Introduction
Diarrhea is categorized into four types based on its
mechanism of action: osmotic diarrhea, secretory diarrhea,
inflammatory and infectious diarrhea, and diarrhea related to
motility disorders. Clinically, diarrhea-predominant irritable
bowel syndrome and functional diarrhea are associated with
alterations in intestinal motility; therefore, some anti-diarrheal
agents decreasing intestinal transit, such as opioids and
5-HT3 antagonist, have been used to manage these
diseases (1).
Scutellaria baicalensis is widely used as a
herbal medicine in East Asia and Europe for gastrointestinal tract
(GI) disorders including dysentery, diarrhea, and abdominal pain
(2). Experimentally, it has
anti-diarrheal effect on irrinotecan-induced diarrhea in rats
(3). Clinically, it demonstrated an
improvement in the diarrhea toxicity grade and reduction of
frequency of severe diarrhea in lung cancer patients with
irrinotecan-induced diarrhea (4).
Baicalin, a component of S. baicalensis has anti-diarrheal
action through its anti-inflammatory effects inhibiting
cyclooxygenase-2 (COX-2) activity and colonic prostaglandin
E2 (PGE2) (5,6).
However, it is still unknown whether S. baicalensis directly
modulates bowel motility.
Bowel motility is the result of a coordinated
contraction and relaxation of intestinal smooth muscles, which is
regulated by various neurotransmitters. The neuroeffector apparatus
in the GI muscles is conceptualized as the SIP syncytium composed
of electrically coupled smooth muscle cells (SMC), interstitial
cells of Cajal (ICC), and platelet-derived growth factor receptor α
positive (PDGFRα+) cells. Various receptors and ion
channels are expressed in SIP cells, and conductance changes in any
type of SIP cells affect the integrated excitability of muscle
layers and responses to neurotransmitters (7).
We hypothesized that if the components of S.
baicalensis could alter the bowel motility, it is conceivable
that they affect the neuromuscular transmission and/or the
excitability of SIP cells. Therefore, we examined the effect of the
major components of S. baicalensis on colonic motility, and
investigated the mechanism of action of the components on smooth
muscle of rat colon.
Materials and methods
Animal and tissue preparation
Male Sprague-Dawley rats (250~300 g), provided ad
libitum standard chow and water, were used for this study. The
rats were housed in stainless steel hanging cages in a colony room
maintained under a 12 h light/dark cycle with a room temperature of
21–23°C and humidity of 65–70%. Their care and handling were in
accordance with the NIH Guide for the Care and Use of Laboratory
Animals. All procedures were approved by the Institutional Animal
Use and Care Committee at the Seoul National University (Seoul,
Korea; approval no. SNU-101872-04). Rats were anesthetized with
isoflurane followed by cervical dislocation. From these rats, 2.5–3
cm distal colons were removed and flushed clean with Krebs
solution. The colonic segments were immediately placed in a 20 ml
organ bath containing Krebs solution bubbled with a mixture of 5%
CO2−95% O2 (pH 7.4) at 37°C. The preparations
were given a minimum of 60–90 min to equilibrate before commencing
the experiments. During this period, spontaneous rhythmic giant
contractions (GCs) were developed and stabilized.
Measurement of colonic motility
The distal end of the colonic segment was tied to a
fixed mount, and the ligated proximal end was secured with silk
thread to an isometric force displacement transducer (FT-03;
Grass-Telefactor; Astro Med, Inc., Slough, UK). Changes in
mechanical signals were detected as isometric tension and recorded
as an index of the longitudinal muscle response. After the
equilibrium period, spontaneous GCs were recorded. The components
of S. baicalensis and chemicals were added to the organ
bath, after which spontaneous GCs were recorded.
Solutions and chemicals
Krebs solution contained (mM) 10.10 glucose, 115.48
NaCl, 21.90 NaHCO3, 4.61 KCl, 1.14
NaH2PO4, 2.5 CaCl2, and 1.16
MgSO4. The following chemicals were used:
Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME;
300 µM), 1H-(1,2,4)-oxadiazolo (4,2-a) quinoxalin-1-one (ODQ;
10 µM), tetradotoxin (TTX; 1 µM), ω-conotoxin (300 nM), apamin (100
nM), and iberiotoxin (100 nM). Each drug was administered 15 min
before adding wogonin. Concentrated stock solutions of L-NAME, TTX,
ω-conotoxin, apamin, iberiotoxin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) were prepared by dissolving the chemicals in
distilled water. ODQ (Sigma-Aldrich; Merck KGaA) was dissolved in
dimethyl sulfoxide (DMSO). Baicalin, baicalein and wogonin
(Sigma-Aldrich; Merck KGaA) were prepared in 0.2 N NaOH. When DMSO
was previously used as a solvent for concentrated stock solutions
of baicalin, baicalein, and wogonin, they recrystallized at high
doses in Krebs solution and we were unable to measure the exact
working concentration. Therefore, in this study, 0.2 N NaOH was
used as the solvent to overcome the problem. The dose of baicalin,
baicalein, and wogonin was chosen between subminimal and submaximal
inhibitory effect of drugs. Even though 500 µM was not submaximal
dose of baicalin, highest dose of baicalin was determined at 500 µM
due to be recrystallized at over 500 µM.
TTX was used to block the enteric impulse in the rat
colon smooth muscle. ω-conotoxin was used to block the
neurotransmitter release from nerve terminals by blocking the
N-type Ca2+ channels (8).
Apamin was used to block a small-conductance
Ca2+-activated K+ (SK) channel, which are
located in PDGFRα+ cells dominantly in the smooth muscle
apparatus. Iberiotoxin was used to block a large-conductance
Ca2+-activated K+ (BK) channel, expressed in
the SMCs.
Analysis of data
The recorded amplitude and frequency of GCs were
analyzed. Two segments from one mouse colon were prepared because
some of pretreated drugs were not washed completely. Therefore, we
separately analyzed each dataset. To measure the effects of the
drug on GCs, each experimental measurement was matched with a
control measurement in the same tissue. The amplitude and frequency
of GCs in the control state were considered 100% and the parameters
of GCs in the presence of baicalin, baicalein, and wogonin were
expressed as percentage of the control. For the fitting of the
concentration response curves of the S. baicalensis
components, the following logisticcal function was used;
Y=100/{1+10^[(IC50-C)h]}, where Y is the normalized (%)
GC amplitude or frequency at a given concentration (C) of each
substance, and h is the slope factor of the curve.
