Introduction
The heart is an important target organ involved in
inflammatory rheumatic diseases (IRD) and cardiovascular death is
one of the most frequent causes of mortality among patients with
IRD (1,2). Heart involvement may occur during the
acute phase of IRD, including myocarditis or coronary artery macro-
or micro-vasculitis, which probably leads to acute heart failure
(AHF) (3). On the other hand,
infection, anemia, tachycardia, renal dysfunction, anxiety,
glucocorticoid usage and other IRD-associated clinical conditions
may increase the heart rate (HR) and exacerbate AHF among these
patients. In either of these two scenarios, a high incidence of
cardiac arrhythmias has been reported and sinus tachycardia is one
of the major manifestations in IRD patients (4).
Although an increase in HR may be a compensatory
response to reduced left ventricular (LV) stroke volume and cardiac
output in the setting of AHF, it may also deteriorate LV systolic
function by causing tachycardiomyopathy (TCM) (5). At present, TCM is mainly defined by the
following clinical criteria: Sustained HR >100 bpm, exclusion of
other causes of HF, and partial or complete recovery of LV function
after restoration of the sinus rhythm or rate control (6,7).
Accordingly, it may be implied that HR control, in addition to the
application of diuretics and/or inotropes, is crucial for the
treatment of decompensated AHF patients without any previously
diagnosed heart disease. Low doses of β-blockers are usually
applied for the initial treatment of tachycardia in IRD patients
with systolic HF. Subsequently, the dose is slowly titrated to
tolerable levels to achieve the target HR. This process may take up
to 2–3 months. As a selective sinus node I(f) current inhibitor,
ivabradine significantly prolongs the diastolic phase, effectively
reduces the HR (8,9) and subsequently improves the prognosis
of patients with chronic HF (10).
However, clinical evidence to support the application of ivabradine
in patients with acute systolic HF is currently limited (11). Therefore, the present study aimed to
investigate the safety and potential efficacy of early and
short-term use of ivabradine in IRD patients with sinus tachycardia
and new-onset AHF.
Patients and methods
Patient population
The present study was a retrospective observational
study, in which IRD patients who experienced new-onset acute
systolic HF as well as sinus tachycardia and received ivabradine
therapy in Peking Union Medical College Hospital (Beijing, China)
between February 2017 and May 2017 were enrolled. All patients
received ivabradine for HR control in addition to the standard
treatment for AHF, including loop diuretics and/or mechanical
ventilation when required. Selection and dosages of other
medications, including β-blockers, were determined by individual
clinical settings.
The inclusion criteria were as follows: i) New-onset
AHF; ii) LV ejection fraction (EF) <50% determined by
transthoracic echocardiography (Simpson's method); iii) sinus
tachycardia with HR >100 bpm; and iv) unequivocal diagnosis of
IRD prior to or concurrent with presentation of the new-onset AHF.
New-onset AHF was defined as a diagnosis of AHF with presence of
pulmonary edema for the first time with a reduction in EF by
<50% but without evidence of history of HF in the previous 3
months. The exclusion criteria included the following: i)
Previously diagnosed or documented HF in the 3 months before the
study; ii) other heart rhythm indicated by in-hospital
electrocardiogram (e.g., supraventricular, atrial, ventricular);
iii) cardiac shock; iv) acute coronary syndrome; v) previous
treatment with ivabradine; and vi) hemodynamically significant
valvular disease. All patients had systolic blood pressures of
>90 mmHg without inotrope/vasopressor usage.
A total of 156 patients with IRD were admitted to
our hospital during the enrolment period, of which 14 patients
(9.0%) met the eligibility criteria. A total of 12 patients (10
females and 2 males; age range, 16–63 years; mean age, 33.8±12.5
years) provided written informed consent and received subsequent
ivabradine treatment, while the other two patients refused. All
patients or their legal guardians provided written informed consent
for administration of ivabradine, since this medication had not
been indicated for treating AHF clinically. The present study
complied with the ethical guidelines of the 1964 Declaration of
Helsinki and its revision from 2002. The study protocol was
reviewed and approved by the ethics committee of Peking Union
Medical College Hospital (Beijing, China).
