Introduction
Lower extremity arteriosclerosis obliterans (ASO) is
an important manifestation of atherosclerosis. The lower
extremities are common for a series of physical disorders caused by
the leg arterial blood supply disorders, and atherosclerosis occur
in the peripheral arteries (1). The
main symptoms in the early stage are intermittent claudication and
the distal arterial pulsation gradually weakens or even disappears.
As the symptoms worsen in the later stages, patients may experience
rest pain, and skin temperature decreases significantly with the
development of diseases, cyanosis, toe ulcers and gangrene. Severe
cases can even lead to amputation and affect patients' quality of
life. With the continuous improvement of living standards the
social population is developing an aging trend. ASO is more common
in the lower limbs due to diseases, lower extremity arterial blood
pressure and vulnerable intima, which has become the main cause of
limb loss in adulthood worldwide (2).
Common methods for medical treatment of ASO include
antihypertensive, lipid-lowering and anti-platelet aggregation.
However, these treatments can only delay the progression of the
disease (3). Trimetazidine is a
clinically used drug for the treatment of coronary insufficiency,
and is a 3-KAT inhibitor and an anti-ischemic drug (4), which can inhibit tissue damage caused
by oxidation and tissue fibrosis (5–7) and
reduce fat metabolism caused by intracellular acidosis and
anaerobic metabolism of ischemic cells (8). Cilostazol is a drug that can
effectively inhibit the aggregation of platelets, inhibit the
activity of phosphodiesterase III and blocking the degradation and
transformation of adenylate cyclase, and can increase the content
of adenylate cyclase in platelets and vascular smooth muscle cells,
endothelial cells, cardiomyocytes and adipocytes and exerts
anti-platelet aggregation and vasodilatation of cilostazol
(9,10). Cilostazol can inhibit platelet
aggregation, the proliferation of vascular smooth muscle cells and
protects endothelial cells during inflammation (11), and has anti-thrombotic effect, which
is mainly used for the treatment of local diseases such as chronic
arterial occlusive ulcer, pain and cold sensation.
Currently, there are only a few studies on the
difference between the efficacy and long-term survival rate of
trimetazidine and cilostazol in the treatment of ASO patients. This
study compared the therapeutic effects of trimetazidine and
cilostazol in the treatment of patients with ASO to identify a
better-acting drug for the treatment of this disease, and intended
to provide a basis for the treatment of ASO.
Materials and methods
General information
A retrospective analysis of the medical records of
206 ASO patients from January 2011 to May 2013 in The Central
Hospital of Wuhan (Wuhan, China) was performed. The Fontaine stage
cases were: 40 cases (19.42%) in stage I, 58 cases (28.16%) in
stage II, 72 cases in stage III (34.95%) and 36 cases (17.48%) in
stage IV. According to the clinical treatment, 94 patients were
treated with trimetazidine (group A) and 112 patients with
cilostazol (group B). There were 62 males and 32 females in group
A, with an age range of 40–75 years, with an average of (63.5±18.8)
years, 22 with diabetes, 28 with hypertension and 21 with
hyperlipidemia. There were 77 males and 35 females in group B, with
an age range of 40–83 years, with an average of (69.5±17.3) years,
32 with diabetes, 22 with hypertension, and 33 with
hyperlipidemia.
Inclusion and exclusion criteria
Inclusion criteria
The primary inclusion criterion was complying with
the diagnostic criteria for ASO patients in the guidelines for the
Treatment of Lower Extremity Arteriosclerosis Obliterans. Other
criteria were intermittent ischemic symptoms such as lameness,
numbness, pain and fatigue, and no drugs taken recently to treat
ASO.
Exclusion criteria
Patients with clinical manifestations but not
diagnosed with ASO after relevant examinations; patients with
severe liver and kidney related disease, malignant tumors, active
infections, chronic respiratory diseases and hematopoietic
dysfunction; patients who had a history of myocardial infarction;
pregnant; patients with contraindications to anticoagulant
thrombolysis and with a history of mental illness or family mental
illness and bleeding (Hemophilia, capillary fragility, intracranial
hemorrhage, gastrointestinal bleeding, urinary tract bleeding,
hemoptysis and vitreous hemorrhage) were excluded from the present
study.
The study was approved by Ethics Committee of The
Central Hospital of Wuhan, and patients or their families signed a
full informed consent form.