IC50 was defined as the concentration of the substance
at which Y was inhibited to 50% of the GC amplitude or frequency in
the control state. Data were expressed as means ± SEM of n, the
number of tissues. Statistical analysis was primarily performed by
2-way repeated measures ANOVA followed by Holm-Sidak multiple
comparison test. P<0.05 was considered significant
differences.
Results
Inhibitory effect of baicalin,
baicalein, and wogonin on GCs in the rat colon
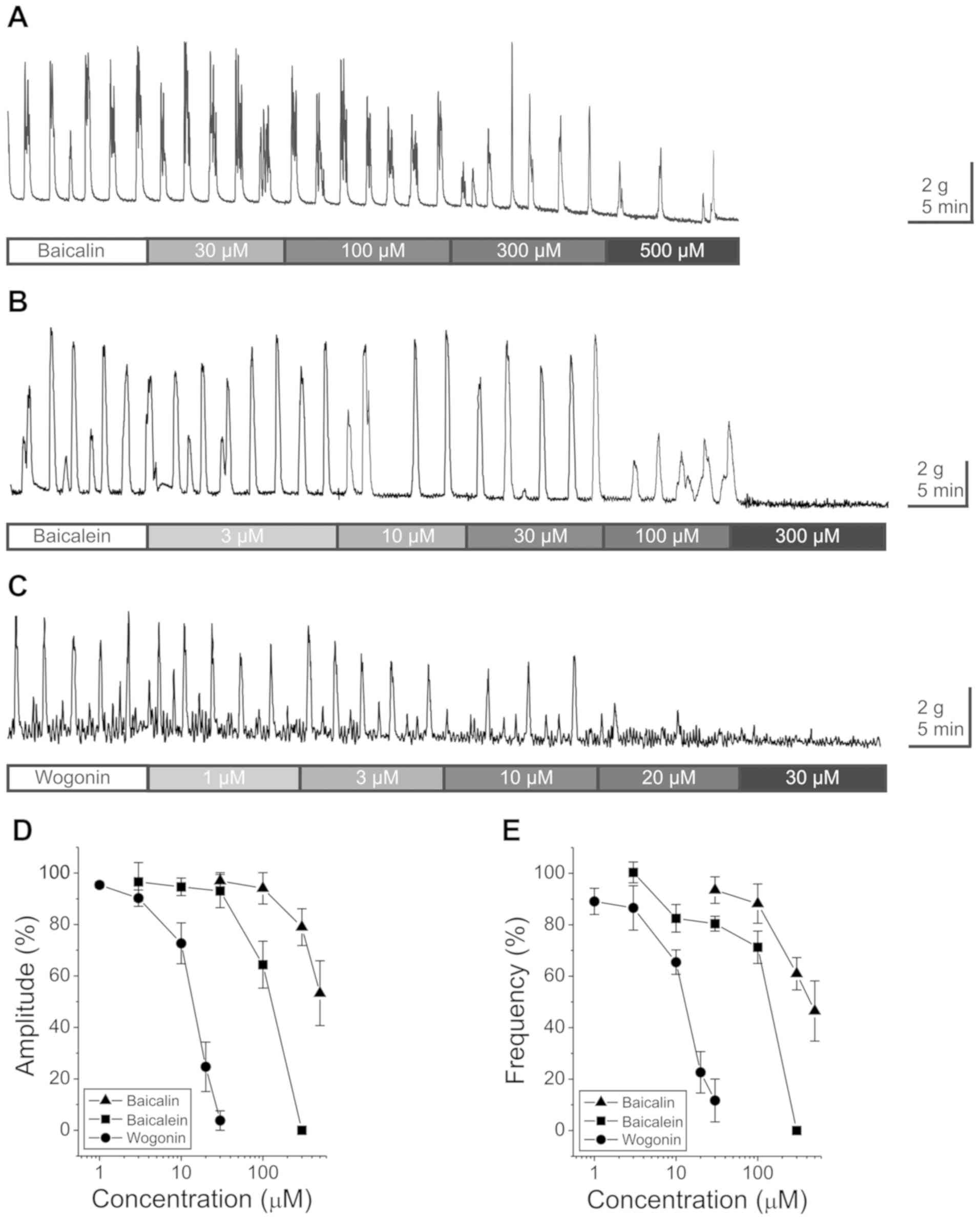
Spontaneous GCs occurred at a mean amplitude of
3.7±0.3 g with a frequency of 0.8±0.1/min in the distal colon of
rats (n=38). As shown in Fig. 1,
three major components of S. baicalensis, namely baicalin
(n=14), baicalein (n=12) and wogonin (n=12), reduced the amplitude
and frequency of GCs in a dose-dependent manner. After washing off
the drugs, the GCs showed complete recovery. The IC50
value of baicalin was 545.4 µM in amplitude and 463.3 µM in
frequency. The IC50 value of baicalein was 112.9 µM in
amplitude and 123.9 µM in frequency. The IC50 value of
wogonin was 14.6 µM in amplitude and 14.2 µM in frequency (Table I). These results show that wogonin is
the most effective agent among the three substances in inhibiting
GCs in the distal colon of rats.
 | Table I.Effects of baicalin, baicalein, and
wogonin on the amplitude and frequency of the giant contractions in
rat colon (mean ± standard error of the mean). |
Table I.
Effects of baicalin, baicalein, and
wogonin on the amplitude and frequency of the giant contractions in
rat colon (mean ± standard error of the mean).
| A. Amplitude |
|---|
|
|---|
| Dose (µM) | Baicalin (%) | Dose (µM) | Baicalein (%) | Dose (µM) | Wogonin (%) |
|---|
| 30 | 97.0±3.2 | 3 | 96.6±7.5 | 1 | 95.4±1.1 |
| 100 | 94.0±6.1 | 10 | 94.6±3.4 | 3 | 90.2±3.2 |
| 300 | 79.0±7.2 | 30 | 93.0±6.4 | 10 | 72.7±8.0 |
| 500 | 53.3±12.6 | 100 | 64.4±9.1 | 20 | 24.7±9.6 |
|
|
| 300 | 0 | 30 | 3.8±3.8 |
| P-value | P<0.001 |
| P<0.001 |
| P<0.001 |
| IC50
(µM) | 545.4 |
| 112.9 |
| 14.6 |
|
| B.