Medication
A special group of cardiologists were responsible
for the patients' medications for HF, including the administration,
dosing and timing of ivabradine. Classic medical treatment for AHF,
including diuretics, oxygen therapy and fluid restrictions, were
selected according to the patients' clinical situations.
Application of β-adrenergic receptor blockers was selected
according to the clinicians' preference. Most patients received no
or moderate dosages of β-blockers as initial therapy for AHF. Use
or discontinuation of glucocorticoids and other immunosuppressant
drugs for IRD were determined by clinical rheumatologists
independent of AHF therapy.
When HR reduction was considered necessary by the
cardiologists and it was not possible to rapidly titrate β-blockers
for this purpose, ivabradine administration was selected for these
AHF patients according to the inclusion and exclusion criteria. Due
to the uncertainty of efficacy and safety of ivabradine treatment
in decompensated AHF patients, most patients were given a low
dosage of 2.5 mg twice daily, and not 5 mg twice daily as applied
in the initial therapy for chronic HF patients (10). If a target HR of 80 bpm was not
achieved within the first three days of treatment, the dosage was
increased to 5 mg twice daily. The application or dose increase of
a β-blocker in combination with ivabradine depended on the
clinician's judgement and/or the patient's general condition. If
the patient's LVEF during the follow-up echocardiogram exceeded
50%, the ivabradine dosage was not further increased, because the
HR would then controllable by a rapid increase in the β-blocker
dose.
Observational indexes
The primary outcome was the improvement of the EF,
which was measured using the biplane Simpson's method on
transthoracic echocardiography. Initial echocardiographic
evaluation was performed within 3 days prior to initiation of
ivabradine treatment, and the follow-up evaluation was performed ~2
weeks after the first ivabradine administration. The secondary
endpoints included HR changes at 48 h after the first ivabradine
administration vs. baseline, changes in B-type natriuretic peptide
(BNP)/N-terminal proBNP (NT-proBNP) levels and cardiac function
according to the New York Heart Association (NYHA) classification
of HF at 2 weeks after the first ivabradine use vs. baseline.
Furthermore, blood pressure, liver and renal function, and plasma
lactic acid changes prior to and after ivabradine use were
repeatedly measured for safety assessment.
Statistical analysis
All analyses were performed using SPSS 19.0
statistical software (IBM Corp.). Values are expressed as the mean
± standard deviation. The Kolmogorov-Smirnov test was used to
evaluate whether the distributions of continuous variables were
normal. Comparisons of LVEF, HR and BNP/NT-proBNP prior to and
after ivabradine administration were performed using paired
t-tests. Comparisons of the NYHA function classification prior to
and after ivabradine administration were performed using the
Wilcoxon signed-rank test, which is a paired non-parametric test.
P<0.05 was considered to indicate statistical significance.
Results
Patient characteristics
The basic clinical characteristics of the patients
are presented in Table I.
Concomitant IRDs included systemic lupus erythematosus (SLE),
secondary antiphospholipid syndrome (APS), granulomatosis with
polyangiitis (GPA) and polymyositis/dermatomyositis (PM/DM).
 | Table I.Clinical characteristics of 12
patients with autoimmune disease and sinus tachycardia. |
Table I.
