Introduction
Hirschsprung's disease (HD), also known as
aganglionosis, is a common pediatric surgical digestive tract
malformation disease (1). Some
studies (2) have suggested that the
lesional intestine of HD may be caused by the abnormality of nerve
ridge cells in the process of migration and differentiation into
the intestinal wall, resulting in the loss of ganglion cells in the
sphincter and submucosal plexus. Other studies (3) have reported that as long as any process
of biological function, such as proliferation and differentiation
of intestinal nerve ridge cells, is impeded it can lead to the
occurrence of HD. In recent years, the cause of HD has been more
related to the congenital malformation of multiple genes, and has
certain family hereditary origin (4,5).
However, there is currently no detailed study on the pathogenesis
of HD.
In recent years, with the continuous development of
molecular biology, a variety of genes have been found to be
associated with HD pathogenesis (6).
Although some studies (7) have
reported that the abnormalities of these genes may be responsible
for the pathogenesis of HD, subsequent studies have found that the
mutations of these genes have certain limitations, and the etiology
of all HD cases cannot be explained. However, as proteins and
genes, diagnosis and treatment of diseases are deeply studied
investigating etiology, a secreted polypeptide signaling molecule
and transforming growth factor-β (TGF-β) super family has been
discovered. This is a class of cytokines with versatility and
extensive biological activity, which have an impact on biological
processes, such as cell growth, proliferation and apoptosis
(8). Bone morphogenetic proteins
(BMPs) are members of the TGF-β superfamily (9). Their mechanism of action is that
R-Smads are used as signal transducers by BMPs. BMP-4 forms a
complex with phosphorylated Smad1. Then, it enters the nucleus from
the cytoplasm and binds to DNA, which regulates the transcription
of BMP target genes (10). Smad is a
very important step in the TGF-β signaling pathway, as it regulates
the progression of TGF-β signaling from the cell membrane into the
nucleus (11). As a member of the
BMP family, BMP-4 has been found to be related to the development
and maturation of the enteric nervous system (12), while Smad1 has been found to be
expressed in the mitosis of spinal cord neurons and play an
important role in the development of the nervous system (13).
Currently, there are no studies on the specific
roles and mechanisms of BMP-4 and Smad1 in HD etiology. Therefore,
in the present study, the expression and clinical significance of
BMP-4 and Smad1 protein and mRNA in HD patients were investigated
in order to provide new methods for the treatment of clinical HD
and a new theoretical basis for the its pathogenesis.
Subjects and methods
General information
A retrospective analysis of 96 HD patients
(experimental group) admitted to Xuzhou Children's Hospital, Xuzhou
Medical University (Xuzhou, China) from June 2015 to June 2017 was
performed. The average age of all children was 2.03±1.28 years,
including 37 males and 59 females. Among them, 38 cases were
typical short-segment HD patients with rectal or sigmoid colon, 35
cases were of long-segment type, and 23 cases of whole colon type.
According to the samples taken, the experimental group was divided
into the stenosis group, the transition group and the expansion
group. Forty-seven children with colostomy due to intestinal
obstruction were selected as the control group. There was no
significant difference in sex, age, BMI and height between the
experimental and control group (P>0.05) (Table I). Inclusion criteria: children
having undergone rectal mucosal biopsy and X-ray barium enema
(diagnosed as HD). Exclusion criteria: children with other serious
organ diseases or other gastrointestinal disorders. All the
families of the children agreed to participate in the experiment
and signed an informed consent form. The study was approved by the
Ethics Committee of Xuzhou Children's Hospital, Xuzhou Medical
University.
 | Table I.General information. |
Table I.