Drugs
The drug used in the experiment was: trimetazidine,
produced by Servier Tianjin Pharmaceutical Co., Ltd. (Tianjin,
China), specification 20 mg × 30 tablets. Product batch number was:
national medicine quasi-word H20055465; cilostazol, Zhejiang Daxie
Pharmaceutical Co., Ltd. (Zhejian, China). Specification was: 50 mg
× 12 tablets, national medicine quasi-word H10960014; Alprostadil
injection, produced by Xi'an Libang Pharmaceutical Co., Ltd.
(Shaanxi, China); Specifications 2 ml: 10 µg × 5 pcs/box, national
medicine quasi-word H20103100.
Method
To control the blood pressure, blood sugar, and diet
(low-salt, low-fat diet) in both groups of patients alprostadil was
used to inhibit platelet aggregation. In addition, plaque was
stabilized against infection, informing patients to keep warm,
protecting the lower limbs from trauma, avoiding causing skin
surface rupture, pus discharge and guided lower limb exercises
conducted by doctors in the rehabilitation department, and
promoting basic treatment such as lower limb circulation. Group A
was given trimetazidine, orally, 30 mg/time, 3 times/day and group
B was given cilostazol, orally, 100 mg twice daily. Each course of
treatment was two weeks and both groups received two courses of
treatment.
Observation indexes
We observed and compared the clinical therapeutic
effect, painless walking distance (PFWD), pedis artery blood flow,
anterior femoral artery, posterior tibial artery blood flow,
brachial artery index (pedis artery systolic pressure/brachial
artery systolic pressure, ABI), toe-brachial index (the ratio of
pressure between toe artery pressure and limb artery, TBI), maximum
walking distance, 5-year survival rate and other adverse reactions
between the groups. Efficacy evaluation criteria were: Fully
recovered: disease symptoms disappeared and regaining health;
Significant effect: no symptoms of numbness and coldness in the
lower extremities, intermittent claudication and disappearance of
resting pain; Effective: The clinical symptoms are changed from
phase I to phase II as per Fontaine staging, and the intermittent
claudication distance was extended to >500 m; Invalid: There
were no improvements in the items that were required to be observed
in the experiment. Total efficiency was calculated as: (healing +
markedly effective) / number of patients.
Statistical analysis
Statistical analysis was performed on the research
data using SPSS 17.0 (Tianjin Soft Network Technology Co., Ltd.,
Tianjin, China) statistical software. The measurement data were
expressed as the mean standard (mean ± SD) using the t-test and
Chi-squrare test. The three time-points before and after the
treatment in the group were compared using repeated measures of
variance analysis and the LSD post hoc test. The data counting was
expressed as a rate (%) by using Chi-square test. Survival rates
were calculated by using the Kaplan-Meier method and were compared
by using the log-rank test. P<0.05 indicated that the difference
was statistically significant.
Results
Comparison of basic information
between the groups
There was no significant difference in sex, age,
ethnicity, height, weight, smoking, blood pressure, pathogenesis
time, Fontaine stages or body mass index (BMI) between the groups
(P>0.05) (Table I).
 | Table I.Basic information between the groups
of ASO patients [n (%)]/mean ± SD. |
Table I.
Basic information between the groups
of ASO patients [n (%)]/mean ± SD.
