Introduction
Hand-foot-and-mouth disease (HFMD) is an infantile
disease characterized by herpes on the hands, feet and mouth, and
associated neurological syndrome (1,2). The
major viruses that cause HFMD are EV71 and CVA16. EV71-induced HFMD
is more serious out of the two because it causes a neurological
syndrome of the central nervous system and may lead to mortality
(3). CVA16-induced HFMD usually
leads to milder symptoms, and the morbidity and mortality are lower
compared with EV71-induced HFMD (4).
However, EV71 is not the only major cause of HFMD outbreak. Zhu
et al (5) conducted a
12-month follow-up of 1,704 patients with clinically confirmed HFMD
and revealed that only 36 cases (2.1%) were identified as
EV71-induced HFMD, 577 cases (33.9%) were CVA16-induced HFMD, 588
cases (34.5%) were caused by other enteroviruses and 503 cases
(29.5%) were not associated with any enterovirus. Furthermore, some
patients with severe and fatal CVA16-induced HFMD have been
reported in the United States (6),
France (7), Japan (8) and China (9,10).
The experimental treatment for HFMD includes
inactivated virus vaccine (5,11), DNA
vaccine (12), synthetic peptide
vaccine (13,14), recombinant VP1 vaccine (15), live attenuated vaccines (16), neutralizing antibodies (17) and antiviral compounds (18). Inspired by previous inactivated polio
vaccines, the development of an active immunoassay for inactivated
EV71 vaccine has been making rapid progress (19). In December 2015, the China Food and
Drug Administration approved two inactivated EV71 vaccines for the
prevention of severe HFMD (20), and
a CVA16 vaccine is presently being developed in China (21). However, these vaccines only provide
protection against HFMD caused by a single enterovirus. The
clinical symptoms of HFMD caused by CVA16 and EV71 strains are
indistinguishable, and they may cause outbreaks alternately or
simultaneously in Asian countries (22). EV71 can recombine viral genes with
CVA16 and produce novel viral variants. In 2008, a large-scale
outbreak of HFMD caused by EV71 and CVA16 recombinant virus
occurred in the city of Fuyang, China (23). Therefore, it is necessary to develop
effective therapeutic agents or therapies for treatment of EV71-
and CVA16-induced HFMD.
In passive immunization, intravenous injection of
human immunoglobulins has been widely used to provide immunological
protection with passive immunity for immunocompromised individuals
(24); however, the therapeutic
efficacy is unstable (25), and the
risk of adverse drug effects is high (26). The monoclonal antibody produced by
hybridoma cells is another form of passive immunity that is
effective (27). However, when the
antibody is injected into humans, immune rejection typically occurs
(28). Immunoglobulin Y (IgY), which
is extracted from the egg yolk of immunized poultry, is an
excellent antibody source for passive immunity (29). Compared with the IgG from mammals,
IgY is more stable (30,31), easy to collect (32), has a high yield (33) and does not react with rheumatoid
factors, complement components or mammalian Fc receptors in human
serum (34). At present, the
application of IgY in the diagnosis and treatment of human diseases
has become a research hotspot, particularly regarding infections
caused by Helicobacter pylori (35), Vibrio cholerae (36) and other bacterial infections, as well
as the infections by human rotavirus (37), severe acute respiratory syndrome
coronavirus (38) and other viruses.
IgY can also be used for the preparation of diagnostic antibodies
for immunohistochemistry, ELISA (39), western blotting and other diagnostic
techniques.
In the present study, the cross antiviral activity
of IgY against EV71 and CVA16 was assessed. White Leghorn
specific-pathogen-free (BWEL-SPF) chickens were immunized with
inactivated EV71 strains and a specific IgY was prepared from egg
yolk. Inhibitory activity of the IgY against EV71 and CVA16 strains
was indicated in vitro. Furthermore, the purity and titer of
the IgY was determined by SDS-PAGE, indirect ELISA and western
blotting. The long-term aim of the present project was to develop a
specific IgY that could potentially act as an antiviral agent for
cross-passive immunotherapy of EV71- or CVA16-induced HFMD.
Materials and methods
Cells and viruses
Rhabdomyosarcoma (RD) cells were obtained from the
China Center for Type Culture Collection (Wuhan, China). EV71,
CVA16, coxsackievirus B1 (CVB1), coxsackievirus B2 (CVB2),
coxsackievirus B3 (CVB3), coxsackievirus B4 (CVB4), coxsackievirus
B5 (CVB5) and coxsackievirus B6 (CVB6) were purchased from the
State Key Laboratory of Virology (Wuhan, China) and diluted (1:5)
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Corning Inc., Corning, NY, USA), 100 U/ml
penicillin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 100
µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). RD cells were
cultured in virus-containing medium at 37°C in a 5%
CO2-humidified incubator. When RD cells containing the
viruses reached ~80% confluence, cells were frozen-thawed at −80°C,
centrifuged at 2,000 × g for 5 min at 4°C and filtered on a 0.22-µM
filter for storage.
Chicken immunization
A total of 6 39-week-old single-comb BWEL-SPF
chickens were purchased from the SPF Experimental Animal Center of
Guangdong Emerging Dahua Agriculture Poultry Co., Ltd. (Guangzhou,
China). Chickens were raised in 3 super-clean benches (Suzhou Antai
Airtech Co. Ltd., Suzhou, China) and had access to food and water
(pH 6.2). After 1 week, laying chickens were divided into two
groups: Group A and Group B. After mixing the EV71 antigen
(109TCID50/ml) with an equal amount of
Freund's incomplete adjuvant and fully emulsifying, 3 chickens were
randomly selected for Group A and injected intramuscularly in both
sides of the chicken wings and left and right sides of the breast
(0.25 ml/site). In Group B, 3 chickens were given a mixture (1 ml)
of saline and Freund's incomplete adjuvant (0.25 ml/site). In
Groups A and B, the injections were performed once/week for 4
weeks. Eggs were collected once a day, labeled and stored at 4°C.
Chickens were euthanized at week 31, following the first
immunization.
Isolation and purification of IgY from
chicken egg yolk
Once the fresh eggs were cleaned with 75% alcohol
and cotton balls, the egg yolks were separated and the extra
albumen was rolled off with filter paper. The egg yolk without the
membrane was diluted with cold deionized water (1:9), mixed
(adjusted to pH 5.0 with 0.1 mol HCl) and then stored at 4°C
overnight. The solution was centrifuged at 4,000 × g for 40 min at
4°C and the supernatant was added to ammonium sulphate to make a
final saturation of 45%. Following this, the solution was incubated
at 4°C for 3 h. After centrifugation at 4,000 × g for 10 min at
4°C, the supernatant was discarded and deionized water (9 times the
volume of egg yolk without the membrane) was used to dissolve the
protein precipitate. Subsequently, sodium sulfate was added to make
a final mass fraction of 13%. The mixture was incubated at 4°C for
3 h and centrifuged again under the same conditions. The sediment
was dissolved in phosphate-buffered saline (PBS). The solution was
dialyzed for 4–5 h (water changed every hour), soaked in PBS
overnight and stored at −20°C. The IgY purified from eggs of group
A after immunization were the specific IgY (S-IgY), and those from
group B were the negative control IgY (C-IgY). The purified IgY was
subjected to centrifugal ultrafiltration at 4,000 × g for 10 min at
4°C using an Amicon Ultra-15 centrifugal filter unit (EMD
Millipore, Billerica, MA, USA) to desalt and concentrate the
antibody. The purified IgY was used in subsequent in vitro
neutralization assays and western blotting.
Quantitation of the purfied IgY
The antibody titer of the S-IgY and C-IgY (purified
IgY) was determined using indirect ELISA. The purified IgY from egg
yolk was diluted to 1:5,000 in PBS, and 50 µl was added to the
ELISA plate, which had been coated with the purified EV71 antigen.