Clinical characteristics of 12
patients with autoimmune disease and sinus tachycardia.
|
|
|
|
|
|
|
|
| Concomitant
medication |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Gender | Age (years) | IRD | NYHA | Mechanical
ventilation | Disease activity | Loop diuretics | β-blockers | Ivabradine | Glucocorticoids |
Immunosuppressants |
|---|
| 1 | F | 34 | SLE | III | No | SLEDAI, 10; ESR, 20
mm; hsCRP, 0.32 mg/l | Furosemide 80
mg/d | Metoprolol 12.5
mg/d | 5 mg/d | MP 24 mg/d | No |
| 2 | M | 29 | SLE/secondary
APS | IV | No | SLEDAI, 2; ESR, 31
mm; hsCRP, 1.52 mg/l | Torasemide 5
mg/d | Metoprolol 37.5
mg/d | 5 mg/d | No | No |
| 3 | F | 17 | GPA | IV | Yes | ESR, 34 mm; hsCRP,
55.01 mg/l | Furosemide 20
mg/d | No | 5 mg/d | MP 80 mg/d | No |
| 4 | F | 16 | PM/DM | IV | No | ESR, 6 mm; hsCRP,
1.10 mg/l | Torasemide 20
mg/d | Metoprolol 50
mg/d | 5 mg/d | MP 80 mg/d | CTX 200 mg/w |
| 5 | F | 33 | SLE | III | No | SLEDAI, 4; ESR, 16
mm; hsCRP, 5.12 mg/l | Furosemide 40
mg/d | Metoprolol 25
mg/d | 5 mg/d | No | No |
| 6 | F | 62 | SLE | IV | Yes | SLEDAI, 4; ESR, 43
mm; hsCRP, 44.53 mg/l | Furosemide 100
mg/d | Metoprolol 12.5
mg/d | 5–15 mg/d | Hydrocortisone 150
mg/d | No |
| 7 | F | 34 | SLE | IV | Yes | SLEDAI, 10 | Furosemide 40