| Basic
information | Group A (n=94) | Group B (n=112) | t/Chi-square
test | P-value |
|---|
| Age (years) |
|
| 0.099 | 0.753 |
| ≥55 | 73 (77.66) | 89 (79.46) |
|
|
|
<55 | 21 (22.34) | 23 (20.54) |
|
|
| Sex |
|
| 0.182 | 0.670 |
| Male | 62 (65.96) | 77 (68.75) |
|
|
|
Female | 32 (34.04) | 35 (31.25) |
|
|
| Ethnicity |
|
| 0.004 | 0.953 |
| Han | 85 (90.43) | 101 (90.18) |
|
|
|
Others | 9
(9.57) | 11 (9.82) |
|
|
| Height (cm) |
|
| 0.062 | 0.803 |
|
<165 | 58 (61.70) | 71 (63.39) |
|
|
| ≥165 | 36 (38.30) | 41 (36.61) |
|
|
| Weight (kg) |
|
| 0.129 | 0.719 |
|
<50 | 59 (62.77) | 73 (65.18) |
|
|
| ≥50 | 35 (37.23) | 39 (34.82) |
|
|
| Smoking history
(years) |
|
| 0.088 | 0.957 |
|
<5 | 29 (30.85) | 36 (32.14) |
|
|
| ≥5 | 33 (35.11) | 40 (35.71) |
|
|
| None | 32 (34.04) | 36 (32.14) |
|
|
| Blood pressure
(mmHg) |
|
|
|
|
| Systolic
pressure |
145.48±20.21 |
141.35±19.92 | 1.472 | 0.143 |
| Diastolic
pressure |
98.82±15.55 |
96.76±16.35 | 0.921 | 0.358 |
| Pathogenesis time
(months) |
|
| 0.077 | 0.781 |
|
<12 | 31 (32.98) | 39 (34.82) |
|
|
|
>12 | 63 (67.02) | 73 (65.18) |
|
|
| Fontaine stages |
|
| 1.113 | 0.774 |
| Stage
I | 18 (19.15) | 22 (19.64) |
|
|
| Stage
II | 29 (30.85) | 29 (25.89) |
|
|
| Stage
III | 33 (35.11) | 39 (34.82) |
|
|
| Stage
IV | 14 (14.89) | 22 (19.64) |
|
|
| BMI | 25.82±7.43 | 24.48±10.11 | 1.066 | 0.288 |
Comparison of efficacy between the
groups of patients
In group A, there were 8 cases of fully recovered
patients (8.51%), 25 cases of significant effective patients
(26.60%), 41 cases of effective patients (42.62%), 20 non-effective
cases (21.28%) and a total of 74 effective cases (78.72%). In group
B, there were 15 cases of fully recovered patients (13.39%), 45
cases of significantly effective patients (40.18%), 41 cases of
effective patients (36.61%), 11 non-effective cases (9.82%) and a
total of 101 cases were effective (90.18%). There was no
significant difference in terms of fully recovered, effective and
non-effective between the groups (P>0.05). The significant
effective and total effective rates were lower in group A than
group B, and the difference was statistically significant
(P<0.05) (Table II).
 | Table II.Comparison of the efficacy of ASO
patients between both groups [n (%)]. |
Table II.
Comparison of the efficacy of ASO
patients between both groups [n (%)].
| Item | n | Fully recovered | Significant
effective | Effective | Non-effective | Total
effective |
|---|
| Group A | 94 | 8
(8.51) | 25 (26.60) | 41 (43.62) | 20 (21.28) | 74
(78.72) |
| Group B | 112 | 15 (13.39) | 45 (40.18) | 41 (36.61) | 11 (9.82) | 101 (90.18) |
| χ2 | – | 1.228 | 4.203 | 1.048 | 5.246 | 5.246 |
| P-value | – | 0.268 | 0.040 | 0.306 | 0.022 | 0.022 |
Comparison of changes in
arteriosclerosis indexes of lower extremities before and after the
treatment between the groups
Before the treatment, there was no difference in
terms of the flow of dorsalis pedis artery, superficial femoral
artery or posterior tibial artery in the groups. There was no
difference between ABI and TBI (P>0.05). After two courses of
treatment, compared with the treatment before, the flow of dorsalis
pedis artery, superficial femoral artery, posterior tibial artery,
ABI and TBI were significantly higher. The increase of group B and
group A was statistically significant (P<0.05) (Table III).
 | Table III.Comparison of lower extremity
arterial blood flow, ABI and TBI before and after the treatment
between the groups (mean ± SD). |
Table III.
Comparison of lower extremity
arterial blood flow, ABI and TBI before and after the treatment
between the groups (mean ± SD).