General information.
| Factors | Experimental group
(n=96) | Control group
(n=47) | χ2
value | P-value |
|---|
| Sex |
|
| 0.076 | 0.784 |
| Male | 37 (38.54) | 17 (36.17) |
|
|
|
Female | 59 (61.46) | 30 (63.83) |
|
|
| Age (years) |
|
| 0.068 | 0.795 |
| ≥2 | 41 (42.71) | 19 (40.43) |
|
|
|
<2 | 55 (57.29) | 28 (59.57) |
|
|
| BMI
(kg/m2) |
|
| 0.061 | 0.805 |
| ≥10 | 45 (46.88) | 21 (44.68) |
|
|
|
<10 | 51 (53.13) | 26 (55.32) |
|
|
| Height (cm) |
|
| 0.064 | 0.800 |
| ≥70 | 43 (44.79) | 20 (42.55) |
|
|
|
<70 | 53 (55.21) | 27 (57.45) |
|
|
| Type |
|
| – | – |
| Short
segment | 38 (39.58) | – |
|
|
| Long
segment | 35 (36.46) | – |
|
|
| Full
colon | 23 (23.96) | – |
|
|
| Family genetic
history |
|
| – | – |
|
Existent | 72 (75.00) | – |
|
|
|
Non-existent | 24 (25.00) | – |
|
|
Experimental materials and
reagents
BMP-4 immunohistochemistry kit and Smad1
immunohistochemistry kit were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The microscope used was
purchased from Olympus Corp. (Tokyo, Japan). DAB kit was purchased
from MXB Biotechnologies (Fuzhou, China), TRIzol reagent was
purchased from Applied Biosystems (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), SYBR-Green RT-qPCR kit
and 2X PCR Master Mix reverse transcription kit were purchased from
Takara Biotechnology Co., Ltd. (Dalian, China), quantitative PCR
instrument was purchased from Bio-Rad Laboratories, Inc. (Hercules,
CA, USA), and all primers were synthesized by Shanghai Jima Biotech
Co., Ltd. (Shanghai, China).
Immunohistochemistry detection of
BMP-4 and Smad1 expression in different groups of children
Firstly, each group of samples, obtained after
operation, was made into paraffin sections with a thickness of ~3
µm for further use, and then detected by immunohistochemistry. The
details of the specific detection method are as follows: every
sample was fixed in 4% paraformaldehyde buffer at 4°C for 24 h.
Paraffin samples were routinely dewaxed and then rinsed 3 times
with PBS for 5 min each. After rinsing, the antigen retrieval
solution was incubated at room temperature for 10 min and washed 3
times with PBS. Then, 10% milk protein was added to each section
for sealing and incubation was continued for 10 min at room
temperature. A total of 50 µl of primary antibodies (rabbit
anti-human BMP-4 and Smad1 monoclonal antibodies; dilution,
1:1,000; cat. nos. ab124715 and ab63356; Abcam, Cambridge, MA, USA)
were added and the samples were left overnight at 4°C. Then, PBS
was used for rinsing, and the secondary antibody (biotinylated goat
anti-rabbit IgG; dilution, 1:500; cat. no. ab6720; Abcam) was added
after removing the PBS solution. Next, it was incubated at room
temperature for 30 min and rinsed with PBS. After being incubated
at room temperature for 10 min, freshly prepared DAB (20:1:1) was
applied for coloring. Every sample was washed with BPS to terminate
coloring, and finally the samples were colored with hematoxylin for
1 min. Next, water was dehydrated with 80, 90 and 100% alcohol.
After the neutral gum was sealed, it was clustered and photographed
with a microscope. The samples of the primary antibody were
replaced with PBS as a negative control, and the following
procedures were the same as above. The expression levels of BMP-4
and Smad1 protein in different groups of children were compared
when brown-to-brown particles in the cytoplasm were identified.
