Introduction
Intracerebral hemorrhage (ICH), a common stroke
subtype with high morbidity and mortality, has become a topic of
increasing interest (1). The
majority of reports on ICH have focused on the association between
blood accumulation and mechanical factors, including blood pressure
(BP), intracranial pressure (ICP) and hematoma pressure (2–5).
However, there remain uncertainties regarding these factors,
particularly in the early stages of ICH. The intracranial
environment is a complex, multi-material composite system
containing the skull (a rigid body), the parenchyma (between solid
and liquid phases), cerebrospinal fluid (CSF; physical properties
similar to water), blood (a highly viscous liquid) and blood
vessels (elastic or hyperelastic solid). In a steady state, there
are two main circulation systems: Blood and CSF; they are driven by
pressure differentials that are markedly altered in patients with
ICH. When ICH occurs, blood flows into the parenchyma and oppresses
it, causing the steady state to break down and the pressure
differentials to change correspondingly.
Considering the difficulty in directly measuring the
changes in mechanical parameters caused by hemorrhagic sites,
mathematical and physical analyses based on medical images are
effective and convenient ways to investigate such parameters.
Finite element analysis (FEA) is a commonly used method that
simulates tissue deformation under a load. FEA provides a reliable
method to evaluate the role that mechanical factors may serve,
which may be otherwise difficult to test and verify. The present
study aimed to demonstrate that hemostasis in ICH depends on
biochemical rather than mechanical factors. It is clear, however,
that mechanical changes in the course of cerebral hemorrhage
precede biochemical changes. For this purpose, the mechanical
properties of brain tissue, which undergoes large mechanical
deformation induced by bleeding, should be given priority. The
mechanical responses of brain tissue have attracted increasing
attention over the past five decades (6–10).
Numerous published studies (11–14) have
investigated uniaxial compression and tension on brain tissues in
various species, but only a few studies (15,16) have
utilized the human brain due to resource scarcity and ethical
issues. However, a constitutive relationship, which is usually
expressed by constitutive equation and can be broadly applicable in
various fields, has not yet been obtained through previous studies.
Existing studies that involved the modeling of the brain have used
simplified models according to special demands, such as
cerebrospinal fluid and brain dynamics (17) or hydrocephalus (18). The intracranial environment is
complex and therefore requires a more complex model to accurately
provide substantial amounts of data. In the present study, several
assumptions were generated to simplify the complexity of the
brain.
The present study investigated whether mechanical
factors, particularly ICP and BP, serve an important role in
inhibiting the accumulation of blood in ICH. An Ogden model based
on the strain energy function was applied to describe the
constitutive relationship. Geometric and material parameters were
acquired from computed tomography (CT) images and tissue tension
data, respectively, as previously described (15). Material parameters were then
corrected by using experimental stress-strain data from a study by
Jin et al (16). The
published strain-stress association of human brain tissue was
adopted to determine the deformation and load threshold (15). Different initial amounts for blood
mass were included in the models as the contact edges. The results
of stress distribution from the preloading conditions and different
initial blood amounts were then compared. The results indicated
that mechanical factors (BP and ICP) do not contribute to
hemostasis in the early stages of ICH.
Materials and methods
Assumptions
As previous studies have simple and specific models,
a variety of assumptions were generated to simplify the complexity
of the brain. Thus the present study produced a generalized model.
The assumptions were as follows: i) The parenchyma was considered
to be an isotropic, homogenous and incompressible material; ii) the
skull was a boundary constraint, and CSF and blood masses were
loading conditions; and iii) the model was formulated without
time-dependent material behavior. In preloading case (PLC)1, no
consideration was given to the physiological cyclical variation of
ICP, thus ICP was set to a constant 1,300 Pa (as blood accumulates
the corresponding CSF volume flows out of the system); and in the
PLC2, no consideration was given to the physiological cyclical
variation of ICP (ICP changed its value dynamically with the blood
accumulation).