| Variables | Group A (n=94) | Group B
(n=112) | t | P-value |
|---|
| Dorsalis pedis
artery [m/(s.cm2)] |
|
|
|
|
| Before
treatment | 0.51±0.11 | 0.52±0.10 |
0.683 |
0.495 |
| After
the first course of treatment |
0.57±0.15a |
0.73±0.21a |
6.180 | <0.001 |
| After
the second course of treatment |
0.62±0.20a,b |
0.80±0.25a,b |
5.630 | <0.001 |
| F | 11.470 | 61.190 |
|
|
|
P-value | <0.001 | <0.001 |
|
|
| Superficial femoral
artery [m/(s.cm2)] |
|
|
|
|
| Before
treatment | 6.12±1.13 | 6.46±1.57 |
1.753 |
0.081 |
| After
the first course of treatment |
6.69±1.92a |
7.31±2.01a |
2.251 |
0.026 |
| After
the second course of treatment |
7.18±2.23a,b |
7.84±2.27a,b |
2.095 |
0.037 |
| F | 7.987 | 13.970 |
|
|
|
P-value | <0.001 | <0.001 |
|
|
| Posterior tibial
artery [m/(s.cm2)] |
|
|
|
|
| Before
treatment | 6.41±1.98 | 6.35±1.77 |
0.230 |
0.819 |
| After
the first course of treatment |
6.59±1.79a |
7.12±1.99a |
1.993 |
0.048 |
| After
the second course of treatment |
7.03±2.01a,b |
7.73±2.88a,b |
1.985 |
0.049 |
| F | 2.570 | 10.440 |
|
|
|
P-value | 0.078 | <0.001 |
|
|
| ABI |
|
|
|
|
| Before
treatment | 0.58±0.23 | 0.59±0.19 |
0.342 |
0.733 |
| After
the first course of treatment |
0.84±0.26a |
1.03±0.32a |
4.617 | <0.001 |
| After
the second course of treatment |
1.06±0.25a,b |
1.25±0.31a,b |
4.779 | <0.001 |
| F | 88.97 | 161.7 |
|
|
|
P-value | <0.001 | <0.001 |
|
|
| TBI |
|
|
|
|
| Before
treatment | 0.32±0.09 | 0.33±0.10 |
0.748 |
0.455 |
| After
the first course of treatment |
0.43±0.16a |
0.61±0.24a | 19.540 | <0.001 |
| After
the second course of treatment |
0.53±0.13a,b |
0.77±0.25a,b |
8.401 | <0.001 |
| F | 61.49 | 128.1 |
|
|
|
P-value | <0.001 | <0.001 |
|
|
Comparison of PFWD and MVD before and
after treatment
There was no significant difference in PFWD and MVD
between the groups before the treatment (P>0.05). The walking
distance between the groups was significantly increased after the
first course of treatment compared to before the treatment. After
the second course of treatment, the walking distance between the
groups increased significantly compared with that before the
treatment and after the first course of treatment. The walking
distance of group B after both the first and second course of
treatment was significantly greater than that of group A, and the
difference was statistically significant (P<0.05) (Table IV).
 | Table IV.Comparison of PFWD and MVD before and
after treatment (mean ± SD). |
Table IV.
Comparison of PFWD and MVD before and
after treatment (mean ± SD).
|
| PFWD (m) |
|
|
|---|
|
|
|
|
|
|---|
|
| Before
treatment | After the first
course of treatment | After the second
course of treatment | F | P-value |
|---|
| Group A (n=94) | 147.47±37.46 |
201.58±30.46a |
320.48±44.68a,b | 510.4 | P<0.001 |
| Group B
(n=112) | 145.58±36.37 |
290.19±50.48a |
490.34±100.37a,b | 722.2 | P<0.001 |
| t | 0.367 | 14.890 | 15.190 |
|
|
| P-value | 0.714 | P<0.001 | P<0.001 |
|
|
|
|
| MVD (m) |
|
|
|
|
|
|
|
|
| Before
treatment | After the first
course of treatment | After the second
course of treatment | F | P-value |
|
| Group A (n=94) |
694.53±190.23a |
822.35±220.12a |
1,067.45±300.56a,b | 57.88 | P<0.001 |
| Group B
(n=112) |
689.25±201.57a |
1,098.27±268.53a |
1,410.45±320.45a,b | 204 | P<0.001 |
| t | 0.192 | 7.965 | 7.871 |
|
|
| P-value | 0.848 | P<0.001 | P<0.001 |
|
|
Comparison of adverse reactions of
patients between the groups
Group A had 5 cases of digestive tract symptoms
(5.32%), 15 cases of lethargy hypodynamia (15.96%), 3 cases of
allergic symptoms (3.19%), 6 cases of leukopenia (6.38%), 11 cases
of liver dysfunction (11.70%) and 9 cases of dizziness and headache
(9.57%). Group B had 5 cases of digestive tract symptoms (4.46%), 7
cases of lethargy hypodynamia (6.25%), 5 cases of allergic symptoms
(4.46%), 9 cases of leukopenia (8.04%), 5 cases of liver
dysfunction (4.46%) and 3 cases of dizziness headache (2.68%).
