Introduction
Renal cell carcinoma (RCC) is the most common type
of malignancy of the kidney, accounts for 2–3% of all malignancies
in adults (1) and its incidence and
mortality are currently on the rise (2). In the USA, RCC is the 6th leading cause
of cancer-associated deaths in men and the 8th leading cause in
women (3). At present, surgery is
the standard treatment for primary RCC, while seven targeted
therapies have been approved for metastatic RCC by the Food and
Drug Association (4). However, the
treatment of RCC remains a huge challenge due to the generally poor
response to chemotherapy and radiotherapy (5–7). Despite
persistent research efforts in the past several decades, only
little progress has been made regarding the early diagnosis and
treatment of RCC (8). To reach this
goal, it is necessary to identify early diagnostic biomarkers and
therapeutic targets using novel approaches, such as a
bioinformatics search.
Cancer stem cells (CSCs) were first identified in 3
types of solid tumor in the early 2000s (9). Now, CSCs have been identified in
various cancer types, including RCC (10). Targeting of CSCs has become an
important strategy to treat cancer. RCC exhibits a poor response to
chemotherapy and radiotherapy due to the survival of CSCs (5–7), and it
is important to identify molecular markers to isolate and
characterize the CSCs among the tumor cells; of note, targeting of
CSCs in RCC has provided a novel treatment strategy, particularly
for metastatic RCC (11).
Recently, CD105 has been described as a novel RCC
CSC marker (12). Therefore, the
present study aimed to assess whether tumoral CD105 has a
prognostic value in RCC. CD105 (endoglin) is the receptor for
transforming growth factor (TGF). CD105 regulates TGF-β signaling
by interacting with TGF-β receptors I and/or II. Several studies
have indicated that CD105 contributes to the development of blood
vessels and angiogenesis, and is essential for tumor growth and the
development of metastasis. In addition, CD105 is a prominent marker
for mesenchymal stem cells (MSCs) (13–15). In
RCC, CD105 has been reported as a potential prognostic marker and
CSC marker. Bussolati et al (16), Hasmim et al (17) and Dallas et al (15) indicated that CD105+ RCC
cells had stronger features of CSCs compared with CD105−
cells. Furthermore, these CD105+ cells expressed MSC
markers including CD44, CD146 and CD73, embryonic stem cell markers
including Nanog and Oct4, and embryonic renal marker paired box-2,
but lacked differentiated epithelial markers. Recently, Saeednejad
Zanjani et al (13) performed
an analysis of 186 clear cell (cc)RCC samples, revealing that CD105
expression was associated with more aggressive tumor behavior, more
advanced disease and worse prognosis. However, in other types of
RCC, including papillary (p)RCC, it was not possible to confirm
CD105 as a CSC marker (12). Further
studies questioned the use of CD105 as a renal CSC marker, as
CD105− cells also exhibited CSC-like features (10,18).
These inconclusive and conflicting results suggest that CD105 in
different types of RCC requires further study.
The Cancer Genome Atlas (TCGA) database includes
gene expression data obtained by RNA-sequencing (seq) for cohorts
of 604 ccRCC, 320 pRCC and 89 chromophobe (ch)RCC cases with the
clinical outcome data available. In the present study, it was
attempted to assess the expression and function of CD105 in RCC
based on mining of TCGA data. The possible functional role of CD105
in RCC may have clinical implications. If CD105 has a function in
RCC, strategies to target CD105 in RCC may represent a novel
therapeutic strategy.
Materials and methods
Data retrieval from TCGA
Gene expression data obtained by RNA-seq for cohorts
of 604 ccRCC, 320 pRCC and 89 chRCC cases that have clinical
outcome data available were extracted from TCGA (http://xena.ucsc.edu/). These datasets included
~20,500 data-points each for ccRCC, pRCC and chRCC. Clinical
information for each patient, including survival status, time to
last follow-up and gender, was also extracted from TCGA. CD105 mRNA
and protein expression data and matching clinical information were
also retrieved from TCGA for these patients. In addition, data on
the CD105 copy number and matching clinical information were
retrieved from TCGA for 526 ccRCC patients.
Survival analysis
Patients were stratified into two groups (high and
low) based on the mean levels of CD105 mRNA expression or copy
number. Kaplan-Meier analysis was performed using GraphPad prism
(version 7; GraphPad Software Inc., La Jolla, CA, USA) or Xena
(http://xena.ucsc.edu/).
CD105-associated gene expression and
enriched pathway analysis
The whole gene expression (RNA-seq) data of the 10
patients with the highest CD105 expression and 10 patients with
lowest CD105 expression was obtained from TCGA. The differentially
expressed genes of these two groups were analysis by webMeV
(version 1.0; http://mev.tm4.org/#/datasets/tcga). Then the pathways
enriched by the upregulated and downregulated genes associated with
CD105 from the TCGA dataset were analysis by webMeV and the dataset
was calculated using the voom function.
