Introduction
Lower extremity arteriosclerosis obliterans is a
chronic occlusion of lower extremity arteries, mainly common iliac
artery and superficial femoral artery, caused by atherosclerosis,
with a main clinical manifestation of insufficient blood supply to
lower extremities (1,2). One of its main risk factors is age, and
the incidence increases with age. Its incidence in people over 60
years can reach 20%. As the population ages and the dietary
structure changes, lower extremity arteriosclerosis obliterans has
become a worldwide public health problem (3,4). Its
prognosis is poor, especially in patients with intermittent
claudication, rest pain, ulcer and gangrene, with higher 5-year
mortality (5,6). Therefore, it is very important to find
suitable and effective treatment methods.
Surgery and interventional treatment can effectively
improve arterial occlusion, but their long-term treatment effects
remain unsatisfactory, especially for peripheral arterial lesion
and polyplanar stenosis, and older patients are less tolerant to
surgery and interventional treatment (7,8). Drug
treatment is a reasonable and feasible means. Drugs improving cell
ischemia and energy metabolism, inhibiting platelet aggregation and
improving microcirculation, such as trimetazidine, alprostadil and
plasmin, have good efficacy (9–11).
Alprostadil is a natural prostaglandin that inhibits platelet
aggregation, promotes erythrocyte deformation and improves
hemorheology (12). Trimetazidine, a
piperazine derivative that can protect cell energy metabolism in
ischemic conditions and ischemic cells, maintains the stability of
the intracellular environment and improves the exercise tolerance
of patients (13). As a proteolytic
enzyme extracted from Agkistrodon blomhoffii ussurensis
venom with monoclonal antibody purification technology, plasmin can
directly dissolve the thrombogenic fibrin without causing excessive
fibrinolysis, having excellent antithrombotic function (14). However, there are few comparative
studies on the efficacy of trimetazidine, alprostadil and plasmin
in lower extremity arteriosclerosis obliterans.
In this study, the medical records of 132 patients
with lower extremity arteriosclerosis obliterans were
retrospectively analyzed, and the efficacy of trimetazidine and
plasmin combined with alprostadil in lower extremity
arteriosclerosis obliterans was compared, in order to clinically
provide reference for the drug use in the treatment of lower
extremity arteriosclerosis obliterans.
Patients and methods
Study object
A retrospective analysis was performed on the
medical records of 132 patients with lower extremity
arteriosclerosis obliterans treated in Yantai Yuhuangding Hospital
(Yantai, China) from March 2015 to August 2017. Among them, 68
patients were treated with trimetazidine combined with alprostadil
(group A), 64 patients treated with plasmin combined with
alprostadil (group B). The patients were in compliance with the
2015 edition of guidelines for the diagnosis and treatment of lower
extremity arteriosclerosis obliterans, with obvious clinical
features of lower extremity arteriosclerosis obliterans and left
ankle brachial index ≤0.9, and aged 40–75 years. None of the
patients had autoimmune disease, severe inflammatory reaction or
other parts of arteriovenous occlusion, and they had complete
records. Patients with severe angina pectoris, diabetes mellitus,
recurrent infection, cerebrovascular disease and peptic ulcer were
excluded, as were patients with anticoagulant drugs; patients with
heart failure, kidney or liver diseases; patients who had been
treated with depressurization and other methods with treatment
drugs affecting vascular function and coagulation mechanism.
The study was approved by the Ethics Committee of
Yantai Yuhuangding Hospital. Patients or their families signed an
informed consent form.
Treatment methods
All 132 patients were routinely treated, followed a
controlled diet, no smoking and drinking, controlled blood lipid
and blood pressure, stabilized atherosclerotic plaque, actively
controlled platelet aggregation, anti-infective treatment and
rehabilitation training. Patients in group A were treated with
trimetazidine in combination with alprostadil, patients in group B
were treated with plasmin in combination with alprostadil on this
basis. The dose of alprostadil was 10 µg + 100 ml of saline,
intravenously dripped after being mixed evenly, once a day. The
dose of trimetazidine was 30 mg orally, 3 times a day. The dose of
plasmin was 100 units + 250 ml of saline, infused once a day. The
treatments were a course followed over 14 days, for a total of 2
consecutive courses of treatment.