Frequency |
|
| Dose
(µM) | Baicalin
(%) | Dose
(µM) | Baicalein
(%) | Dose
(µM) | Wogonin
(%) |
|
| 30 | 93.5±25.2 | 3 | 100.3±4.1 | 1 | 89.1±5.1 |
| 100 | 88.2±7.6 | 10 | 82.5±5.4 | 3 | 86.5±8.6 |
| 300 | 61.0±6.3 | 30 | 80.4±2.9 | 10 | 65.5±4.8 |
| 500 | 46.5±11.7 | 100 | 71.3±6.3 | 20 | 22.6±8.0 |
|
|
| 300 | 0 | 30 | 11.7±8.3 |
| P-value | P<0.001 |
| P<0.001 |
| P<0.001 |
| IC50
(µM) | 463.3 |
| 123.9 |
| 14.2 |
No effect of nitrergic inhibitory
pathway on the wogonin-induced reduction of GCs
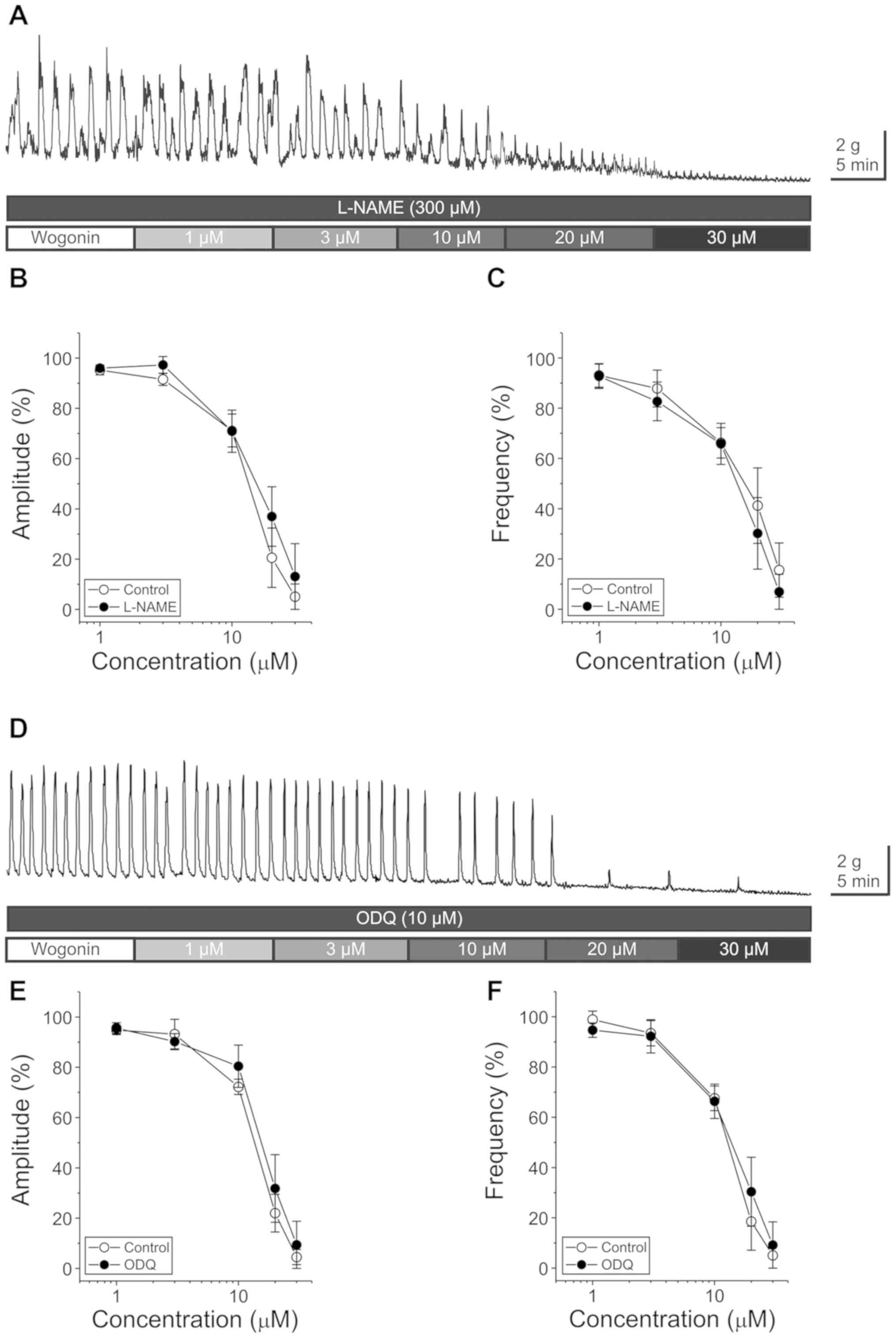
In order to evaluate whether wogonin-induced
reduction of GCs was mediated by nitric oxide (NO), the NO
synthesis inhibitor, L-NAME (300 µM), was used for pre-treatment
prior to applying increasing concentrations of wogonin. When
comparing the presence and absence of L-NAME, no significant
difference in the wogonin-induced reduction of GC amplitude
(F(1,10)=0.99, P=0.341) and frequency
(F(1,11)=1.01, P=0.336) (Fig.
2A-C) (n=6–7) could be observed.
An inhibitor of soluble guanylyl cyclase, ODQ, was
used to examine the involvement of cGMP in the wogonin-induced
reduction of GCs. The pretreatment with ODQ (10 µM) did not affect
the inhibitory effect of wogonin on the amplitude
(F(1,9)=0.485, P=0.504) and the frequency
(F(1,9)=0.259, P=0.623) of GCs (Fig. 2D-F) (n=5–6).
