Introduction
Early spontaneous abortion is a common complication
in early pregnancy. Abortion before 12 weeks of pregnancy is called
early abortion (1). Relevant
statistics show that the incidence of abortion accounts for 11–16%
of all pregnancies, with that of early spontaneous abortion
accounting for more than 79% of the total number of abortion
(2). Reasons for abortion are very
complicated, such as virus, infection of protozoa, pregnant women
with cervical incompetence, ischemia and hypoxia, abnormal rise in
blood sugar, abnormal thyroid function, and unhealthy living habits
such as smoking, drug use and drinking (3–5). Studies
have shown that genetic factors, immune factors, embryonic
chromosomal abnormalities and endocrine hormone imbalance of
pregnant women during pregnancy can affect pregnancy. Immune
factors and endocrine hormone imbalance during pregnancy are
important causes of early spontaneous abortion, accounting for
48–59% of them (6). Hormones during
pregnancy such as β-human chorionic gonadotropin (β-HCG),
progesterone and estrogen all play important roles in maintaining
pregnancy (7,8).
A growing number of studies have found that certain
factors can cause changes in the expression of microRNA (miRNA),
which affects their regulated target genes, leading to changes in
hormone levels (9). Studies have
found that miR-99a is highly expressed in endometrial cancer
tissues, presumably related to abnormal hormone levels, but its
specific role in early pregnancy is still unclear (10). With the continuous development of
cytokine research, scholars believe that TGF-β1, a cytokine with
strong immunosuppressive effect that is closely related to
pregnancy, can protect the embryo (11). In the present study, the expression
of TGF-β1 and miR-99a in early spontaneous abortion and the
correlation with the levels of serum β-HCG, progesterone and
estrogen during pregnancy were investigated.
Patients and methods
Specimen collection
A total of 70 pregnant women with early spontaneous
abortion diagnosed in Jining No. 1 People's Hospital (Jining,
China) from February 1, 2015 to May 1, 2018 were selected as the
study group, with an age range of 22–40 years and an average age of
25.79±7.38 years. Another 83 normal pregnant women who chose
abortion in the same period were selected as the control group,
with an age range of 22–38 years and an average age of 26.04±5.24
years.
The inclusion and exclusion criteria were: i) Only
pregnant women who were admitted to Jining No. 1 People's Hospital
were included as subjects, without abortion caused by chromosomes,
anatomy, endocrine abnormalities, reproductive system infections
and autoimmune diseases; ii) In this study, patients with
hypertension, hepatitis B virus, gallstone, AIDS and various blood
diseases were excluded. The control group excluded pregnant women
with abnormal pregnancy history. Subjects signed an informed
consent form. This study was approved by the Ethics Committee of
Jining No. 1 People's Hospital.
Main reagents and instruments
TGF-β1 ELISA detection kit (Shanghai Guyan
Biotechnology Co., Ltd., Shanghai, China), progesterone ELISA
detection kit (Qingdao Jieshikang Biotechnology Co., Ltd., Qingdao,
China), estrogen ELISA detection kit (Qingdao Jieshikang
Biotechnology Co., Ltd.), serum β-HCG ELISA detection kit (Qingdao
Jieshikang Biotechnology Co., Ltd.), and enzyme microplate reader
(Shanghai Haozhuang Instrument Co., Ltd., Shanghai, China) were
used in the study.
RNA extraction reagent TRIzol (Nanjing Kebai
Biotechnology Co., Ltd., Nanjing, China), reverse transcription kit
(Nanjing Kebai Biotechnology Co., Ltd.), miR-99a PCR kit (Thermo
Fisher Scientific Co., Ltd., Shanghai, China), and a fluorescence
quantitative PCR instrument (Xian Tianlong Technology Co., Ltd.,
Xian, China) were used in the study. The primers for miR-99a and
its internal reference U6 in the kit are shown in Table I.
 | Table I.Primers for miR-99a and its internal
reference U6. |
Table I.