RT-qPCR detection of BMP-4 and Smad1
mRNA expression in different groups of children
The obtained tissues were first treated and then
total RNA of BMP-4 and Smad1 was extracted by adding TRIzol
reagent. The purity and concentration of RNA were detected by
ultraviolet spectrophotometer (Shanghai Metash Instruments Co.,
Ltd., Shanghai, China) and 1 µg of total RNA was obtained. cDNA was
synthesized by reverse transcription according to the 2X PCR Master
Mix, and the reaction conditions were as follows: 37°C for 15 min,
85°C for 5 sec. After reverse transcription, 2 µl of the
synthesized cDNA were taken for qPCR. The reaction conditions were:
pre-denaturation at 95°C for 30 sec, 95°C for 5 sec, 65°C for 30
sec, and finally 65°C for 15 sec. Reaction conditions:
pre-denaturation at 95°C for 30 sec, 95°C for 5 sec, 65°C for 30
sec, finally 65°C for 15 sec and 95°C for 5 sec as extension,
repeated 40 times. β-actin was used as an internal reference
substance. Primer sequences are shown in Table II. The relative expression of the
gene was quantified using the 2−ΔCq method (14), and fluorescent qPCR was performed by
a qPCR instrument. The experiment was repeated 3 times.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Genes | Upstream primers | Downstream
primers |
|---|
| BMP-4 |
5′-TCCTGGTAACCGAATGCT-3′ |
5′-CACCTGCTCCCGAAATAG-3′ |
| Smad1 |
5′-CAGCAGAGGAGATGTTCAGGCA-3′ |
5′-TGCACGAAGATGCTGCTGTCAC-3′ |
| β-actin |
5′-AGATGTGGACAGCAAGCAG-3′ |
5′-GCGCAAGTTAGGTTTTGTCA-3′ |
Statistical analysis
In this study, SPSS 18.0 software package
(Bizinsight, Beijing, China) was used for the statistical analysis
of the experimental data. Measurement data were expressed as the
mean ± standard deviation. t-test was used for the comparison
between two groups, one-way analysis of variance was used for the
comparison between multiple groups and LSD was the post hoc test.
Chi-square test was used for count data. The experimental graphs
were produced using GraphPad Prism 6 software (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical detection of the
positive expression levels of BMP-4 and Smad1 proteins in the four
groups of children
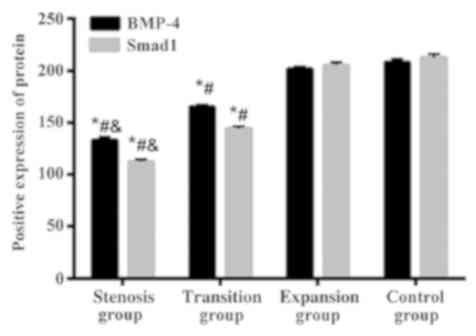
According to the location of the specimens taken,
the subjects were divided into stenosis group (n=31), transition
group (n=33), and expansion group (n=32). There was no significant
difference in the expression of BMP-4 and Smad1 proteins between
the control and the expansion group (P>0.05). The positive
expression levels of BMP-4 and Smad1 proteins in the transition
group were decreased compared with those in the expansion and
control groups (P<0.05), and the positive expression levels of
BMP-4 and Smad1 proteins in the stenosis group were decreased
compared with those in the transition, expansion, and control
groups (P<0.05) (Table III,
Fig. 1).
 | Table III.Comparison of BMP-4 and Smad1 positive
protein expression levels among four groups. |
Table III.
Comparison of BMP-4 and Smad1 positive
protein expression levels among four groups.