Observation indicators
Subsequent to the 2 courses of treatment the two
groups of patients were observed with regard to therapeutic
effects, changes in blood flow perfusion indicators (vascular peak
velocity and blood flow, DCU12 full digital color Doppler
ultrasound diagnostic apparatus, Xuzhou Kaixin Electronic Equipment
Co., Ltd., Xuzhou, China) of the superficial femoral artery,
posterior tibial artery and dorsalis pedis artery, in endothelial
function (fasting blood collected by nurses in the morning and
detected in the laboratory medicine of Yantai Yuhuangding
Hospital), in left ankle brachial index [PeriFlux 5000 laser
Doppler flowmeter; Swedish Parry Medicine (China) Co., Beijing,
China], in pain-free walking distance, and in maximum walking
distance.
Efficacy evaluation criteria
Evaluation criteria were as follows: Recovery:
Symptoms disappeared, and dorsal blood flow, skin color, skin
temperature and dorsal arterial pulse returned to normal. The
maximum walking distance increased by >100%, and the left ankle
brachial index was between 0.9 and 1.3. Markedly effective:
Conscious symptoms, skin color, skin temperature and dorsal artery
pulse were improved significantly. Pain-free and maximum walking
distance increased by >90%, but ≤100%. The left ankle brachial
index was ≥0.9, and the blood flow of dorsalis pedis artery
increased by >80%. Effective: Conscious symptoms, skin color,
skin temperature and dorsal artery pulse were improved better than
those before treatment. Pain-free and maximum walking distance
increased by 50–89%. Left ankle brachial index increased by
0.2–0.3, and blood flow increased by >50%. Non-effective:
Conscious symptoms, skin color, skin temperature and dorsal artery
pulse was not improved compared to those before treatment.
Pain-free and maximum walking distance increased by ≤50%, and no
change in left ankle brachial index. The total effective rate was
calculated as: recovery + markedly effective + effective.
Statistical analysis
SPSS19.0 (AsiaAnalytics Formerly SPSS China,
Shanghai, China) was used. Enumeration data were expressed as [n
(%)], and χ2 test was used for the comparison of ratio.
Measurement data were expressed as mean ± SD, and t-test was used
for comparison between the two groups. ANOVA and LSD post hoc test
for repeated measurement were used for comparison at different
times in the group. P<0.05 indicated the difference was
statistically significant.
Results
General data
There were 68 patients in group A, including 42 male
patients and 26 female patients, aged (62.45±9.26) years, and 64
patients in group B, including 41 male patients and 23 female
patients, aged (63.33±9.17) years. There were no statistically
significant differences in sex and age between the two groups of
patients (P>0.05), and in other basic data such as BMI, smoking
history, proportion of hypertension patients, proportion of
diabetes mellitus patients, platelet count, activated partial
thromboplastin time, D-dimer and bleeding time (P>0.05)
(Table I).
 | Table I.General data. |
Table I.
General data.
| Item | Group A (n=68) | Group B (n=64) | χ2/t | P-value |
|---|
| Sex [n (%)] |
|
| 0.075 | 0.785 |
| Male | 42
(61.76) | 41
(64.06) |
|
|
|
Female | 26
(38.24) | 23
(35.94) |
|
|
| Age (years) |
62.45±9.26 |
63.33±9.17 | 0.548 | 0.585 |
| BMI
(kg/m2) |
25.41±4.15 |
26.18±4.23 | 1.055 | 0.293 |
| Smoking history [n
(%)] |
|
| 0.065 | 0.799 |
| Yes | 45
(66.18) | 41
(64.06) |
|
|
| No | 23
(33.82) | 23
(35.94) |
|
|
| Hypertension [n
(%)] |
|
| 0.068 | 0.795 |
| Yes | 41
(60.29) | 40
(62.50) |
|
|
| No | 27
(39.71) | 24
(37.50) |
|
|
| Diabetes mellitus [n
(%)] |
|
| 0.531 | 0.466 |
| Yes | 22
(32.35) | 17
(26.56) |
|
|
| No | 46
(67.65) | 47
(73.44) |
|
|
| Platelet count
(×109/l) | 221.45±61.53 | 219.68±62.56 | 0.164 | 0.870 |
| Activated partial
thromboplastin time (sec) |
29.18±2.14 |
28.94±2.31 | 0.620 | 0.537 |
| D-dimer (µg/l) | 362.44±128.17 | 359.18±124.62 | 0.148 | 0.883 |
| Bleeding time
(min) |
6.38±1.06 |
6.71±1.14 | 1.723 | 0.087 |
Efficacy analysis
The results of the efficacy analysis of the two
groups of patients after 2 courses of treatment showed that there
were no statistically significant differences in recovery rate,
markedly effective rate, effective rate, non-effective rate and
total effective rate between the two groups of patients (P>0.05)
(Table II).
 | Table II.Efficacy analysis of the two groups of
patients after two courses of treatment [n (%)]. |
Table II.