mg/d | No | 5 mg/d | MP 80 mg/d | No |
| 8 | F | 36 | SLE | III | No | SLEDAI: 4 ESR, 21
mm; hsCRP, 2.51 mg/l | Furosemide 40
mg/d | Carvedilol 50
mg/d | 5 mg/d | MP 40 mg/d | No |
| 9 | F | 32 | SLE | III | No | SLEDAI, 7; ESR, 3
mm; hsCRP, 0.13 mg/l | No | Metoprolol 25
mg/d | 5–10 mg/d | MP 24 mg/d | CTX 400 mg/w |
| 10 | F | 48 | GPA | IV | Yes | ESR, 8 mm; hsCRP,
180.64 mg/l | Furosemide 80
mg/d | Metoprolol 25
mg/d | 5 mg/d | MP pulse + 80
mg/d | CTX 400 mg/w |
| 11 | F | 21 | PM/DM | III | No | ESR, 86 mm; hsCRP,
1.47 mg/l; | No | Metoprolol 25
mg/d | 5–10 mg/d | MP 40 mg/d | Tacrolimus 2–3
mg/d |
| 12 | M | 43 | PM/DM | III | No | ERS, 25 mm; hsCRP,
0.95 mg/l | Furosemide 20
mg/d | Metoprolol 25
mg/d | 5–10 mg/d | Prednisone 60
mg/d | CTX 400 mg/w |
HR reduction
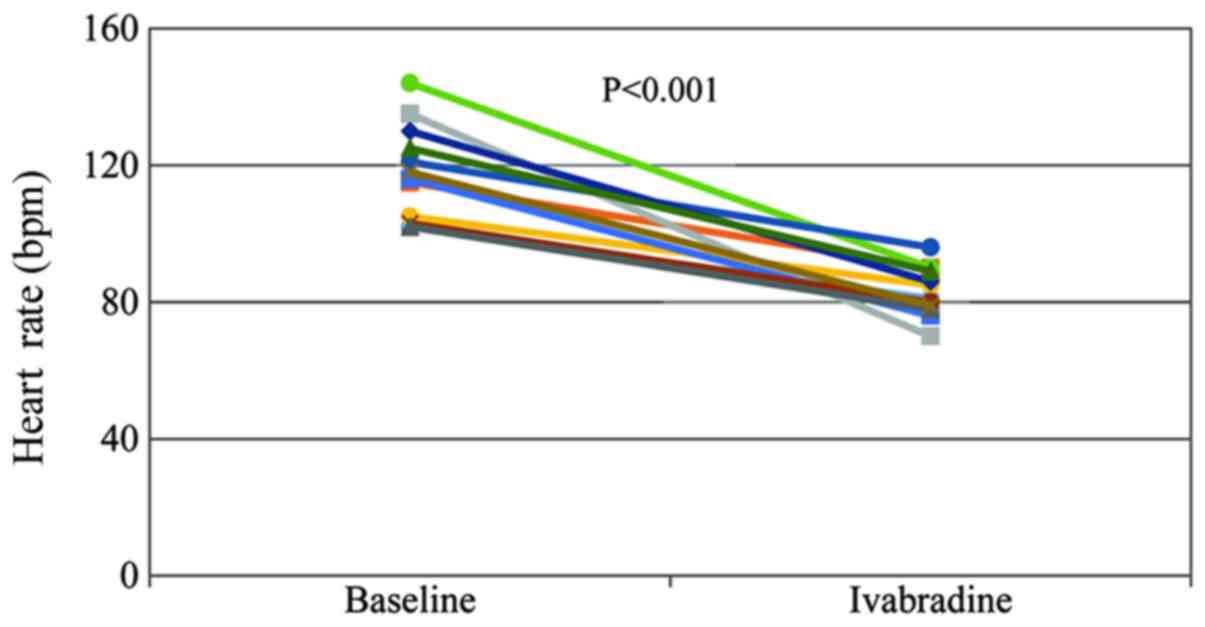
The patients of the present study presented with a
significant HR reduction at 48 h after ivabradine treatment
(Fig. 1), and the mean resting HR
was decreased from 118.0±13.8 to 83.3±7.3 bpm (P<0.001).
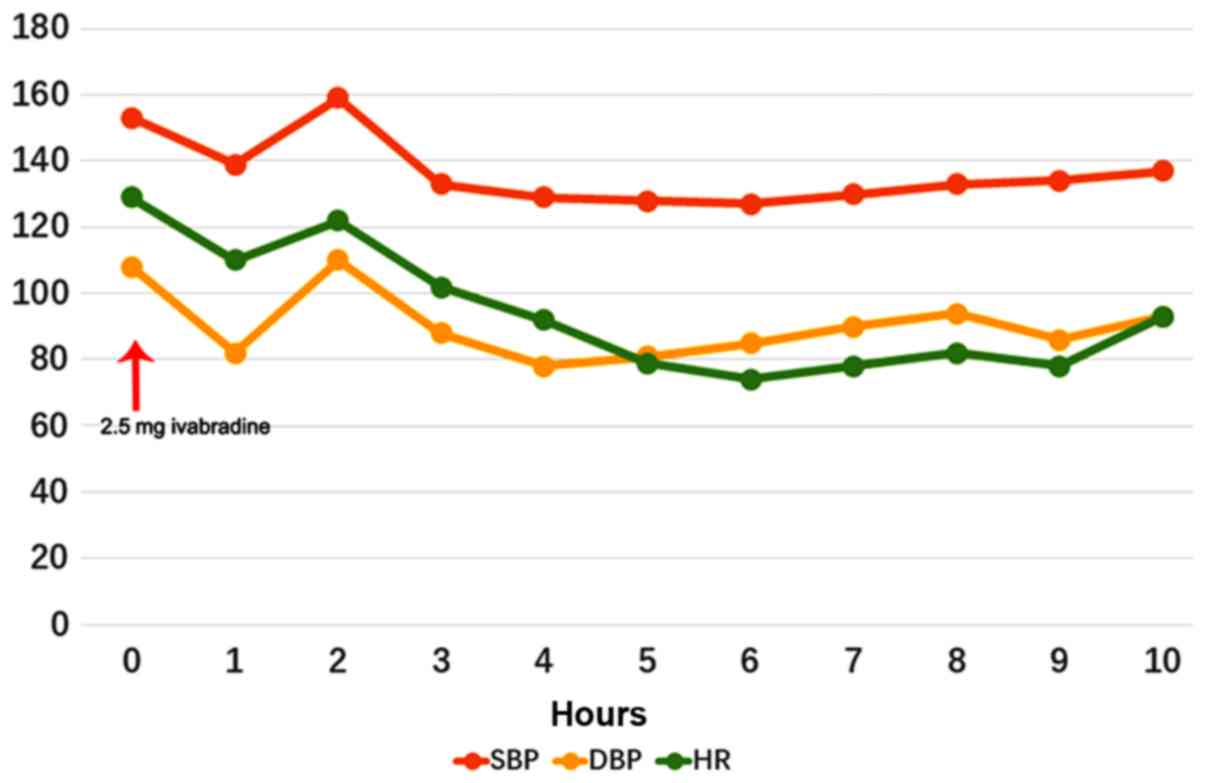
Fig. 2 provides the changes in HR
and blood pressure in a typical IRD patient (case no. 3; female;
age, 17 years), who received mechanical ventilation due to
pulmonary edema and hypoxemia caused by AHF, over the first 10 h.