No involvement of enteric
neurotransmission in the wogonin-induced reduction of GCs
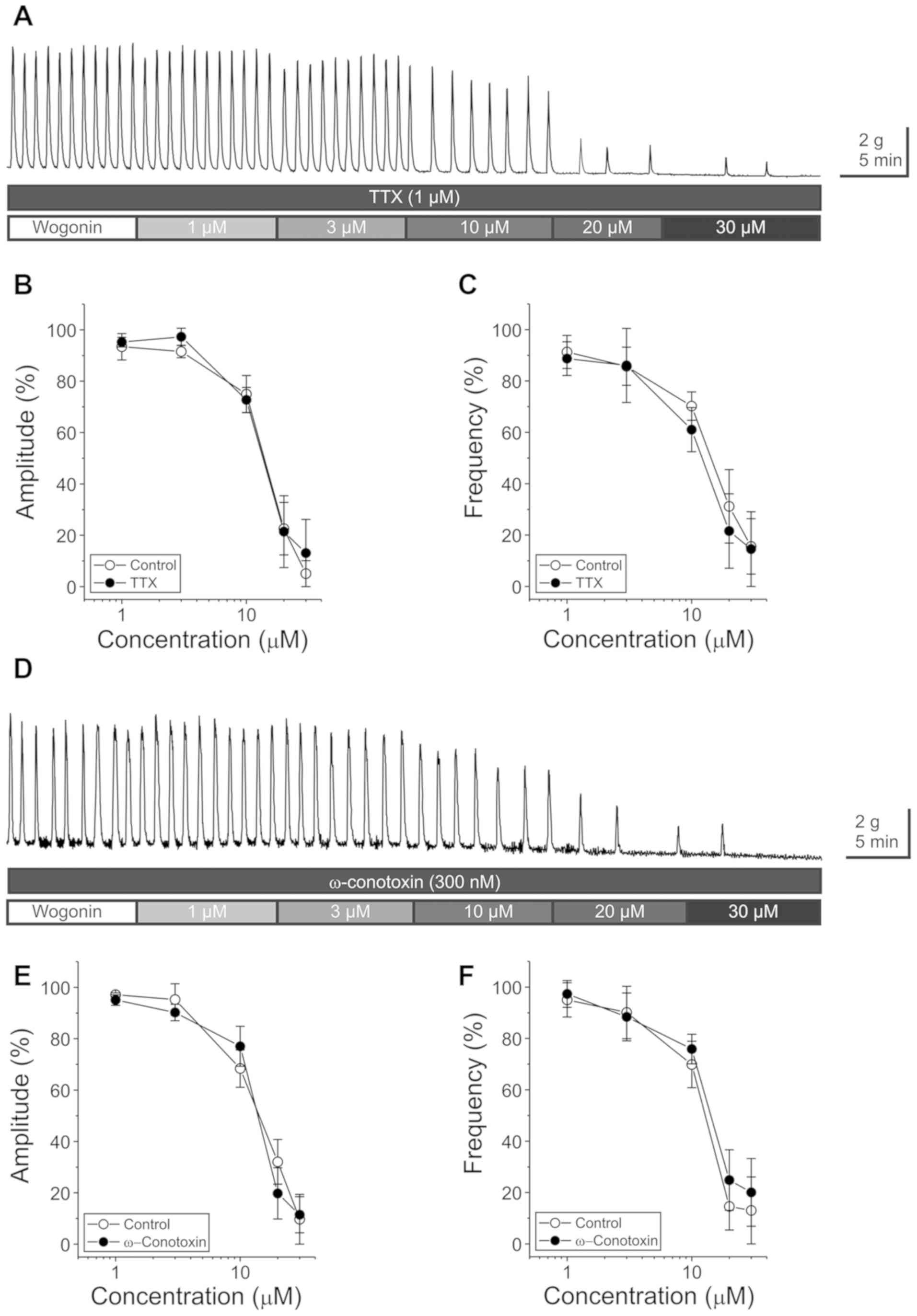
To evaluate whether wogonin-induced reduction of GCs
was dependent on the activation of the enteric nervous system, TTX
or ω-conotoxin were pretreated before applying wogonin. In the six
distal colon pretreated with TTX (1 µM), the application of wogonin
at 1, 3, 10, 20, and 30 µM did not show any significant changes in
the amplitude (F(1,10)=0.181, P=0.679) and frequency
(F(1,9)=0.498, P=0.498), compared to those observed in
the control devoid of pretreatment with TTX (Fig. 3A-C).
In the five distal colon pretreated with ω-conotoxin
(300 nM), the application of wogonin at 1, 3, 10, 20, and 30 µM did
not cause any significant changes in the amplitude
(F(1,8)=0.118, P=0.740) and frequency
(F(1,8)=0.757, P=0.409), compared to those observed in
the control devoid of pretreatment with ω-conotoxin (Fig. 3D-F).