Efficacy analysis of the two groups of
patients after two courses of treatment [n (%)].
| Criteria | Group A (n=68) | Group B (n=64) | χ2 | P-value |
|---|
| Recovery | 15 (22.06) | 11 (17.19) | 0.498 | 0.482 |
| Markedly
effective | 32 (47.06) | 33 (51.56) | 0.268 | 0.605 |
| Effective | 18 (26.47) | 15 (23.44) | 0.162 | 0.688 |
| Non-effective | 3
(4.41) | 5
(7.81) | 0.670 | 0.413 |
| Total effective
rate | 65 (95.59) | 59 (92.19) | 0.670 | 0.413 |
Changes in blood flow perfusion
indicators
Before treatment, there were no statistically
significant differences in the vascular peak velocity and blood
flow of the superficial femoral artery, posterior tibial artery and
dorsalis pedis artery between the two groups (P>0.05). After
treatment, the vascular peak velocity in group B was lower than
that in group A (P<0.05), but the blood flow was higher than
that in group A (P<0.05). The vascular peak velocity and blood
flow were improved in the two groups. The vascular peak velocity of
those after treatment was lower than that before treatment of
patients in the two groups (P<0.05), but the blood flow was
higher than that before treatment (P<0.05) (Tables III and IV).
 | Table III.Changes in vascular peak velocity of
patients in two groups before and after treatment (m/sec). |
Table III.
Changes in vascular peak velocity of
patients in two groups before and after treatment (m/sec).
| Artery | Group A (n=68) | Group B (n=64) | t | P-value |
|---|
| Superficial femoral
artery |
| Before
treatment | 1.54±0.33 | 1.56±0.34 | 0.342 | 0.732 |
| After
treatment | 1.24±0.21 | 1.32±0.19 | 2.290 | 0.024 |
| Posterior tibial
artery |
| Before
treatment | 1.35±0.22 | 1.34±0.23 | 0.255 | 0.799 |
| After
treatment | 1.13±0.14 | 1.19±0.15 | 2.377 | 0.019 |
| Dorsalis pedis
artery |
| Before
treatment | 1.01±0.15 | 1.00±0.14 | 0.395 | 0.693 |
| After
treatment | 0.78±0.08 | 0.81±0.09 | 2.027 | 0.045 |
 | Table IV.Changes in blood flow of patients in
the two groups before and after treatment (ml/min). |
Table IV.
Changes in blood flow of patients in
the two groups before and after treatment (ml/min).
| Artery | Group A (n=68) | Group B (n=64) | t | P-value |
|---|
| Superficial femoral
artery |
| Before
treatment | 364.73±93.56 | 368.45±92.88 | 0.231 | 0.818 |
| After
treatment | 386.18±101.24 | 425.66±102.17 | 2.229 | 0.028 |
| Posterior tibial
artery |
| Before
treatment | 365.89±96.54 | 358.77±94.43 | 0.428 | 0.669 |
| After
treatment | 398.18±105.46 | 431.15±103.74 | 2.303 | 0.023 |
| Dorsalis pedis
artery |
| Before
treatment |
22.46±8.17 |
21.59±7.92 | 0.621 | 0.536 |
| After
treatment |
28.03±10.58 |
32.25±11.02 | 2.245 | 0.027 |
Changes in endothelial function
There were no statistically significant differences
in endothelial esterase, high-sensitivity C-reactive protein,
nitric oxide and circulating endothelial cell count levels between
the two groups before and after treatment (P>0.05). After
treatment, the levels were improved in the two groups. Endothelial
esterase, high-sensitivity C-reactive protein and circulating
endothelial cell count levels after treatment were lower than those
before treatment (P<0.05), but nitric oxide level was higher
than that before treatment (P<0.05) (Table V).
 | Table V.Changes in endothelial function of
patients in the two groups before and after treatment. |
Table V.