There was no significant difference between the groups in digestive
tract symptoms, allergic symptoms, leucopenia or liver dysfunction
(P>0.05). There were differences in lethargy hypodynamia,
dizziness and headache (P<0.05), which was statistically
significant (Table V).
 | Table V.Comparison of adverse reactions of
patients between the groups [n, (%)]. |
Table V.
Comparison of adverse reactions of
patients between the groups [n, (%)].
|
| Group A (n=94) | Group B
(n=112) | Chi-square
test | P-value |
|---|
| Digestive tract
symptoms | 5
(5.32) | 5 (4.46) | 0.809 | 0.776 |
| Lethargy
hypodynamia | 15 (15.96) | 7 (6.25) | 5.049 | 0.025 |
| Allergic
symptoms | 3
(3.19) | 5 (4.46) | 0.222 | 0.638 |
| Leukopenia | 6
(6.38) | 9 (8.04) | 0.207 | 0.649 |
| Liver
dysfunction | 11 (11.70) | 5 (4.46) | 3.738 | 0.053 |
| Dizziness and
headache | 9
(9.57) | 3 (2.68) | 4.430 | 0.035 |
Comparison of 5-year survival rates
between the groups
The 1- to 5-year survival rates in group A was 90
cases (95.74%), 84 cases (89.36%), 75 cases (79.79%), 63 cases
(67.02%), and 49 cases (52.13%), respectively. The 1–5-year
survival rates in group B was 109 cases (97.32%), 105 cases
(93.75%), 98 cases (87.50%), 90 cases (80.36%) and 78 cases
(69.64%), respectively. There was no significant difference in 1-
to 3-year survival rates between the groups (P>0.05), the
difference in 4–5-year survival rates was statistically significant
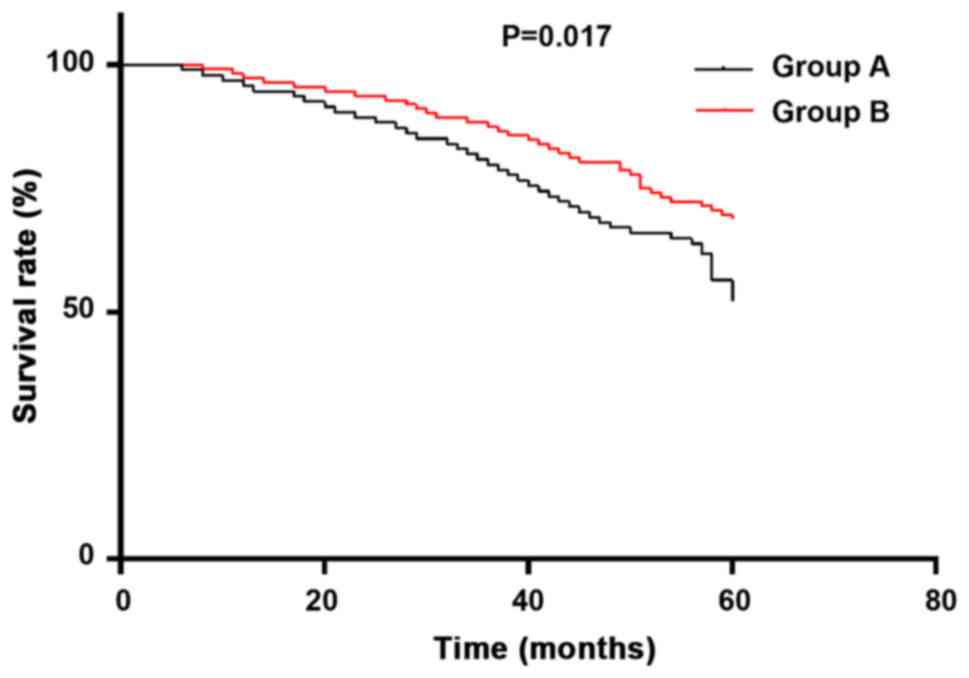
(P<0.05) (Table VI). Survival
curve diagram shows the 5-year survival of group B treated with
cilostazol was more significant than the same group treated with
trimetazidine, and the overall survival rate was P=0.017 (Fig. 1).
 | Table VI.Comparison of 5-year survival rates
between the groups [n (%)]. |
Table VI.