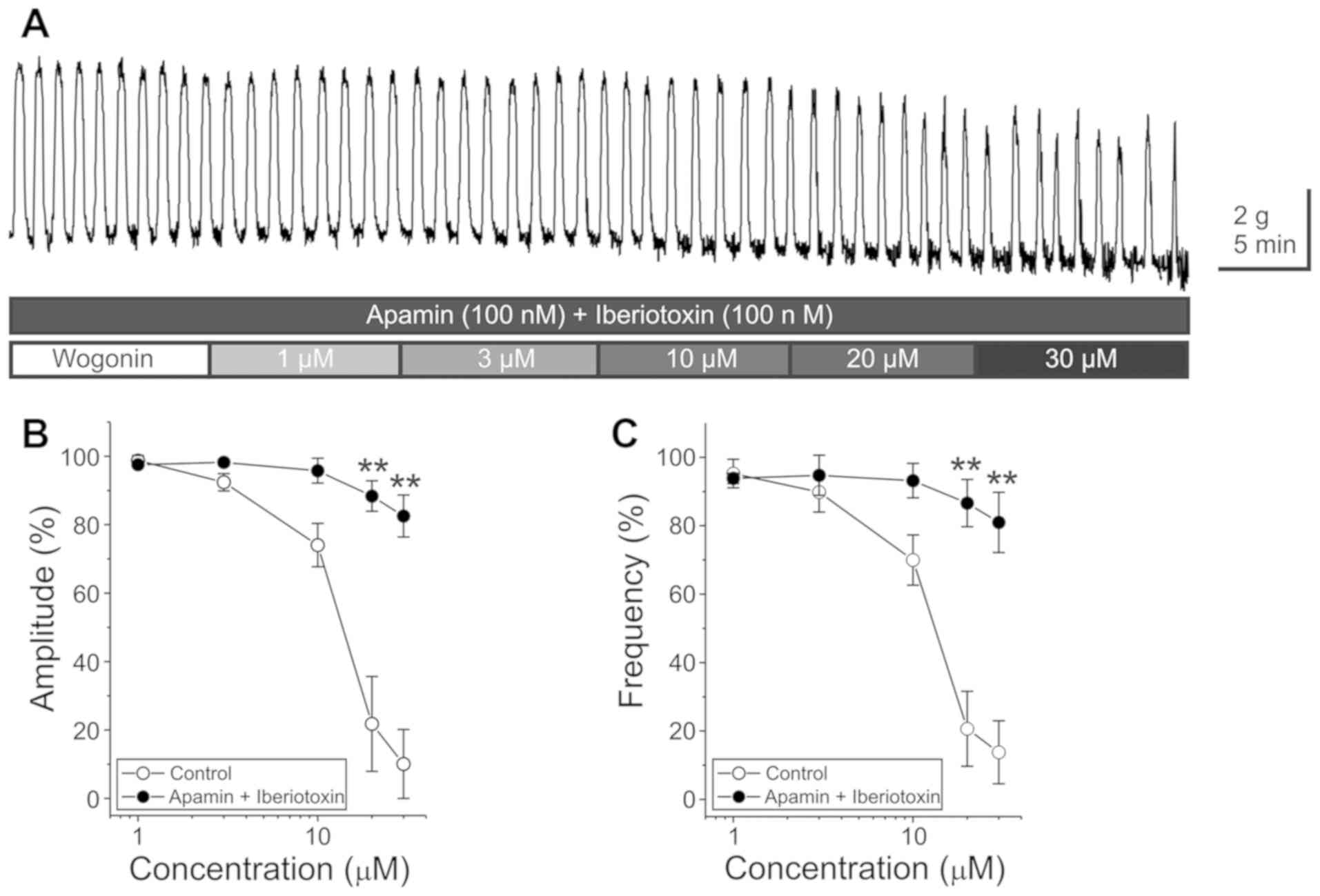
Involvement of
Ca2+-activated K+ channels in the
wogonin-induced reduction of GCs
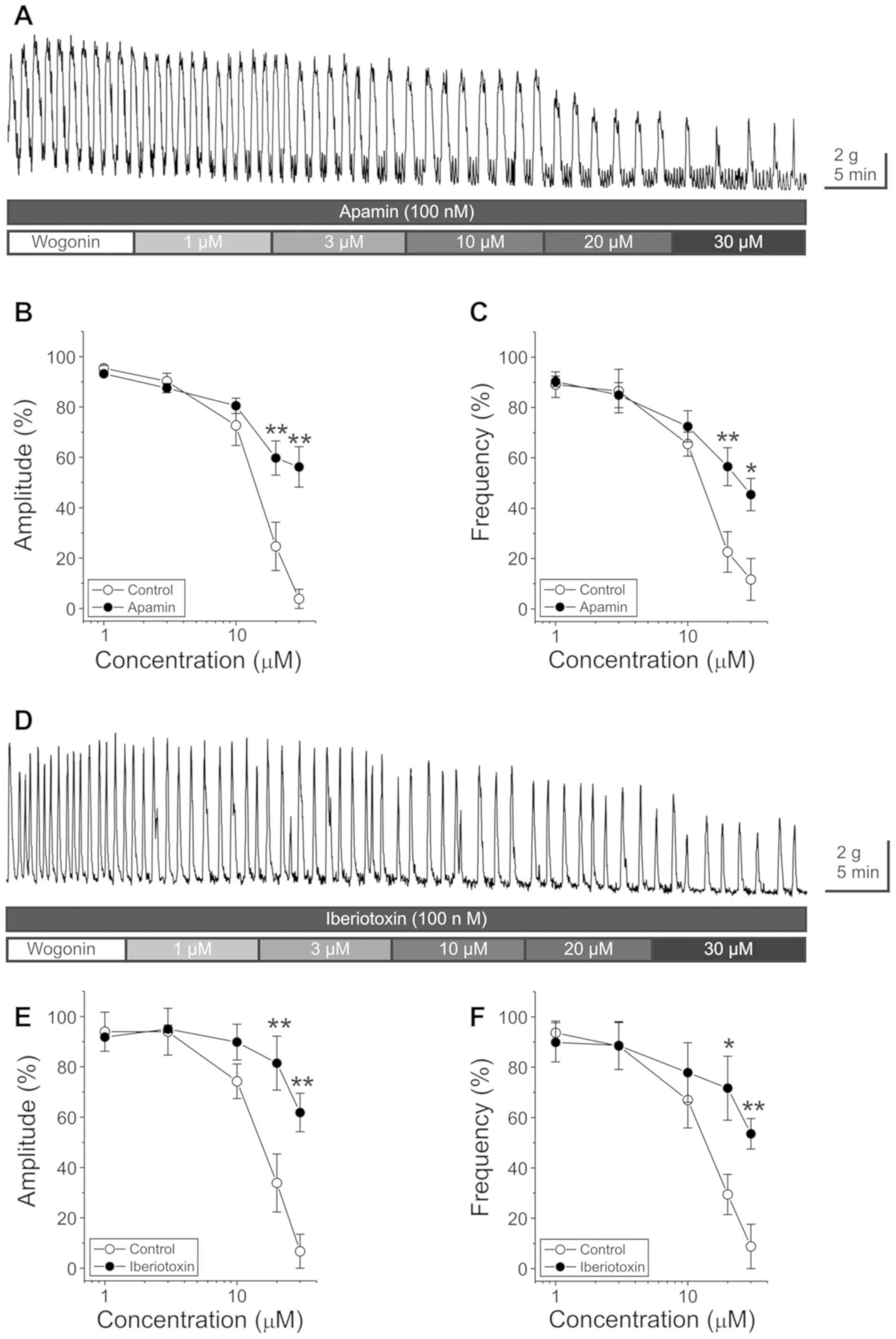
The Ca2+-activated K+ channel
(KCa) channel blockers, apamin and iberiotoxin were used
to investigate the participation of KCa channels in
wogonin-induced reduction of GCs. The pretreatment with apamin (100
nM) partially but significantly inhibited the wogonin (20 and 30
µM)-induced reduction of GCs (Tables
II and III) (Fig. 4A-C) (n=12–15). Similarly, the
pretreatment with iberiotoxin (100 nM) also partially inhibited the
wogonin (20 and 30 µM)-induced reduction of GCs (Tables II and III) (Fig.