Within 6 h of 2.5 mg ivabradine administration, her HR dropped from
129 to 74 bpm without significant changes in blood pressure.
Simultaneously, her LVEF increased from 28 to 40%. There were no
dosage modifications of ivabradine or new prescriptions of
β-blockers within the first 48 h for all patients, and the dosage
of sedatives was not modified if the patients were intubated. If
the patient's HR persistently remained >80 bpm, the ivabradine
dose was titrated to 10 mg/day after 3 days or even to 15 mg/day
thereafter.
Transthoracic echocardiography
changes
The interval between the first dose of ivabradine
and echocardiography re-evaluation ranged from 10 to 20 days (mean,
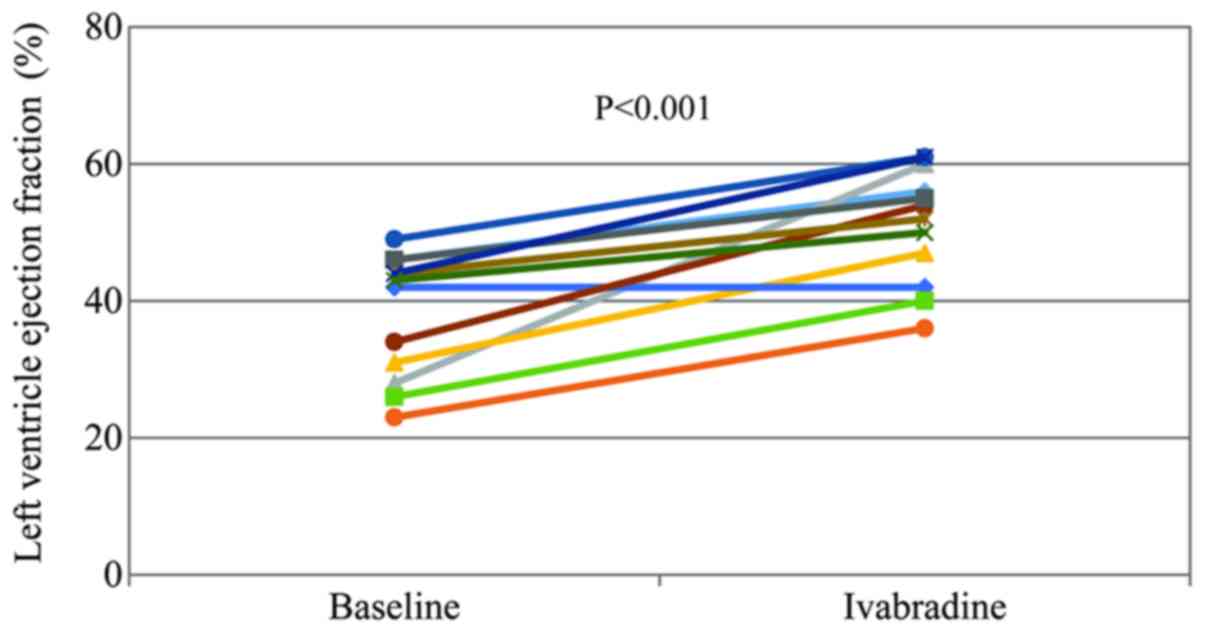
14.0±2.6 days). A significant improvement in LVEF was seen after
ivabradine treatment (38.0±9.0 vs. 51.2±8.4%; P<0.001; Fig. 3). Of the 12 patients, 8 only received
5 mg/day of ivabradine and the HR was <80 bpm at the time of
echocardiography re-evaluation. Another 3 patients received 10
mg/day, and 1 patient required 15 mg/day of ivabradine to reach the
target HR. After the increase in EF was recognized, the β-blocker
dose was rapidly increased to reduce the HR. The average period of
ivabradine administration ranged from 5 to 30 days (mean, 18.1±8.8
days), depending on the HR control, β-blocker usage and mitigation
of systolic AHF.
