Introduction
Pelvic inflammatory disease (PID) is a gynecological
disease that is common among young and sexually active females with
upper genital tract infections, including endometritis,
salpingitis, tubal ovarian abscesses or pelvic peritonitis
(1). Clinical features of PID
include adnexal pain, abnormal vaginal discharge, fever, menstrual
irregularities and dyspareunia (2).
Long-term and repeated infections lead to chronic pelvic pain,
tubal infertility and ectopic pregnancy, which have a serious
impact on health and quality of life (3,4).
The pathophysiological mechanism of PID includes the
inflammatory response (5,6). The inflammatory response causes the
release and maturation of interleukin (IL)-1β (5,6), which
is mediated by nucleotide-binding domain-like receptor protein 3
(NLRP3) inflammasome (7,8). NLRP3 inflammasome is composed of sensor
NLRP3, adaptor protein apoptosis-associated speck-like protein and
procaspase-1 (7,8). The nuclear factor-κB (NF-κB) pathway
comprises important transcription factors that regulate
inflammatory cytokines (9,10) and chemokines (11,12),
including IL-1β, IL-6, tumor necrosis factor-α (TNF-α) and monocyte
chemotactic protein 1 (MCP-1), which are associated with the
inflammatory responses of PID (13,14). The
clinical use of antibiotics is the preferred choice for PID
treatment according to the Centers for Disease Control and
Prevention guidelines in the United States (15); however, the majority of patients
often experience subsequent bacterial drug-resistance (13). Therefore, the development of
effective natural drugs for the prevention of PID is of great
importance.
Asiatic acid (AA) is a natural triterpenoid
extracted from Centella asiatica (16,17). It
has many beneficial properties, including anti-inflammatory
(16) and antioxidant (18) effects. Recently, AA has been
demonstrated to control inflammation and exert protective effects
via inhibiting NLRP3 inflammasome activation (16) and the NF-κB (19) pathway. However, the effects of AA on
PID remain unknown. Therefore, the aim of present study was to
investigate the protective effects and underlying mechanisms of AA
in a rat model of PID.
Materials and methods
Chemicals and reagents
AA was purchased from Sigma-Aldrich (Merck KGaA;
Darmstadt, Germany). Pentobarbital was purchased from Chengdu XiYa
Chemical Technology Co., Ltd. (Chengdu, China). Progesterone
injections were obtained from Zhejiang Xianju Pharmaceutical Co.,
Ltd. (Taizhou, China). Absorbable gelatin sponges were purchased
from Jinling Pharmaceutical Co., Ltd. (Nanjing, China). The
pathogenic Escherichia coli strain and Ureaplasma
urealyticum strain (t-strain mycoplasma) were obtained from
Nanjing Bianzhen Biology Science and Technology Co., Ltd. (Nanjing,
China).
Animal selection and group
allocation
A total of 75 female specific pathogen-free Sprague
Dawley rats (aged 9 weeks; weighing 220–240 g; Shanghai SLAC
Laboratory Animal Co., Ltd., Shanghai, China), which were
maintained under the controlled conditions at 23°C with a 12-h
light/dark cycle (60% humidity) and access to food and water ad
libitum, were assigned randomly to the following five equal
groups: A control group, consisting of rats that received vehicle
only (propylene glycol, 1.0 mg/kg) via intragastric gavage once
daily; a PID group consisting of rats that underwent the model of
PID and received an equal volume of vehicle (propylene glycol, 1.0
mg/kg) via intragastric gavage once daily; a PID + AA 5 group
consisting of rats that underwent the model of PID and received 5
mg/kg AA in vehicle (1.5 ml) via intragastric gavage once daily; a
PID + AA 35 group consisting of rats that underwent the model of
PID and received 35 mg/kg AA in vehicle via intragastric gavage
once daily; and a PID + AA 75 group consisting of rats that
underwent the model of PID and received 75 mg/kg AA in vehicle via
intragastric gavage once daily. All procedures were approved by the
Animal Ethics Committee of Zhejiang Chinese Medical University
(Hangzhou, China).
Establishment of the PID model
A PID model in rats was established based on
previously published protocols (13,14).