Geometry and meshing
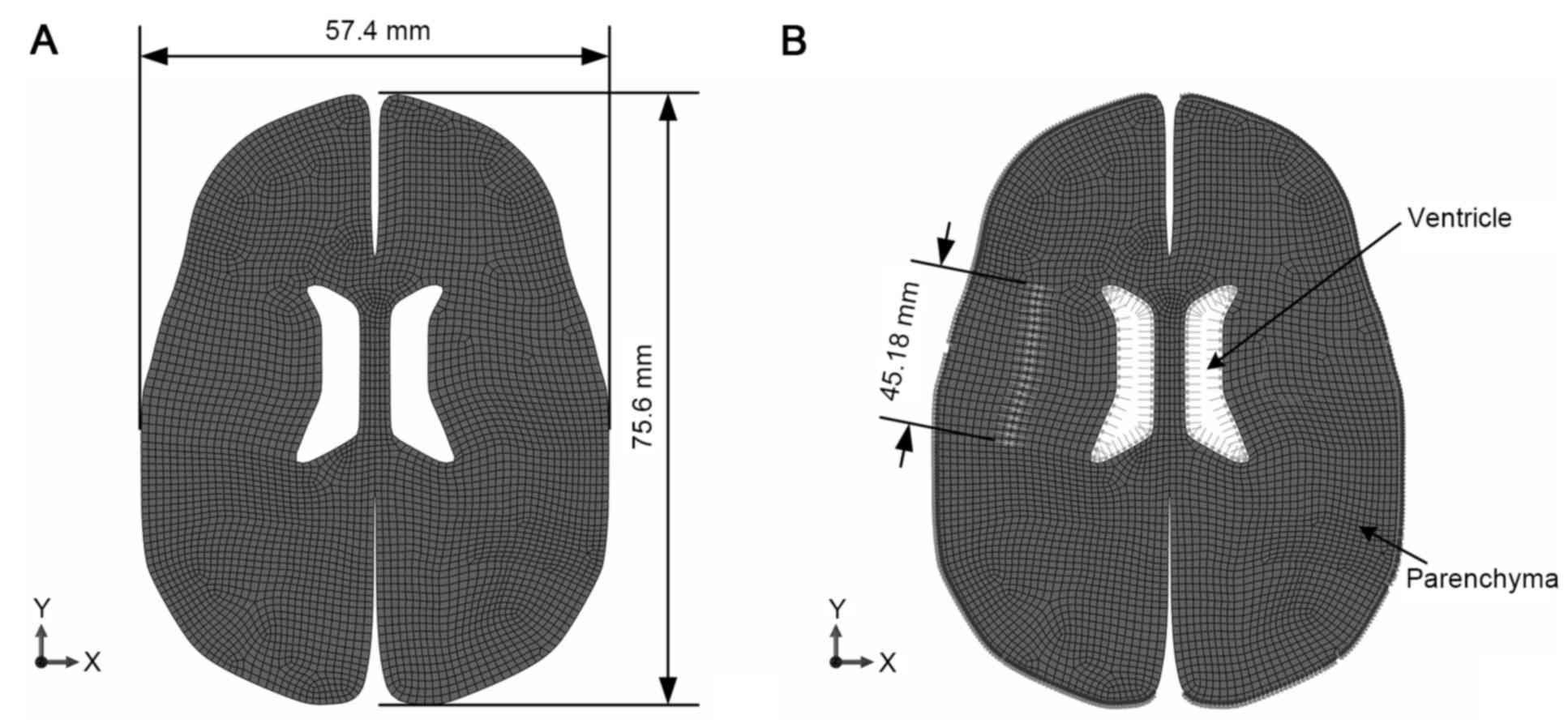
The establishment of a digital model was performed
in accordance with the study by Wittek et al (19). Patient geometric data for the
parenchymal mesh were universal features obtained from preoperative
CT data from the database of the First Affiliated Hospital of
Chongqing Medical University (Chongqing, China). The model
consisted of 4,313 8-node quadrilateral elements and 13,373 nodes,
as shown in Fig. 1. The two
hemispheres were analyzed, taking into account the possibility of
midline migration caused by a jostle effect from a hematoma.
Elements were detached along the maximum diameter of the blood mass
on the cross-section and the position of the mass was referred to
on CT images. The free edges were preset in the model to represent
the contact edges between the blood mass and the parenchyma, with a
range set between 7.407 and 43.682 mm.
Material model for brain
parenchyma
The hyperelastic Ogden model for strain energy was
applied to explain the deformation of the brain parenchyma. The
material behavior can be described by the Ogden hyperelastic
formula:
W=∑i=1N2μiαi2(λ1αi+λ2αi+λ3αi-3)
Where λi are the principal stretch
ratios, and µi and αi are
material coefficients, which are parameters determined by
experimentation. It was assumed that the tissue kept a fixed volume
and initial form during tension deformation. Thus,
λ1λ2λ3=1 andλ1=λT,λ2=λ3=λT-12
Where λT is the principal stretch
ratio in the stretching direction. Then, in uniaxial tension,
W=∑i=1N2μ1αi2(λTαi+2λT-12αi-3)
The equation yields the following uniaxial tension
stress component, σ11, along the x1-axis:
σ11=(λT)=∑i=1N2μiαi2(λTαi-1-λT-12αi-1)
For N=3,
σ11=W′(λT)=2μ1α1(-λT-1-α12+λT-1+α1)+2μ2α2(-λT-1-α22+λT-1+α2)+2μ3α3(-λT-1-α32+λT-1+α3).
This equation can be utilized to calculate the
required material parameters.