Primers for miR-99a and its internal
reference U6.
| Group | Upstream primer
sequence | Downstream primer
sequence |
|---|
| miR-99a |
5′-ACACTCCAGCTGGGAACCCGTAGATCCG-3′ |
5′-GGTGTCGTGCAGTCG-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACATATACT-3′ |
5′-ACGCTTCACGAATTTGCGTGTC-3′ |
Detection methods
ELISA detection of TGF-β1 and levels
of serum β-HCG, progesterone and estrogen during pregnancy
Elbow venous blood was extracted from all the
subjects in a fasting state with a vacuum blood tube before 9:00 in
the morning, centrifuged at 3,010 × g for 5 min at 4°C, and then
placed in a cryogenic refrigerator at 4°C for use. The experimental
procedure was based on ELISA detection kit instructions related to
TGF-β1, serum β-HCG, progesterone and estrogen during pregnancy. A
sample well to be tested, a standard well and a blank well were
set. A total of 100 µl of sample diluent was added to the blank
well, 100 µl of sample to be tested or standard sample added to
remaining wells, mixed well and sealed with a microplate sealer,
prior to incubatoin at 37°C for 30 min. The microplate sealer was
carefully removed, with the liquid discarded, and dried. Each well
was filled with washing solution, discarded after standing for 30
sec, repeating 5 times and pat dried. A total of 50 µl of the
enzyme-labeled reagent was added to each well except for the blank
well, washed again after incubation at 37°C for 30 min. The
developer was added to each well, mixed well, coloring at 37°C for
15 min in the dark. A total of 50 µl of stop solution was added to
each well. The OD value of each well was measured immediately at a
wavelength of 450 nm using an enzyme microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The concentrations of
TGF-β1, serum β-HCG, progesterone and estrogen during pregnancy
were calculated.
RT-qPCR detection of miR-99a
The TRIzol method was used to extract total RNA from
blood serum which was then stored in a refrigerator at −80°C until
use. The total RNA was reverse-transcribed using Reverse
Transcription kit. The reaction system was: 1 µl of M-MLV, 1 µl of
Olig (dT), 0.5 µl of RNase inhibitor, 1 µl of dNTPs, RNAse-free
water added to 15 µl. Incubation was performed at 38°C for 60 min,
and 1 µl of cDNA was taken at 85°C for 5 sec, stored at 4°C.
RT-qPCR was used to detect the level of miR-99a in the serum, with
its operations referring to the miR-99a SYBR-Green PCR kit protocol
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The reaction
procedure was: at 95°C for 1 min, at 95°C for 15 sec, and at 60°C
for 20 sec, for a total of 39 cycles. Three replicate wells were
set up for each sample. This experiment was repeated 3 times, with
U6 as an internal control. All reactions were run 3 times, with U6
as a control. The 2−ΔCq method was used to calculate the
expression of mir-99a (12).
Statistical analysis
SPSS 17.0 (Asia Analytics Formerly SPSS China)
statistical analysis software was used to analyze the experimental
data. The t-test was used for the comparison of mean between two
groups, and partial correlation analysis for the correlation.
P<0.05 indicates the data difference is statistically
significant.
Results
Comparison of levels of β-HCG,
progesterone and estrogen between the study and control group
The expression of β-HCG in the study group and the
control group was (4802.00±1694.00) mU/l and (10315.00±3999.00)
mU/l, respectively, and that of β-HCG was significantly lower in
the study group than that in the control group, with a
statistically significant difference (P<0.01). Expression of
progesterone in the study group and the control group was
(30.19±10.57) nmol/l and (108.60±40.60) nmol/l, respectively, and
that of progesterone was significantly lower in the study group
than that in the control group, with a statistically significant
difference (P<0.01). Expression of estrogen in the study group
and the control group was (96.33±10.76) nmol/l and (289.14±20.16)
nmol/l, respectively, and that of estrogen was significantly lower
in the study group than that in the control group, with a
statistically significant difference (P<0.01) (Table II).
 | Table II.Comparison of levels of β-HCG,
progesterone and estrogen and body mass index between the study and
control groups. |
Table II.