| Factors | Stenosis group
(n=31) | Transition group
(n=33) | Expansion group
(n=32) | Control group
(n=47) | F value | P-value |
|---|
| BMP-4 |
133.54±2.76a–c |
165.03±2.42a,b | 201.67±2.16 | 208.32±3.19 |
6,080 | <0.001 |
| Smad1 |
112.46±2.02a–c |
144.29±2.17a,b | 205.33±2.63 | 212.41±3.58 | 10,636 | <0.001 |
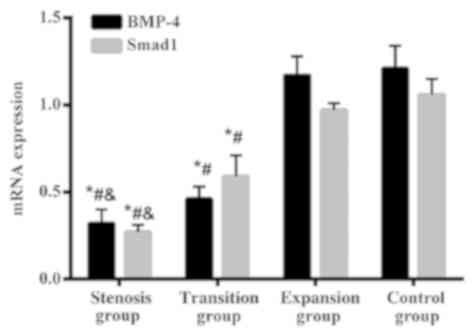
RT-qPCR mRNA detection results
The expression of BMP-4 gene in the stenotic and
transitional segments of HD was significantly lower than that in
the control group and the expansion group and the differences were
statistically significant (P<0.05). The data in expansion group
were slightly lower than that in the control group, but there was
no significant difference (P>0.05). The expression level of
BMP-4 gene was gradually decreased from the expansion group to the
stenosis group, and the differences were statistically significant
(P<0.05). The expression of Smad1 gene in the stenotic and
transitional segments of HD was significantly lower than that in
the control group and the expansion group, and the differences were
statistically significant (P<0.05). The data in expansion group
were slightly lower than that in the control group, but there was
no significant difference (P>0.05). The expression level of
Smad1 gene was gradually decreased from the expansion group to the
stenosis group, and the differences were statistically significant
(p<0.05) (Table IV and Fig. 2).
 | Table IV.Comparison of BMP-4 and Smad1 mRNA
expression levels among four groups. |
Table IV.
Comparison of BMP-4 and Smad1 mRNA
expression levels among four groups.
| Factors | Stenosis group
(n=31) | Transition group
(n=33) | Expansion group
(n=32) | Control group
(n=47) | F value | P-value |
|---|
| BMP-4 |
0.32±0.08a–c |
0.46±0.07a,b | 1.17±0.11 | 1.21±0.13 | 711.8 | <0.001 |
| Smad1 |
0.27±0.04a–c |
0.59±0.12a,b | 0.97±0.04 | 1.06±0.09 | 739.7 | <0.001 |
Discussion
The enteric nervous system is part of the direct
control of the gastrointestinal tract by the autonomic nervous
system. Cells called neural crest can differentiate and derive cell
populations. However, once the neural crest cells are missing, this
may lead to intestinal ganglion neurons, which is Hirschsprung
disease (HD) (15). HD is a
digestive tract malformation disease that occurs in children with
benign hyperplasia. HD's usual symptoms are constipation and
bloating in children, and may lead to nutritional malabsorption and
hypoevolutism (16). Currently, many
studies have shown that the occurrence of HD has complex genetic
factors. The idea is that the pathogenesis is more unified, because
when the migration process of neural crest cells is affected, the
intestinal wall cannot colonize the neural crest cells derived from
the enteric nervous system causing HD to occur (17). In a study of the molecular biology of
HD, it was found that the BMP/Smad pathway plays a very important
role in the development of the nervous system at various stages and
sites (18). As a member of the BMP
family, it has been found that BMP-4 is critical for the intestinal
development (19). Smad1 is a
receptor-activated Smad, an intracellular signaling molecule
belonging to BMPs (20). Some
studies (21) have found the
presence of phosphorylated Smad1 in enteric ganglion cells.
Abnormal regulation of the BMP/Smad signaling pathway leads to a
variety of development-related diseases (22), and some studies (23) have demonstrated that BMP-4 and Smad1
play key roles in intestinal development. However, the specific
expression and effect of BMP-4 and Smad1 proteins and mRNAs in HD
patients have rarely been studied, therefore the present study
explored the expression and clinical significance of BMP-4 and
Smad1 protein and mRNA in HD patients.
Pathogenesis is a complex multi-factorial process.
In the present study, we first used immunohistochemistry to detect
BMP-4 and Smad1 proteins in samples from different parts of HD
patients and children with intestinal obstruction. There was no
significant difference in the expression of BMP-4 and Smad1
proteins between the control and the expansion group (P>0.05).