Three duplicated wells were set in the same sample, the C-IgY group
or the S-IgY group. The plate was covered, incubated at 37°C for 30
min and the wells were washed 5 times (30 min each time) with a
washing buffer provided in an EV71 Ab ELISA kit (cat. no.
SBJ-H2014; Nanjing SenBeiJia Biological Technology Co., Ltd.,
Nanjing, China). The plate was incubated with horseradish
peroxidase (HRP)-conjugated goat anti-chicken IgY antibody
(1:2,000; Abcam, Cambridge, MA, USA) for 30 min at 37°C, washed 5
times with a washing buffer (30 sec each time) and subsequently
incubated with 100 µl freshly prepared TMB color liquid (50 µl each
of color liquid A and B) at 37°C in the dark for 15 min. Following
color development, 50 µl of 2 mol H2SO4 was
added to stop the reaction and the absorbance was measured
immediately at 450 nm using a Bio-Tek EL 309 microplate reader
(Omega Bio-Tek, Inc., Norcross, GA, USA). The concentration of the
IgY was obtained according to the standard curves.
SDS-PAGE analysis
Samples of IgY and 5X loading buffer (4:1) were
heated in a metal water bath at 95°C for 10 min and placed on ice
for an additional 10 min. Samples (10 µl/lane) was separated via
SDS-PAGE on a 10% gel at a constant current of 45 mA for 50 min.
Following protein separation, the gel was stained with Coomassie
Brilliant Blue R250 for 30 min and then de-stained with a
de-staining solution (30 min each time). Coomassie-stained gels
were imaged and protein bands were analyzed using BandScan 5.0
software (ProZyme., Hayward, CA, USA).
Western blotting
Total protein from the purified EV71 and CVA16 virus
strains and the EV71 VP1 vaccine (cat. no. DAG1665; Creative
Diagnostics Co., New York, NY, USA) was quantified using a
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.), 5X
loading buffer (cat. no. P0015L; Beyotime Institute of
Biotechnology, Haimen, China) was added to samples (4:1) and
denatured by heating in a metal water bath at 95°C for 10 min.
Samples were placed on ice for 10 min. Samples (20 µg protein/lane)
were separated via SDS-PAGE on 10 and 5% gels. The separated
proteins were transferred onto polyvinylidene fluoride membranes
and blocked with 5% skimmed milk at room temperature for 90 min.
The membranes were washed five times with Tris-buffered saline
containing 0.05% Tween-20 (TBST). The membranes were incubated with
primary antibodies overnight on a shaking incubator at 4°C. The
membranes were washed five times with TBST. Following primary
incubation, membranes were incubated with secondary antibodies for
90 min on a shaking incubator at room temperature and washed four
times with TBST. The primary and secondary antibodies used are
summarized in Table I. Protein bands
were visualized using an Enhanced Chemiluminescence Western
Blotting kit (Thermo Fisher Scientific, Inc.).
 | Table I.Primary and secondary antibodies. |
Table I.
Primary and secondary antibodies.
| Protein | Primary
antibody | Dilution used | Catalogue
number | Secondary
antibody | Dilution used | Catalogue
number |
|---|
| EV71 | C-IgY | 1:20,000 |
| HRP goat
anti-chicken IgY | 1:5,000 |
ab20572b |
|
| S-IgY | 1:20,000 |
| HRP goat
anti-chicken IgY | 1:5,000 |
ab20572b |
|
| EV71 VP1 monoclonal
antibody | 1:10,000 | MAB
1255-M05a | HRP goat anti-mouse
IgG | 1:5,000 |
ab97023b |
| CVA16 | C-IgY | 1:5,000 |
| HRP goat
anti-chicken IgY | 1:2,500 |
ab20572b |
|
| S-IgY | 1:5,000 |
| HRP goat
anti-chicken IgY | 1:2,500 |
ab20572b |
|
| EV71 VP1 monoclonal
antibody | 1:10,000 | MAB
1255-M05a | HRP goat anti-mouse
IgG | 1:5,000 |
ab97023b |
| EV71 VP1
vaccine | C-IgY | 1:20,000 |
| HRP goat
anti-chicken IgY | 1:5,000 |
ab20572b |
|
| S-IgY | 1:20,000 |
| HRP goat
anti-chicken IgY | 1:5,000 |
ab20572b |
Bidirectional immune agar diffusion
test
A 10 g/l agarose plate was prepared, and plum-shaped
holes (aperture, 3–5 mm; hole distance, 3–4 mm) were punched out on
the agarose plate. The inactivated EV71 virus was added to the
central well and different dilutions (1:2, 1:4, 1:8, 1:16 and 1:32)
of the purified IgY were added into the peripheral 6 wells. At the
same time, a blank group (PBS only) was also assessed. Following
this, the plate was placed upside down in a 60°C-wet box and
incubated for 24–48 h. The formation of the precipitation line was
observed.
50% tissue culture infective dose
(TCID50) assay
Virus strains were serially diluted
(10−1−10−10) with DMEM supplemented with 10%
FBS and titrated 100 µl/well on RD monolayer cells that had been
seeded in a 96-well plate at a density of 1×104
cells/well. The virus-infected cells were incubated for 48 h at
37°C in a 5% CO2-humidified incubator before the
presence of cytopathic effect (CPE) was observed under a microscope
(IX73; Olympus Corporation, Tokyo, Japan). The TCID50 of
the virus strains were determined according to the Reed-Muench
formula (40).
In vitro neutralization assay
RD cells were seeded into a 48-well plate at a
concentration of 6×104/well and cultured overnight at
37°C. S-IgY and C-IgY were diluted to 1 mg/ml with PBS. Enviroxime
(Purity, 98%; Toronto Research Chemicals Inc., North York, ON,
Canada) was dissolved in dimethyl sulfoxide at 10 mg/ml and diluted
1:10 into culture media. The experiment was divided into 4 groups:
The blank control group (PBS only), the negative control IgY
(C-IgY), the S-IgY groups and the Envrioxime group. Subsequently,
S-IgY and C-IgY were incubated at 56°C for 30 min. S-IgY, C-IgY and
Enviroxime were serially diluted (1:25, 1:50, 1:100, 1:200, 1:400,
1:800, 1:1,600 and 1:3,200) with culture media, and mixed with an
equal volume of EV71 (200 TCID50) or CVA16
(200TCID50). The mixtures were shaken for 5 min and
incubated at 37°C in a 5% CO2-humidified incubator for
30 min. Subsequently, RD cells were inoculated with 300 µl of the
mixture and incubated O2 at 37°C in a 5%
CO2-humidified incubator for 48 h to promote the
antibody binding to the viruses. Neutralization titers were
determined as the highest dilutions of antibody that protected at
least half of RD cells in one well from CPE.
To detect the antiviral activity of the IgY against
different enteroviruses, the S-IgY, C-IgY and EV71 VP1 monoclonal
antibodies (Table II) were
incubated at 56°C for 30 min. Antibodies were diluted (1:25) and
mixed with an equal volume of 200 TCID50 of EV71, CVA16,
CVB1, CVB2, CVB3, CVB4, CVB5 and CVB6 strains, respectively.
Oscillation and incubation were performed as mentioned above. The
inhibition rate was based on the CPE.
 | Table II.Concentration of IgY from different
chickens. |
Table II.