Comparison of levels of β-HCG,
progesterone and estrogen and body mass index between the study and
control groups.
| Group | Study group
(n=70) | Control group
(n=83) | t | P-value |
|---|
| β-HCG (mU/l) | 4802.00±1694.00 | 10315.00±3999.00 | 10.750 | <0.001 |
| Progesterone
(nmol/l) | 30.19±10.57 | 108.60±40.60 | 15.710 | <0.001 |
| Estrogen (ng/l) | 96.33±10.76 | 289.14±20.16 | 71.830 | <0.001 |
Difference in expression of TGF-β1 and
miR-99a between the two groups
The expression of TGF-β1 in the study group and the
control group was (20.45±10.78) ng/l and (96.01±11.59) ng/l,
respectively, and that of TGF-β1 was significantly lower in the
study group than that in the control group, with a statistically
significant difference (P<0.001). Expression of miR-99a in the
study group and the control group was (1.71±0.15) and (0.70±0.03),
respectively, and that of miR-99a was significantly higher in the
study group than that in the control group, with a statistically
significant difference (P<0.001) (Table III).
 | Table III.Difference in expression of TGF-β1 and
miR-99a in the study and control groups. |
Table III.
Difference in expression of TGF-β1 and
miR-99a in the study and control groups.
| Factor | Study group
(n=70) | Control group
(n=8) | t | P-value |
|---|
| TGF-β1 (ng/l) | 20.45±10.78 | 96.01±11.59 | 41.470 | <0.001 |
| miR-99a | 1.71±0.15 | 0.70±0.03 | 59.970 | <0.001 |
Correlation of TGF-β1 and miR-99a with
levels of serum β-HCG, progesterone and estrogen in study group of
patients
Correlation of TGF-β1 with levels of
serum β-HCG, progesterone and estrogen in the study group of