Comparison of NT-proBNP and BNP levels
prior to and after treatment
NT-proBNP and BNP levels were examined at baseline
and at an average of 14.2±2.7 days (5–20 days) after the
commencement of ivabradine treatment. A total of 8 patients were
subjected to NT-proBNP testing and the remaining 4 were subjected
to BNP testing due to their mild to severe renal dysfunction. As
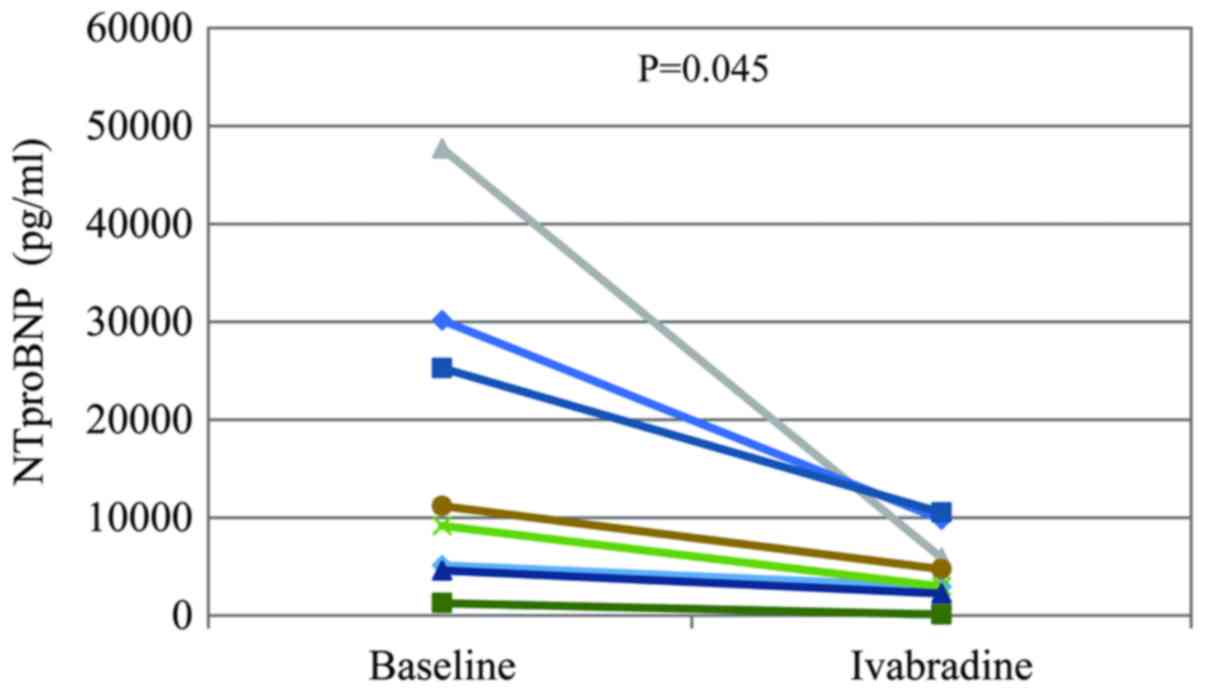
indicated in Fig. 4, the NT-proBNP
level after ivabradine treatment was significantly lower than the
initial level (4,900±3,672 vs. 16,806±16,130 pg/ml; P=0.045). There
was no significant difference between BNP levels prior to and after
treatment with ivabradine (427±282 vs. 318±145 ng/l; P=0.528;
Table II).