The positive expression levels of BMP-4 and Smad1 proteins in the
transition group were decreased compared with those in the
expansion and control groups (P<0.05), and the positive
expression levels of BMP-4 and Smad1 proteins in the stenosis group
were decreased compared with those in the transition, expansion,
and control groups (P<0.05). Then we used RT-qPCR to detect the
expression of BMP-4 and Smad1 mRNA in the samples from different
parts of HD patients and children with intestinal obstruction. The
results showed that there was no significant difference in the
expression of BMP-4 and Smad1 genes between the control and the
expansion group (P>0.05), but the expression levels of BMP-4 and
Smad1 in the transition and stenosis group decreased successively,
and the differences were statistically significant (P<0.05). The
results above suggest that there is an abnormal expression of BMP-4
and Smad1 in children with HD. Some studies (24) have shown that abnormal expression of
BMP-4 and Smad1 has an effect on the expression of downstream
signaling molecules, therefore the growth and differentiation of
ganglion cells are effected. So we hypothesized that BMP-4 and
Smad1 may interact with each other during the development of
ganglion cells, influencing the development and function of the
intestinal nervous system. An in vitro study of BMP-4
(25) has found that the
overexpression of BMP-4 in mice can cause intestinal development
defects in mice, which is also confirmed by our conclusion of
BMP-4. However, there is no relevant study on the expression of
Smad1 in children with HD.
In summary, the expression levels of BMP-4 and Smad1
in the plexus of HD lesions were significantly reduced, indicating
that BMP-4 and Smad1 are closely related to the occurrence of HD,
and it is speculated that they have a certain influence on the
intestinal development of congenital digestive tract malformations,
which provides a theoretical basis for the subsequent research on
the pathogenesis of HD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ assisted with immunohistochemistry and was
responsible for the drafting of the manuscript. JZ and FL performed
PCR. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou,
China). Signed informed consents were obtained from the parents of
the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kenny SE, Tam PK and Garcia-Barcelo M:
Hirschsprung's disease. Semin Pediatr Surg. 19:194–200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tam PK and Garcia-Barceló M: Genetic basis
of Hirschsprung's disease. Pediatr Surg Int. 25:543–558. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heanue TA and Pachnis V: Enteric nervous
system development and Hirschsprung's disease: Advances in genetic
and stem cell studies. Nat Rev Neurosci. 8:466–479. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Z, Jiang Q, Li Q, Cheng W, Qiao G,
Xiao P, Gan L, Su L, Miao C and Li L: Genotyping analysis of 3 RET
polymorphisms demonstrates low somatic mutation rate in Chinese
Hirschsprung disease patients. Int J Clin Exp Pathol. 8:5528–5534.
2015.PubMed/NCBI
|
|
5
|
Kapur RP: Practical pathology and genetics
of Hirschsprung's disease. Semin Pediatr Surg. 18:212–223. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson SR, Lee I, Ebeling C, Stephenson
DA, Schweitzer KM, Baxter D, Moon TM, LaPierre S, Jaques B, Silvius
D, et al: Disrupted SOX10 function causes spongiform
neurodegeneration in gray tremor mice. Mamm Genome. 26:80–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferreira R, Nilsson JR, Solano C,
Andréasson J and Grøtli M: Design, synthesis and inhibitory
activity of photoswitchable RET kinase inhibitors. Sci Rep.