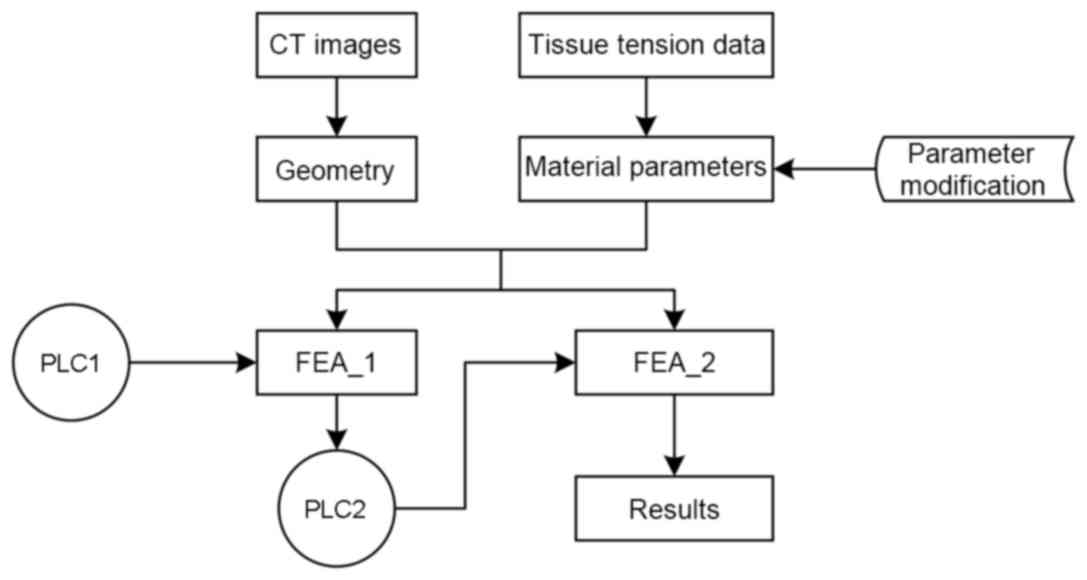
Boundary conditions
Given that the displacement of the outer brain
surface is confined by the skull, all nodes were constrained on the
outer edge with the exception of the gap edge between the left and
right hemispheres, as shown in Fig.
1. In order to configure the terminal load condition, 4,676 Pa
von Mises stress was adopted as the threshold, as previously shown
(20), which was obtained through
performing FEA on the data from Franceschini et al (15).
Loading
Two types of loads were applied in the simulations.
First, a load was applied to the different lengths of the contact
edge; this load was used to simulate the pressure applied on the
edge by a blood mass in ICH. Two types of determined preloading
conditions were applied on the ventricular edge as ICP. The brain
model was analyzed using two distinct definitions of the prescribed
nodal motion (referred to as PLC1 and PLC2). A stable load
environment test (PLC1) was performed to estimate incremental
pressure increases and establish a volume-pressure association. The
nonlinear parameter estimation was achieved by the least square
method using MATLAB 7.10 software (MathWorks, Inc., Natick, MA,
USA). In the mutative load environment test (PLC2), the nonlinear
FEA of blood accumulation was analyzed using Abaqus/Standards v.
6.12 software (Dassaut Systems, Waltham, MA, USA).
Preloading
PLC1
Using the normal ICP range (21), pressure was applied to the edge of
the ventricles at 1,300 Pa to simulate ICP. A cavity in the
parenchyma, which was formed by the simulated pressure, represented
blood accumulation following parenchyma deformation. Applying the
ABC/2 formula for ICH volume, the volume of the blood mass in the
parenchyma was calculated using the following function:
Vellipsoid=(π×A×B×C)/6. Where Vellipsoid is
the ellipsoid volume representing the blood mass volume, and A, B
and C represent the X/Y/Z axial dimensions of the ellipsoid in the
Cartesian coordinate system, respectively. The length of the minor
axis was set equal to the height of the ellipsoid.
PLC2
Similar to PLC1, pressure was exerted on the edge
that was equal to the ICP. In the PLC2 simulation, the pressure
increased with blood accumulation. As the CSF circulation was
omitted, there were not enough conditions to apply a
pressure-volume index method (22).
Thus, using data acquired from the pressure-volume curve between
ICP and incremental changes in intracranial content, as previously
described (21), the pressure acting
on the edge of the ventricles increased with increasing volume of
blood in the skull.
Loading on the detached edge
Loads were applied to different lengths of detached
edges, thus simulating hematoma pressure. The pressure was
increased until a threshold stress occurred on the areas of partial
stress concentration. The experimental procedure is demonstrated in
Fig. 2.