Statistical analysis
All statistical analyses were carried out with SPSS
19.0 (IBM Corp., Armonk, NY, USA). Differences in mean values
between two groups were analyzed by a two-tailed Student's t-test
and the mean values of >2 groups were compared with one-way
analysis of variance. Multiple comparison between the groups was
performed using Student-Newman-Keuls method. P≤0.05 was considered
statistically significant.
Results
CD105 expression in tumor and normal
tissue in different cancer types
To confirm the expression of CD105, the expression
of CD105 in tumor and normal tissue from the TCGA database was
analyzed in different types of cancer using firebrowse software
(http://firebrowse.org/#). The results indicated
that the expression of CD105 is different in different cancer types
(Fig. 1A). The expression of CD105
in tumor tissue is higher compared normal tissue in patients with
thyroid carcinoma, while in patients with liver hepatocellular
carcinoma, the expression of CD105 was higher in normal tissue
compared with tumor tissue. Furthermore, it was identified that the
expression of CD105 in ccRCC tumor tissue was significantly higher
compared with that of normal renal tissue (P=0.03). However, for
pRCC and chRCC, the expression of CD105 in normal tissue was
significantly higher compared with that in the tumor tissue (P=0.04
and P=0.01; Fig. 1B). These results
suggest that CD105 may play an important role in ccRCC, but not in
pRCC and chRCC.
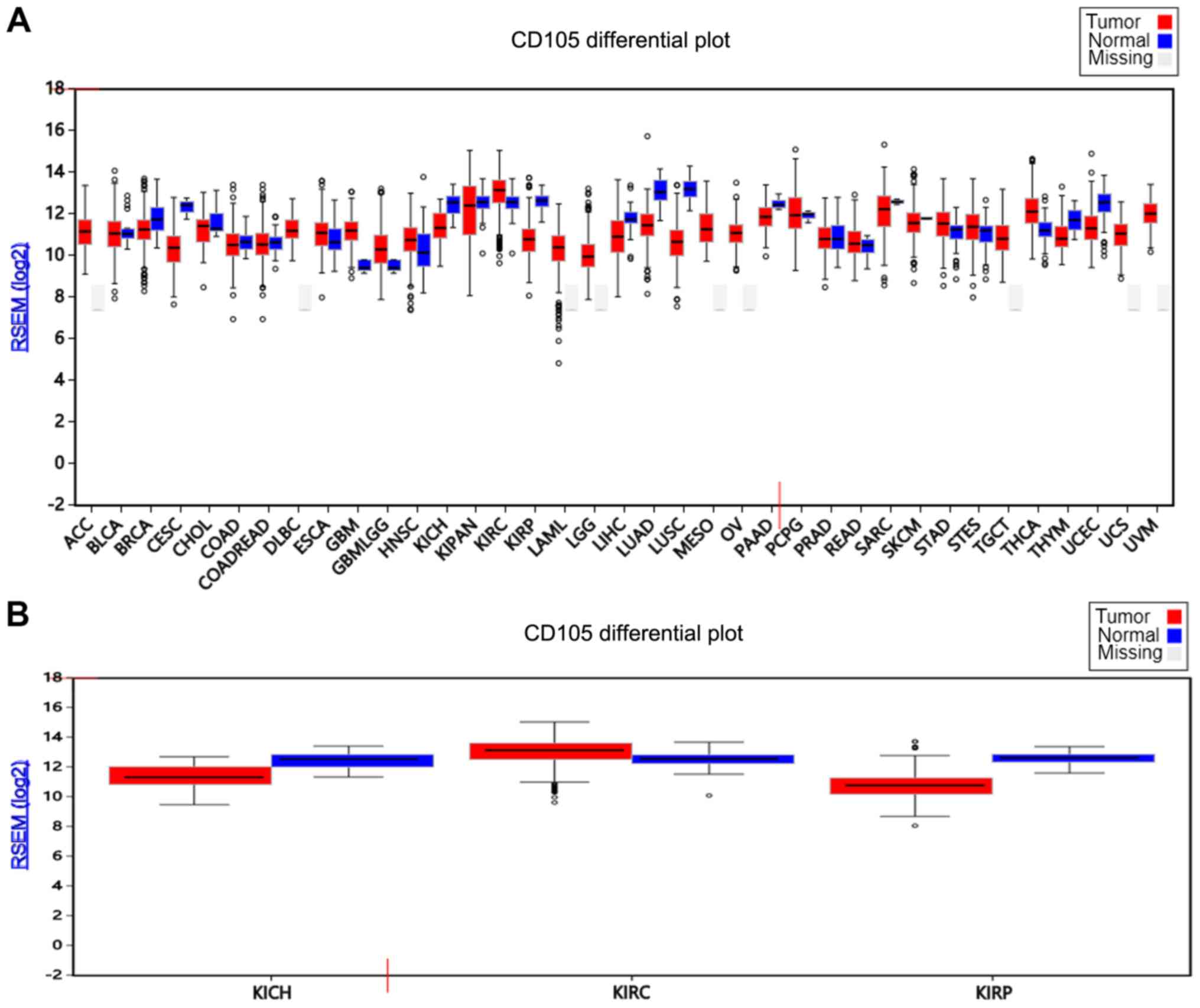 | Figure 1.CD105 expression in normal and tumor
tissues in different cancer types. (A) CD105 expression in
different cancer and normal tissues. (B) CD105 expression in three
renal cell carcinoma and normal kidney tissues. The top, middle and
bottom lines of the boxes indicate the third quartiles, median and
first quartiles, respectively. The whiskers indicate the standard
deviations and the circles indicate the values beyond the standard
deviations. The different cancer types are displayed on the x-axis.
ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma;
BRCA, breast invasive carcinoma; CESC, cervical squamous cell
carcinoma and endocervical adenocarcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; COADREAD,
colorectal cancer; DLBC, Lymphoid Neoplasm Diffuse Large B-cell
Lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme;
GBMLGG, glioma; HNSC, head and neck squamous cell carcinoma; KICH,
kidney chromophobe; KIPAN, pan-kidney cohort (KICH+KIRC+KIRP);
KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal
papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain
lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD,
lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO,
mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD,
pancreatic adenocarcinoma; PCPG, pheochromocytoma and
paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum
adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD,
stomach adenocarcinoma; STES, stomach and esophageal carcinoma;
TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM,
thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine
carcinosarcoma; UVM, uveal melanoma; RSEM, RNA-seq by
expectation-maximization. |
Prognostic value of CD105 expression
in RCC
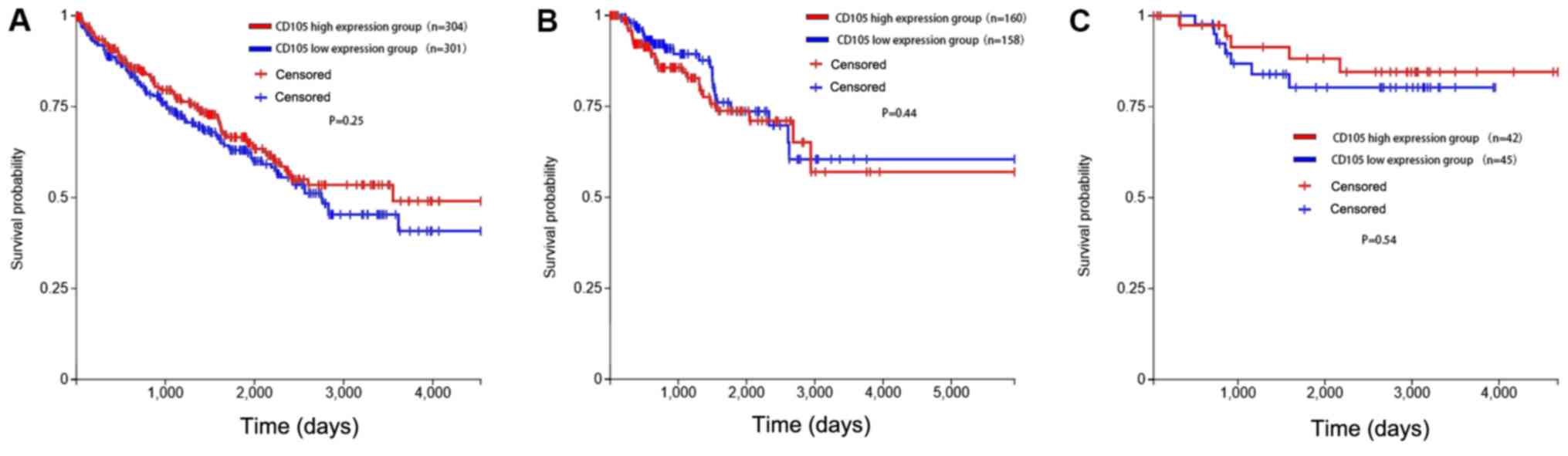
CD105 mRNA expression levels and clinical follow-up
data of 604 cases of ccRCC, the most common subtype of RCC, were
obtained from TCGA using Xena. The clinicopathological information
of the patients are listed in Table
I. Cases were assigned to CD105-high or CD105-low groups using
the median CD105 mRNA expression as a cutoff. Time to death was
plotted in a Kaplan-Meier curve for those cases exhibiting
expression of CD105 transcripts above the median (n=304) and equal
to or below the median (n=301). The results indicated no
significant difference between the two groups (P=0.25), although
the curves exhibited a trend, with those cases with a higher
expression of CD105 surviving for longer (Fig. 2A).
 | Table I.Clinicopathological information. |
Table I.
Clinicopathological information.
| Variable | ccRCC | pRCC | chRCC |
|---|
| Total number of
patients | 604 | 320 | 89 |
| Female | 210 | 79 | 41 |
| Male | 394 | 241 | 48 |
| Sex ratio
(male:female) | 1.88 | 2.81 | 1.22 |
| Age (mean ± standard
deviation) | 61±0.48 | 62±0.43 | 52±0.39 |
Similar analyses were performed for pRCC (n=318) and
chRCC patients (n=87). Among patients with pRCC, there was also no
significant difference in survival between the CD105 high and low
expression groups (P=0.44). Similar results were also obtained for
chRCC with a P-value of 0.54. These results suggest that, although
there was a trend of the CD105 high expression group surviving for
longer in the three types of RCC, CD105 expression had no
significant influence on survival.
