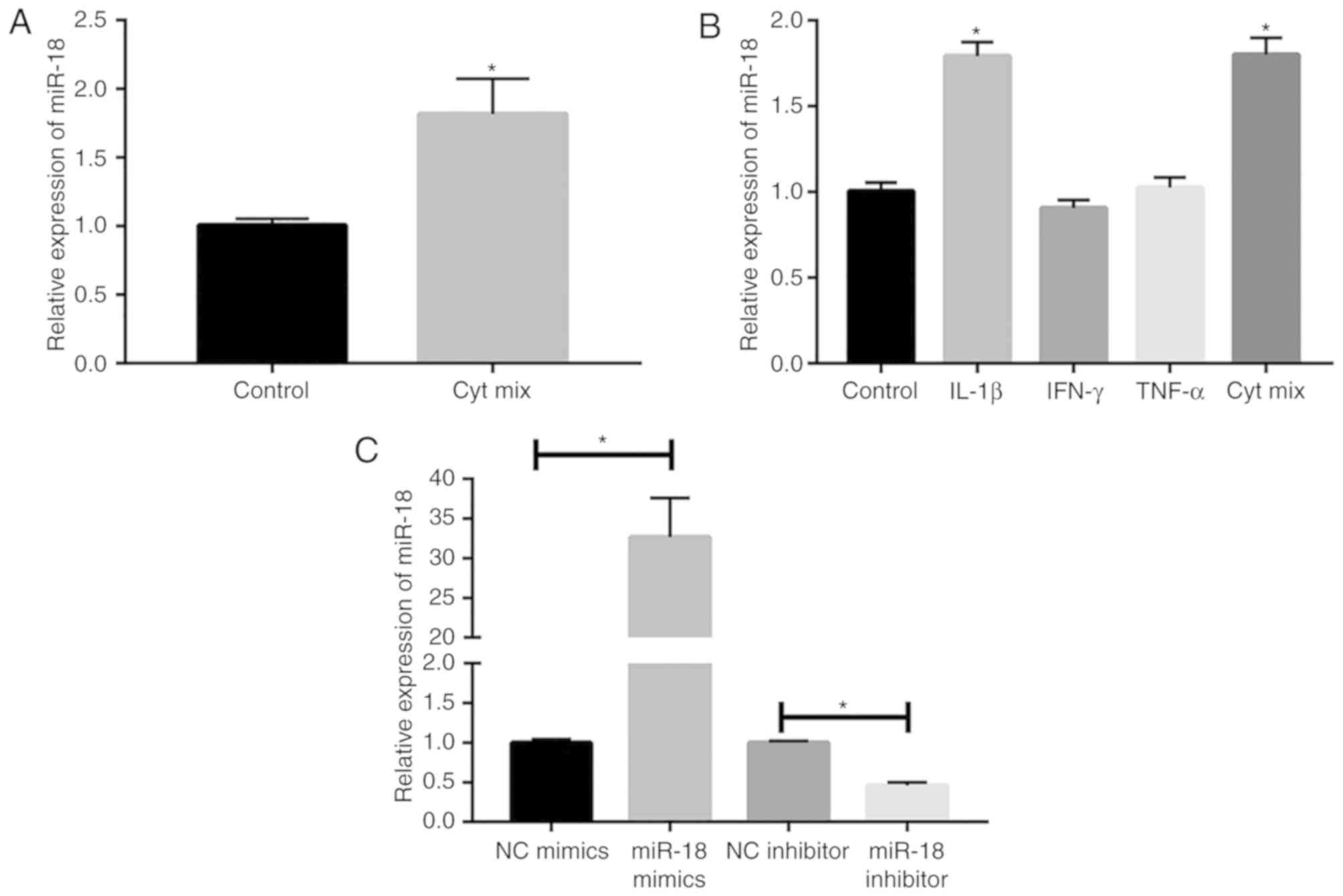
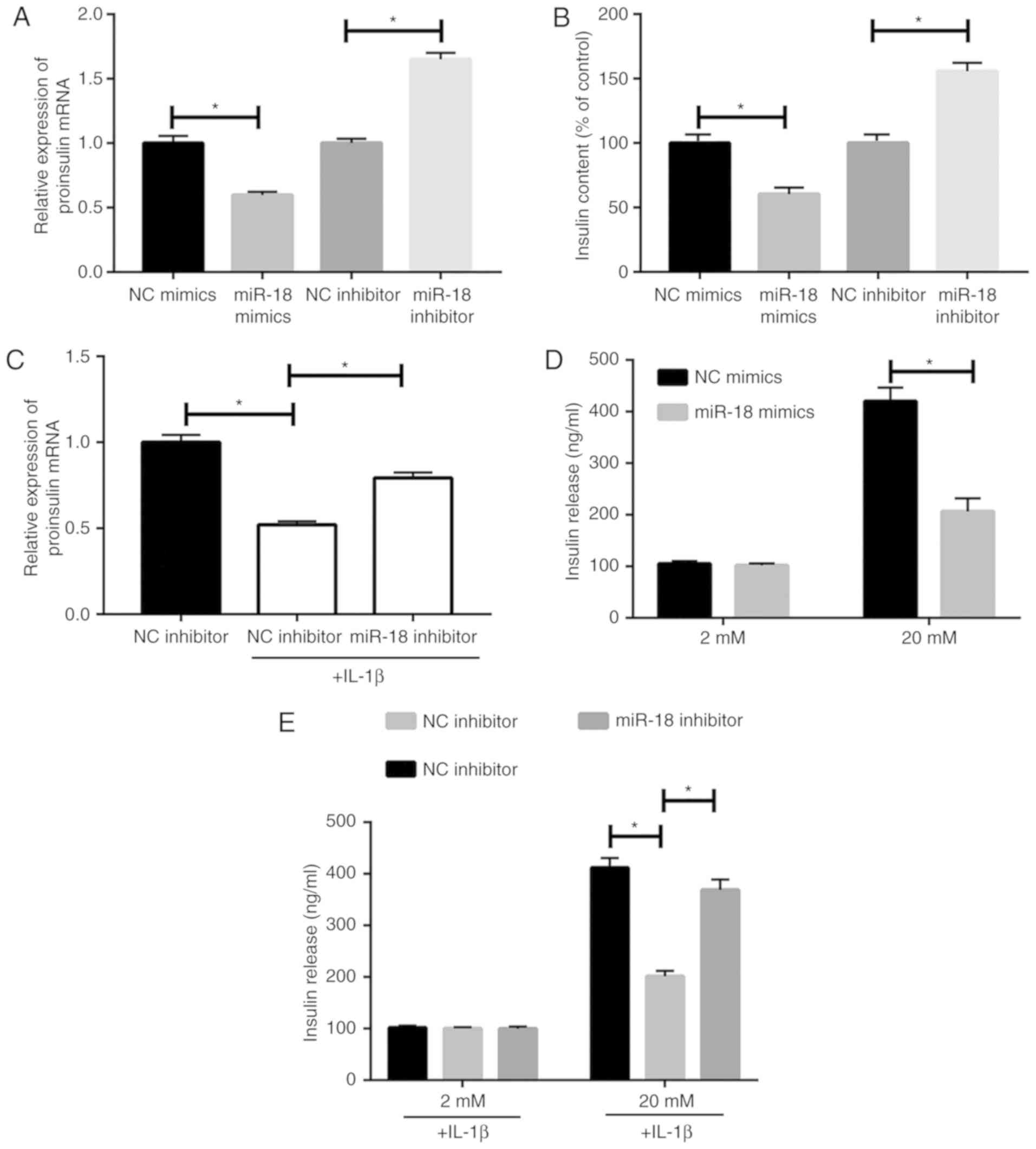
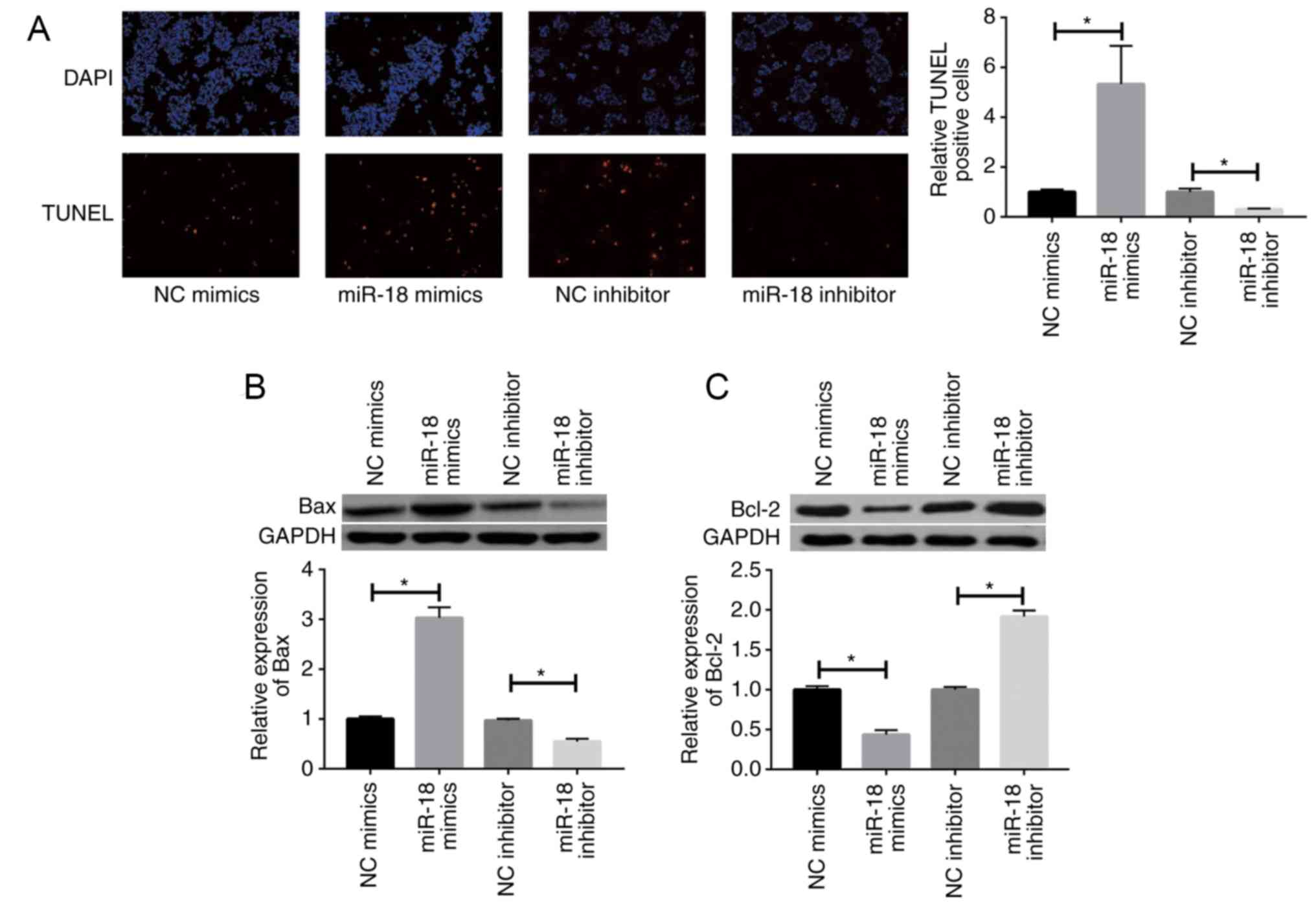
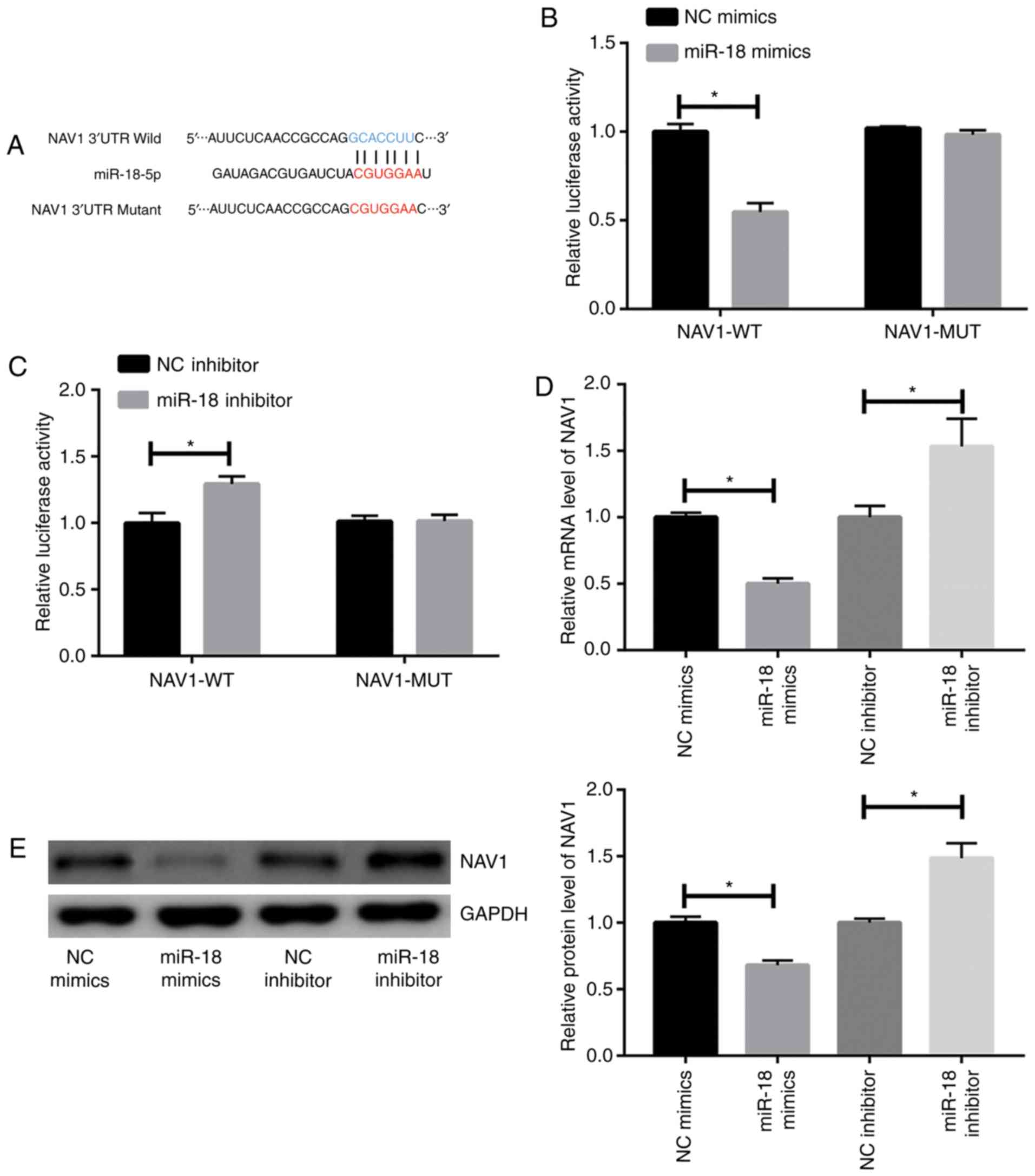
|
1
|
Report of the expert committee on the
diagnosis and classification of diabetes mellitus, . Diabetes Care.
20:1183–1197. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomis R, Artola S, Conthe P, Vidal J,
Casamor R and Font B; investigadores del Grupo de Estudio OBEDIA, :
Prevalence of type 2 diabetes mellitus in overweight or obese
outpatients in Spain. OBEDIA Study. Med Clin (Barc). 142:485–492.
2014.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Runkel M, Muller S, Brydniak R and Runkel
N: Downgrading of type 2 diabetes mellitus (T2DM) after obesity
surgery: Duration and severity matter. Obes Surg. 25:494–499. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen
S and Eizirik DL: Mechanisms of pancreatic beta-cell death in type
1 and type 2 diabetes: Many differences, few similarities.
Diabetes. 54 (Suppl 2):S97–S107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergman RN, Finegood DT and Kahn SE: The
evolution of beta-cell dysfunction and insulin resistance in type 2
diabetes. Eur J Clin Invest. 32 (Suppl 3):S35–S45. 2002. View Article : Google Scholar
|
|
6
|
Li X, Li X, Wang G, Xu Y, Wang Y, Hao R
and Ma X: Xiao Ke Qing improves glycometabolism and ameliorates
insulin resistance by regulating the PI3K/Akt pathway in KKAy mice.