Rats in the study were immediately administered intramuscular
injections of buprenorphine (0.01 mg/kg; bid; Tianjin Medicine
Research Institute Pharmaceutical Co., Ltd., Tianjin, China) to
relieve pain following infection (17). All rats were acclimated for 7 days
and subcutaneously injected with 45 mg/kg progesterone (Zhejiang
Xianju Pharmaceutical Co., Ltd., Taizhou, China) prior to
infection. An absorbable gelatin sponge was saturated with
microbe-mixing solution (Nanjing Bianzhen Biology Science and
Technology Co., Ltd., Nanjing, China) with U. urealyticum
(1×108 cfu/ml) and pathogenic Escherichia coli
(1×108 cfu/ml). The upper genital tract of each rat in
the PID group was then implanted with a microbe-containing gelatin
sponge and rats were inverted for 3 min. The cervixes of rats in
the control group were implanted with microbe-free gelatin sponges.
A total of four infections were performed every 2 days. Following
the first infection, the experimental groups were administrated
with AA via intragastric gavage and the PID and control groups were
gavaged with an equal volume of vehicle. At 8 days following the
first infection, rats were intravenously anesthetized with
pentobarbital at a dose of 30 mg/kg. Following anesthesia, the
right fallopian tube and uterus were harvested and stored at −80°C.
When rats exhibited severe symptoms e.g. convulsion for a period of
>10 min, they were euthanized with via intravenous
administration of pentobarbital at a dose of 140 mg/kg as
previously described (20).
Western blot analysis
The right fallopian tube and right uterine horn (n=5
from each group) were used for western blotting. Samples were
homogenized and extracted using radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Nanjing, China), and
then centrifuged at 5,000 × g at 4°C for 30 min. The protein
concentration in the supernatant was measured using a bicinchoninic
acid assay kit. Soluble lysates (30 µg/lane) were resolved using
10% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
blocked with 5% non-fat dry milk at 25°C for 2 h and incubation was
performed with the following specific primary antibodies:
Anti-NLRP3 (1:1,000; sc-34410), anti-caspase-1 (1:1,000; sc-1597
all Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-p-NF-κB
p65 (1:500; ab10859369), anti-p-inhibitor of NF-κB (IκB)-α (1:500;
ab331284), anti-caspase-3 (1:1,000; ab9664; all Abcam, Cambridge,
UK) and anti-β-actin (1:1,000; sc-1616; Santa Cruz Biotechnology,
Inc.) at 4°C overnight. Membranes were then rinsed with
Tris-buffered saline with Tween and further incubated with the
secondary antibody (goat anti-mouse IgG; 1:500; bs12478; Bioworld,
Biogottechnology, Co., Ltd., Nanjing, China) for 1 h at room
temperature. Detection of specific proteins was performed with an
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The levels of protein were analyzed using ImageJ
(v2.1.4.7, National Institutes of Health, Bethesda, MD, USA)
software.
Biochemical analysis
Samples of the right uterus and fallopian tube were
collected and homogenized, and then centrifuged at 5,000 × g at 4°C
for 30 min. The concentration of IL-1β (ERC007.96; Neobioscience,
Beijing, China), IL-6 (ERC003.96; Neobioscience), TNF-α
(ERC102a.96; Neobioscience), MCP-1 (ERC113.48; Neobioscience),
chemokine C-X-C motif ligand 1 (CXCL-1; ab219044, Abcam) and
chemokine C-C motif ligand 5 (RANTES; ERC105.96; Neobioscience,)
supernatants was determined using ELISA kits according to the
manufacturer's protocol. Malondialdehyde (MDA; S0131) production
and superoxide dismutase (SOD; S0060) activity was measured by
commercial kit (Beyotime Institute of Biotechnology) following the
manufacturer's protocol. Myeloperoxidase (MPO) activity was
assessed using an MPO assay kit (A044; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China).
Statistical analysis
Data are expressed as the mean + standard error of
the mean and were analyzed through SPSS software (version 16.0,
SPSS Inc., Chicago, IL, USA). Statistical analysis was performed
using a one-way analysis of variance followed by Dunnett's post hoc
test. P<0.05 was determined to indicate a statistically
significant difference.
Results
AA reduces the level of inflammatory
cytokines and chemokines
Levels of proinflammatory cytokines IL-1β (Fig. 1A), IL-6 (Fig. 1B) and TNF-α (Fig. 1C), as well as chemokines MCP-1
(Fig. 1D), RANTES (Fig. 1E) and CXCL-1 (Fig. 1F) were detected. The results indicate
that IL-1β, IL-6, TNF-α, CXCL-1, MCP-1 and RANTES expression was
significantly increased following infection in the PID group
compared with the control group, whereas AA significantly decreased
the expression of inflammatory cytokines and chemokines in the PID
+ AA 35 and PID + AA 75 groups compared with the PID group
(Fig. 1). No significant differences
were observed between the PID and PID + AA 5 groups, PID + AA 35
and PID + AA 75 groups, PID + AA 35 and control groups, and PID +
AA 75 and control groups.