5:97692015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McGehee AM, Moss BJ and Juo P: The
DAF-7/TGF-β signaling pathway regulates abundance of the
Caenorhabditis elegans glutamate receptor GLR-1. Mol Cell
Neurosci. 67:66–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dias JM, Alekseenko Z, Applequist JM and
Ericson J: TGFβ signaling regulates temporal neurogenesis and
potency of neural stem cells in the CNS. Neuron. 84:927–939. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XB, Wang W, Zhu XC, Ye WJ, Cai H, Wu
PL, Huang XY and Wang LX: The potential of asiaticoside for
TGF-β1/Smad signaling inhibition in prevention and progression of
hypoxia-induced pulmonary hypertension. Life Sci. 137:56–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hatakeyama Y, Nguyen J, Wang X, Nuckolls
GH and Shum L: Smad signaling in mesenchymal and chondroprogenitor
cells. J Bone Joint Surg Am 85-A. (Suppl 3):13–18. 2003. View Article : Google Scholar
|
|
12
|
Fukuda S and Taga T: Roles of BMP in the
development of the central nervous system. Clin Calcium.
16:781–785. 2006.(In Japanese). PubMed/NCBI
|
|
13
|
Weng Q, Chen Y, Wang H, Xu X, Yang B, He
Q, Shou W, Chen Y, Higashi Y, van den Berghe V, et al: Dual-mode
modulation of Smad signaling by Smad-interacting protein Sip1 is
required for myelination in the central nervous system. Neuron.
73:713–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohelnikova-Duchonova B, Oliverius M,
Honsova E and Soucek P: Evaluation of reference genes and
normalization strategy for quantitative real-time PCR in human
pancreatic carcinoma. Dis Markers. 32:203–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pontarelli EM, Ford HR and Gayer CP:
Recent developments in Hirschsprung's-associated enterocolitis.
Curr Gastroenterol Rep. 15:3402013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohammed AA and Gahukamble DB: Concordant
expression of Hirschsprung's disease in monozygous twins. Saudi Med
J. 21:200–201. 2000.PubMed/NCBI
|
|
17
|
Jiao CL, Chen XY and Feng JX: Novel
insights into the pathogenesis of Hirschsprung's-associated
enterocolitis. Chin Med J (Engl). 129:1491–1497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JH, Liu YZ, Yin LJ, Chen L, Huang J,
Liu Y, Zhang RX, Zhou LY, Yang QJ, Luo JY, et al: BMP9 and COX-2
form an important regulatory loop in BMP9-induced osteogenic
differentiation of mesenchymal stem cells. Bone. 57:311–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen K, Xie W, Luo B, Xiao W, Teitelbaum
DH, Yang H, Zhang K and Zhang C: Intestinal mucosal barrier is
injured by BMP2/4 via activation of NF-κB signals after ischemic
reperfusion. Mediators Inflamm. 901530:20162014.
|
|
20
|
Arnold SJ, Maretto S, Islam A, Bikoff EK
and Robertson EJ: Dose-dependent Smad1, Smad5 and Smad8 signaling
in the early mouse embryo. Dev Biol. 296:104–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hegarty SV, O'Keeffe GW and Sullivan AM:
BMP-Smad 1/5/8 signalling in the development of the nervous system.
Prog Neurobiol. 109:28–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aubin J, Davy A and Soriano P: In vivo
convergence of BMP and MAPK signaling pathways: Impact of
differential Smad1 phosphorylation on development and homeostasis.
Genes Dev. 18:1482–1494. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blank U, Seto ML, Adams DC, Wojchowski DM,
Karolak MJ and Oxburgh L: An in vivo reporter of BMP signaling in
organogenesis reveals targets in the developing kidney. BMC Dev
Biol. 8:862008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Henningfeld KA, Rastegar S, Adler G and
Knöchel W: Smad1 and Smad4 are components of the bone morphogenetic
protein-4 (BMP-4)-induced transcription complex of the Xvent-2B
promoter. J Biol Chem. 275:21827–21835. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chalazonitis A, Pham TD, Li Z, Roman D,
Guha U, Gomes W, Kan L, Kessler JA and Gershon MD: Bone
morphogenetic protein regulation of enteric neuronal phenotypic
diversity: Relationship to timing of cell cycle exit. J Comp
Neurol. 509:474–492. 2008. View Article : Google Scholar : PubMed/NCBI
|