Comparison of 5-year survival rates
between the groups [n (%)].
|
| Survival time |
|---|
|
|
|
|---|
|
| 1 year | 2 years | 3 years | 4 years | 5 years |
|---|
| Group A (n=94) | 90 (95.74) | 84 (89.36) | 75 (79.79) | 63 (67.02) | 49 (52.13) |
| Group B
(n=112) | 109 (97.32) | 105 (93.75) | 98 (87.50) | 90 (80.36) | 78 (69.64) |
| Method of
detection | 0.387 | 1.300 | 2.260 | 4.757 | 6.632 |
| P-value | 0.534 | 0.254 | 0.133 | 0.029 | 0.010 |
Discussion
ASO is a degenerative disease that often occurs in
the elderly, and the most common symptom is intermittent
claudication, and the incidence rate has increased in recent years
(12). Its incidence rates,
mortality and quality of life are associated with vascular injury
(13). It is estimated that there
are 202 million individuals worldwide suffering from ASO (14). Impaired perfusion in the femoral
artery and arteries are due to stenosis or complete obstruction
(occlusion) of the arterial lumen in the distal part of the aorta
and/or pelvis. The most important cause of ASO is atherosclerosis
(15). If patients do not receive
treatment in time, and only start the treatment after the condition
becomes severe, then it will cause an unsatisfactory therapeutic
effect and may seriously affect the quality of life (16). This reduces the cardiovascular risk
of patients, improves the functional performance and quality of
life for patients (17).
The current findings showed that the total effective
rate of clinical efficacy in patients treated with cilostazol was
90.18%. which was improved compared to that in the patients treated
with trimetazidine (78.72%) (P<0.05). It can be concluded that
cilostazol is more effective in the treatment of ASO. To further
prove this conclusion, we re-checked and analyzed many aspects of
ASO indicators, adverse reactions and long-term survival rates.
There was no significant difference in lower extremity arterial
blood flow, ankle brachial index, toe-brachial index, painless
walking distance and maximum walking distance between the groups
(P>0.05). After the first course of treatment, the above
indicators have increased in both groups. The difference was
statistically significant compared with before the treatment
(P<0.05). After the end of the second course of treatment, the
above-mentioned index values in both groups were significantly
increased (P<0.05). However, the improvement of the above
indicators in patients treated with cilostazol was better than the
trimetazidine group in the first and second course of treatment
(P<0.05). Cilostazol can effectively dilate vascular smooth
muscle, promoting blood circulation in patients. The resistance of
peripheral arteries is greatly reduced and the fluctuation index is
greatly increased. When the blood flow of the surrounding blood
vessels is effectively improved, the pain of the lower limbs is
reduced, and the maximum walking distance and the painless walking
distance of patients are increased. The fluctuation index is
greatly increased. When the blood flow of the surrounding blood
vessels is effectively improved, and the pain of the lower limbs is
reduced, and the maximum walking distance and the painless walking
distance of the patient are increased. The main mechanism in
addition to being able to expand peripheral blood vessels, inhibits
the proliferation of vascular smooth muscles and improve body
inflammation while inhibiting thrombosis (18). O'Donnell, et al (19) also stated that cilostole is a safe,
effective treatment for sexual well-being. It improves the
patients' symptoms and quality of life, and has beneficial effects
on arterial compliance through its lipid-lowering properties.
Thompson et al (20) pointed
out that cilostazol significantly increased patient walking
distance and quality of life indicators. Also there are no serious
side effects, and this view is consistent with the findings of this
study. There was no significant difference between the groups in
terms of the number of adverse reactions, the digestive tract
symptoms, allergic symptoms, liver function, and leukopenia
(P>0.05). In the trimetazidine group, there were 15 cases of
lethargy hypodynamia and 9 cases of dizziness and headache, which
were significantly different compared to the cilostazol group (7
cases, 3 cases) (P<0.05). Patients with cilostazol had fewer
symptoms such as lethargy hypodynamia, dizziness and headaches, and
these symptoms are related to the role of drugs in dilating
cerebral blood vessels. It is suggested that cilostazol is more
obvious than the tromethazine in the function of dilating blood
vessels. Due to platelets having an important effect in the
development of atherosclerosis in patients, cilostazol inhibits
phosphodiesterase activity, decreases cAMP degradation, and
increases cAMP levels in platelets and cells (21). Therefore, it acts as an anti-platelet
aggregation and dilating blood vessels to prevent thrombotic
vascular occlusion. Syrkin et al (22) reported that trimetazidine has a
certain anti-ischemic effect in the treatment of intermittent
claudication and cardiac diseases. It has a beneficial effect on
extending the intermittent claudication distance; however, the side
effects cannot be ignored and further research is required. Through
the observation of the long-term survival rates of patients between
the groups, the 5-year survival rate of patients taking cilostazol
was 69.64%, whereas the 5-year survival rate of the trimetazidine
group was 52.13%, which was much lower than the cilostazol group,
and there was a significant difference between the groups
(P<0.05). Based on the above results, the significant high
survival rate further confirmed that cilostazol has a better drug
efficacy. Also the only two drug treatments approved by the US Food
and Drug Administration for use in the United States, include
pentoxifylline and cilostazol (23).