4D-F) (n=5–6). Following pretreatment with apamin plus
iberiotoxin (Table IV) (Fig. 5), wogonin had no statistically
significant inhibitory effect on the amplitude and frequency of
GC.
 | Table II.Effects of SK channel blockers on the
wogonin-induced reduction of the GCs (mean ± standard error of the
mean). |
Table II.
Effects of SK channel blockers on the
wogonin-induced reduction of the GCs (mean ± standard error of the
mean).
|
| Amplitude | Frequency |
|---|
|
|
|
|
|---|
| Dose (µM) | Control (%) | Apamin (%) | P-value | Control (%) | Apamin (%) | P-value |
|---|
| 1 | 95.4±1.1 | 93.2±1.0 | 0.769 | 89.1±5.1 | 90.4±2.1 | 0.865 |
| 3 | 90.2±3.2 | 87.5±1.9 | 0.915 | 86.5±8.6 | 84.9±5.0 | 0.602 |
| 10 | 72.7±8.0 | 80.5±3.0 | 0.26 | 65.5±4.8 | 72.5±6.2 | 0.739 |
| 20 | 24.7±9.6 | 59.7±6.8 |
<0.001b | 22.6±8.0 | 56.5±7.5 |
<0.001b |
| 30 | 3.8±3.8 | 56.2±8.0 | 0.003b | 11.7±8.3 | 45.4±6.4 | 0.049a |
 | Table III.Effects of BK channel blockers on the
wogonin-induced reduction of the GCs (mean ± standard error of the
mean). |
Table III.
Effects of BK channel blockers on the
wogonin-induced reduction of the GCs (mean ± standard error of the
mean).
|
| Amplitude | Frequency |
|---|
|
|
|
|
|---|
| Dose | Control (%) | Iberiotoxin
(%) | P-value | Control (%) | Iberiotoxin
(%) | P-value |
|---|
| 1 | 93.9±7.6 | 91.8±0.7 | 0.845 | 93.6±4.7 | 89.9±7.8 | 0.787 |
| 3 | 94.0±9.3 | 95.0±1.5 | 0.925 | 88.4±9.3 | 88.6±9.5 | 0.989 |
| 10 | 74.3±6.8 | 89.8±7.1 | 0.164 | 66.9±11.1 | 77.9±11.9 | 0.44 |
| 20 | 33.9±11.5 | 81.5±10.7 |
<0.001b | 29.5±8.0 | 71.7±12.7 | 0.012a |
| 30 | 6.7±6.7 | 61.8±7.6 |
<0.001b | 8.8±8.8 | 53.5±6.1 | 0.009b |
 | Table IV.Effects of the combined pre-treatment
of SK and BK channel blockers on the wogonin-induced reduction of
the giant contractions (mean ± standard error of the mean). |
Table IV.
Effects of the combined pre-treatment
of SK and BK channel blockers on the wogonin-induced reduction of
the giant contractions (mean ± standard error of the mean).
|
| Amplitude | Frequency |
|---|
|
|
|
|
|---|
| Dose (µM) | Control (%) | Apa+Ibe (%) | P-value | Control (%) | Apa+Ibe (%) | P-value |
|---|
| 1 | 98.7±1.5 | 97.6±0.5 | 0.914 | 95.3±4.2 | 93.9±1.5 | 0.941 |
| 3 | 92.4±2.5 | 98.2±1.0 | 0.592 | 89.8±5.8 | 94.7±5.9 | 0.823 |
| 10 | 74.0±6.3 | 95.8±3.6 | 0.053 | 70.0±7.3 | 93.2±5.0 | 0.072 |
| 20 | 21.8±13.9 | 88.4±4.5 |
<0.010a | 20.7±11.0 | 86.6±7.0 |
<0.001a |
| 30 | 10.1±10.1 | 82.6±6.1 |
<0.010a | 13.8±9.2 | 81.0±8.8 |
<0.001a |
Discussion
This study demonstrates that the three major
flavonoids of S. baicalensis, baicalin, baicalein and
wogonin, inhibit the colonic spontaneous motility in a
dose-dependent manner in the rat colon. Of the three substances,
wogonin was the most effective in inhibiting GCs. This inhibitory
effect of wogonin is associated with KCa channels in the
smooth muscle.
In the aortic and mesenteric arterial smooth
muscles, baicalin and baicalein were found to be related to
NO-mediated responses and cGMP regulation (9). NO/cGMP pathway is the main
physiological mechanism that modulates intestinal motility of both,
the duration and amplitude of GCs. NO activates soluble guanylyl
cyclase and produces cGMP to relax the smooth muscles by lowering
the cytosolic calcium levels and/or by reducing the sensitivity of
the contractile elements to calcium (10–13). The
association with NO in GCs raised the possibility that wogonin
suppresses GCs by increasing the NO release. However, in our study,
L-NAME did not affect the wogonin-induced reduction of GCs, which
is consistent with a previous report in the aorta (14). Furthermore, a soluble guanylyl
cyclase inhibitor, ODQ, also did not affect the inhibitory action
of wogonin on GCs. These results indicate that wogonin neither
stimulates NO production nor enhances the cGMP-dependent mechanisms
in the suppression of GCs.
Other inhibitory enteric neurotransmitters also
appear to not mediate the wogonin-induced reduction of GCs. In our
study, wogonin still effectively reduced the GCs in the presence of
either TTX or ω-conotoxin. These findings suggest that the
inhibitory effect of wogonin is not associated with activating the
inhibitory enteric neural pathways such as NO/cGMP system. Rather,
wogonin seems to directly inhibit the smooth muscle contractility
to suppress GCs.