Concentration of IgY from different
chickens.
| Chicken | Week after
immunization | Concentration of
IgY (mg/ml) |
|---|
| A1 | 0 W | 3.56±0.184 |
|
| 4 W | 4.73±0.159 |
|
| 8 W | 7.44±0.396 |
|
| 12 W | 6.85±0.283 |
| A2 | 0 W | 1.97±0.934 |
|
| 4 W | 3.31±0.131 |
|
| 8 W | 4.57±0.098 |
|
| 12 W | 5.07±0.042 |
| A3 | 0 W | 2.261±0.013 |
|
| 4 W | 2.91±0.109 |
|
| 8 W | 7.11±0.117 |
|
| 12 W | 6.46±0.294 |
| B1 | 0 W | 2.24±0.018 |
|
| 4 W | 4.66±0.181 |
|
| 8 W | 5.73±0.011 |
|
| 12 W | 7.71±0.006 |
| B2 | 0 W | 5.95±0.044 |
|
| 4 W | 5.26±0.024 |
|
| 8 W | 8.75±0.008 |
|
| 12 W | 11.16±0.109 |
| B3 | 0 W | 4.39±0.156 |
|
| 4 W | 6.55±0.113 |
|
| 8 W | 6.50±0.085 |
|
| 12 W | 6.27±0.052 |
To determine the stability of the purified IgY under
different physical conditions, the IgY was diluted to 1 mg/ml with
PBS. The diluted IgY was incubated at different temperatures (4°C,
room temperature, 37°C or 60°C) for 48 h or was frozen-thawed
(frozen at −20°C and thawed at 4°C) five times. Serially diluted
IgY (1:300, 1:600 and 1:1,200) was mixed with an equal volume of
EV71 strains (200 TCID50). The experiment was divided
into 5 groups, the 4°C group, the room temperature (RT) group, the
37°C group, the 60°C group and the freeze-thaw group. Following
this, oscillation, incubation and calculation of inhibition rate
were performed as described above.
To determine the time-dependent effect of the
purified IgY on EV71 infection in RD cells, the S-IgY and C-IgY
were diluted to 6 mg/ml with PBS and were incubated at 56°C for 30
min. The culture supernatants of the RD cells in 48-well plates
were replaced by 300 µl EV71 strains (100 TCID50). A
total of 1 µl IgY was added to the RD cells at 0, 1, 2, 3, 4 or 5 h
post-infection. RD cells were incubated under 5% CO2 at
37°C. After 48 h of cell culture, the inhibition rate for RD cells
was obtained according to CPE.
Statistical data analysis
All data were presented as the mean ± standard
deviation. Statistical analysis was performed using Graphpad Prism
software (version 7.0; GraphPad software, La Jolla, CA, USA) and
SPSS 21.0 software (IBM Corp., Armonk, NY, USA). The Student's
t-test was used to evaluate differences between two groups, and
one-way analysis of variance followed by Dunnett's post hoc test
was used to evaluate differences among different groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Levels of IgY significantly increase
in EV71-immunized chicken egg yolks
The present study was performed to isolate and
purify S-IgY from EV71-immunized chicken egg yolks. As indicated in
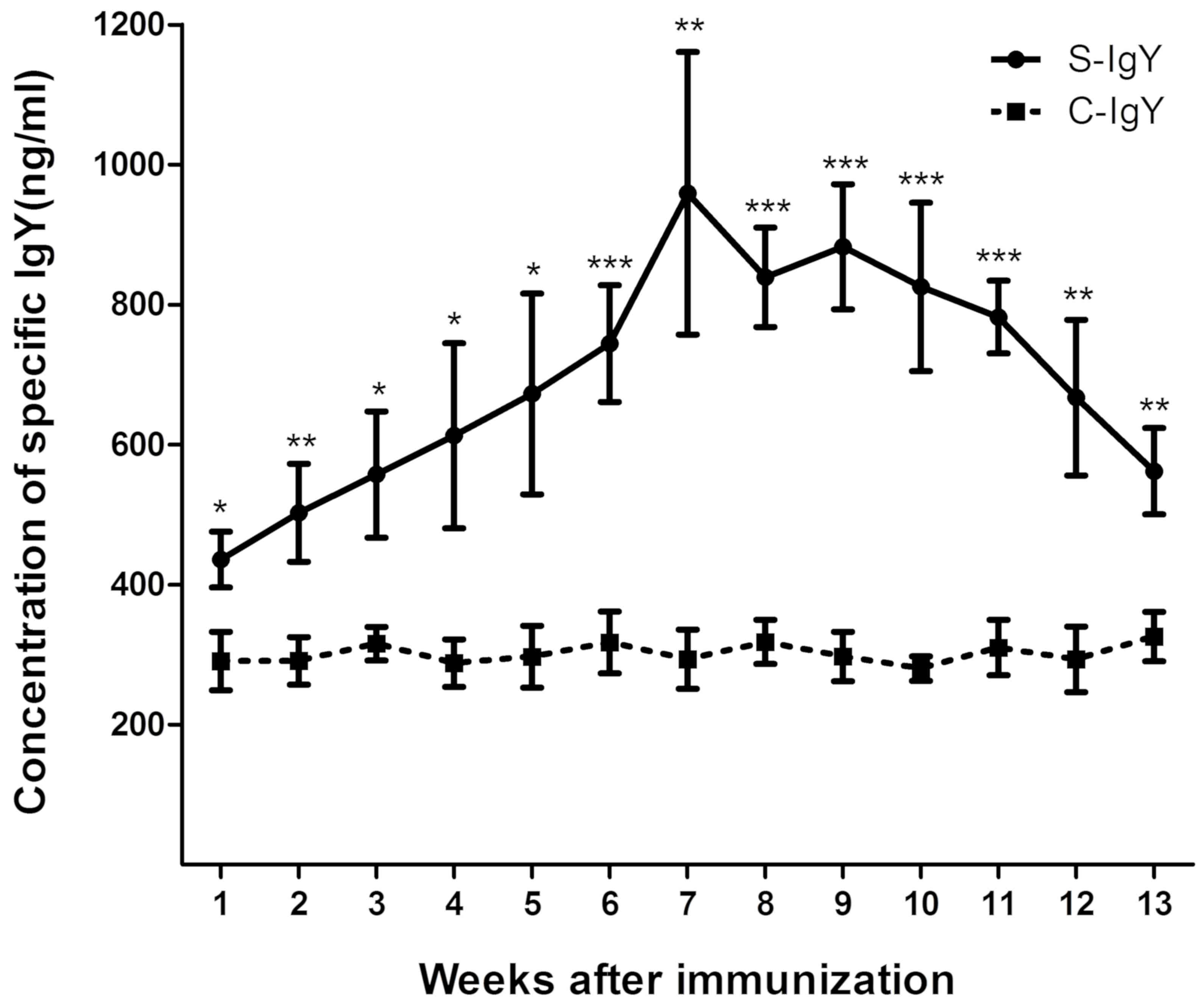
Fig. 1, the levels of S-IgY were
detected at day 7, peaked at week 7 and were maintained at a higher
level compared with C-IgY for a total of 4 weeks. The levels of
S-IgY began to decrease gradually at week 11 following the initial
immunization, whereas the levels of the C-IgY group did not change
significantly over the experimental period, suggesting that the
isolated IgY is specific in its response to EV71 immunization.
Furthermore, the levels of IgY in group A and group B chicken egg
yolks were elevated after immunization (Table II). From these findings it was
concluded that the S-IgY from week 7 chicken egg yolks after EV71
antigen immunization would be purified and used for the following
experiments.