In 2016, the American College of Cardiology/American Heart
Association regarding the management of patients with lower
extremity arterial disease (24)
indicated that cilostazol may be used as a drug of choice for the
treatment of lower extremity peripheral arterial disease.
Cilostazol has been shown to be effective in improving intermittent
claudication in patients with PAD in several clinical trials
(25), showing that the efficacy of
cilostazol is worthy of recognition.
There was no difference between the experimental
group and the control group in terms of sex, age, and lifestyle and
other general clinical baseline data. This improved the
authenticity and reliability of our experiment. In addition to the
efficacy of trimetazidine and cilostazol in the treatment of ASO,
the disease index and other adverse reactions were observed
statistically. The 5-year survival rate between the groups of
patients were recorded in detail and the data were proved by the
results, which reflects the rigor of the experiment. The purpose of
our experimental research is to extend the research results
obtained to a larger population and make a valuable contribution.
However, given the limited medical resources in The Central
Hospital of Wuhan, the number of selected cases is insufficient.
This may result in a lack of wide range of representations and the
research conclusion obtained in the laboratory cannot be widely
applied.
In conclusion, cilostazol has a good clinical effect
in the treatment of ASO, with few adverse reactions and high
long-term survival rate, and it is worthy of being promoted in
clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH drafted the manuscript. MH and DW were
responsible for analysis of the observation indexes. MH and TH
analyzed and interpreted the patient data regarding the
arteriosclerosis obliterans in lower extremity. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Central Hospital of Wuhan, Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim TD, Rea D, Schwarz M, Grille P,
Nicolini FE, Rosti G, Levato L, Giles FJ, Dombret H, Mirault T, et
al: Peripheral artery occlusive disease in chronic phase chronic
myeloid leukemia patients treated with nilotinib or imatinib.
Leukemia. 27:1316–1321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He XM, Zheng YQ, Liu SZ, Liu Y, He YZ and
Zhou XY: Altered plasma microRNAs as novel biomarkers for
arteriosclerosis obliterans. J Atheroscler Thromb. 23:196–206.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki J, Shimamura M, Suda H, Wakayama K,
Kumagai H, Ikeda Y, Akazawa H, Isobe M, Komuro I and Morishita R:
Current therapies and investigational drugs for peripheral arterial
disease. Hypertens Res. 39:183–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, He S, Wang X and Wang D: Effect
of trimetazidine on heart rate variability in elderly patients with
acute coronary syndrome. Pak J Med Sci. 32:75–78. 2016.PubMed/NCBI
|
|
5
|
Chang J, Im GJ, Chae SW, Lee SH, Kwon SY,
Jung HH, Chung AY, Park HC and Choi J: Protective role of
trimetazidine against neomycin-induced hair cell damage in
zebrafish. Clin Exp Otorhinolaryngol. 6:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Atilgan D, Parlaktas BS, Uluocak N,
Erdemir F, Markoc F, Saylan O and Erkorkmaz U: The effects of
trimetazidine and sildenafil on bilateral cavernosal nerve injury
induced oxidative damage and cavernosal fibrosis in rats.
ScientificWorldJournal. 2014:9703632014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aksoy F, Dogan R, Ozturan O, Eren SB,
Veyseller B, Pektas A and Hüseyinbas Ö: Protective effect of
trimetazidine on amikacin-induced ototoxicity in rats. Int J
Pediatr Otorhinolaryngol. 78:663–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stadnik M, Handzlik-Orlik G, Sarnecki K,
Krysiak R and Okopień B: Clinical aspects of the use of
trimetazidine in the prevention and treatment of myocardial
diseases. Przegl Lek. 70:730–734. 2013.(In Polish). PubMed/NCBI
|
|
9
|
Rondina MT and Weyrich AS: Targeting
phosphodiesterases in anti-platelet therapy. Handb Exp Pharmacol.