patients
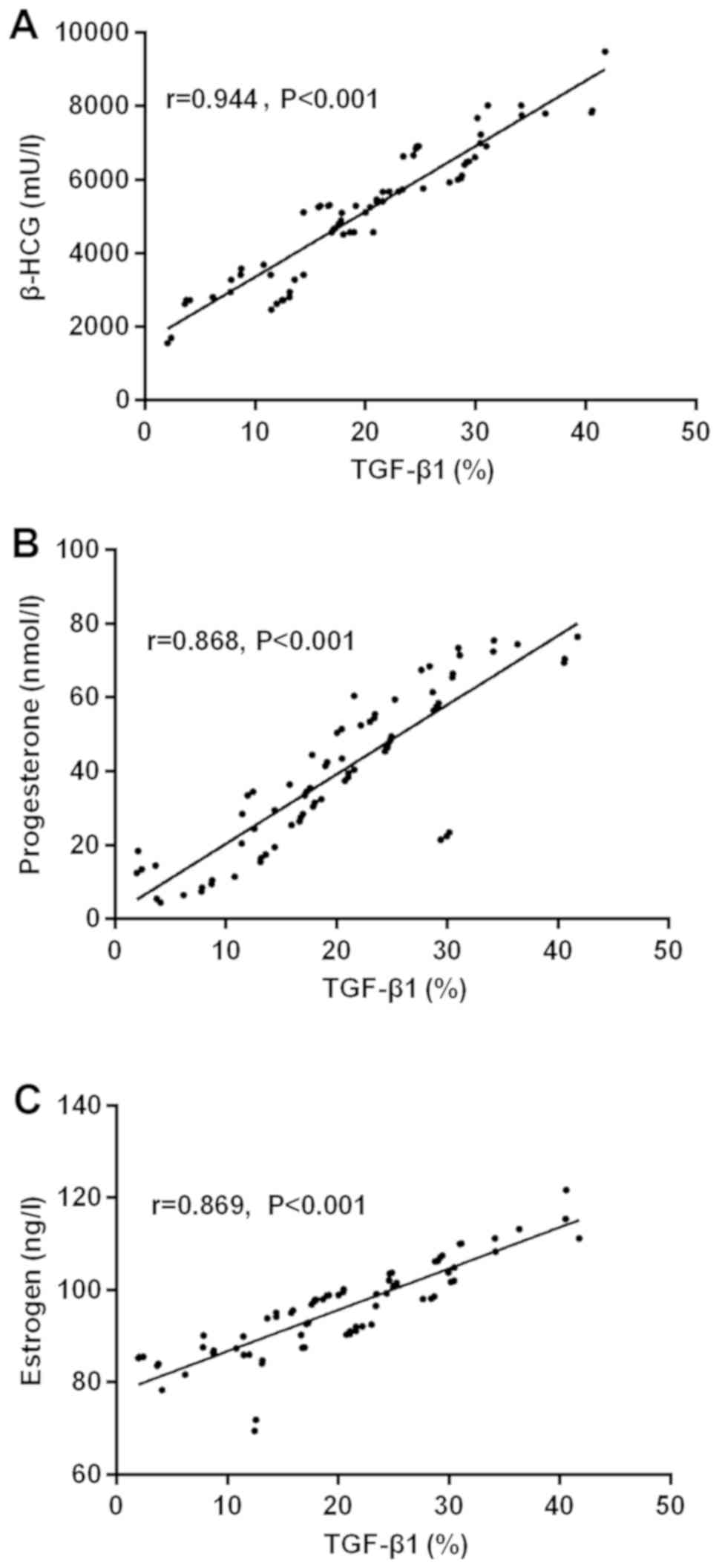
The results of partial correlation analysis showed
that the expression of TGF-β1 was positively correlated with that
of β-HCG in the serum of pregnant women with early spontaneous
abortion (r=0.944, P<0.001). Expression of TGF-β1 was positively
correlated with that of progesterone in the serum of pregnant women
with early spontaneous abortion (r=0.868, P<0.001). TGF-β1 was
positively correlated with that of estrogen in the serum of
pregnant women with early spontaneous abortion (r=0.869,
P<0.001) (Fig. 1).
Correlation of miR-99a with levels of
serum β-HCG, progesterone and estrogen in the study group of
patients
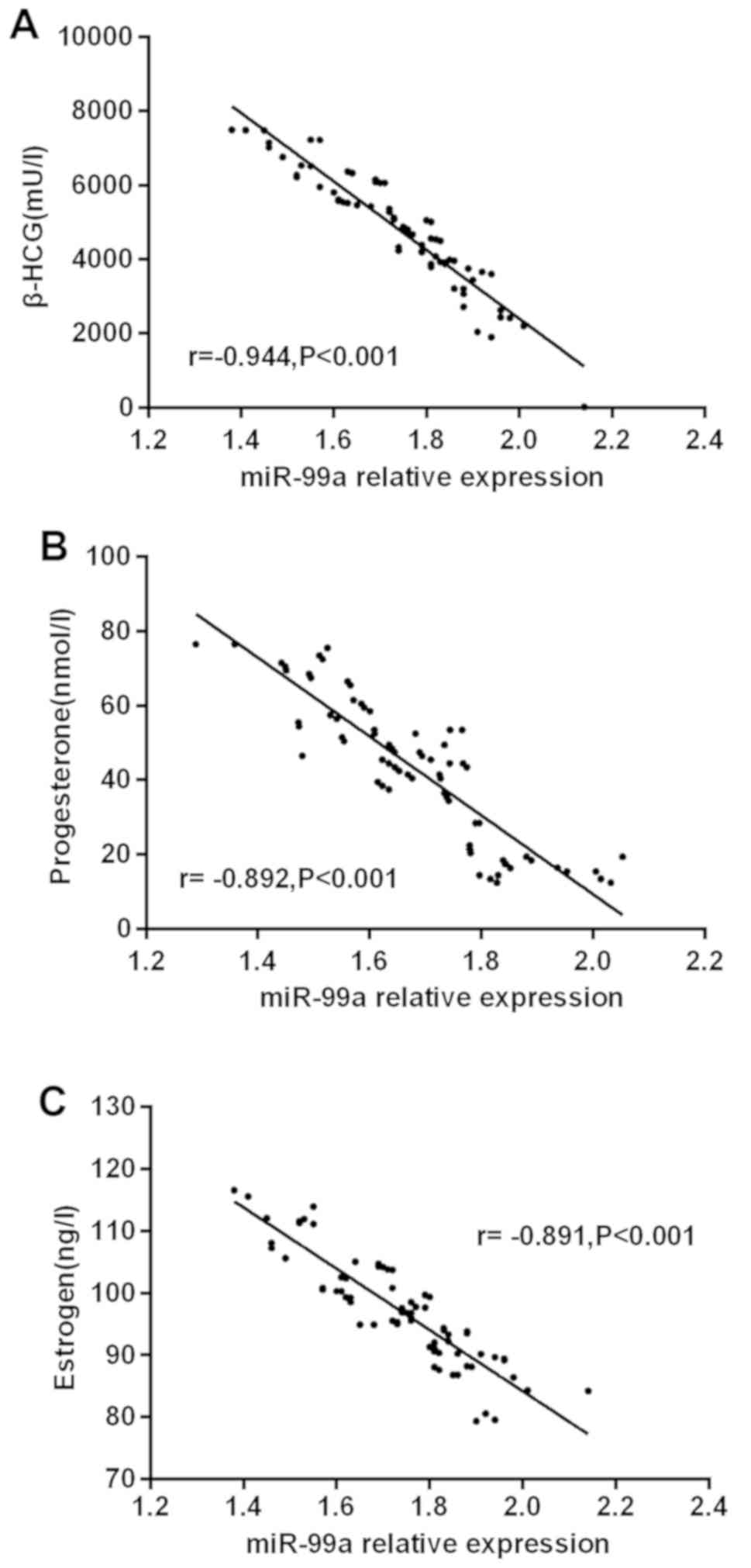
The results of partial correlation analysis showed
that the expression of miR-99a was negatively correlated with that
of β-HCG in the serum of pregnant women with early spontaneous
abortion (r=−0.944, P<0.001). Eexpression of miR-99a was
negatively correlated with that of progesterone in the serum of
pregnant women with early spontaneous abortion (r=−0.892,
P<0.001), and that of miR-99a was negatively correlated with
that of estrogen in the serum of pregnant women with early
spontaneous abortion (r=−0.891, P<0.001) (Fig. 2).
Discussion
The main reasons of abortion in early pregnancy are
the lack of nutrition provided by the mother causes the fetal
development in the uterus to cease, or the abdomen of the pregnant
woman is squeezed and injured, causing the fetal position to be
unstable or the development of the fetus itself is unhealthy. In