Characterization of the isolated
IgY
The titers of the viruses were determined using the
TCID50 assay. Results indicated that the
TCID50 for the EV71 strain was 107.1
TCID50/ml, whereas the TCID50 for CVA16
strain was 106.3 TCID50/ml. As indicated in
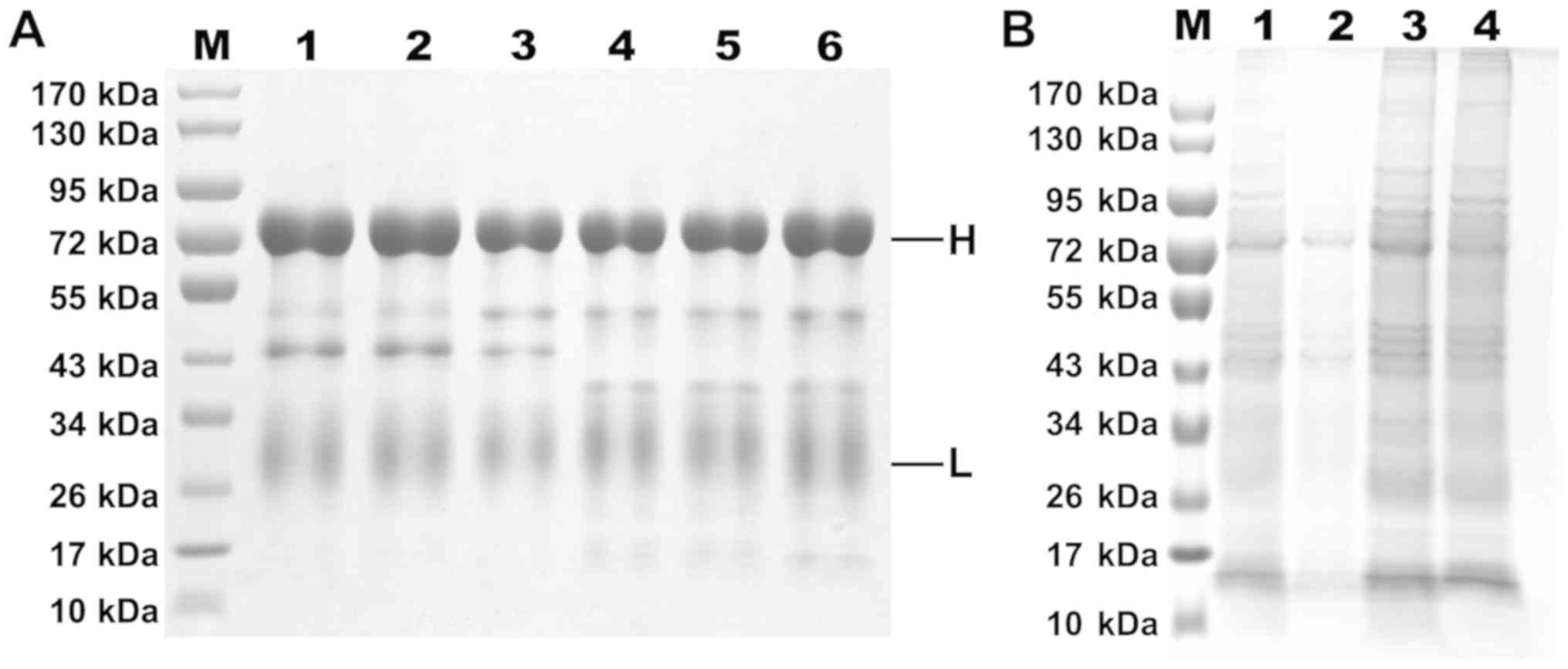
Fig. 2, the results of SDS-PAGE
demonstrated that the disulfide bond of the target protein was
opened under reduction conditions, and the presence of the two
dominant bands, a 70-kDa-sized species that represents the H chain
and a 30-kDa-sized species corresponding to the L chain, was
noted.
As the water-soluble fraction (WSF) contains a large
amount of heteroprotein after egg yolk acid-isolation, water was
used to dissolve the egg yolk, which was then salted out twice by
45% saturation of (NH4)2SO4 and
13% mass fraction of Na2SO4. This markedly
improved the purity of IgY, however, the SDS-PAGE results indicated
that some impurity bands were still present in the samples, which
may include some low density lipoproteins and active proteins. The
purity of the IgY was determined to be over 85% according to gel
system software Bandscan 5.0.
IgY cross binds to the structural
proteins VP0, VP1 and VP3 of EV71 and CVA16
The titer of the purified IgY was measured with a
bidirectional immune agar diffusion test. As indicated in Fig. 3A and B, a white precipitation line
was identified between the antigen hole in the center and the
antibody wells with dilution ratios of 1:2, 1:4, and 1:8, but no
precipitation line was indicated between the PBS wells and the
antigen wells. These data suggested that the purified S-IgY has the
ability to specifically cross bind to the antigens of EV71 and
CVA16.
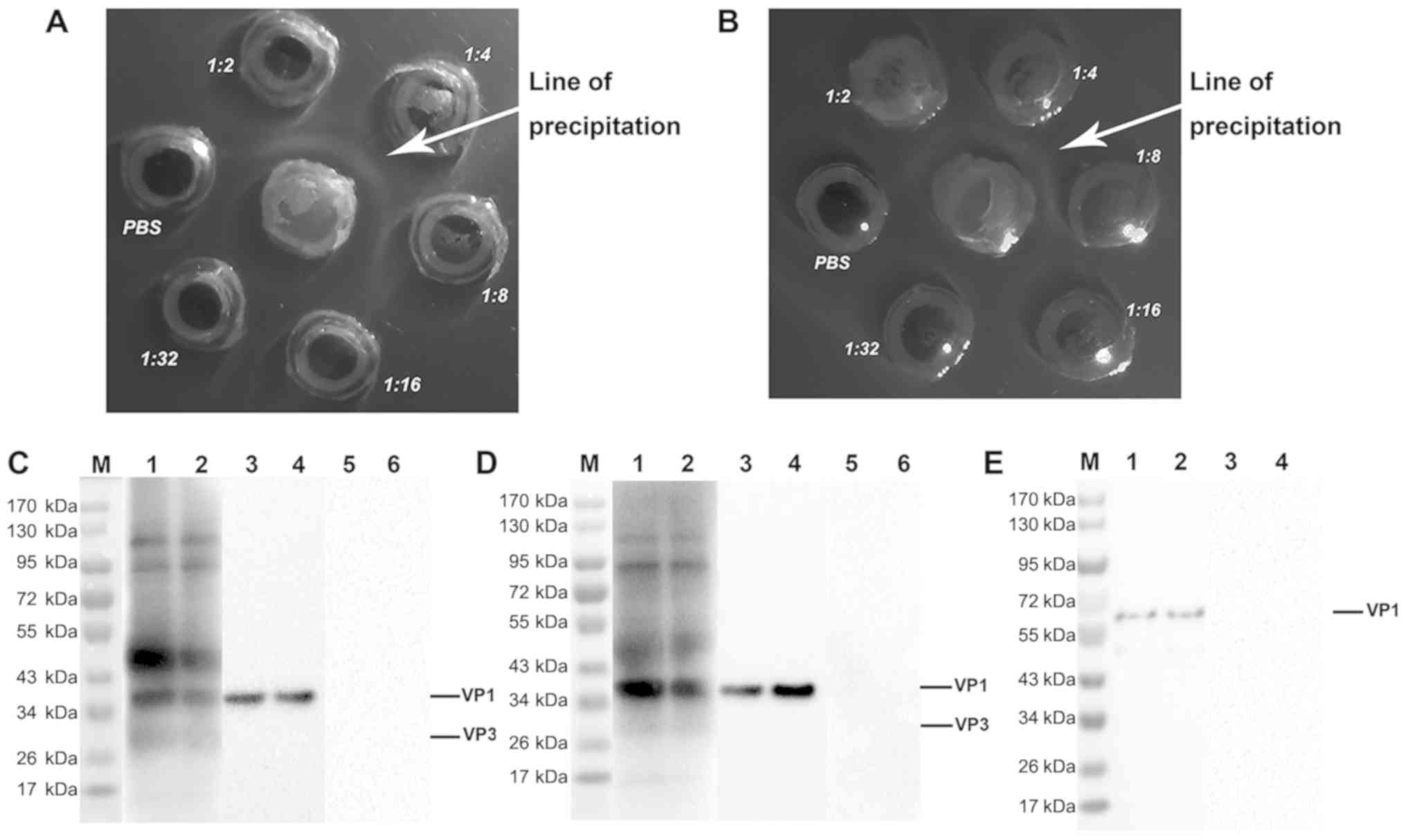 | Figure 3.Characterization of the specific IgY
against EV71 and CVA16 strains. (A) Bidirectional immune agar
diffusion test for EV71. (B) Bidirectional immune agar diffusion
test for CVA16. (C and D) Western blotting of the immunoreactivity
of the specific IgY. (C) EV71 and (D) CVA16 were assessed. The EV71
and CVA16 viral extracts were subjected to SDS-PAGE, transferred to
polyvinylidene fluoride membranes and detected with the specific
IgY, VP1 monoclonal antibody, or the negative control IgY. Lanes 1
and 2, the isolated IgY; lanes 3 and 4, VP1 monoclonal antibody;
lanes 5 and 6, negative control IgY. (E) The VP1 recombinant
protein was subjected to SDS-PAGE, transferred to a polyvinylidene
fluoride membrane and detected with the specific IgY or negative
control IgY. Lane M, protein markers; Lanes 1 and 2, the specific
IgY; lanes 3 and 4, the negative control IgY. IgY, immunoglobulin
Y; EV71, enterovirus 71; CVA16, coxsackievirus A16. |
The results of western blotting further confirmed
that the S-IgY exhibited a good immunological binding reaction with
the viral proteins of EV71 and CVA16, whereas neither EV71 nor
CVA16 had immunoreactivity with C-IgY (Fig. 3C and D), suggesting that the purified
IgY specifically recognizes the proteins of EV71 and CVA16
strains.