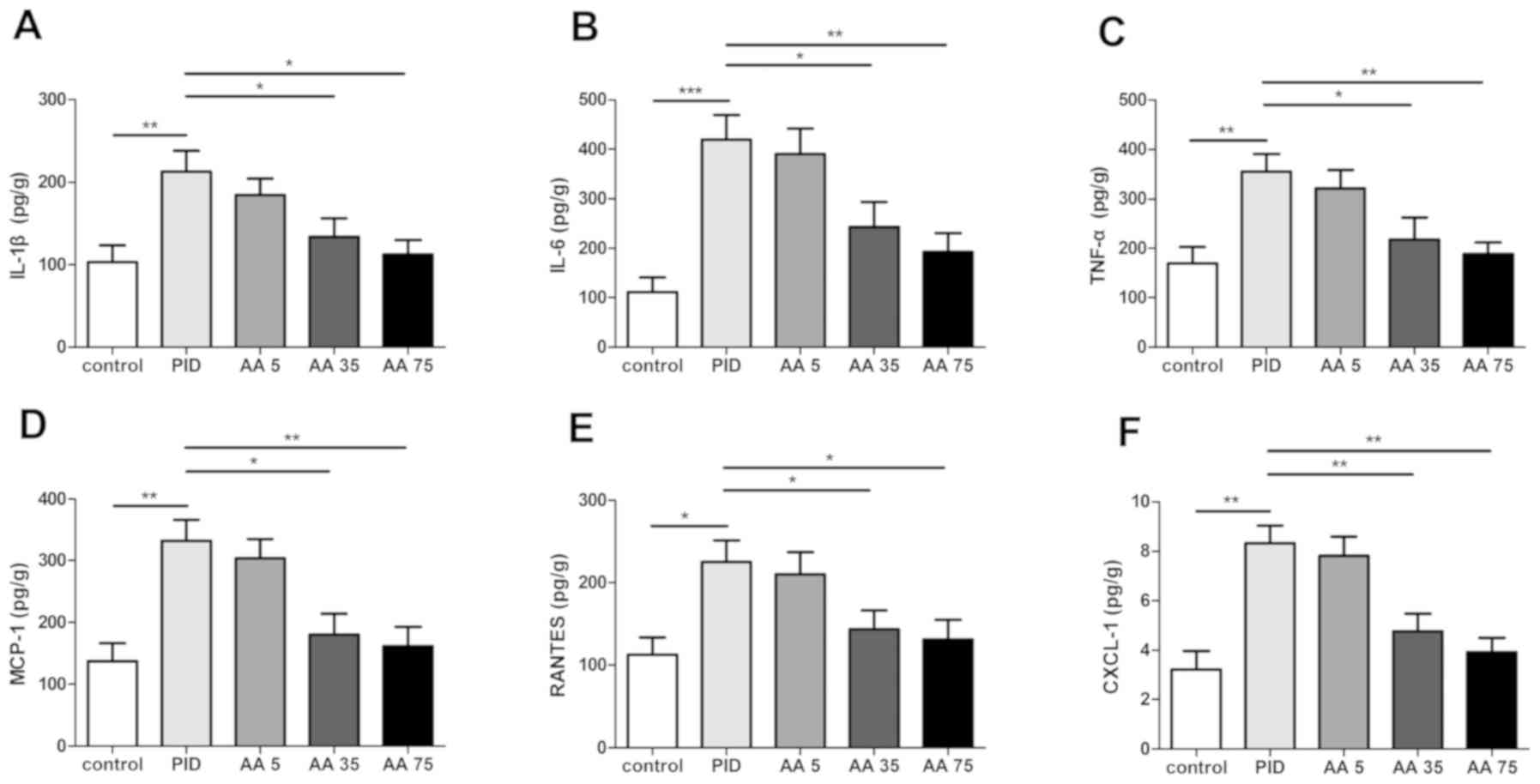 | Figure 1.AA administration reduces the level of
inflammatory cytokines and chemokines following the establishment
of a PID model in rats. Effects of AA on the levels of (A) IL-1β,
(B) IL-6, (C) TNF-α, (D) MCP-1, (E) RANTES and (F) CXCL-1.