Smooth muscle can generate spontaneous GC without
neural input, which is termed myogenic contraction. In the
tunica muscularis of the colon, SMCs contact ICC and
PDGFRα+ cells through a gap junction, resulting in the
formation of a SIP syncytium. The SIP syncytium is responsible for
the motility of GI muscles classically referred to in the
literature as ‘myogenic’ (15).
K+ channels play an important role in the regulation of
contractility, and opening of K+ channels contribute to
the relaxing effect of various relaxants (16–19).
Among the K+ channels, KCa channels in the
smooth muscles are the important negative feedback elements in
limiting the extracellular Ca2+ influx-mediated smooth
muscle contraction (20, 21). Two types of KCa are
identified in smooth muscles: SK channels sensitive to apamin and
BK channels sensitive to iberiotoxin (22,23).
Considering the roles of KCa channels in
smooth muscle contractility, it could be hypothesized that
KCa channels might participate in the wogoinin-induced
reduction of GCs. Based on the results of the experiment to test
this hypothesis, we found that apamin and iberiotoxin significantly
attenuated the inhibitory effect of wogonin on GCs. These data
indicate that activation of both SK and BK channels are the pivotal
processes in the wogonin-induced reduction of GCs. Previous studies
have shown that SK channel expression is dominant in
PDGFRα+ cells, although this channel is also present in
SMCs (24–28). The current density of SK channels is
much higher in PDGFRα+ cells than in the SMCs (29,30).
Unlike the SK channel, the BK channel is known to be expressed in
SMCs (31–33). Therefore, it is a possibility that
wogonin hyperpolarizes PDGFRα+ cells via activation of
SK channels to lead to SMC relaxation indirectly, while it
hyperpolarizes SMC via activation of BK channels to relax SMC
directly. These direct and indirect effects on SMC through
different channels result in a reduction of GCs. Wogonin may
transiently regulate the intracellular Ca2+
concentration underneath of the membrane through Ca2+
influx or Ca2+ release from the intracellular
Ca2+ store in PDGFRα+ cells and SMCs
(35). Therefore, reduction of GCs
by wogonin might be induced by the opening of KCa
channels directly and/or increase of intracellular Ca2+
concentration underneath of the membrane, and the subsequent
opening of KCa channels, which is closely associated
with Ca2+ influx channels and/or Ca2+ release
site, indirectly.
The components of S. baicalensis exert
various biological actions, especially anti-inflammation in soft
tissues (mucosa, submucosa, and skin), but not in smooth muscle
(34,35). It has anti-diarrheal effects on
irrinotecan-induced diarrhea (3,4).
Baicalin has anti-diarrheal action through inhibiting COX-2
activity and PGE2 (5,6). In our
study, wogonin, one of components of S. baicalensis,
directly inhibited intestinal smooth muscle motility, but not
through a mechanism associated with inflammatory pathway. Taken
together, this study may provide a completely novel concept of
identifying the mechanism of the anti-diarrheal effect of Chinese
Skullcap, S. baicalensis. Therefore, the results of this
study will serve as a guide to a new therapeutic approach for
treatment of acute or chronic diarrhea associated with dysmotility,
such as diarrhea-predominant IBS.
In conclusion, our in vitro experiments
demonstrated that baicalin, baicalein and wogonin, the components
of S. baicalensis, inhibited colonic motility. Wogonin, in
particular, directly inhibited the colonic smooth muscle through
both SK and BK channels, but not via the activation of inhibitory
enteric nerves such as the nitrergic nerves. These findings suggest
that wogonin may be a candidate drug for the treatment of increased
colon motility diseases, such as diarrhea-predominant IBS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HJK, JHL, ISY and TSS conceived the experiments and
experimental plan. HJK and TSS performed the experiments. JHL, HMK
and TSS collected and analyzed the data. JHL, HMK and TSS wrote the
paper. All authors approve the final version of these manuscript
and figures.
Ethics approval and consent to
participate
Animal care and handling were in accordance with the
NIH Guide for the Care and Use of Laboratory Animals. All
procedures were approved by the Institutional Animal Use and Care
Committee at the Seoul National University (Seoul, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest to disclose with regards to this study.
References
|
1
|
Tack J: Functional diarrhea. Gastroenterol
Clin North Am. 41:629–637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Q, Chen XY and Martin C: Scutellaria
baicalensis, the golden herb from the garden of Chinese medicinal
plants. Sci Bull (Beijing). 61:1391–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui DN, Wang X, Chen JQ, Lv B, Zhang P,
Zhang W, Zhang ZJ and Xu FG: Quantitative evaluation of the
compatibility effects of huangqin decoction on the treatment of
irinotecan-induced gastrointestinal toxicity using untargeted
metabolomics. Front Pharmacol. 8:2112017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mori K, Kondo T, Kamiyama Y, Kano Y and
Tominaga K: Preventive effect of Kampo medicine (Hangeshashin-to)
against irinotecan-induced diarrhea in advanced non-small-cell lung
cancer. Cancer Chemother Pharmacol. 51:403–406. 2003.PubMed/NCBI
|
|
5
|
Kase Y, Saitoh K, Makino B, Hashimoto K,
Ishige A and Komatsu Y: Relationship between the antidiarrhoeal
effects of Hange-Shashin-To and its active components. Phytother
Res. 13:468–473. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kase Y, Hayakawa T, Aburada M, Komatsu Y
and Kamataki T: Preventive effects of Hange-shashin-to on
irinotecan hydrochloride-caused diarrhea and its relevance to the
colonic prostaglandin E2 and water absorption in the rat. Jpn J
Pharmacol. 75:407–413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanders KM, Ward SM and Koh SD:
Interstitial cells: Regulators of smooth muscle function. Physiol
Rev. 94:859–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams DJ, Smith AB, Schroeder CI, Yasuda T
and Lewis RJ: Omega-conotoxin CVID inhibits a pharmacologically
distinct voltage-sensitive calcium channel associated with
transmitter release from preganglionic nerve terminals. J Biol
Chem. 278:4057–4062. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Y, Wong CM, Lau CW, Yao X, Tsang SY,
Su YL and Chen ZY: Inhibition of nitric oxide/cyclic GMP-mediated
relaxation by purified flavonoids, baicalin and baicalein, in rat
aortic rings. Biochem Pharmacol. 67:787–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schlossmann J, Feil R and Hofmann F:
Signaling through NO and cGMP-dependent protein kinases. Ann Med.