Changes in endothelial function of
patients in the two groups before and after treatment.
|
| Group A (n=68) | Group B (n=64) | t | P-value |
|---|
| Endothelial
esterase (ng/l) |
| Before
treatment | 71.62±7.25 | 72.33±7.46 | 0.555 | 0.580 |
| After
treatment | 62.59±6.24 | 63.78±6.51 | 1.072 | 0.286 |
| High-sensitivity
C-reactive protein (mg/l) |
| Before
treatment |
3.25±1.26 |
3.33±1.21 | 0.372 | 0.711 |
| After
treatment |
2.28±1.13 |
2.19±1.06 | 0.471 | 0.638 |
| Nitric oxide
(µmol/l) |
| Before
treatment | 50.72±5.25 | 52.13±5.18 | 1.552 | 0.123 |
| After
treatment | 59.64±5.85 | 60.77±6.12 | 1.085 | 0.280 |
| Circulating
endothelial cell count |
| Before
treatment |
7.82±3.06 |
7.76±3.12 | 0.111 | 0.911 |
| After
treatment |
4.25±1.58 |
4.17±1.46 | 0.302 | 0.763 |
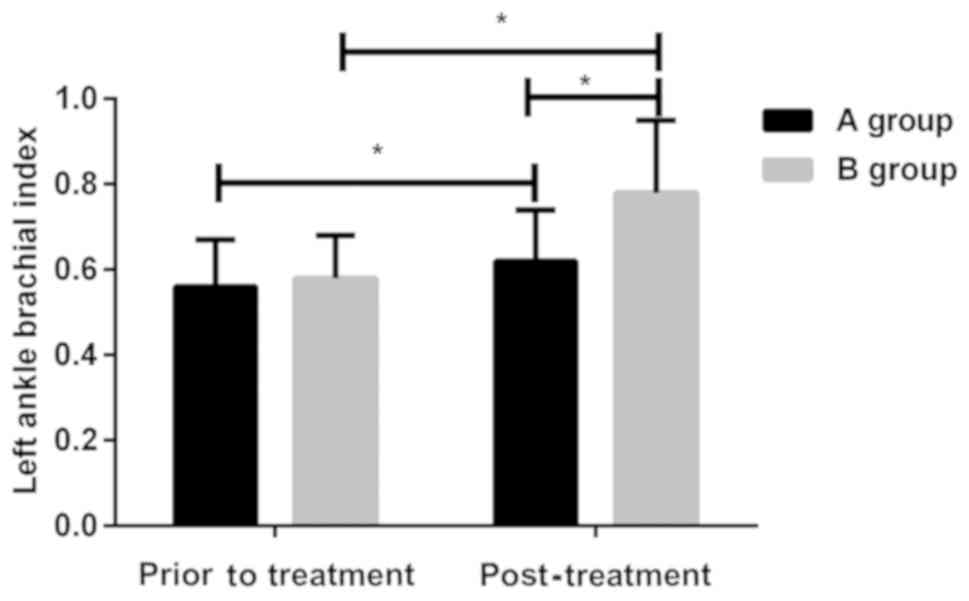
Changes in left ankle brachial
index
There was no statistically significant difference in
left ankle brachial index between the two groups of patients before
treatment (P>0.05). After treatment, the left ankle brachial
index was lower in group A than that in group B (P<0.05), and
was improved in the two groups. The index was higher after
treatment than that before treatment in the two groups (P<0.05)
(Fig. 1).
Changes in pain-free and maximum
walking distance change
There were no statistically significant differences
in pain-free walking distance between the two groups before and
after treatment (P>0.05), and in maximum walking distance before
treatment (P>0.05). After treatment, the maximum walking
distance was significantly higher in group A than that in group B
(P<0.05). After treatment, the pain-free walking distance and
maximum walking distance of the two groups of patients were
improved and were higher than those before treatment (P<0.05)
(Table VI).
 | Table VI.Changes in pain-free and maximum
walking distance of patients in two groups before and after
treatment. |
Table VI.