The proteins of EV71 and CVA16 viruses in the
samples were identified to be 36 kDa for VP1 and 28 kDa for VP3.
The predicted molecular weights of the VP1 protein is 35 kDa and 26
kDa for VP3 (41,42). VP1 protein of EV71 and CVA16 was
verified with VP1 monoclonal antibody (Fig. 3C and D, lanes 3 and 4), and the
purified IgY was verified with a commercial VP1 protein (Fig. 3E). Taken together, these data
indicate that the IgY isolated from the egg yolks of EV71-immunized
BWEL-SPF chickens specifically cross binds to EV71 and CVA16
viruses, and these data are consistent with the results of
ELISA.
IgY cross blocks the CPE induced by
EV71 and CVA16 in vitro
The protective effect of the purified IgY on
enterovirus-induced CPE in RD cells was assessed by neutralization
assay in vitro. As indicated in Fig. 4A, RD cells in the EV71 non-infected
groups were normal and healthy, indicating that the IgY and the
positive control drug Envrioxime themselves had no cytotoxic effect
in RD cells. Once the cells were infected with EV71 strains,
different degrees of CPE (atrophied, rounded, shedding and
apoptosis) appeared in the blank control group, the C-IgY group and
the Envrioxime group, whereas RD cells did not exhibit CPE in the
S-IgY group, suggesting a protecting effect of the specific IgY on
EV71 infection in RD cells (Fig.
4A). Fig. 4B indicated that the
IgY had a strong anti-EV71 activity in vitro at the
concentrations of 1.25, 2.5, 5, 10, and 20 µg/ml. The results in
Fig. 4C revealed that IgY had a
strong anti-CVA16 activity in vitro at the concentrations of
2.5, 5, 10, and 20 µg/ml, which substantially inhibited EV71- or
CVA16-induced CPE and blocked infectivity of the virus; however,
lower concentrations of the IgY did not prevent from the virus
infection of RD cells.
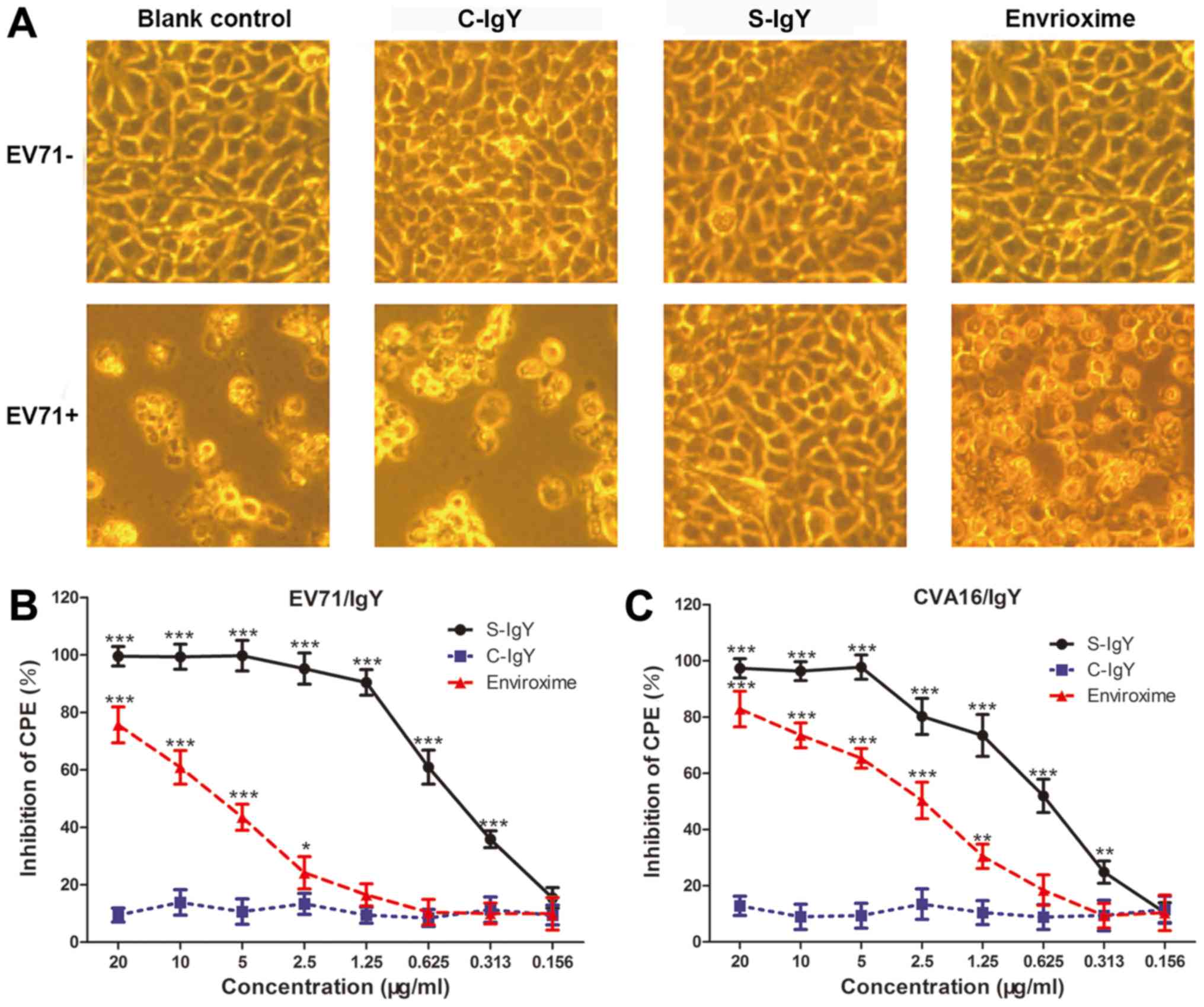 | Figure 4.In vitro neutralization assays
in RD cells. (A) RD cells were infected with or without EV71, and
the cells were treated with PBS (blank control), negative control
IgY (C-IgY), S-IgY or Envrioxime. A representative image from each
treatment group is indicated (magnification, ×400). (B and C)
Dose-response inhibitory effect of IgY on CPE in RD cells. (B)
EV71-infected RD cells and (C) CVA16-infected RD cells were treated
with different concentrations of the C-IgY, the isolated S-IgY or
Envrioxime as described in (A). The ability of the antibodies to
inhibit CPE was determined using the neutralization assay. CPE
values were expressed relative to those for cells with no antibody
treatment (control CPE value, 0%). Data were presented as the mean
± standard deviation of three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001 vs. C-IgY. RD,
rhabdomyosarcoma; IgY, immunoglobulin Y; EV71, enterovirus 71;
CVA16, coxsackievirus A16; C-IgY, negative control IgY; S-IgY,
specific IgY; CPE, cytopathic effect. |
To understand the time-dependent protection of the
S-IgY against enterovirus infection, RD cells were infected with
EV71 and then IgY was added to the cells at different time points
after infection. The present data indicated that the IgY inhibited
>70% of EV71-induced CPE in RD cells when the IgY was added 2 h
post-infection, and inhibited >40% of EV71-induced CPE when the
IgY was added 3 h post-infection (Fig.