Results
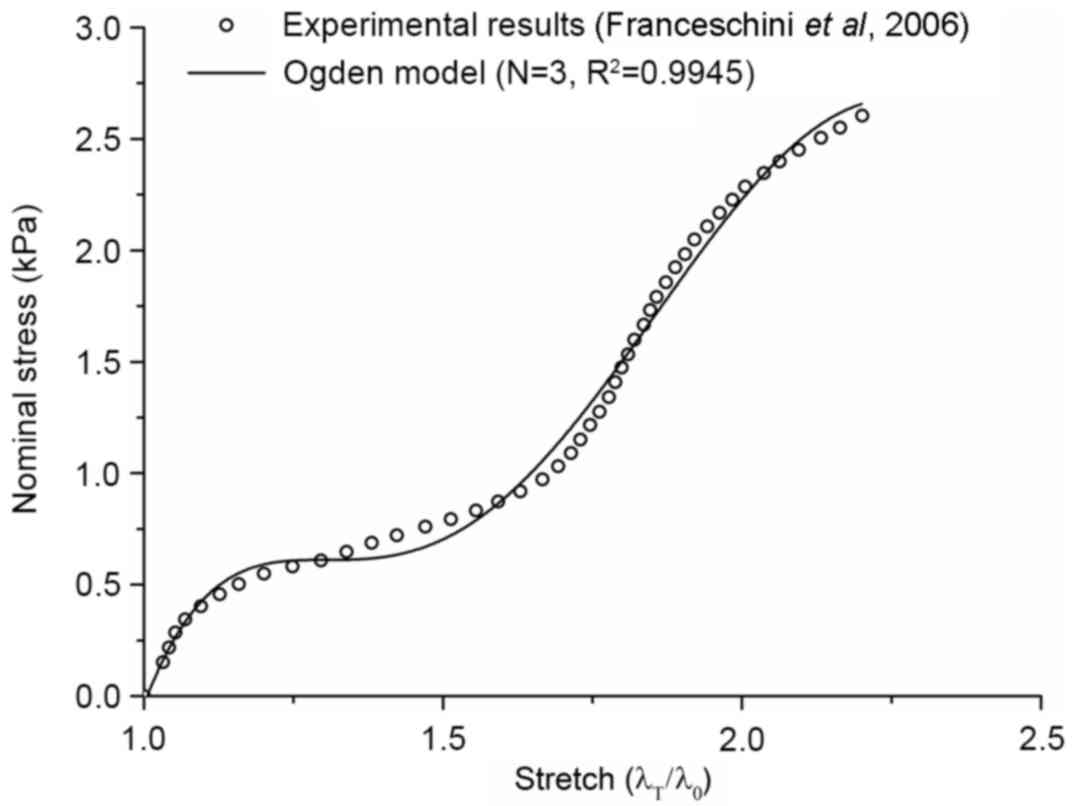
Material parameters
The Ogden model has previously been confirmed to be
highly consistent with the results of Franceschini et al
(15), as shown by the goodness of
fit index (Fig. 3). The parameters
for weighted material were obtained via examining the experimental
data of Jin et al (16). The
parameters for weighted material of the parenchyma are presented in
Table I.
 | Table I.Coefficients defining the Ogden
hyperelastic material for the parenchyma. |
Table I.
Coefficients defining the Ogden
hyperelastic material for the parenchyma.
|
| Factor |
|---|
|
|
|
|---|
| i | µi | αi | D1 |
|---|
| 1 | −310.990 | −0.537 |
|
| 2 | 132.611 | −0.086 | 0.308 |
| 3 | 180.541 | −1.032 |
|