The prognostic value of gender in RCC was also
assessed, as it was reported that RCC has a gender bias in
incidence with a male-to-female ratio of 2.3:1 (19). In the current study, the
male-to-female ratio was depicted in Table I. However, no significant impact of
gender on survival was identified in ccRCC, pRCC and chRCC (data
not shown). These results suggest that while the incidence of RCC
is higher in males, the outcome of RCC in males and females is
similar.
CD105 expression in different stages
and grades of RCC
The present study also assessed the association of
CD105 expression with different tumor (T) and metastasis (M)
stages, as well as the pathological stage, in the three types of
RCC. The results indicated that the expression of CD105 exhibited
no significant difference between M1 and M0 (P=0.90). Similarly, no
significance was obtained regarding the differences between
different T-stages (T1 vs. T2, P=0.24). However, CD105 was
significantly higher expressed in stage II than in stage I tumors
(P=0.04), while CD105 expression in stage IV tumors was lower than
that in stage II tumors, but with no statistical significance
(P=0.18). The same result was obtained for pRCC (Fig. 3B), where CD105 was higher expressed
in stage II than in stage I tumors (P=0.04). Furthermore, in pRCC,
CD105 was higher expressed in T3 than in T2 tumors (P=0.02).
However, the expression of CD105 exhibited no difference between
different T, M and pathological stages in chRCC (Fig. 3C). These results suggest that the
function of CD105 in RCC at different stages is complex.