*P<0.05, **P<0.01, ***P<0.001. n=5. AA, Asiatic acid; PID,
pelvic inflammatory disease; IL, interleukin; TNF-α, tumor necrosis
factor-α; MCP-1, monocyte chemotactic protein 1; RANTES, chemokine
C-C motif ligand 5; CXCL-1, chemokine (C-X-C motif) ligand 1; AA 5,
PID + 5 mg/kg AA group; AA 35, PID + 35 mg/kg AA group; AA 75, PID
+ 75 mg/kg AA group. |
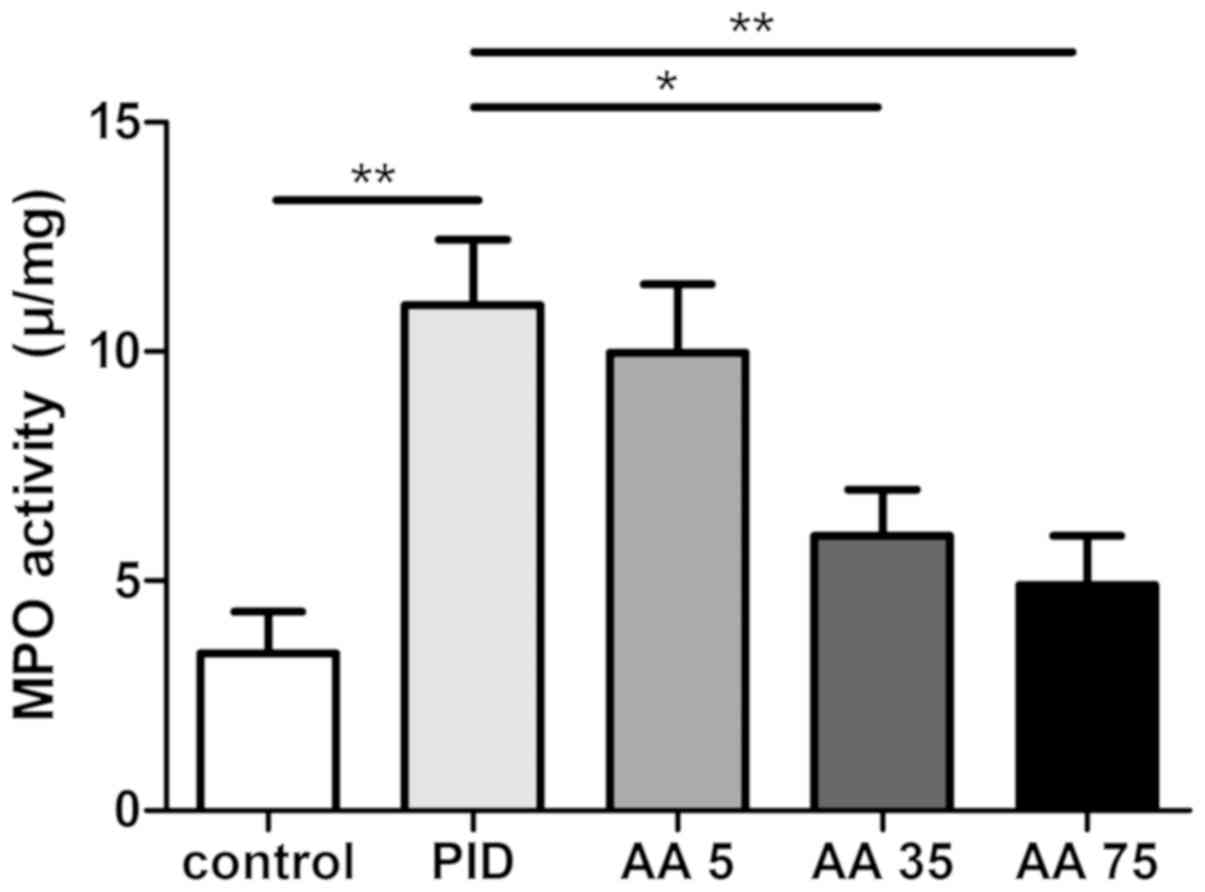
AA inhibits neutrophil
infiltration
MPO activity was assessed, as it is an oxidative
enzyme in neutrophils and acts as a specific marker of neutrophil
infiltration (16,17,21)
(Fig. 2). The results demonstrated
that MPO activity was significantly increased in the PID group
compared with the control group, whereas AA significantly inhibited
MPO activity in the PID + AA 35 and PID + AA 75 groups compared
with the PID group. The differences observed between the PID and
PID + AA 5 groups, PID + AA 35 and PID + AA 75 groups, PID + AA 35
and control groups, and PID + AA 75 and control groups were not
significant.