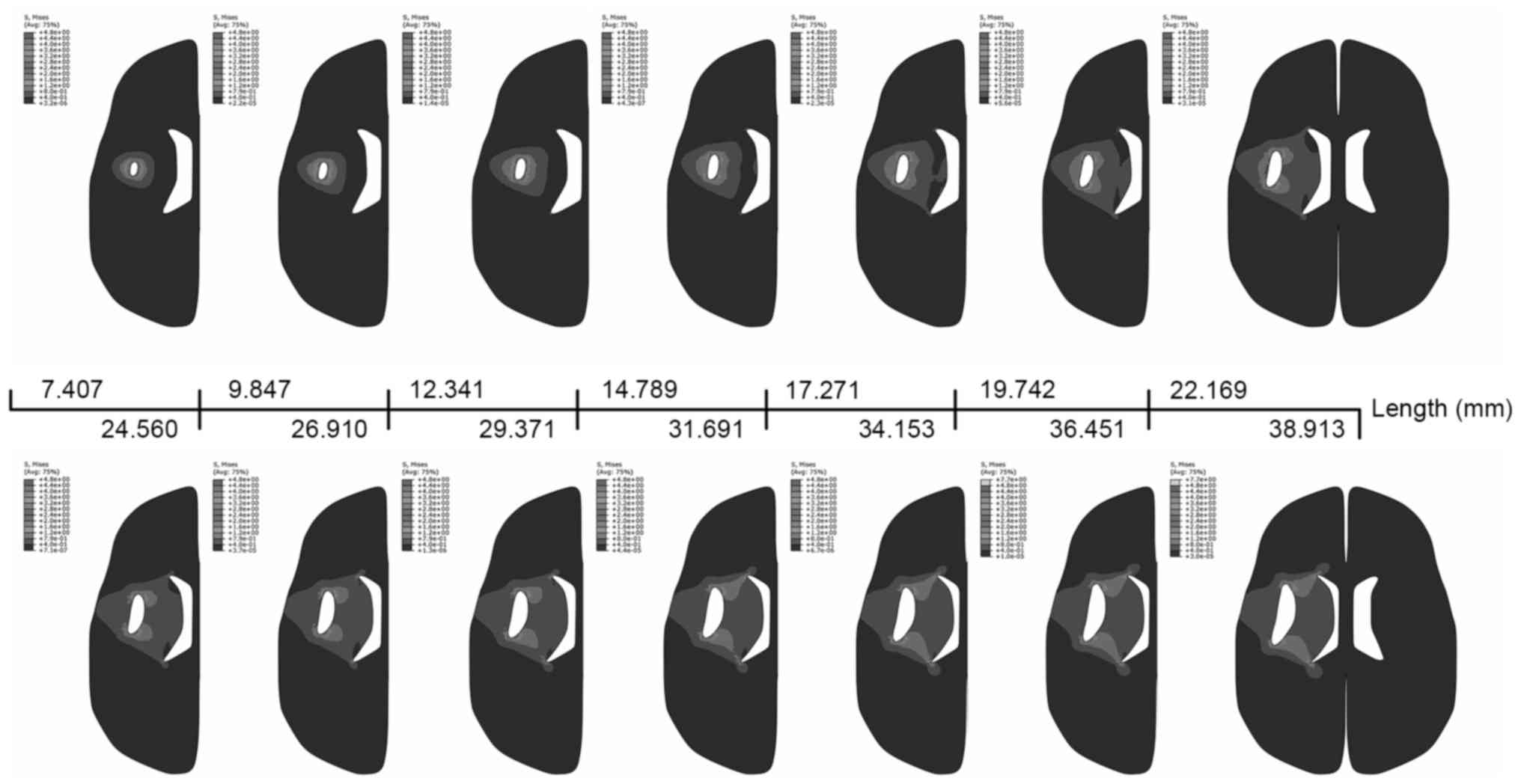
Finite element analyses
Following the application of a load under conditions
described for PLC1, the volume of the cavity increased from 105 to
3,822 mm3 (data not shown). These volume data were used
to compute ICP incremental changes in PLC2 (data not shown). PLC2
simulated ICP increases with increasing contents in the skull,
represented by the von Mises stress distribution in PLC2 (Fig. 4). Maximal stress was located at the
two ends of the cavity, with the main stresses distributed in the
surrounding zone. The ventricle began to be impacted when the
length of the detached edge reached 19.741 mm, but brain midline
shift had not been observed in the simulations. A large level of
stress was concentrated on the side of the ventricle when the