Changes in pain-free and maximum
walking distance of patients in two groups before and after
treatment.
|
| Group A (n=68) | Group B (n=64) | t | P-value |
|---|
| Pain-free walking
distance (m) |
| Before
treatment |
145.67±21.26 |
147.62±22.37 | 0.514 | 0.609 |
| After
treatment |
448.59±93.34 |
471.63±94.11 | 1.412 | 0.160 |
| Maximum walking
distance (m) |
| Before
treatment |
953.65±348.27 |
962.49±351.64 | 0.145 | 0.885 |
| After
treatment |
1,347.63±321.48 |
1,223.65±268.72 | 2.396 | 0.018 |
Discussion
Lower extremity arteriosclerosis obliterans is a
very common systemic peripheral vascular disease in clinical
practice. The formation of atherosclerotic plaque causes different
degrees of stenosis in the arterial tube of patients and even
complete occlusion, blocking blood flow and leading to limb
ischemic injury, so the condition of lower extremity
arteriosclerosis obliteration is complicated, with high disability
rate and mortality (15,16). Many studies have reported that
trimetazidine or plasmin in combination with alprostadil has better
efficacy in the treatment of arteriosclerosis obliterans (17,18).
However, there are few comparative studies on the efficacy of
trimetazidine and plasmin in combination with alprostadil. In this
study, the efficacy of trimetazidine and plasmin in combination
with alprostadil was analyzed, and differences in the efficacy of
the two methods were explored, in order to provide reference for
clinical treatment.
In the study, the medical records of 132 patients
with lower extremity arteriosclerosis obliterans were selected in
strict accordance with inclusion and exclusion criteria. There was
no statistically significant difference in the general data of
patients between the two groups. Their data are comparable in this
study, and the results have a certain degree of credibility. The
results of the efficacy analysis of the two groups of patients
showed that there were no significant differences in the recovery
rate, markedly effective rate, effective rate, non-effective rate
and total effective rate between the two groups, suggesting that
the two treatments have similar efficacy. Then, changes in blood
flow perfusion-related indicators were further analyzed in the two
groups. It was found that the degree of improvement of the vascular
peak velocity and blood flow of the superficial femoral artery,
posterior tibial artery and dorsalis pedis artery, and the left
ankle brachial index of patients in B group was better than that in
group A. The results of the pain-free and maximum walking distance
analysis of the two groups showed that the maximum walking distance
after treatment in group A was higher than that in group B, but
there was no difference in pain-free walking distance and in the
comparison results of endothelial function between the two groups
after treatment in this study.
At present, there are few studies on the efficacy of
trimetazidine or plasmin in combination with alprostadil in the
treatment of lower extremity arteriosclerosis obliterans worldwide.
Therefore, there is still uncertainty in the efficacy comparison
between the two treatments in this study. It is hoped that this
study will promote more scholars to participate in the analysis of
the efficacy of trimetazidine and plasmin combined with alprostadil
in lower extremity arteriosclerosis obliterans, providing better
reference for clinical treatment. The mechanisms of action of
trimetazidine, alprostadil and plasmin in the treatment of lower
extremity arteriosclerosis obliterans are not the same (12–14). It
can be seen from the results that both treatments can improve blood
flow perfusion in patients, which may be caused by alprostadil
promoting fibrinolysis. In some studies of vascular diseases, it
can improve tissue plasminogen activator and its inhibitor levels,
promoting fibrinolysis, and alprostadil also has vasodilation
effects (19,20); thus, blood flow perfusion indicators
in group A are improved compared to those before treatment.
However, there are few reports on the pro-fibrinolytic effect of
trimetazidine. The pro-fibrinolytic effect of plasmin is obvious,
and can effectively clear blocked blood vessels, so blood flow
perfusion indicators in group B of patients are better than those
in group A. This may also be the reason for the left ankle brachial
index in group B being better than that in group A. The effect of
trimetazidine on improving cell energy metabolism has been
recognized, so this may be the main reason why patients in group A
have longer maximum walking distance after treatment than patients
in group B. Although plasmin can dissolve thrombus and improve
blood flow perfusion, this improvement may not be as obvious as
that of trimetazidine due to the duration of treatment (21). This is the shortcoming of this study,
i.e., that only the short-term efficacy was evaluated. Further
follow-up reports are to be performed to analyze the long-term
efficacy of the two treatments. Differences in pain-free walking
distance and endothelial function-related indicators between the
two groups after treatment were not found. Both treatments are
effective in improving symptoms, but neither trimetazidine nor
plasmin has any analgesic effect. This may be the reason why there
is no difference in pain-free walking distance between the two
groups of patients after treatment. Trimetazidine has been reported
to be able to improve endothelial function (22), and plasmin also to be able to promote
endothelial progenitor cell production, repair vascular damage and
improve endothelial function (23).