5A).
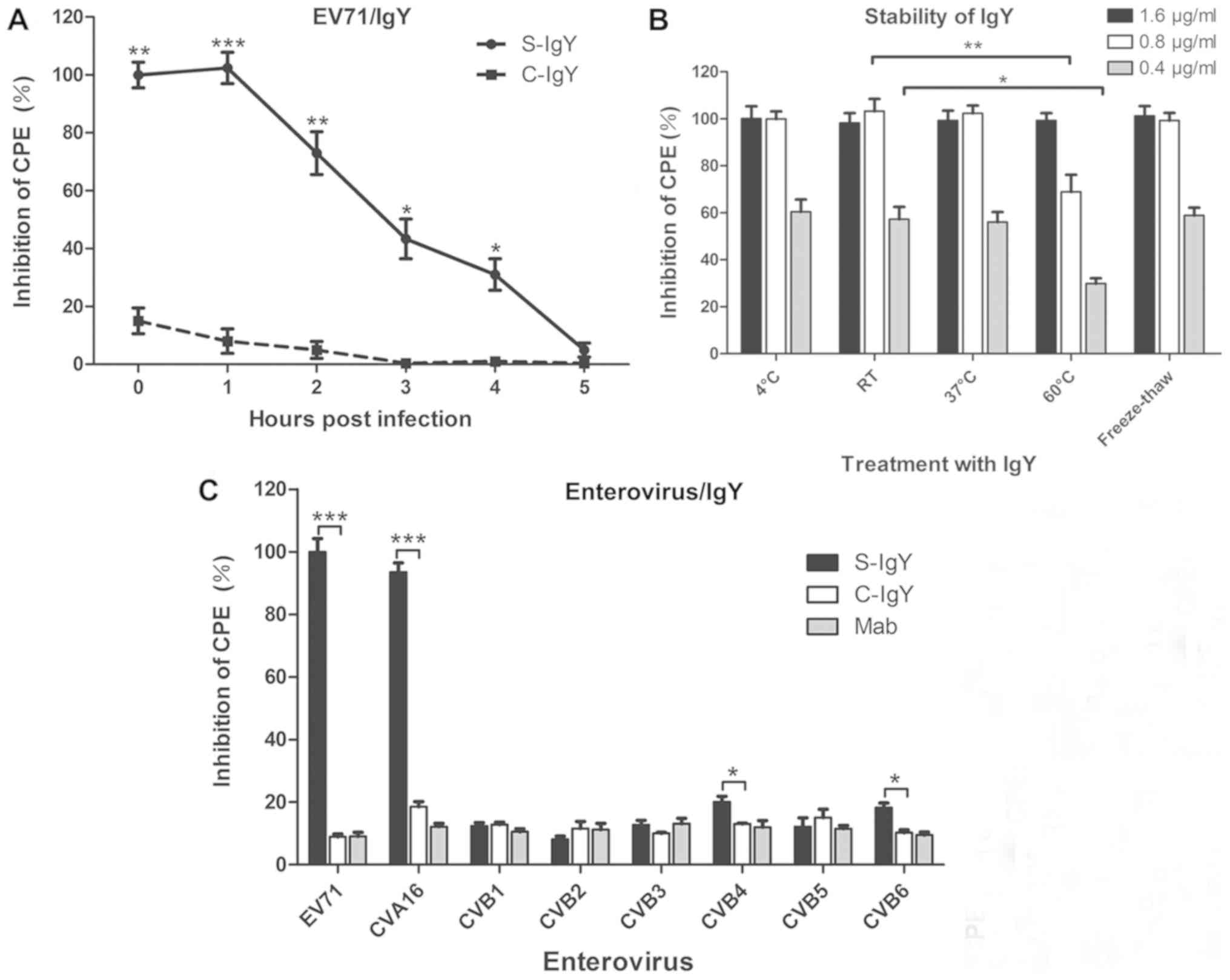 | Figure 5.Characterization of the IgY
bioactivity by in vitro neutralization assay. (A)
Time-dependent inhibitory effect of the S-IgY on EV71-induced CPE
in RD cells. RD cells were infected with EV71 and treated with
C-IgY or S-IgY at different time points following EV71 infection.
CPE values were expressed relative to those for cells with no
antibody treatment (control CPE value, 0%). *P<0.05, **P<0.01
and ***P<0.001 vs. C-IgY (Student's t-test). (B) Effect of
physical factors (temperature and freeze-thaw) on the S-IgY
bioactivity in RD cells. RD cells were infected with EV71 and
treated with IgY (0.4, 0.8, or 1.6 µg/ml) following exposure of the
IgY to different temperatures for 48 h, or by freezing-thawing for
five times. CPE values were expressed relative to those for cells
with no antibody treatment (control CPE value, 0%). *P<0.05 and
**P<0.01 * vs. RT IgY. (C) Inhibitory effect of the S-IgY on CPE
induced by different enterovirus strains. RD cells were infected
with eight different enterovirus strains, and the cells were
treated with the C-IgY, the S-IgY or Mab. CPE values were expressed
relative to those for cells with no antibody treatment (control CPE
value, 0%). Data were presented as the mean ± standard deviation of
three independent experiments. *P<0.05 and ***P<0.001 as
indicated. Mab, IgY monoclonal antibody; RD, rhabdomyosarcoma; IgY,
immunoglobulin Y; EV71, enterovirus 71; C-IgY, negative control
IgY; S-IgY, specific IgY; CPE, cytopathic effect. |
The stability of antiviral activity was determined
for the isolated IgY. As observed in Fig. 5B, purified IgY was stable after 48 h
at room temperature at 4°C and 37°C, and the inhibition rate was
almost 100% when the cells were treated with 1.6 and 0.8 µg/ml IgY
after 48 h at 60°C. Furthermore, there was no decrease in antiviral
activity observed with S-IgY after freeze thawing five times
(Fig. 5B), indicating that the
freeze-thaw cycle did not impair IgY activity.
The antiviral activity of the purified IgY in 8
different strains of enterovirus demonstrated that the S-IgY had a
strong inhibitory activity against EV71 and CVA16 strains
(P<0.001), but had only marginal or no antiviral activity in the
6 other strains of the enterovirus examined, including CVB1, CVB2,
CVB3, CVB4, CVB5, and CVB6 (Fig.
5C). The results revealed that the S-IgY has a differential
antiviral activity among different types of enterovirus, further
suggesting that the IgY isolated from EV71-immunized chicken egg
yolks has a specific protection against EV71- and CVA16-induced
infections.
Discussion
EV71 and CVA16 belong to the small RNA family and
enterovirus genus and have approximately the same structure
(43). Of the four structural
proteins of EV71 and CVA16, VP4 is located inside the capsid and
connects with RNA (44); VP1, VP2
and VP3 are located on the surface of the capsid; and VP2 and VP4
are cleaved from the VP0 protein by autocatalytic action that
involves virion stability and infectivity (45,46).