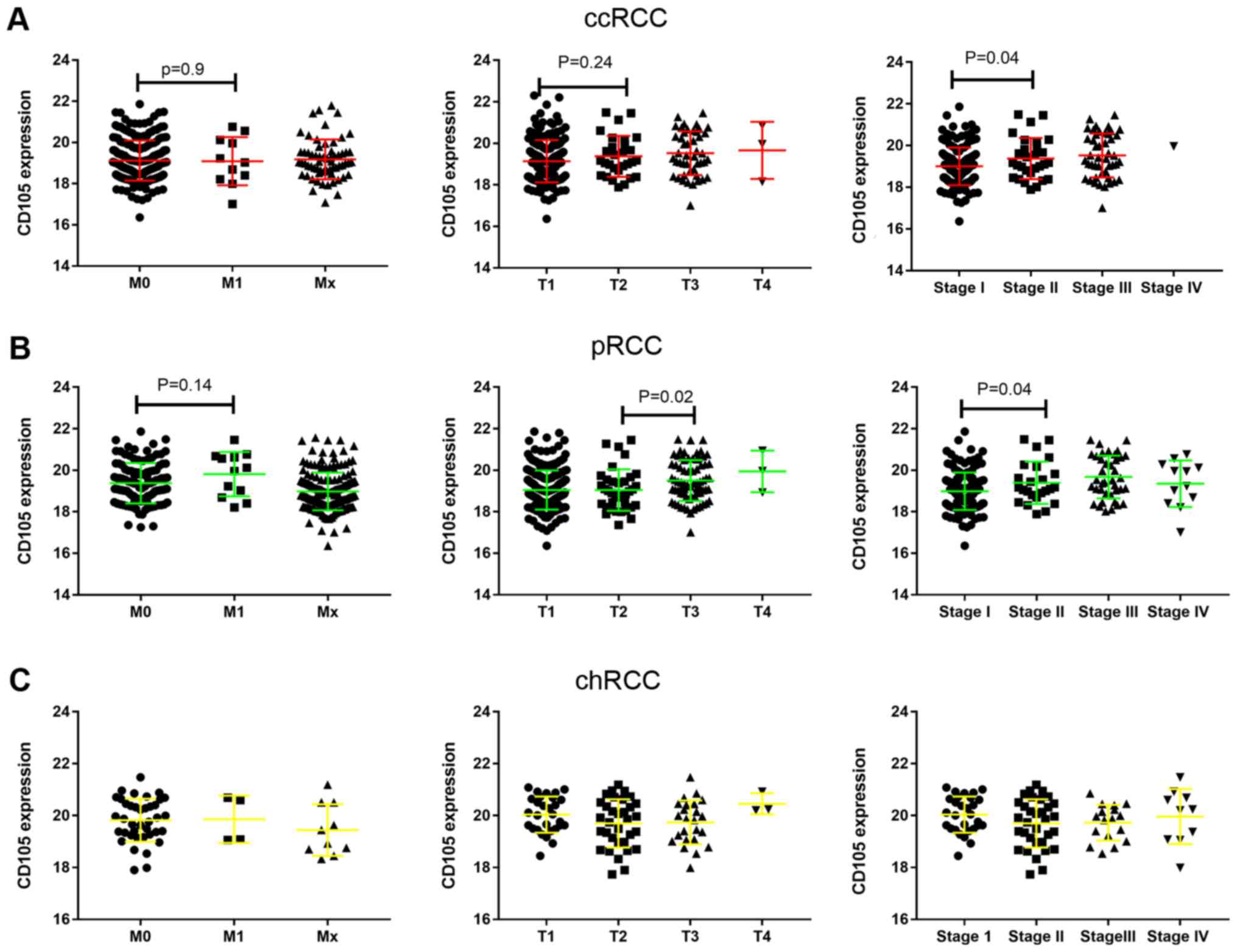 | Figure 3.mRNA expression of CD105 and its
association with M and T stage, as well as pathological stage, in
ccRCC, pRCC and chRCC. (A-C) mRNA expression of CD105 in (A) ccRCC,
(B) pRCC and (C) chRCC tissues with different M stage (left panel),
T stage (middle panel) and pathological stage (right panel). RCC,
renal cell carcinoma; ccRCC, clear cell RCC; pRCC, papillary RCC;
chRCC, chromophobe RCC; M, metastasis; T, tumor. |
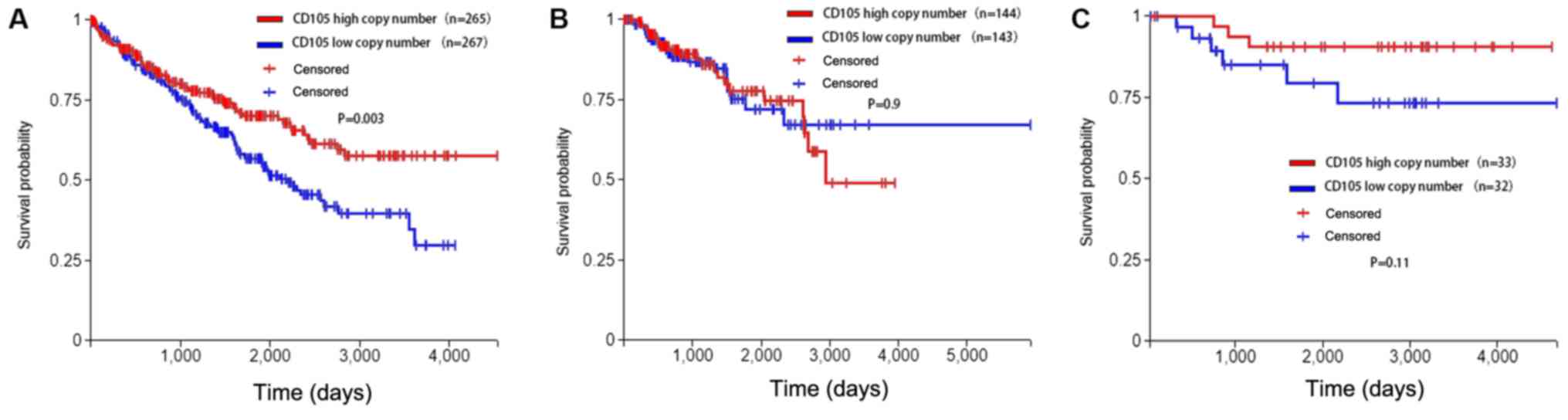
Gene copy number of CD105 in RCC
A possible mechanism for the high expression of
CD105 in RCC is the gene copy number. Therefore, the copy number of
CD105 in RCC samples was analysed in the present study. Data on
CD105 copy number, expression levels and clinical follow-up of 526
cases of ccRCC were obtained from TCGA using Xena (http://xena.ucsc.edu/). Cases were assigned into
CD105-high or CD105-low copy number groups using the median CD105
copy number as the cutoff. Time to death was plotted in a
Kaplan-Meier curve for those cases with a CD105 copy number above
the median (n=265) and equal to or below the median (n=267). A
significant difference in survival was identified between the two
groups (P=0.003). These results demonstrated that ccRCC patients
with a higher copy number of CD105 in their tumor tissues survive
for longer. In chRCC, the same trend was identified, but it was not
significant. However, in pRCC and chRCC, no significant impact of
the CD105 copy number on survival was noted, but there was a trend,
with those pRCC cases with a lower copy number of CD105 surviving
for longer (Fig. 4).