The results of the current study showed that the degree of
improvement of the two drugs in endothelial function was similar,
but all the results had not been verified. Therefore, it is hoped
that this study will promote more scholars to participate in the
analysis of the efficacy of trimetazidine and plasmin combined with
alprostadil in lower extremity arteriosclerosis obliterans, to
validate our results and provide better reference for clinical
treatment.
In conclusion, both trimetazidine and plasmin
combined with alprostadil can effectively treat lower extremity
arteriosclerosis obliterans. The former is better than the latter
in improving exercise capacity, but the latter is better than the
former in improving blood flow perfusion in patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and YW drafted the manuscript and worked on the
treatment methods. SX and YB were responsible for observation
indicators analysis. NL and SZ assisted with efficacy evaluation
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Yantai Yuhuangding Hospital (Yantai, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Conte MS, Pomposelli FB, Clair DG,
Geraghty PJ, McKinsey JF, Mills JL, Moneta GL, Murad MH, Powell RJ,
Reed AB, et al Society for Vascular Surgery Lower Extremity
Guidelines Writing Group; Society for Vascular Surgery, : Society
for Vascular Surgery practice guidelines for atherosclerotic
occlusive disease of the lower extremities: Management of
asymptomatic disease and claudication. J Vasc Surg. 61
(Suppl):S2.S41–41S.e1. 2015. View Article : Google Scholar
|
|
2
|
Gerhard-Herman MD, Gornik HL, Barrett C,
Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG,
Hamburg NM, Kinlay S, et al: 2016 AHA/ACC Guideline on the
Management of Patients With Lower Extremity Peripheral Artery
Disease: Executive summary: A report of the American College of
Cardiology/American Heart Association Task Force on Clinical
Practice Guidelines. J Am Coll Cardiol. 69:1465–1508. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hinchliffe RJ, Brownrigg JR, Apelqvist J,
Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE
and Schaper NC; International Working Group on the Diabetic Foot, :
IWGDF guidance on the diagnosis, prognosis and management of
peripheral artery disease in patients with foot ulcers in diabetes.
Diabetes Metab Res Rev. 32 (Suppl 1):37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaff MR, White CJ, Hiatt WR, Fowkes GR,
Dormandy J, Razavi M, Reekers J and Norgren L: An update on methods
for revascularization and expansion of the TASC lesion
classification to include below-the-knee arteries: A supplement to
the Inter-Society Consensus for the Management of Peripheral
Arterial Disease (TASC II): The TASC Steering Comittee. Ann Vasc
Dis. 8:343–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McDermott MM: Lower extremity
manifestations of peripheral artery disease: The pathophysiologic
and functional implications of leg ischemia. Circ Res.
116:1540–1550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, Hu Y, Zeng H, Li L, Zhao J, Zhao
J, Liu F, Bao Y and Jia W: Serum fibroblast growth factor 21 levels
is associated with lower extremity atherosclerotic disease in
Chinese female diabetic patients. Cardiovasc Diabetol. 14:322015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aboyans V, Ricco JB, Bartelink MEL, Björck
M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S,
et al ESC Scientific Document Group, : 2017 ESC Guidelines on the
Diagnosis and Treatment of Peripheral Arterial Diseases, in
collaboration with the European Society for Vascular Surgery
(ESVS): Document covering atherosclerotic disease of extracranial
carotid and vertebral, mesenteric, renal, upper and lower extremity
arteries. Endorsed by the European Stroke Organization (ESO)The
Task Force for the Diagnosis and Treatment of Peripheral Arterial
Diseases of the European Society of Cardiology (ESC) and of the
European Society for Vascular Surgery (ESVS). Eur Heart J.
39:763–816. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel MR, Conte MS, Cutlip DE, Dib N,
Geraghty P, Gray W, Hiatt WR, Ho M, Ikeda K, Ikeno F, et al:
Evaluation and treatment of patients with lower extremity
peripheral artery disease: Consensus definitions from Peripheral
Academic Research Consortium (PARC). J Am Coll Cardiol. 65:931–941.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu YS, Li DX, Zhang M and Jiang TM:
Trimetazidine hydrochloride as a new treatment for patients with
peripheral vascular disease - an exploratory trial. Eur Rev Med
Pharmacol Sci. 20:188–193. 2016.PubMed/NCBI
|
|
10
|
Lawall H, Pokrovsky A, Checinski P,
Ratushnyuk A, Hamm G, Randerath O, Grieger F and Bentz JWG:
Efficacy and safety of alprostadil in patients with peripheral
arterial occlusive disease Fontaine Stage IV: Results of a placebo
controlled randomised multicentre trial (ESPECIAL). Eur J Vasc
Endovasc Surg. 53:559–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang PP, Li SZ, Han MZ, Xiao ZJ, Yang RC,
Qiu LG and Han ZC: Autologous transplantation of peripheral blood
stem cells as an effective therapeutic approach for severe
arteriosclerosis obliterans of lower extremities. Thromb Haemost.