addition to the mothers nutritional supply to the fetus and
external physical factors, the chromosome of the mother to the
fetus and endocrine imbalance are also main reasons of spontaneous
abortion in early pregnancy (13,14).
Synthesizing and secreting endocrine hormones and different
hormones to enter the blood, endocrine cells have different effects
on different tissue cell metabolism of the human body, producing
different physiological activities (15). The important hormones during
pregnancy are β-HCG, progesterone and estrogen, which have
important effects on physiological activities in pregnant women and
the fetus (16). β-HCG is a
glycoprotein that has a great influence on maintaining the
stability of the uterine environment (17). Estrogen prepares the implantation of
the embryo in the uterus (18). On
the basis of estrogenic effects during pregnancy, progesterone
keeps the fertilized egg implanted in the uterus and maintains a
smooth pregnancy (19). The
detection of β-HCG, progesterone and estrogen is important for the
diagnosis of early pregnancy. Studies have shown that TGF-β1 and
miR-99a are associated with changes in pathological phenomena of
pregnancy and hormone levels during pregnancy. TGF-β1 expression is
low in the serum of pregnant womens peripheral blood, and the
elevated expression level of miR-99a will inhibit hormone
expression levels during pregnancy (16,20). In
this study, the expression of TGF-β1 and miR-99a in early
spontaneous abortion and the correlation with the levels of serum
β-HCG, progesterone and estrogen during pregnancy were
investigated.