AA suppresses NLRP3 inflammasome
activation and the NF-κB pathway
To determine the potential mechanisms of AA, the
expression of NLRP3, caspase-1 p-NF-κB p65, p-IκB-α, and cleaved
caspase-3 were measured. PID induced a significantly higher
expression of NLRP3, active caspase-1, p-NF-κB p65, p-IκB-α and
cleaved caspase-3 protein in the PID group compared with the
control group (Fig. 3). By contrast,
these changes were significantly reversed in the PID + AA 35 and
PID + AA 75 groups compared with the PID group. However, the levels
of active caspase-1, p-NF-κB p65, p-IκB-α and cleaved caspase-3
(only in the PID + AA 35 group) were significantly increased in the
PID + AA 35 and PID + AA 75 groups relative to control animals.
Furthermore, the differences observed between PID + AA 35 and
control groups, and PID + AA 75 and control group on the level of
NLRP3 were not significant. No significant differences were
observed between the PID and PID + AA 5 groups or the PID + AA 35
and PID + AA 75 groups.
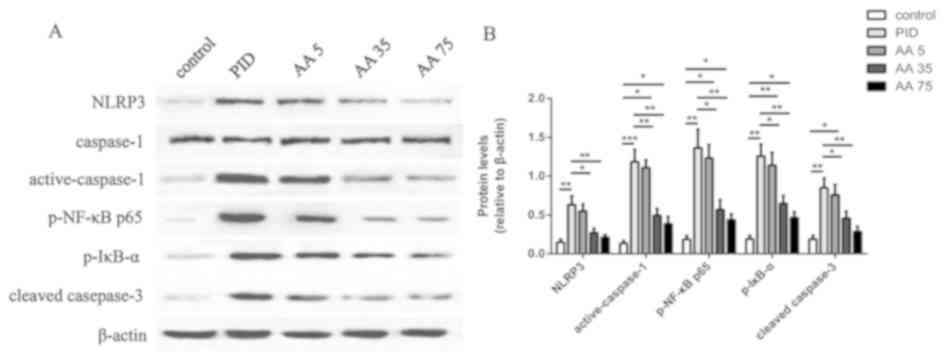 | Figure 3.AA administration suppresses
activation of NLRP3 inflammasome, the NF-κB pathway and caspase-3.
(A) Levels of NLRP3, caspase 1, active-caspase-1, p-NF-κB p65,
p-IκB-α, cleaved caspase-3 and β-actin indicated by western
blotting. (B) Quantification of western blotting. *P<0.05,
**P<0.01, ***P<0.001. n=5. AA, Asiatic acid; NLRP3,
nucleotide-binding domain-like receptor protein 3; p-NF-κB p65,
phosphorylated-nuclear factor-κB p65; p-IκB-α, phosphorylated-
inhibitor of NF-κB; PID, pelvic inflammatory disease; AA 5, PID + 5
mg/kg AA group; AA 35, PID + 35 mg/kg AA group; AA 75, PID + 75
mg/kg AA group. |
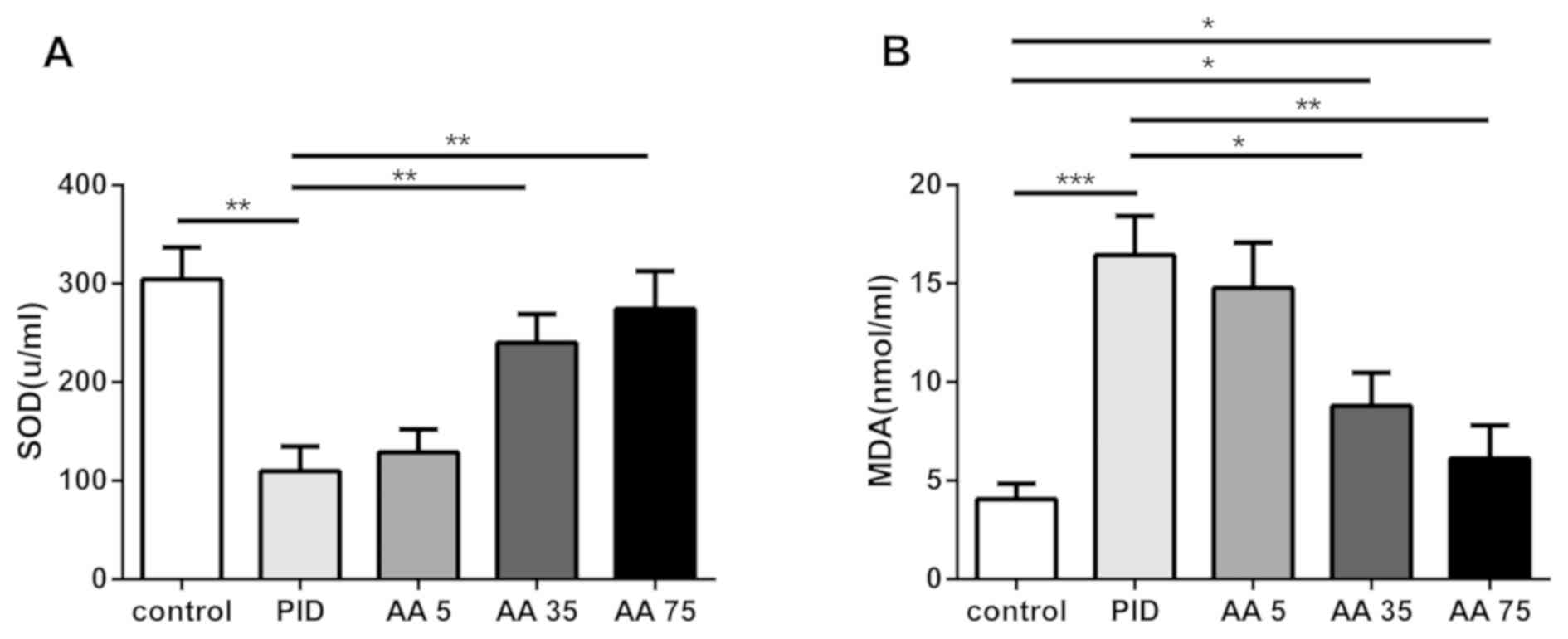
AA attenuates oxidative stress
The effects of AA on SOD activity and MDA production
were assessed (Fig. 4). There was a
significant decrease in SOD activity in the PID group compared with
the control group, whereas the MDA level was significantly
increased. However, treatment with AA in the PID + AA 35 and PID +
AA 75 groups significantly increased the SOD activity and decreased
the production of MDA compared with the PID group. However, the
levels of MDA were significantly increased in the PID + AA 35 group
relative to control animals. No significant differences were
observed between the PID and PID + AA 5 groups, the PID + AA 35 and
PID + AA 75 groups, and PID + AA 75 and control groups.
Discussion