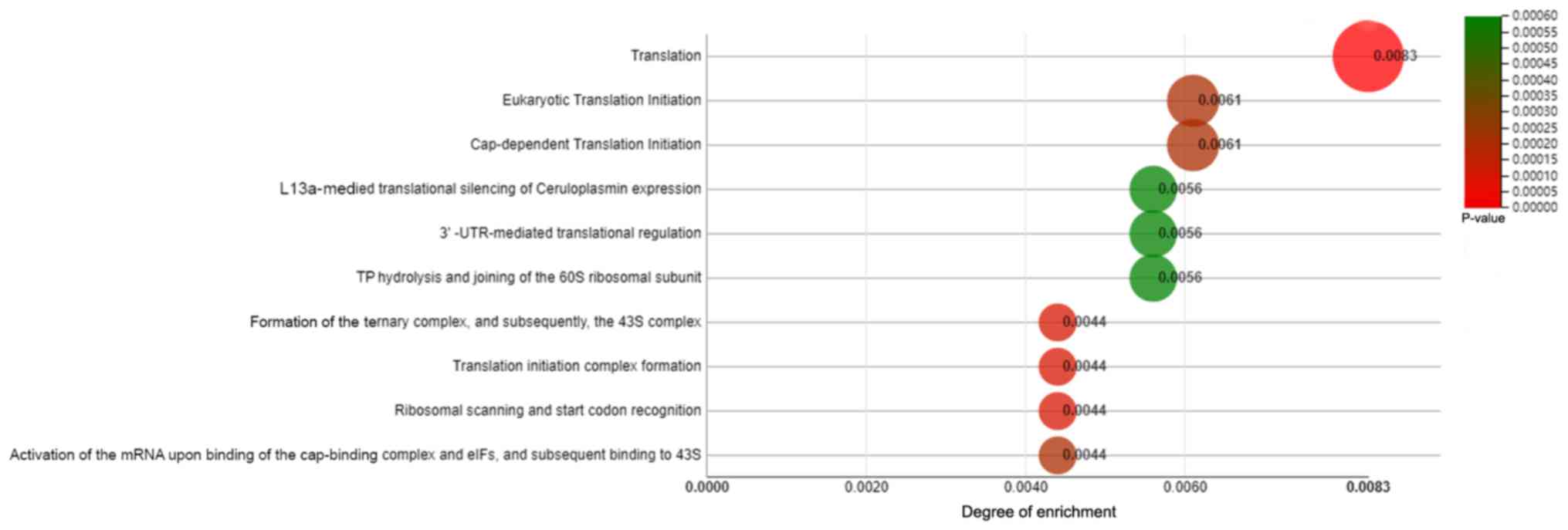
Enriched pathways by genes associated
with CD105 expression in RCC
Pathways enriched by genes positively and negatively
correlated with CD105 expression in ccRCC were identified using
webMeV. The 10 most enriched pathways by genes positively and
negatively associated with the expression of CD105 were identified
by analysis of the TCGA data for ccRCC (Fig. 5). The top 3 pathways are translation,
eukaryotic translation initiation and cap-dependent translation
initiation. These results suggest that CD105 may be involved in
translation pathway.
A total of 2,674 genes whose expression was
correlated with CD105 expression in ccRCC were identified using
webMeV. The top 20 of these genes are listed in Table II. Of note, CSC marker genes,
including Nanog, sex-determining region Y box 2 and Oct4, were not
among them.
 | Table II.Top 20 genes whose expression is
associated with CD105. |
Table II.
Top 20 genes whose expression is
associated with CD105.
| Gene name | logFC | AveExpr | P-value | Adj. P-value | t | B |
|---|
| UMPS | −2.4428 | 1.1268 |
3.22×10−0.2 | 0.166 | −2.1469 | −3.8354 |
| RAB25 | −2.1835 | 0.8057 |
1.00×10−0.3 | 0.0223 | −3.3118 | −0.8953 |
| SERPINA5 | −2.1075 | 2.8337 |
3.77×10−0.2 | 0.1824 | −2.083 | −3.8258 |
| KNG1 | −2.0606 | 0.8968 |
3.39×10−0.2 | 0.1712 | −2.1264 | −3.866 |
| ATP6V0A4 | −1.9947 | 0.5582 |
1.49×10−0.2 | 0.1053 | −2.4422 | −3.2189 |
| SSU72 | −1.9548 | 2.0074 |
3.37×10−0.2 | 0.1706 | −2.1287 | −3.7714 |
| CLDN8 | −1.8909 | 0.0309 |
1.99×10−0.2 | 0.1249 | −2.3351 | −3.4854 |
| FXYD4 | −1.8854 | 0.3206 |
3.24×10−0.2 | 0.1666 | −2.1447 | −3.8588 |
| SCNN1B | −1.7532 | 1.0789 |
8.90×10−0.3 | 0.0761 | −2.6263 | −2.7293 |
| ATP6V1G3 | −1.7428 | −0.287 |
1.83×10−0.2 | 0.119 | −2.366 | −3.4324 |
| SLC6A4 | 1.9869 | 4.3231 |
2.08×10−0.2 | 0.1279 | 2.3183 | −3.6399 |
| PTHLH | 1.8662 | 3.3825 |
5.00×10−0.3 | 0.0541 | 2.8193 | −2.3873 |
| DOC2A | 1.7541 | 3.6568 |
1.00×10−0.4 | 0.0066 | 3.9825 | 1.316 |
| MSLN | 1.7275 | 1.1849 |
1.04×10−0.2 | 0.084 | 2.5701 | −2.8679 |
| ABCC2 | 1.684 | 3.1759 |
1.00×10−0.4 | 0.0075 | 3.8833 | 0.9659 |
| ADCY2 | 1.6699 | 0.2704 |
1.00×10−0.4 | 0.0057 | 4.0427 | 1.5383 |
| PABPC1L | 1.6646 | 3.1852 |
0.00×10−0 | 0.0033 | 4.3249 | 2.6437 |
| RAB42 | 1.6581 | 4.0105 |
7.25×10−6 | 0.0021 | 4.5307 | 3.4781 |
| ATHL1 | 1.6556 | 5.3775 |
2.40×10−3 | 0.035 | 3.0554 | −1.7662 |
| GPR143 | 1.648 | 2.1146 |
3.00×10−4 | 0.013 | 3.5988 | 0.012 |
Discussion
CD105 is a tumor marker expressed in vascular
endothelial cells and has a role in new blood vessel formation
(14); furthermore, CD105 is
associated with high tumor microvessel density and is a predictor
of poor prognosis in several solid tumor types (15). Recently, CD105 has been described as
a renal CSC marker. Saeednejad Zanjani et al (13) reported that CD105 may serve as a
useful prognostic molecular marker and potentially a target
molecule for targeted therapy only in ccRCC, but possibly not in
other subtypes of RCC. The present study identified that the
expression of CD105 in tumor tissues is higher than that in normal
tissues only in ccRCC, while, in pRCC and chRCC, the expression of
CD105 is lower in tumor tissue compared with that in normal tissue.