length reached 36.451 mm. The results from PLC1 demonstrated a
similar stress distribution (data not shown).
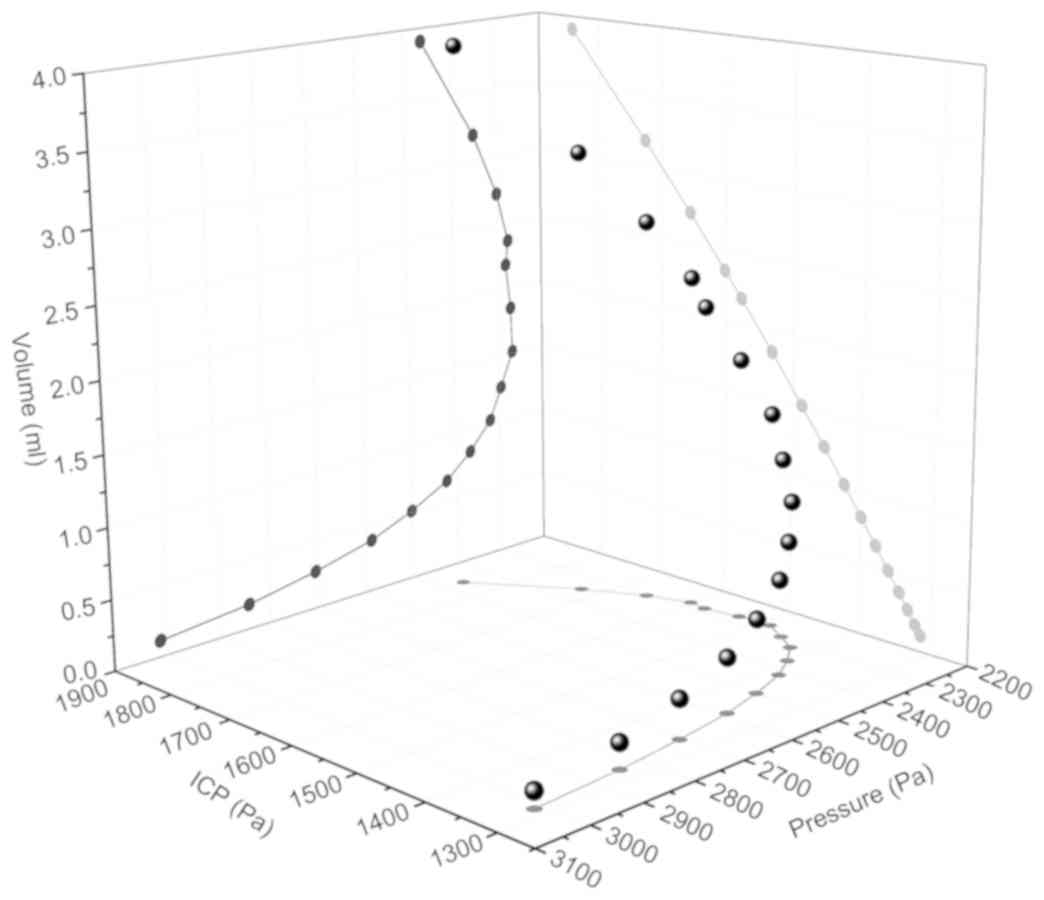
Association between hematoma volume
and pressure
Fig. 5 presents the
association between blood mass pressure, blood mass volume and ICP
in PLC2. With accumulating blood, the cavity volume of the
parenchyma increased. The load demand for reaching a stress
threshold on a stress concentrated position declined until ICP
rapidly increased with cavity volume (from 2,180 to 3,907
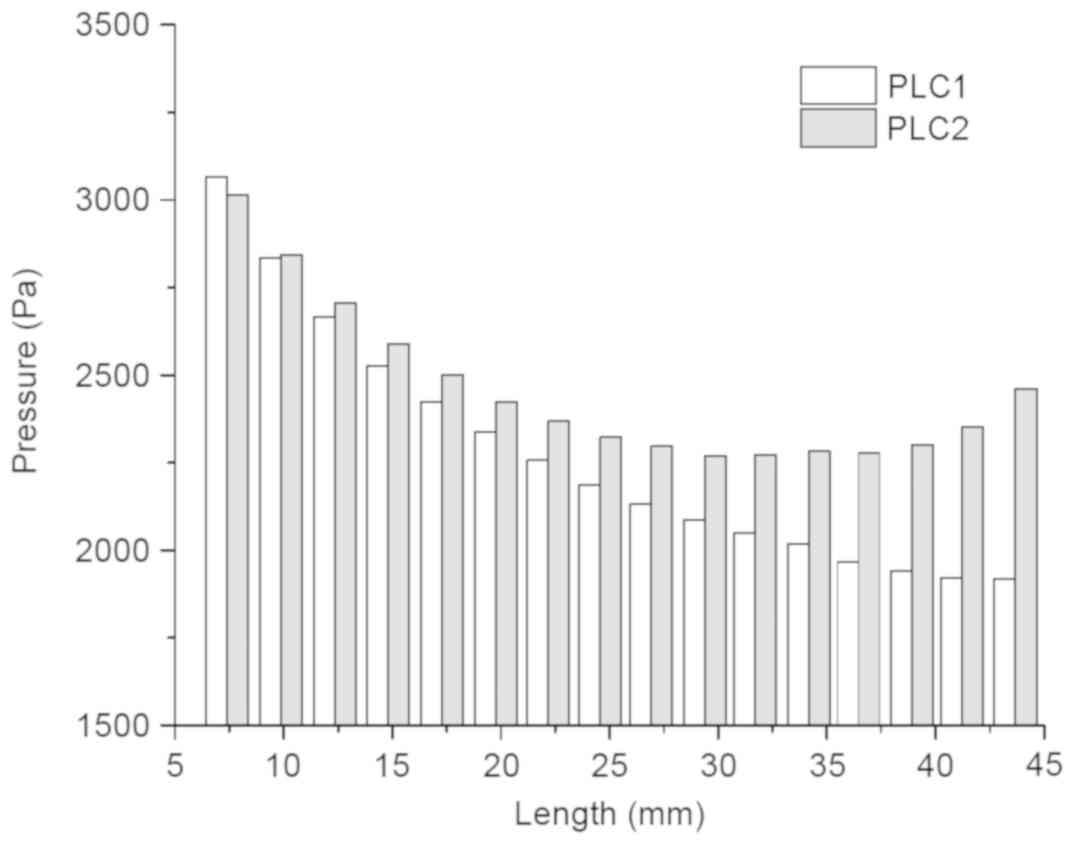
mm3). Comparing the trends observed in PLC1 and PLC2
revealed that there was no distinct difference between the two
preload cases if ICP was ignored (Fig.
