endocrinol.
25:1699–1709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calvisi DF, Ladu S, Conner EA, Seo D,
Hsieh JT, Factor VM and Thorgeirsson SS: Inactivation of Ras
GTPase-activating proteins promotes unrestrained activity of
wild-type Ras in human liver cancer. J Hepatol. 54:311–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knösel T, Chen Y, Hotovy S, Settmacher U,
Altendorf-Hofmann A and Petersen I: Loss of desmocollin 1–3 and
homeobox genes PITX1 and CDX2 are associated with tumor progression
and survival in colorectal carcinoma. Int J Colorectal Dis.
27:1391–1399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hamidov Z, Altendorf-Hofmann A, Chen Y,
Settmacher U, Petersen I and Knösel T: Reduced expression of
desmocollin 2 is an independent prognostic biomarker for shorter
patients survival in pancreatic ductal adenocarcinoma. J Clin
Pathol. 64:990–994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwok SC, Liu X, Mangel P and Daskal I:
PTX1(ERGIC2)-VP22 fusion protein upregulates interferon-beta in
prostate cancer cell line PC-3. DNA Cell Biol. 25:523–529. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Osaki M, Chinen H, Yoshida Y, Ohhira T,
Sunamura N, Yamamoto O, Ito H, Oshimura M and Kugoh H: Decreased
PITX1 gene expression in human cutaneous malignant melanoma and its
clinicopathological significance. Eur J Dermatol. 23:344–349.
2013.PubMed/NCBI
|
|
24
|
Kong G, Liu Z, Wu K, Zhang Y, Deng Z, Feng
W, Chen S and Wang H: Strong expression of paired-like homeodomain
transcription factor 1 (PITX1) is associated with a favorable
outcome in human osteosarcoma. Tumour Biol. 36:7735–7741. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakabayashi M, Osaki M, Kodani I, Okada F,
Ryoke K, Oshimura M, Ito H and Kugoh H: PITX1 is a reliable
biomarker for predicting prognosis in patients with oral epithelial
dysplasia. Oncol Lett. 7:750–754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takenobu M, Osaki M, Fujiwara K, Fukuhara
T, Kitano H, Kugoh H and Okada F: PITX1 is a novel predictor of the
response to chemotherapy in head and neck squamous cell carcinoma.
Mol Clin Oncol. 5:89–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao F, Gong P, Song Y, Shen X, Su X, Li
Y, Wu H, Zhao Z and Fan H: Downregulated PITX1 modulated by
MiR-19a-3p promotes cell malignancy and predicts a poor prognosis
of gastric cancer by affecting transcriptionally activated PDCD5.
Cell Physiol Biochem. 46:2215–2231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klopocki E, Kähler C, Foulds N, Shah H,
Joseph B, Vogel H, Lüttgen S, Bald R, Besoke R, Held K, et al:
Deletions in PITX1 cause a spectrum of lower-limb malformations
including mirror-image polydactyly. Eur J Hum Genet. 20:705–708.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Infante CR, Park S, Mihala AG, Kingsley DM
and Menke DB: Pitx1 broadly associates with limb enhancers and is
enriched on hindlimb cis-regulatory elements. Dev Biol.
374:234–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gajewska A, Herman AP, Wolinska-Witort E,
Kochman K and Zwierzchowski L: In vivo oestrogenic modulation of
Egr1 and Pitx1 gene expression in female rat pituitary gland. J Mol
Endocrinol. 53:355–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marullo R, Werner E, Degtyareva N, Moore
B, Altavilla G, Ramalingam SS and Doetsch PW: Cisplatin induces a
mitochondrial-ROS response that contributes to cytotoxicity
depending on mitochondrial redox status and bioenergetic functions.
PLoS One. 8:e811622013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ozols RF, Bookman MA, du Bois A, Pfisterer
J, Reuss A and Young RC: Intraperitoneal cisplatin therapy in
ovarian cancer: Comparison with standard intravenous carboplatin
and paclitaxel. Gynecol Oncol. 103:1–6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burger AM, Double JA and Newell DR:
Inhibition of telomerase activity by cisplatin in human testicular
cancer cells. Eur J Cancer. 33:638–644. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kies MS, Rosen ST, Tsang TK, Shetty R,
Schneider PA, Wallemark CB and Shields TW: Cisplatin and
5-fluorouracil in the primary management of squamous esophageal
cancer. Cancer. 60:2156–2160. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Declercq W, Vanden Berghe T and
Vandenabeele P: RIP kinases at the crossroads of cell death and
survival. Cell. 138:229–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Fan C and Zhang H, Zhao Q, Liu Y,
Xu C, Xie Q, Wu X, Yu X, Zhang J and Zhang H: MLKL and FADD are
critical for suppressing progressive lymphoproliferative disease
and activating the NLRP3 inflammasome. Cell Rep. 16:3247–3259.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oliver Metzig M, Fuchs D, Tagscherer KE,
Gröne HJ, Schirmacher P and Roth W: Inhibition of caspases primes
colon cancer cells for 5-fluorouracil-induced TNF-α-dependent
necroptosis driven by RIP1 kinase and NF-κB. Oncogene.
35:3399–3409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ratovitski EA: Phospho-ΔNp63α-responsive
microRNAs contribute to the regulation of necroptosis in squamous
cell carcinoma upon cisplatin exposure. FEBS Lett. 589:1352–1358.
2015. View Article : Google Scholar : PubMed/NCBI
|