35:21–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ignarro LJ, Lippton H, Edwards JC, Baricos
WH, Hyman AL, Kadowitz PJ and Gruetter CA: Mechanism of vascular
smooth muscle relaxation by organic nitrates, nitrites,
nitroprusside and nitric oxide: Evidence for the involvement of
S-nitrosothiols as active intermediates. J Pharmacol Exp Ther.
218:739–749. 1981.PubMed/NCBI
|
|
12
|
Rybalkin SD, Yan C, Bornfeldt KE and Beavo
JA: Cyclic GMP phosphodiesterases and regulation of smooth muscle
function. Circ Res. 93:280–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Crombruggen K and Lefebvre RA:
Nitrergic-purinergic interactions in rat distal colon motility.
Neurogastroenterol Motil. 16:81–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu JT, Zhang DX, Liu F, Mao HP, Ma YK,
Yang Y, Li CX, Qiu LZ, Geng X, Zhang JM, et al: Vasodilatory effect
of wogonin on the rat aorta and its mechanism study. Biol Pharm
Bull. 38:1873–1878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blair PJ, Rhee PL, Sanders KM and Ward SM:
The significance of interstitial cells in neurogastroenterology. J
Neurogastroenterol Motil. 20:294–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gokce G, Bagcivan I, Kilicarslan H,
Yildirim S, Gultekin YE and Sarioglu Y: Relaxation effects of
adrenomedullin in isolated rabbit corpus cavernosum smooth muscle.
BJU Int. 93:859–862. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anwer K, Oberti C, Perez GJ, Perez-Reyes
N, McDougall JK, Monga M, Sanborn BM, Stefani E and Toro L:
Calcium-activated K+ channels as modulators of human myometrial
contractile activity. Am J Physiol. 265:C976–C985. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brayden JE: Potassium channels in vascular
smooth muscle. Clin Exp Pharmacol Physiol. 23:1069–1076. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirber MT, Ordway RW, Clapp LH, Walsh JV
Jr and Singer JJ: Both membrane stretch and fatty acids directly
activate large conductance Ca(2+)-activated K+ channels in vascular
smooth muscle cells. FEBS Lett. 297:24–28. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imai T, Okamoto T, Yamamoto Y, Tanaka H,
Koike K, Shigenobu K and Tanaka Y: Effects of different types of K+
channel modulators on the spontaneous myogenic contraction of
guinea-pig urinary bladder smooth muscle. Acta Physiol Scand.
173:323–333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herrera GM and Nelson MT: Differential
regulation of SK and BK channels by Ca(2+) signals from Ca(2+)
channels and ryanodine receptors in guinea-pig urinary bladder
myocytes. J Physiol. 541:483–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gebremedhin D, Kaldunski M, Jacobs ER,
Harder DR and Roman RJ: Coexistence of two types of
Ca(2+)-activated K+ channels in rat renal arterioles. Am J Physiol.
270:F69–F81. 1996.PubMed/NCBI
|
|
23
|
Pérez GJ, Toro L, Erulkar SD and Stefani
E: Characterization of large-conductance, calcium-activated
potassium channels from human myometrium. Am J Obstet Gynecol.
168:652–660. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurahashi M, Mutafova-Yambolieva V, Koh SD
and Sanders KM: Platelet-derived growth factor receptor-α-positive
cells and not smooth muscle cells mediate purinergic
hyperpolarization in murine colonic muscles. Am J Physiol Cell
Physiol. 307:C561–C570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vogalis F and Goyal RK: Activation of
small conductance Ca(2+)-dependent K+ channels by purinergic
agonists in smooth muscle cells of the mouse ileum. J Physiol.
502:497–508. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koh SD, Dick GM and Sanders KM:
Small-conductance Ca(2+)-dependent K+ channels activated by ATP in
murine colonic smooth muscle. Am J Physiol. 273:C2010–C2021. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bayguinov O, Hagen B, Bonev AD, Nelson MT
and Sanders KM: Intracellular calcium events activated by ATP in
murine colonic myocytes. Am J Physiol Cell Physiol. 279:C126–C135.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peri LE, Sanders KM and
Mutafova-Yambolieva VN: Differential expression of genes related to
purinergic signaling in smooth muscle cells, PDGFRα-positive cells,
and interstitial cells of Cajal in the murine colon.
Neurogastroenterol Motil. 25:e609–e620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hwang SJ, Blair PJ, Durnin L,
Mutafova-Yambolieva V, Sanders KM and Ward SM: P2Y1 purinoreceptors
are fundamental to inhibitory motor control of murine colonic
excitability and transit. J Physiol. 590:1957–1972. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee H, Koh BH, Peri LE, Sanders KM and Koh
SD: Functional expression of SK channels in murine detrusor PDGFR+
cells. J Physiol. 591:503–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dick GM, Rossow CF, Smirnov S, Horowitz B
and Sanders KM: Tamoxifen activates smooth muscle BK channels
through the regulatory beta 1 subunit. J Biol Chem.
276:34594–34599. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hagen BM and Sanders KM: Deglycosylation
of the beta1-subunit of the BK channel changes its biophysical
properties. Am J Physiol Cell Physiol. 291:C750–C756. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Huang H, Hou D, Liu P, Wei H, Fu X
and Niu W: Mechanosensitivity of STREX-lacking BKCa channels in the
colonic smooth muscle of the mouse. Am J Physiol Gastrointest Liver
Physiol. 299:G1231–G1240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon SB, Lee YJ, Park SK, Kim HC, Bae H,
Kim HM, Ko SG, Choi HY, Oh MS and Park W: Anti-inflammatory effects
of Scutellaria baicalensis water extract on LPS-activated RAW 264.7
macrophages. J Ethnopharmacol. 125:286–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cuéllar MJ, Giner RM, Recio MC, Máñez S
and Rios JL: Topical anti-inflammatory activity of some Asian
medicinal plants used in dermatological disorders. Fitoterapia.
72:221–229. 2001. View Article : Google Scholar : PubMed/NCBI
|