6). Therefore, the load, applied on the detached edges and
resulting in destruction at the end of the crack, decreased with
increasing detached length when the stress threshold was
reached.
Discussion
During ICH, blood accumulation in the parenchyma
compresses surrounding tissues and eventually induces deformation.
Discovering where stress may be distributed by this deformation may
help predict locations of damage and the direction in which blood
may continue to accumulate. The present study revealed that maximal
stress occurred near the two ends of the detached edges of the
blood mass in the direction of the major axis, indicating that the
direction of blood accumulation was associated with the initial
shape of the blood mass without consideration of anisotropy and
inhomogeneity. The von Mises stress distribution in all the
simulations was similar to hematoma geometry as observed on CT and
magnetic resonance imaging (MRI). However, the volume of the blood
mass in this simulation was far smaller than the volume calculated
directly from CT images. Cells exposed to an abnormal mechanical
environment may undergo apoptosis or necrocytosis (23–25).
Under mechanical stress or stimulation, the physiological response
of the cells may accelerate the spread of bleeding. The majority of
medical images are generally acquired within 4 h of ICH onset, when
erythrocyte lysis has not yet begun (26), but physiological changes in the
tissue surrounding the blood mass are inevitable. The von Mises
stress distribution may help predict the final shape of the
hematoma as observed on CT and MRI images.
In ICH, blood accumulates as a result of continued
bleeding following vessel rupture. The reasons for blood to stop
accumulating are typically considered to include biochemical
factors and mechanical factors. Lowering blood pressure quickly
following ICH so as to reduce blood accumulation is considered a
potentially effective method to minimize ICH-induced brain damage.
However, there is no conclusive evidence to prove the efficacy of
this strategy (1). Under normal
physiological conditions, terminal arteriolar pressure is ~40% of
systemic arterial blood pressure (27). Intravascular pressure can be ≥6,500
Pa under physiological conditions, even without considering
patients with hypertension (28,29). In
the simulations performed in the present study, the pressure
applied to the cavity edges increased continuously until a critical
stress level was reached, with a maximum pressure value of 3,066
Pa, which was still less than typical intravascular pressure. Thus,
the stress caused by deformation in the parenchyma was not
sufficient to stop blood from accumulating. Comparing the load
change with the cavity edge between the two preloading conditions
demonstrated that the critical pressure required for blood mass
enlargement in the two cases decreased with increasing cavity
volume. The change in the pressure had a similar trend when the
weighted effect of ICP was not taken into consideration.
In conclusion, the results of the present study
suggest that mechanical factors (BP and ICP) do not serve a
decisive role in stopping blood from accumulating in the early
stages of ICH. Therefore, stress may be the primary contributing
factor to the final shape of the hematoma in patients with ICH.
Acknowledgements
The authors of the present study would like to
acknowledge Professor Zhan-Fang Liu of the College of Aerospace
Engineering at Chongqing University (Chongqing, China) for
providing the Abaqus/Standards software.
Funding
The present study was supported by the National
Basic Research 973 Program of China (grant no. 2014CB541600), the
Visiting Scholar Foundation of Key Laboratory of Biorheological
Science and Technology at Chongqing University (Chongqing, China),
and the Ministry of Education (grant no. CQKLBST-2018-019).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
PR performed the modeling, the finite element
analysis and was a major contributor in writing the manuscript. BCW
provided substantial contributions to the conception of the work.
YZW and HJX provided the acquisition, analysis, and interpretation
of data for the work. TWG and XFL performed the simulations and
contributed in the preparation of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xi G, Strahle J, Hua Y and Keep RF:
Progress in translational research on intracerebral hemorrhage: Is
there an end in sight? Prog Neurobiol. 115:45–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu G, Xi G and Huang F: Spontaneous
intracerebral hemorrhage in humans: Hematoma enlargement, clot
lysis, and brain edema. Acta Neurochir Suppl. 96:78–80. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steiner T, Al-Shahi Salman R, Beer R,
Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn
CJ, Krieger D, et al: European stroke organisation (ESO) guidelines
for the management of spontaneous intracerebral hemorrhage. Int J
Stroke. 9:840–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalita J, Misra UK, Vajpeyee A, Phadke RV,
Handique A and Salwani V: Brain herniations in patients with
intracerebral hemorrhage. Acta Neurol Scand. 119:254–260. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiploylee C and Colbourne F: Intracranial
pressure measured in freely moving rats for days after
intracerebral hemorrhage. Exp Neurol. 255:49–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fung YCB: Elasticity of soft tissues in
simple elongation. Am J Physiol. 213:1532–1544. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zebian B and Critchley G: Spontaneous
intracranial haemorrhage. Surgery (Oxford). 30:136–141. 2012.