210:225–238. 2012. View Article : Google Scholar
|
|
10
|
Kohda N, Tani T, Nakayama S, Adachi T,
Marukawa K, Ito R, Ishida K, Matsumoto Y and Kimura Y: Effect of
cilostazol, a phosphodiesterase III inhibitor, on experimental
thrombosis in the porcine carotid artery. Thromb Res. 96:261–268.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakamoto T, Ohashi W, Tomita K, Hattori K,
Matsuda N and Hattori Y: Anti-inflammatory properties of
cilostazol: Its interruption of DNA binding activity of NF-κB from
the Toll-like receptor signaling pathways. Int Immunopharmacol.
62:120–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Criqui MH and Aboyans V: Epidemiology of
peripheral artery disease. Circ Res. 116:1509–1526. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP,
Fullerton HJ, et al Writing Group Members; American Heart
Association Statistics Committee; Stroke Statistics Subcommittee, :
Executive Summary: Heart disease and stroke statistics - 2016
update: A report from the American Heart Association. Circulation.
133:447–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fowkes FG, Rudan D, Rudan I, Aboyans V,
Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ,
Mensah GA, et al: Comparison of global estimates of prevalence and
risk factors for peripheral artery disease in 2000 and 2010: A
systematic review and analysis. Lancet. 382:1329–1340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lawall H, Huppert P, Espinola-Klein C and
Rümenapf G: The diagnosis and treatment of peripheral arterial
vascular disease. Dtsch Arztebl Int. 113:729–736. 2016.PubMed/NCBI
|
|
16
|
Chong PF, Garratt AM, Golledge J,
Greenhalgh RM and Davies AH: The intermittent claudication
questionnaire: A patient-assessed condition-specific health outcome
measure. J Vasc Surg. 36:764–771; discussion 863–864. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurklinsky AK and Levy M: Effect of
ramipril on walking times and quality of life among patients with
peripheral artery disease and intermittent claudication: a
randomized controlled trial. Journal of the American Medical
Association 2013; 309: 453–460. Vasc Med. 18:234–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chao TH, Chen IC, Lee CH, Chen JY, Tsai
WC, Li YH, Tseng SY, Tsai LM and Tseng WK: Cilostazol enhances
mobilization of circulating endothelial progenitor cells and
improves endothelium-dependent function in patients at high risk of
cardiovascular disease. Angiology. 67:638–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Donnell ME, Badger SA, Sharif MA, Young
IS, Lee B and Soong CV: The vascular and biochemical effects of
cilostazol in patients with peripheral arterial disease. J Vasc
Surg. 49:1226–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thompson PD, Zimet R, Forbes WP and Zhang
P: Meta-analysis of results from eight randomized,
placebo-controlled trials on the effect of cilostazol on patients
with intermittent claudication. Am J Cardiol. 90:1314–1319. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asal NJ and Wojciak KA: Effect of
cilostazol in treating diabetes-associated microvascular
complications. Endocrine. 56:240–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Syrkin AL, Artiukhina EG, Kanorskiĭ SG and
Chuntyzheva MM: Antiischemic efficacy of trimetazidine in patients
with intermittent claudication and effort angina. Kardiologiia.
43:49–52. 2003.(In Russian). PubMed/NCBI
|
|
23
|
Roset PN: Systematic review of the
efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline
for the treatment of intermittent claudication (Br J Surg 2012; 99:
1630–1638). Br J Surg. 100:18382013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
No authors listed, . Correction to: 2016
AHA/ACC Guideline on the Management of Patients With Lower
Extremity Peripheral Artery Disease: A Report of the American
College of Cardiology/American Heart Association Task Force on
Clinical Practice Guidelines. Circulation. 135:e791–e792.
2017.PubMed/NCBI
|
|
25
|
Pearce L, Ghosh J, Counsell A and
Serracino-Inglott F: Cilostazol and peripheral arterial disease.
Expert Opin Pharmacother. 9:2683–2690. 2008. View Article : Google Scholar : PubMed/NCBI
|