These results demonstrated that as a tumor marker, CD105 may only
have a role ccRCC, but possibly not in other subtypes of RCC, which
consistent with the results of the previous study (13). In the present study, even for ccRCC,
the Kaplan-Meier survival curves of patients stratified by high and
low expression of CD105 exhibited no significant difference. The
reason for this observation may be the fact that the mRNA levels of
CD105 may not represent the protein levels. Therefore, the CD105
copy number was used, based on which the ccRCC patients were
stratified into two groups. It was revealed that the high CD105
copy number group survived for longer and this trend was in
accordance with the in the high CD105 mRNA group. This appears in
contrast with previous experimental results, where higher CD105
expression indicated more invasion and poor prognosis (20). Of note, previous studies also
suggested that CD105 is an independent predictive marker for the
risk of death and unfavourable prognosis in patients with ccRCC
after curative resection (21,22). The
reason for this may be that hypoxia has an important role in ccRCC,
and certain proteins are markedly decreased under hypoxia. In
addition, CD105 may directly or indirectly regulate Hif-1α under
hypoxia, which may lead to more aggressive RCC phenotypes and a
higher risk of recurrence (23).
However, the precise mechanisms require further study.
In the present study, webMeV analysis was employed
to identify pathways enriched by genes positively and negatively
correlated with CD105 expression in ccRCC. The top 3 pathways
identified were translation, eukaryotic translation initiation and
cap-dependent translation initiation. As a CSC marker, CD105
confers self-renewal capacity and contributes to chemoresistance in
RCC, and is associated with cell proliferation (24). Therefore, it is conceivable that
CD105 is associated with translation, eukaryotic translation
initiation and cap-dependent translation initiation. Furthermore,
the genes most associated with CD105 were not cancer stem markers,
which may indicate that CD105 exhibits stem cell-independent
functions.
Several previous studies have indicated that
anti-CD105 monoclonal antibody may effectively reduce or suppress
angiogenesis, tumor growth and metastasis in SCID mice (25). In the present study, it was indicated
that the role of CD105 in different stages of ccRCC is complex. In
addition to the expression, the function of CD105 in RCC should
also be evaluated to elucidate its role. CD105 may have a role in
regulating the tumor microenvironment, such as hypoxia.
Of note, the present study had certain limitations.
First, CD105 not only serves as a CSC marker in RCC, but also
activates angiogenesis-associated factors in RCC. However, in the
present study, the authors only assessed the expression in RCC
tissue and its role in RCC. Furthermore, the present study did not
include any in vitro cell experiment or animal study, and
the expression of CD105 was only evaluated by a bioinformatics
analysis to demonstrate the function of CD105 in RCC. Finally,
hypoxia has an important role in RCC, so it is possible that CD105
has different functions in different microenvironments, which
requires further assessment.
In conclusion, the present results suggest that the
roles of CD105 in RCC are complex. In ccRCC, CD105 mRNA expression
was significantly upregulated and a higher copy number was
significantly associated with a favourable prognosis. To evaluate
the role of CD105 in RCC, it is not reasonable to only assess the
expression and not the function. Of note, the association of CD105
mRNA expression and copy number with various types of RCC, their
association with patient survival and the underlying mechanisms
require further study.
Acknowledgements
The authors would like to thank Mrs. Karen Wolf
(George Whipple Lab for Cancer Research, Departments of Urology and
Pathology, University of Rochester, Rochester, NY, USA), a native
English language speaker, for to checking the manuscript for
grammatical and spelling errors.
Funding
The current study was funded by the National Natural
Science Foundation of China (grant no. 81802518).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in The Cancer Genome Atlas repository
(http://xena.ucsc.edu/).
Authors' contributions
DHS and JPC designed the study, collected the data
and analyzed the data. DHS wrote the manuscript. YY and BP
collected the data. CCG and XDY designed the study and collected
the data.
Ethics approval and informed consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that no competing financial
interests exist.