Previous findings suggest that the antigenic determinant is based
on the surface proteins, VP1 and VP3 (47). In the treatment of HFMD, inactivated
EV71 virus vaccine has been proved to have immunogenicity, but it
has no cross protection against other entericviruses, such as
CVA16, in phase III clinical trials (48). Lim et al (49) screened monoclonal antibodies that can
recognize the N-terminal of VP1 by passive immunization. Their
study demonstrated the cross-neutralization of monoclonal
antibodies against multiple EV71 subtype strains; however, there
was no effect on the CVA16 strains.
In the present study, the results of the
neutralization assay revealed that S-IgY cross blocked CPE induced
by EV71 and CVA16 in a dose-dependent manner in vitro.
Furthermore, bidirectional immune agar diffusion testing and
western blotting further demonstrated that the isolated IgY cross
bound to the envelope proteins VP1 and VP3 of EV71 and CVA16,
suggesting that the inhibitory effect of IgY on EV71- and
CVA16-induced CPE is mediated through targeting VP1 and VP3
structural proteins. Therefore, it was concluded that the isolated
IgY is likely to be effective in recognizing the sequential
epitopes or conformational structure of VP1 and VP3, preventing
EV71 and CVA16 from entering and infecting host cells, and
ultimately preventing and treating EV71- and CVA16-caused
infections. Notably, chicken antibodies may recognize the
sequential epitopes or conformational structure.
IgY is the sum of antibodies extracted from the egg
yolk of immunized BWEL-SPF chickens, of which 2–10% have antigen
specificity (50). In the present
study, 150 mg IgY was extracted from each egg. Notably, the total
IgY produced by an immunized chicken in a year is ~20 times as much
as the IgG produced by a immunized rabbit (51). The protein in egg yolk is
predominantly divided into WSFs and water-insoluble fractions. In
the present study, the results indicated that the levels of the IgY
in the egg yolk were significantly increased following
immunization, which may be due to the increase in the age of the
chickens (33). The SDS-PAGE results
revealed that the purified IgY was composed of a 70-kDa H chain and
a 30-kDa L chain, which is consistent with the literature (52), suggesting that IgY antibody with high
purity can be obtained by the water dilution combined with sulfate
precipitation. The indirect ELISA for measuring the S-IgY titer and
the growth-decline rule indicated that the titer of antibody peaked
at week 7 after the initial immunization and was maintained at a
higher level for ~ 4 weeks compared with C-IgY, the antibody levels
decreased gradually after week 11. The high titer, suitable purity
and long duration of the IgY in the immunized egg yolks make it
possible for manufacturing plants to prepare a large quantity of
IgY.
In conclusion, the present findings indicated that
the levels of S-IgY were significantly increased in chicken egg
yolk following immunization with EV71. It was also revealed that
IgY cross blocked CPE induced by EV71 and CVA16, and that the IgY
cross bound to the envelope proteins VP1 and VP3 of EV71 and CVA16,
suggesting that the cross protection of IgY against EV71 and CVA16
infection may be mediated through targeting VP1 and VP3 structural
proteins of the two viruses. These findings provide a scientific
basis for developing IgY as a cross passive immunotherapy for EV71-
or CVA16-induced HFMD. Further in vivo studies in
preclinical animal models with this cross immunotherapy are
warranted.
Acknowledgements
The authors would like to thank Professor Qingdi
Quentin Li (National Institute of Allergy and Infectious Diseases,
National Institutes of Health, Bethesda, USA) for carefully editing
this manuscript.
Funding
The present study was supported by grants from the
National Science Foundation of China (grant no. 81560574) and the
Bagui Scholar Foundation of He Songqing (grant no.
2016GXNSFFA380003).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, YY, SW and QX contributed to the conception and
design of the study. EG, YZ, NT JW, YY and QX performed the
experiments. JW, YY, SW, QX and EG prepared the manuscript. SW and
SH performed the statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The procedure strictly followed the Guide for the EU
Directive for animal experiments, the protocols use in the current
study were approved by the Institutional Animal Care and Use
Committee of Guilin Medical University (Guilin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Solomon T, Lewthwaite P, Perera D, Cardosa
MJ, McMinn P and Ooi MH: Virology, epidemiology, pathogenesis, and
control of enterovirus 71. Lancet Infect Dis. 10:778–790. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yip CC, Lau SK, Woo PC and Yuen KY: Human
enterovirus 71 epidemics: What's next? Emerg Health Threats J.
6:197802013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu SL, Pan H, Liu P, Amer S, Chan TC,
Zhan J, Huo X, Liu Y, Teng Z, Wang L and Zhuang H: Comparative
epidemiology and virology of fatal and nonfatal cases of hand, foot
and mouth disease in mainland China from 2008 to 2014. Rev Med
Virol. 25:115–128. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anastasina M, Domanska A, Palm K and
Butcher S: Human picornaviruses associated with neurological
diseases and their neutralization by antibodies. J Gen Virol.
98:1145–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao
H, Zhang YT, Yao X, Chu K, Chen QH, et al: Efficacy, safety, and
immunology of an inactivated alum-adjuvant enterovirus 71 vaccine
in children in China: A multicentre, randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet. 381:2024–2032. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright HT Jr, Landing BH, Lennette EH and
McAllister RM: Fatal infection in an infant associated with
Coxsackie virus group A, type 16. N Engl J Med. 268:1041–1044.
1963. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Legay F, Leveque N, Gacouin A, Tattevin P,
Bouet J, Thomas R and Chomelt JJ: Fatal coxsackievirus A-16
pneumonitis in adult. Emerg Infect Dis. 13:1084–1086. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goto K, Sanefuji M, Kusuhara K, Nishimura
Y, Shimizu H, Kira R, Torisu H and Hara T: Rhombencephalitis and
coxsackievirus A16. Emerg Infect Dis. 15:1689–1691. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang CY, Li Lu F, Wu MH, Lee CY and Huang
LM: Fatal coxsackievirus A16 infection. Pediatr Infect Dis J.
23:275–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen WJ, Arnold JC, Fairchok MP, Danaher
PJ, McDonough EA, Blair PJ, Garcia J, Halsey ES, Schofield C,
Ottolini M, et al: Epidemiologic, clinical, and virologic
characteristics of human rhinovirus infection among otherwise
healthy children and adults: Rhinovirus among adults and children.
J Clin Virol. 64:74–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z,
Zhang Y, Li Y, Mao Q, Wang J, et al: An inactivated enterovirus 71
vaccine in healthy children. N Engl J Med. 370:829–837. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tung WS, Bakar SA, Sekawi Z and Rosli R:
DNA vaccine constructs against enterovirus 71 elicit immune
response in mice. Genet Vaccines Ther. 5:62007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foo DG, Alonso S, Chow VT and Poh CL:
Passive protection against lethal enterovirus 71 infection in
newborn mice by neutralizing antibodies elicited by a synthetic
peptide. Microbes Infect. 9:1299–1306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kirk K, Poh CL, Fecondo J, Pourianfar H,
Shaw J and Grollo L: Cross-reactive neutralizing antibody epitopes
against Enterovirus 71 identified by an in silico approach.
Vaccine. 30:7105–7110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foo DG, Alonso S, Phoon MC, Ramachandran
NP, Chow VT and Poh CL: Identification of neutralizing linear
epitopes from the VP1 capsid protein of Enterovirus 71 using
synthetic peptides. Virus Res. 125:61–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou H, Wang G, Li XF, Li Y, Zhu SY, Qin
CF and Tang R: Alumina-encapsulated vaccine formulation with
improved thermostability and immunogenicity. Chem Commun (Camb).