First, the levels of β-HCG, progesterone and
estrogen were detected using ELISA. The results showed that the
expression was significantly lower in the study group than in the
control group, with a statistically significant difference
(P<0.001). It is speculated that hormone levels during pregnancy
in patients with early pregnancy abortion are significantly lower
than those in normal pregnancy. In addition, a large number of
clinical studies show that hormone levels during pregnancy in
normal pregnancy are significantly higher than those in patients
with early pregnancy abortion, consistent with our findings
(21). Next, ELISA and qRT-PCR were
used to detect TGF-β1 and miR-99a, respectively, in the serum of
included patients with early spontaneous abortion. The results
showed that the expression of TGF-β1 was significantly lower in the
study group than that in the control group, with a statistically
significant difference (P<0.001), that of miR-99a was
significantly higher in the study group than that in the control
group, with a statistically significant difference (P<0.001).
This is similar to the findings of Turcatel et al (22). qRT-PCR was used by them to detect
TGF-β1 and miR-99a in the serum of patients with abortion. It is
found that the expression of TGF-β1 in the serum of patients with
abortion is significantly lower than that of normal pregnant women,
while that of miR-99a in the serum of patients with abortion is
significantly higher than that of normal pregnant women, with
statistically significant difference (all P<0.05). Finally, the
correlation of TGF-β1 and miR-99a in the serum with the levels of
β-HCG, progesterone and estrogen was analyzed in patients with
early spontaneous abortion. The results showed that TGF-β1 was
positively correlated with the levels of β-HCG, progesterone and
estrogen in the serum of patients (r=0.944, 0.868, 0.869,
P<0.001), but that of miR-99a was negatively correlated with
them (r=−0.944, −0.892, −0.891, all P<0.001). In the detection
of hormone levels during pregnancy in patients with abortion, Dong
et al (23) found that TGF-β1
and miR-99a in the serum of patients are closely related to hormone
levels during pregnancy. In addition, TGF-β1 in the serum of
patients is positively correlated with hormone levels during
pregnancy, while that of miR-99a is negatively correlated.
In this study, the number of subjects included was
small, which may cause some contingency on the experimental
results.
In summary, miR-99a is highly expressed in the serum
of patients with early spontaneous abortion, but TGF-β1 expression
is low. The expression levels of the two factors are related to
hormone levels during pregnancy. By monitoring the expression
levels of miR-99a and TGF-β1 in the serum of pregnant women,
according to the correlation the hormones during pregnancy, the
concentration in the serum of their peripheral blood can be
adjusted. Compared with monitoring hormone levels during pregnancy,
this adjustment is more convenient and intuitive. miR-99a and
TGF-β1 are expected to be new candidate molecular diagnostic
markers in the diagnosis of early spontaneous abortion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
JX performed the ELISA and wrote the manuscript. JX
and YC were responsible for PCR. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Jining No. 1 People's Hospital (Jining, China). Patients who
participated in the present study had complete clinical data.
Signed informed consents were obtained from the patients or the
guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mekinian A, Cohen J, Kayem G, Carbillon L,
Nicaise-Roland P, Gaugler B, Darai E, Bornes M and Fain O:
Unexplained recurrent early miscarriages: Role of immunomodulation?
Rev Med Interne. 38:264–268. 2017.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tunç E, Tanrıverdi N, Demirhan O,
Süleymanova D and Çetinel N: Chromosomal analyses of 1510 couples
who have experienced recurrent spontaneous abortions. Reprod Biomed
Online. 32:414–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Granat NE, Frolova OG, Iankova MF and
Ilovaĭskaia SF: Causes of miscarriage. Med Sestra. 37:37–40.
1978.(In Russian). PubMed/NCBI
|
|
4
|
Dean DD, Agarwal S and Tripathi P:
Connecting links between genetic factors defining ovarian reserve
and recurrent miscarriages. J Assist Reprod Genet. 35:2121–2128.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Regan L and Rai R: Epidemiology and the
medical causes of miscarriage. Best Pract Res Clin Obstet Gynaecol.