References
|
1
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King SC, Pollack LA, Li J, King JB and
Master VA: Continued increase in incidence of renal cell carcinoma,
especially in young patients and high grade disease: United States
2001 to 2010. J Urol. 191:1665–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linehan WM and Ricketts CJ: Decade in
review-kidney cancer: Discoveries, therapies and opportunities. Nat
Rev Urol. 11:614–616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bedke J, Gauler T, Grünwald V, Hegele A,
Herrmann E, Hinz S, Janssen J, Schmitz S, Schostak M, Tesch H, et
al: Systemic therapy in metastatic renal cell carcinoma. World J
Urol. 35:179–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown C: Targeted therapy: An elusive
cancer target. Nature. 537 (Suppl):S106–S108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang F, Wu Q, Zhang Y, Xiong H, Li X, Li
B, Xie W, Zhang L, Xu M, Zhang K and He F: LncRNA LOC653786
promotes growth of RCC cells via upregulating FOXM1. Oncotarget.
9:12101–12111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choueiri TK and Motzer RJ: Systemic
therapy for metastatic renal-cell carcinoma. N Engl J Med.
376:354–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corrò C and Moch H: Biomarker discovery
for renal cancer stem cells. J Pathol Clin Res. 4:3–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Wang Q, Mao J, Qin T, Sun Y, Yang
J, Han Y, Li L and Li Q: Salinomycin suppresses cancer cell
stemness and attenuates TGF-β-induced epithelial-mesenchymal
transition of renal cell carcinoma cells. Chem Biol Interact.
296:145–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matak D, Brodaczewska KK, Szczylik C, Koch
I, Myszczyszyn A, Lipiec M, Lewicki S, Szymanski L, Zdanowski R and
Czarnecka AM: Functional significance of CD105-positive cells in
papillary renal cell carcinoma. BMC Cancer. 17:212017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saeednejad Zanjani L, Madjd Z, Abolhasani
M, Shariftabrizi A, Rasti A and Asgari M: Expression of CD105
cancer stem cell marker in three subtypes of renal cell carcinoma.
Cancer Biomark. 21:821–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duff SE, Li C, Garland JM and Kumar S:
CD105 is important for angiogenesis: Evidence and potential
applications. FASEB J. 17:984–992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dallas NA, Samuel S, Xia L, Fan F, Gray
MJ, Lim SJ and Ellis LM: Endoglin (CD105): A marker of tumor
vasculature and potential target for therapy. Clin Cancer Res.
14:1931–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bussolati B, Bruno S, Grange C, Ferrando U
and Camussi G: Identification of a tumor-initiating stem cell
population in human renal carcinomas. FASEB J. 22:3696–3705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hasmim M, Bruno S, Azzi S, Gallerne C,
Michel JG, Chiabotto G, Lecoz V, Romei C, Spaggiari GM, Pezzolo A,
et al: Isolation and characterization of renal cancer stem cells
from patient-derived xenografts. Oncotarget. 7:15507–15524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song L, Ye W, Cui Y, Lu J, Zhang Y, Ding
N, Hu W, Pei H, Yue Z and Zhou G: Ecto-5′-nucleotidase (CD73) is a
biomarker for clear cell renal carcinoma stem-like cells.
Oncotarget. 8:31977–31992. 2017.PubMed/NCBI
|
|
19
|
Feldman DR and Motzer RJ: Novel targets
and therapies for metastatic renal cell carcinoma. Oncology
(Williston Park). 20:1745–1756. 2006.PubMed/NCBI
|
|
20
|
Yang X, Zhang D, Chong T, Li Y, Wang Z and
Zhang P: Expression of CK19, CD105 and CD146 are associated with
early metastasis in patients with renal cell carcinoma. Oncol Lett.
15:4229–4234. 2018.PubMed/NCBI
|
|
21
|
Yagasaki H, Kawata N, Takimoto Y and
Nemoto N: Histopathological analysis of angiogenic factors in renal
cell carcinoma. Int J Urol. 10:220–227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saroufim A, Messai Y, Hasmim M, Rioux N,
Iacovelli R, Verhoest G, Bensalah K, Patard JJ, Albiges L, Azzarone
B, et al: Tumoral CD105 is a novel independent prognostic marker
for prognosis in clear-cell renal cell carcinoma. Br J Cancer.
110:1778–1784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Myszczyszyn A, Czarnecka AM, Matak D,
Szymanski L, Lian F, Kornakiewicz A, Bartnik E, Kukwa W, Kieda C
and Szczylik C: The role of hypoxia and cancer stem cells in renal
cell carcinoma pathogenesis. Stem Cell Rev. 11:919–943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu J, Guan W, Liu P, Dai J, Tang K, Xiao
H, Qian Y, Sharrow AC, Ye Z, Wu L and Xu H: Endoglin is essential
for the maintenance of self-renewal and chemoresistance in renal
cancer stem cells. Stem Cell Reports. 9:464–477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seon BK, Matsuno F, Haruta Y, Kondo M and
Barcos M: Long-lasting complete inhibition of human solid tumors in
SCID mice by targeting endothelial cells of tumor vasculature with
antihuman endoglin immunotoxin. Clin Cancer Res. 3:1031–1044.
1997.PubMed/NCBI
|