AA is derived from a Chinese herbal plant, which has
a long medical history in China (16,17). Due
to its low side-effect profile and range of biological properties,
it has been used to treat a wide range of diseases (22–24).
However, the effects of AA on PID remain unknown. The results of
the present study demonstrated that AA decreases the levels of
inflammatory cytokines and chemokines, inhibits neutrophil
infiltration, suppresses the activation of NLRP3 inflammasome,
caspase-3 and the NF-κB pathway, and attenuates oxidative
stress.
Inflammation is a primordial defense against
infection (25); however, an
excessive inflammatory response may induce tissue damage and cause
physiological dysfunction (16,17).
IL-1β is a subtype of IL-1 and a pivotal inflammatory cytokine that
increases levels of proinflammatory cytokines, including IL-6 and
TNF-α, amplifies the inflammatory response and induces apoptosis
(26). IL-6 is another principal
proinflammatory cytokine that serves an important role, regulating
the release of chemotactic mediators and cell adhesion molecules
(27). Furthermore, IL-6 directly
affects tubal transport (28). TNF-α
also contributes to cell death and tissue injury (28,29). In
addition, chemokines CXCL-1, MCP-1 and RANTES serve a role in the
recruitment and activation of inflammatory cells, and activated
neutrophils promote the inflammatory response (13). In the present study, levels of
proinflammatory cytokines, chemokines and neutrophils were
significantly increased following pathogen infection and AA
treatment markedly reversed these changes.
NLRP3 inflammasome serves an important role in
inflammation (7,8,15).
Aberrant NLRP3 inflammasome activation is deleterious and conducive
to the development of a number of inflammatory diseases (30). The aim of the present study was
therefore to evaluate its relevance during PID. To the best of our
knowledge, the current study is the first to demonstrate that PID
induces NLRP3 inflammasome activation, while AA administration
suppresses it.
Oxidative stress, including reactive oxygen species
(ROS), contributes to cell and tissue damage; furthermore, ROS
regulate NLRP3 inflammasome activation (31). SOD is an important antioxidant enzyme
that clears oxygen radicals and inhibits tissue damage (32). MDA is the final product of lipid
peroxidation, which represents the oxidative stress intensity and
oxygen free radical levels (17).