Front Med. 12:388–396. 2018. View Article : Google Scholar
|
|
7
|
Gao L, Li SL and Li YK: Liraglutide
promotes the osteogenic differentiation in MC3T3-E1 cells via
regulating the expression of Smad2/3 through PI3K/Akt and
wnt/β-catenin pathways. DNA Cell Biol. Nov 7–2018.(Epub ahead of
print). View Article : Google Scholar
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie MB, Sui XQ, Pei D, Yao Q and Huang Q:
Study on the expression and mechanism of plasma microRNA-21 in
patients with ischemic cardiomyopathy. Eur Rev Med Pharmacol Sci.
21:4649–4653. 2017.PubMed/NCBI
|
|
10
|
Sayed D and Abdellatif M: MicroRNAs in
development and disease. Physiol Rev. 91:827–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guay C and Regazzi R: Role of islet
microRNAs in diabetes: Which model for which question?
Diabetologia. 58:456–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Plaisance V, Waeber G, Regazzi R and
Abderrahmani A: Role of microRNAs in islet beta-cell compensation
and failure during diabetes. J Diabetes Res. 2014:6186522014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schrimpe-Rutledge AC, Fontes G, Gritsenko
MA, Norbeck AD, Anderson DJ, Waters KM, Adkins JN, Smith RD,
Poitout V and Metz TO: Discovery of novel glucose-regulated
proteins in isolated human pancreatic islets using LC-MS/MS-based
proteomics. J Proteome Res. 11:3520–3532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanchez-Mejias A, Kwon J, Chew XH, Siemens
A, Sohn HS, Jing G, Zhang B, Yang H and Tay Y: A novel
SOCS5/miR-18/miR-25 axis promotes tumorigenesis in liver cancer.
Int J Cancer. 144:311–321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seidell JC: Obesity, insulin resistance
and diabetes-a worldwide epidemic. Br J Nutr. 83 (Suppl 1):S5–S8.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kahn BB and Flier JS: Obesity and insulin
resistance. J Clin Invest. 106:473–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta D, Krueger CB and Lastra G:
Over-nutrition, obesity and insulin resistance in the development
of beta-cell dysfunction. Curr Diabetes Rev. 8:76–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borst SE: The role of TNF-alpha in insulin
resistance. Endocrine. 23:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Febbraio MA: Role of interleukins in
obesity: Implications for metabolic disease. Trends Endocrinol
Metab. 25:312–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donath MY, Størling J, Maedler K and
Mandrup-Poulsen T: Inflammatory mediators and islet beta-cell
failure: A link between type 1 and type 2 diabetes. J Mol Med
(Berl). 81:455–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lynn FC, Skewes-Cox P, Kosaka Y, McManus
MT, Harfe BD and German MS: MicroRNA expression is required for
pancreatic islet cell genesis in the mouse. Diabetes. 56:2938–2945.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lovis P, Roggli E, Laybutt DR, Gattesco S,
Yang JY, Widmann C, Abderrahmani A and Regazzi R: Alterations in
microRNA expression contribute to fatty acid-induced pancreatic
beta-cell dysfunction. Diabetes. 57:2728–2736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark A, Wells CA, Buley ID, Cruickshank
JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR and Turner RC:
Islet amyloid, increased A-cells, reduced B-cells and exocrine
fibrosis: Quantitative changes in the pancreas in type 2 diabetes.
Diabetes Res. 9:151–159. 1988.PubMed/NCBI
|
|
26
|
Butler AE, Janson J, Bonner-Weir S, Ritzel
R, Rizza RA and Butler PC: Beta-cell deficit and increased
beta-cell apoptosis in humans with type 2 diabetes. Diabetes.
52:102–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsang WP and Kwok TT: The miR-18a*
microRNA functions as a potential tumor suppressor by targeting on
K-Ras. Carcinogenesis. 30:953–959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang XL, Cui GH and Zhou KY: Correlation
of PI3K-Akt signal pathway to apoptosis of tumor cells. Ai Zheng.
27:331–336. 2008.(In Chinese). PubMed/NCBI
|