14:839–854. 2000. View Article : Google Scholar
|
|
6
|
Makino T: Recurrent reproductive wastage
and immunologic factors. Am J Reprod Immunol. 48:266–268. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo Y and He GP: Correlative analysis of
postpartum depression. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
32:460–465. 2007.(In Chinese). PubMed/NCBI
|
|
8
|
Yahi D, Ojo NA and Mshelia GD: Effects of
dexamethasone on progesterone and estrogen profiles and uterine
progesterone receptor localization during pregnancy in Sahel goat
in Semi-Arid region. J Anim Sci Technol. 59:122017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferraro L, Ravo M, Nassa G, Tarallo R, De
Filippo MR, Giurato G, Cirillo F, Stellato C, Silvestro S,
Cantarella C, et al: Effects of oestrogen on microRNA expression in
hormone-responsive breast cancer cells. Horm Cancer. 3:65–78. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Zhang Z, Zhang X, Lin Y, Luo T, Xiao
Z and Zhou Q: A dual PI3K/AKT/mTOR signaling inhibitor miR-99a
suppresses endometrial carcinoma. Am J Transl Res. 8:719–731.
2016.PubMed/NCBI
|
|
11
|
Lygnos MC, Pappa KI, Papadaki HA, Relakis
C, Koumantakis E, Anagnou NP and Eliopoulos GD: Changes in maternal
plasma levels of VEGF, bFGF, TGF-beta1, ET-1 and sKL during
uncomplicated pregnancy, hypertensive pregnancy and gestational
diabetes. In Vivo. 20:157–163. 2006.PubMed/NCBI
|
|
12
|
Livak KJ and Scmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HK, Luo FW, Geng Q, Li J, Liu QZ,
Chen WB, Li F and Xie JS: Analysis of fetal chromosomal karyotype
and etiology in 252 cases of early spontaneous abortion. Zhonghua
Yi Xue Yi Chuan Xue Za Zhi. 28:575–578. 2011.(In Chinese).
PubMed/NCBI
|
|
14
|
Tara F, Lotfalizadeh M and Moeindarbari S:
The effect of diagnostic amniocentesis and its complications on
early spontaneous abortion. Electron Physician. 8:2787–2792. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alter RC: Abortion outcome as a function
of sex-role identification. Psychol Women Q. 8:211–233. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gol M, Altunyurt S, Cimrin D, Guclu S,
Bagci M and Demir N: Different maternal serum hCG levels in
pregnant women with female and male fetuses: Does fetal
hypophyseal--adrenal--gonadal axis play a role? J Perinat Med.
32:342–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Lorenzo G, Ceccarello M, Cecotti V,
Ronfani L, Monasta L, Vecchi Brumatti L, Montico M and DOttavio G:
First trimester maternal serum PIGF, free β-hCG, PAPP-A, PP-13,
uterine artery Doppler and maternal history for the prediction of
preeclampsia. Placenta. 33:495–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravindranath N and Moudgal RN: Effect of a
specific estrogen antibody on pregnancy establishment in the bonnet
monkey (Macaca radiata). Fertil Steril. 54:1162–1167. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dobrzyn K, Smolinska N, Szeszko K, Kiezun
M, Maleszka A, Rytelewska E and Kaminski T: Effect of progesterone
on adiponectin system in the porcine uterus during early pregnancy.
J Anim Sci. 95:338–352. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshihara A, Noh JY, Mukasa K, Suzuki M,
Ohye H, Matsumoto M, Kunii Y, Watanabe N, Suzuki N, Kameda T, et
al: Serum human chorionic gonadotropin levels and thyroid hormone
levels in gestational transient thyrotoxicosis: Is the serum hCG
level useful for differentiating between active Graves disease and
GTT? Endocr J. 62:557–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown JB, Evans JH, Beischer NA, Campbell
DG and Fortune DW: Hormone levels in threatened abortion. J Obstet
Gynaecol Br Commonw. 77:690–700. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turcatel G, Rubin N, El-Hashash A and
Warburton D: MIR-99a and MIR-99b modulate TGF-β induced epithelial
to mesenchymal plasticity in normal murine mammary gland cells.
PLoS One. 7:e310322012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong F, Zhang Y, Xia F, Yang Y, Xiong S,
Jin L and Zhang J: Genome-wide miRNA profiling of villus and
decidua of recurrent spontaneous abortion patients. Reproduction.
148:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|