91:606–609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng GY, Zhan JH, Luo JH and Cheng X:
Effect of alprostadil on wound healing of scalded rats and the
mechanism. Zhonghua Shao Shang Za Zhi. 34:380–385. 2018.(In
Chinese). PubMed/NCBI
|
|
13
|
Stary CM, Kohin S, Samaja M, Howlett RA
and Hogan MC: Trimetazidine reduces basal cytosolic Ca2+
concentration during hypoxia in single Xenopus skeletal myocytes.
Exp Physiol. 88:415–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun MZ, Liu S and Greenaway FT:
Characterization of a fibrinolytic enzyme (ussurenase) from
Agkistrodon blomhoffii ussurensis snake venom: Insights into
the effects of Ca2+ on function and structure. Biochim
Biophys Acta. 1764:1340–1348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stoner MC, Calligaro KD, Chaer RA, Dietzek
AM, Farber A, Guzman RJ, Hamdan AD, Landry GJ and Yamaguchi DJ;
Society for Vascular Surgery, : Reporting standards of the Society
for Vascular Surgery for endovascular treatment of chronic lower
extremity peripheral artery disease. J Vasc Surg. 64:e1–e21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reinecke H, Unrath M, Freisinger E,
Bunzemeier H, Meyborg M, Lüders F, Gebauer K, Roeder N, Berger K
and Malyar NM: Peripheral arterial disease and critical limb
ischaemia: Still poor outcomes and lack of guideline adherence. Eur
Heart J. 36:932–938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horie T, Kimura T and Ono K: Emerging
novel biomarkers for arteriosclerosis obliterans. J Atheroscler
Thromb. 23:171–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoshino J, Ubara Y, Hara S, Sogawa Y,
Suwabe T, Higa Y, Nakanishi S, Sawa N, Katori H, Takemoto F, et al:
Quality of life improvement and long-term effects of peripheral
blood mononuclear cell transplantation for severe arteriosclerosis
obliterans in diabetic patients on dialysis. Circ J. 71:1193–1198.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bandinelli F, Bartoli F, Perfetto E, Del
Rosso A, Moggi-Pignone A, Guiducci S, Cinelli M, Fatini C, Generini
S, Gabrielli A, et al: The fibrinolytic system components are
increased in systemic sclerosis and modulated by alprostadil
(alpha1 ciclodestryn). Clin Exp Rheumatol. 23:671–677.
2005.PubMed/NCBI
|
|
20
|
Saigawa T, Kato K, Ozawa T, Toba K,
Makiyama Y, Minagawa S, Hashimoto S, Furukawa T, Nakamura Y, Hanawa
H, et al: Clinical application of bone marrow implantation in
patients with arteriosclerosis obliterans, and the association
between efficacy and the number of implanted bone marrow cells.
Circ J. 68:1189–1193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Z, Chen JM, Huang H, Kuznicki M, Zheng
S, Sun W, Quan N, Wang L, Yang H, Guo HM, et al: The protective
effect of trimetazidine on myocardial ischemia/reperfusion injury
through activating AMPK and ERK signaling pathway. Metabolism.
65:122–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rehberger-Likozar A and Šebeštjen M:
Influence of trimetazidine and ranolazine on endothelial function
in patients with ischemic heart disease. Coron Artery Dis.
26:651–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lacroix R, Sabatier F, Mialhe A, Basire A,
Pannell R, Borghi H, Robert S, Lamy E, Plawinski L, Camoin-Jau L,
et al: Activation of plasminogen into plasmin at the surface of
endothelial microparticles: A mechanism that modulates angiogenic
properties of endothelial progenitor cells in vitro. Blood.
110:2432–2439. 2007. View Article : Google Scholar : PubMed/NCBI
|