52:6447–6450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao R, Han J, Deng Y, Yu M, Qin E and Qin
C: Presence of high-titer neutralizing antibodies against
enterovirus 71 in intravenous immunoglobulin manufactured from
Chinese donors. Clin Infect Dis. 50:125–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang G, Zhou F, Gu B, Ding C, Feng D, Xie
F, Wang J, Zhang C, Cao Q, Deng Y, et al: In vitro and in vivo
evaluation of ribavirin and pleconaril antiviral activity against
enterovirus 71 infection. Arch Virol. 157:669–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chong P, Hsieh SY, Liu CC, Chou AH, Chang
JY, Wu SC, Liu SJ, Chow YH, Su IJ and Klein M: Production of EV71
vaccine candidates. Hum Vaccin Immunother. 8:1775–1783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reed Z and Cardosa MJ: Status of research
and development of vaccines for enterovirus 71. Vaccine.
34:2967–2970. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Bao H, Zhang X, Zhai M, Bao X, Wang
D and Zhang S: Epidemiological and genetic analysis concerning the
non-enterovirus 71 and non-coxsackievirus A16 causative agents
related to hand, foot and mouth disease in Anyang city, Henan
Province, China, from 2011 to 2015. J Med Virol. 89:1749–1758.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizuta K, Aoki Y, Suto A, Ootani K,
Katsushima N, Itagaki T, Ohmi A, Okamoto M, Nishimura H, Matsuzaki
Y, et al: Cross-antigenicity among EV71 strains from different
genogroups isolated in Yamagata, Japan, between 1990 and 2007.
Vaccine. 27:3153–3158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang
Y, Mao N, Xu S, Zhu S, Cui A, et al: An emerging recombinant human
enterovirus 71 responsible for the 2008 outbreak of hand foot and
mouth disease in Fuyang city of China. Virol J. 7:942010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jolles S, Sewell WA and Misbah SA:
Clinical uses of intravenous immunoglobulin. Clin Exp Immunol.
142:1–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ameratunga R, Sinclair J and Kolbe J:
Increased risk of adverse events when changing intravenous
immunoglobulin preparations. Clin Exp Immunol. 136:111–113. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han JF, Cao RY, Deng YQ, Tian X, Jiang T,
Qin ED and Qin CF: Antibody dependent enhancement infection of
enterovirus 71 in vitro and in vivo. Virol J. 8:1062011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Wang EQ and Balthasar JP:
Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin
Pharmacol Ther. 84:548–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sgro C: Side-effects of a monoclonal
antibody, muromonab CD3/orthoclone OKT3: Bibliographic review.
Toxicology. 105:23–29. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tini M, Jewell UR, Camenisch G, Chilov D
and Gassmann M: Generation and application of chicken egg-yolk
antibodies. Comp Biochem Physiol A Mol Integr Physiol. 131:569–574.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee J, Kang HE and Woo HJ: Stability of
orally administered immunoglobulin in the gastrointestinal tract. J
Immunol Methods. 384:143–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nilsson E, Stålberg J and Larsson A: IgY
stability in eggs stored at room temperature or at + 4°C. Br Poult
Sci. 53:42–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schade R, Staak C, Erhard M, Hugl H, Koch
G and Hendriksen C: The production of avian (Egg Yolk) Antibodies:
IgY. ATLA. 24:925–934. 1997.
|
|
33
|
Ulmer-Franco AM, Cherian G, Quezada N,
Fasenko GM and McMullen LM: Hatching egg and newly hatched chick
yolk sac total IgY content at 3 broiler breeder flock ages. Poult
Sci. 91:758–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang WW: The use of gene-specific IgY
antibodies for drug target discovery. Drug Discov Today. 8:364–371.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YH, Park D, Yang G, Lee SH, Bae DK,
Kyung J, Kim D, Choi EK, Son JC, Hwang SY and Kim YB:
Anti-Helicobacter pylori effects of IgY from egg york of
immunized hens. Lab Anim Res. 28:55–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akbari MR, Ahmadi A, Mirkalantari S and
Salimian J: Anti-Vibriocholerae IgY antibody inhibits
mortality in suckling mice model. J Natl Med Assoc. 110:84–87.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thu HM, Myat TW, Win MM, Thant KZ, Rahman
S, Umeda K, Nguyen SV, Icatlo FC Jr, Higo-Moriguchi K, Taniguchi K,
et al: Chicken Egg Yolk antibodies (IgY) for prophylaxis and
treatment of rotavirus diarrhea in human and animal neonates: A
concise review. Korean J Food Sci Anim Resour. 37:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu CY, Huang H, Wang XM, Liu YG, Wang ZG,
Cui SJ, Gao HL, Li Z, Li JP and Kong XG: Preparation and evaluation
of anti-SARS coronavirus IgY from yolks of immunized SPF chickens.
J Virol Methods. 133:112–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao Y, Gao X, Maragh S, Telford WG and
Tona A: Cell lines as candidate reference materials for quality
control of ERBB2 amplification and expression assays in breast
cancer. Clin Chem. 55:1307–1315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee MS, Cohen B, Hand J and Nokes DJ: A
simplified and standardized neutralization enzyme immunoassay for
the quantification of measles neutralizing antibody. J Virol
Method. 78:209–217. 1999. View Article : Google Scholar
|
|
41
|
Feng Q, He Y and Lu J: Virus-like
particles produced in pichia pastoris induce protective immune
responses against coxsackievirus A16 in mice. Med Sci Monit.
22:3370–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu CC, Chou AH, Lien SP, Lin HY, Liu SJ,
Chang JY, Guo MS, Chow YH, Yang WS, Chang KH, et al: Identification
and characterization of a cross-neutralization epitope of
Enterovirus 71. Vaccine. 29:4362–4372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ang LW, Koh BK, Chan KP, Chua LT, James L
and Goh KT: Epidemiology and control of hand, foot and mouth
disease in Singapore, 2001–2007. Ann Acad Med Singapore.
38:106–112. 2009.PubMed/NCBI
|
|
44
|
Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z,
Li X, Yin W, Shen X, Porta C, et al: A sensor-adaptor mechanism for
enterovirus uncoating from structures of EV71. Nat Struct Mol Biol.
19:424–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang YX, Huang YM, Li QJ, Li XY, Zhou YD,
Guo F, Zhou JM and Cen S: A highly conserved amino acid in VP1
regulates maturation of enterovirus 71. PLoS Pathog.
13:e10066252017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Curry S, Fry E, Blakemore W, Abu-Ghazaleh
R, Jackson T, King A, Lea S, Newman J and Stuart D: Dissecting the
roles of VP0 cleavage and RNA packaging in picornavirus capsid
stabilization: The structure of empty capsids of foot-and-mouth
disease virus. J Virol. 71:9743–9752. 1997.PubMed/NCBI
|
|
47
|
Kristensen T, Newman J, Guan SH, Tuthill
TJ and Belsham GJ: Cleavages at the three junctions within the
foot-and-mouth disease virus capsid precursor (P1-2A) by the 3C
protease are mutually independent. Virology. 522:260–270. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chong P, Liu CC, Chow YH, Chou AH and
Klein M: Review of enterovirus 71 vaccines. Clin Infect Dis.
60:797–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lim XF, Jia Q, Khong WX, Yan B, Premanand
B, Alonso S, Chow VT and Kwang J: Characterization of an
isotype-dependent monoclonal antibody against linear neutralizing
epitope effective for prophylaxis of enterovirus 71 infection. PLoS
One. 7:e297512012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mine Y and Kovacs-Nolan J: Chicken egg
yolk antibodies as therapeutics in enteric infectious disease: A
review. J Med Food. 5:159–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Solhi R, Alebouyeh M, Khafri A, Rezaeifard
M and Aminian M: In vitro evaluation of cross-strain inhibitory
effects of IgY polyclonal antibody against H. pylori. Microb
Pathog. 110:682–687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Müller S, Schubert A, Zajac J, Dyck T and
Oelkrug C: IgY antibodies in human nutrition for disease
prevention. Nutr J. 14:1092015. View Article : Google Scholar : PubMed/NCBI
|