The results of the present study suggest that PID reduces SOD and
increase MDA, whereas AA treatment markedly inhibits these changes
and attenuates oxidative stress.
In conclusion, the results of the present study
suggest that AA treatment has potent anti-inflammatory and
antioxidant effects in rats with pathogen-induced PID and the
mechanism of this action may be associated with suppression of
NLRP3 inflammasome activation and the NF-κB pathway. The present
study provides a basis for further research into the potential use
of AA as a clinical treatment for patients with PID.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Famous Old
Chinese Medicine Experts Inheritance Studio Project of State
Administration of Traditional Chinese Medicine (grant no.
2014[20]). Fuping Famous Old Chinese Medicine Experts Inheritance
Studio Research Project of Zhejiang Province (grant no.
GZS2012023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DK and PF were responsible for the experimental
design and data analysis. DK drafted the manuscript. QZ and XM were
responsible for the experiments. PJ analyzed amd interpreted the
data and revised the manuscript critically for important
intellectual content. All authors checked and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures and care were approved by the
guidelines of Animal Ethics Committee of Zhejiang Chinese Medical
University and were in compliance with the relevant laws and
institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gradison M: Pelvic inflammatory disease.
Am Fam Physician. 85:791–796. 2012.PubMed/NCBI
|
|
2
|
Newton D, Bayly C, Fairley CK, Chen M,
Keogh L, Temple-Smith M, Williams H, McNamee K, Fisher J, Henning
D, et al: Women's experiences of pelvic inflammatory disease:
Implications for health-care professionals. J Health Psychol.
19:618–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross JD: Pelvic inflammatory disease. BMJ
Clin Evid. 2013:16062013.PubMed/NCBI
|
|
4
|
Bu X, Liu Y, Lu Q and Jin Z: Effects of
‘Danzhi Decoction’ on chronic pelvic pain, hemodynamics, and
proinflammatory factors in the murine model of sequelae of pelvic
inflammatory disease. Evid Based Complement Alternat Med.
2015:5472512015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dhasmana D, Hathorn E, McGrath R, Tariq A
and Ross JD: The effectiveness of nonsteroidal anti-inflammatory
agents in the treatment of pelvic inflammatory disease: A
systematic review. Syst Rev. 3:792014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tee YT, Wang PH, Yang SF, Tsai HT, Lee SK,
Ko JL, Lin LY and Chen SC: Correlation of plasma osteopontin and
neutrophil gelatinase-associated lipocalin levels with the severity
and clinical outcome of pelvic inflammatory disease. Taiwan J
Obstet Gynecol. 53:158–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin TH, Yao Z, Sato T, Keeney M, Li C,
Pajarinen J, Yang F, Egashira K and Goodman SB: Suppression of
wear-particle-induced pro-inflammatory cytokine and chemokine
production in macrophages via NF-κB decoy oligodeoxynucleotide: A
preliminary report. Acta Biomater. 10:3747–3755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han L, Sun J, Lu CJ, Zhao RZ, Lu Y, Lin HJ
and Wei JA: Formula PSORI-CM01 inhibits the inflammatory cytokine
and chemokine release in keratinocytes via NF-κB expression. Int
Immunopharmacol. 44:226–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karkeni E, Bonnet L, Astier J, Couturier
C, Dalifard J, Tourniaire F and Landrier JF: All-trans-retinoic
acid represses chemokine expression in adipocytes and adipose
tissue by inhibiting NF-κB signaling. J Nutr Biochem. 42:101–107.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burke SJ, Stadler K, Lu D, Gleason E, Han
A, Donohoe DR, Rogers RC, Hermann GE, Karlstad MD and Collier JJ:
IL-1β reciprocally regulates chemokine and insulin secretion in
pancreatic β-cells via NF-κB. Am J Physiol Endocrinol Metab.
309:E715–E726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou W, Xiao Z, Wen X, Luo J, Chen S, Cheng
Z, Xiang D, Hu J and He J: The anti-inflammatory effect of
Andrographis paniculata (Burm. f.) Nees on pelvic inflammatory
disease in rats through down-regulation of the NF-κB pathway. BMC
Complement Altern Med. 16:4832016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou W, Wen X, Sheng X, Zheng YI, Xiao Z,
Luo J, Chen S, Wang Y, Cheng Z, Xiang D and Nie Y: Gas
chromatography-mass spectrometric method-based urine metabolomic
profile of rats with pelvic inflammatory disease. Exp Ther Med.