View Article : Google Scholar
|
|
8
|
Estes MS and McElhane JH: Response of
brain tissue to compressive loading. New York ASME. 1970.
|
|
9
|
Miller K and Chinzei K: Constitutive
modelling of brain tissue: Experiment and theory. J Biomech.
30:1115–1121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goriely A, Geers MA, Holzapfel GA,
Jayamohan J, Jérusalem A, Sivaloganathan S, Squier W, van Dommelen
JA, Waters S and Kuhl E: Mechanics of the brain: Perspectives,
challenges, and opportunities. Biomech Model Mechanobiol.
14:931–965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prevost TP, Balakrishnan A, Suresh S and
Socrate S: Biomechanics of brain tissue. Acta Biomater. 7:83–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bilston LE, Liu Z and Phan-Thien N: Large
strain behaviour of brain tissue in shear: Some experimental data
and differential constitutive model. Biorheology. 38:335–345.
2001.PubMed/NCBI
|
|
13
|
Bayly PV, Black EE, Pedersen RC, Leister
EP and Genin GM: In vivo imaging of rapid deformation and strain in
an animal model of traumatic brain injury. J Biomech. 39:1086–1095.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rashid B, Destrade M and Gilchrist MD:
Mechanical characterization of brain tissue in compression at
dynamic strain rates. J Mech Behav Biomed Mater. 10:23–38. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franceschini G, Bigoni D, Regitnig P and
Holzapfel GA: Brain tissue deforms similarly to filled elastomers
and follows consolidation theory. J Mech Phys Solids. 54:2592–2620.
2006. View Article : Google Scholar
|
|
16
|
Jin X, Zhu F, Mao H, Shen M and Yang KH: A
comprehensive experimental study on material properties of human
brain tissue. J Biomech. 46:2795–2801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linninger AA, Tangen K, Hsu CY and Frim D:
Cerebrospinal fluid mechanics and its coupling to cerebrovascular
dynamics. Ann Rev Fluid Mech. 48:219–257. 2016. View Article : Google Scholar
|
|
18
|
Taylor Z and Miller K: Reassessment of
brain elasticity for analysis of biomechanisms of hydrocephalus. J
Biomech. 37:1263–1269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wittek A, Miller K, Kikinis R and Warfield
SK: Patient-specific model of brain deformation: Application to
medical image registration. J Biomech. 40:919–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren P, Wang BC, Wang YZ, Hao SL, Guo TW
and Li XF: Evaluating tensile damage of brain tissue in
intracerebral hemorrhage based on strain energy. Exp Ther Med.
16:4843–4852. 2018.PubMed/NCBI
|
|
21
|
Marmarou A and Beaumont A: Physiology of
the cerebrospinal fluid and intracranial pressure. Youmans
neurological surgery. Winn HR: 6th. Springer; Philadelphia, PA: pp.
169–182. 2011, View Article : Google Scholar
|
|
22
|
Maset AL, Marmarou A, Ward JD, Choi S,
Lutz HA, Brooks D, Moulton RJ, DeSalles A, Muizelaar JP, Turner H,
et al: Pressure-volume index in head-injury. J Neurosurg.
67:832–840. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai MS, Chou YL, Chang GL and Shen CL:
The effect of magnitudes and duration of pressure on cerebral
cortex in a rat model. J Clin Neurosci. 8:157–163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agar A, Li S, Agarwal N, Coroneo MT and
Hill MA: Retinal ganglion cell line apoptosis induced by
hydrostatic pressure. Brain Res. 1086:191–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tök L, Nazıroğlu M, Uğuz AC and Tök O:
Elevated hydrostatic pressures induce apoptosis and oxidative
stress through mitochondrial membrane depolarization in PC12
neuronal cells: A cell culture model of glaucoma. J Recept Signal
Transduct Res. 34:410–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xi G, Keep RF and Hoff JT: Mechanisms of
brain injury after intracerebral haemorrhage. Lancet Neurol.
5:53–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gore RW: Pressures in cat mesenteric
arterioles and capillaries during changes in systemic arterial
blood pressure. Circ Res. 34:581–591. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lipowsky HH: Microvascular rheology and
hemodynamics. Microcirculation. 12:5–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boas DA, Jones SR, Devor A, Huppert TJ and
Dale AM: A vascular anatomical network model of the spatio-temporal
response to brain activation. Neuroimage. 40:1116–1129. 2008.
View Article : Google Scholar : PubMed/NCBI
|