11:1653–1660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Workowski KA and Bolan GA; Centers for
Disease Control Prevention, : Sexually transmitted diseases
treatment guidelines, 2015. MMWR Recomm Rep 64. 64:1–137. 2015.
|
|
16
|
Jiang W, Li M, He F, Bian Z, He Q, Wang X,
Yao W and Zhu L: Neuroprotective effect of asiatic acid against
spinal cord injury in rats. Life Sci. 157:45–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang W, Li M, He F, Yao W, Bian Z, Wang X
and Zhu L: Protective effects of asiatic acid against spinal cord
injury-induced acute lung injury in rats. Inflammation.
39:1853–1861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pakdeechote P, Bunbupha S, Kukongviriyapan
U, Prachaney P, Khrisanapant W and Kukongviriyapan V: Asiatic acid
alleviates hemodynamic and metabolic alterations via restoring
eNOS/iNOS expression, oxidative diet-induced metabolic syndrome
rats. Nutrients. 6:355–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JW, Park HA, Kwon OK, Jang YG, Kim JY,
Choi BK, Lee HJ, Lee S, Paik JH, Oh SR, et al: Asiatic acid
inhibits pulmonary inflammation induced by cigarette smoke. Int
Immunopharmacol. 39:208–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morton DB and Griffiths PH: Guidelines on
the recognition of pain, distress and discomfort in experimental
animals and an hypothesis for assessment. Vet Rec. 116:431–436.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Yi L, Xiang Z, Zhong J, Zhang H and
Sun T: Resveratrol attenuates spinal cord injury-induced
inflammatory damage in rat lungs. Int J Clin Exp Pathol.
8:1237–1246. 2015.PubMed/NCBI
|
|
22
|
Guo W, Liu W, Jin B, Geng J, Li J, Ding H,
Wu X, Xu Q, Sun Y and Gao J: Asiatic acid ameliorates dextran
sulfate sodium-induced murine experimental colitis via suppressing
mitochondria-mediated NLRP3 inflammasome activation. Int
Immunopharmacol. 24:232–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Xiao X and Yang M: Asiatic acid
inhibits lipopolysaccharide-induced acute lung injury in mice.
Inflammation. 39:1642–1648. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao C, Wu B, Hou Z, Xie Q, Liao T, Wang T
and Ma D: Asiatic acid inhibits LPS-induced inflammatory response
in human gingival fibroblasts. Int Immunopharmacol. 50:313–318.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gilroy DW: Eicosanoids and the endogenous
control of acute inflammatory resolution. Int J Biochem Cell Biol.
42:524–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang W, Huang Y, He F, Liu J, Li M, Sun
T, Ren W, Hou J and Zhu L: Dopamine D1 receptor agonist A-68930
inhibits NLRP3 inflammasome activation, controls inflammation, and
alleviates histopathology in a rat model of spinal cord injury.
Spine (Phila Pa 1976). 41:E330–E334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamimura D, Ishihara K and Hirano T: IL-6
signal transduction and its physiological roles: The signal
orchestration model. Rev Physiol Biochem Pharmacol. 149:1–38. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SA, Tsai HT, Ou HC, Han CP, Tee YT,
Chen YC, Wu MT, Chou MC, Wang PH and Yang SF: Plasma
interleukin-1beta, −6, −8 and tumor necrosis factor-alpha as highly
informative markers of pelvic inflammatory disease. Clin Chem Lab
Med. 46:997–1003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dash SK, Chattopadhyay S, Dash SS,
Tripathy S, Das B, Mahapatra SK, Bag BG, Karmakar P and Roy S: Self
assembled nano fibers of betulinic acid: A selective inducer for
ROS/TNF-alpha pathway mediated leukemic cell death. Bioorg Chem.
63:85–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baldwin AG, Brough D and Freeman S:
Inhibiting the inflammasome: A chemical perspective. J Med Chem.
59:1691–1710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding W, Guo H, Xu C, Wang B, Zhang M and
Ding F: Mitochondrial reactive oxygen species-mediated NLRP3
inflammasome activation contributes to aldosterone-induced renal
tubular cells injury. Oncotarget. 7:17479–17491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jing W, Chunhua M and Shumin W: Effects of
acteoside on lipopolysaccharide-induced inflammation in acute lung
injury via regulation of NF-κB pathway in vivo and in vitro.
Toxicol Appl Pharmacol. 285:128–135. 2015. View Article : Google Scholar : PubMed/NCBI
|