 | Table II.Comparison of BNP levels and NYHA
functional classification prior to and after ivabradine
treatment. |
Table II.
Comparison of BNP levels and NYHA
functional classification prior to and after ivabradine
treatment.
| Parameter | Prior to
treatment | After
ivabradine | P-value |
|---|
| BNP (ng/l) | 427±282 | 318±145 | 0.528 |
| NYHA score | 3.5±0.5 | 2.3±0.6 |
<0.001a |
Comparison of NYHA classification
prior to and after ivabradine treatment
The NYHA classification of HF in the subjects was
compared between baseline and 14 days after ivabradine treatment by
using Wilcoxon's signed-rank test (Table II). With ivabradine treatment, a
significant improvement in the NYHA function classification was
achieved when compared with the initial evaluation (2.3±0.6 vs.
3.5±0.5; P<0.001).
Safety
No incidences of hypertension, hypotension,
bradycardia, atrial fibrillation were recorded in the patients of
the current study. Liver and renal function also remained stable in
these patients. Plasma lactic acid was not elevated prior to or
following ivabradine treatment. Only one of the 12 patients
reported the common ocular side effect of phosphene.
Discussion
New-onset AHF with sinus tachycardia in IRD patients
is commonly encountered in the clinic. According to the present
study, ivabradine significantly decreased the HR, improved the LVEF
and decreased NT-proBNP/BNP, thereby markedly improving cardiac
function in the small sample of patients.
The causes of new-onset AHF in IRD have remained to
be fully elucidated. Heart involvement and elevated HR are
frequently observed in autoimmune diseases, including SLE (12,13),
rheumatoid arthritis (14–16), GPA (17), PM/DM (18) and systemic sclerosis (19). Ischemic cardiomyopathy associated
with coronary artery and microvascular diseases and/or
inflammation-associated myocarditis are two major mechanisms
underlying the elevated risks of AHF. The patients in the present
study had no self-reported or medically recorded history of
myocardial infarction. In addition, there were no acute coronary
events at enrolment in these AHF patients. Evidence of severe
microvascular disease or inflammation-associated myocarditis was
insufficient in the present cohort due to inconvenient functional
tests or high-resolution imaging performed in these critically ill
patients. Two patients in the present study received
gadolinium-enhanced cardiac magnetic resonance and did not to
exhibit any obvious signs of myocarditis, myocardial edema or
fibrosis. However, SLE myocarditis was the most characteristic
feature of myocardial involvement in SLE but was of a subclinical
nature (20,21). No endomyocardial biopsy was performed
in this group of patients.
In the present study, most cases of AHF were
triggered by bacterial or fungal infections. Of the 12 patients, 7
received long-term regular corticosteroid and/or immunosuppressant
therapy and remained stable prior to the onset of AHF. The general
activity evaluations in the 7 SLE patients were mild to moderate
according to the SLE Disease Activity Index score. Of note, other
target organs involved in IRD were relatively ‘silent’, suggesting
that new-onset AHF was unlikely caused by exacerbated IRD. All
patients in the present study shared a common sign of inappropriate
sinus tachycardia prior to the onset of AHF. Thus, it may be
hypothesized that TCM is an important cause for the development of
AHF in these patients. For instance, case no. 3 received
methylprednisolone pulse therapy and high-dose steroid maintenance
treatment due to pulmonary injury by GPA, with a LVEF of 61% at
that time. Her HR constantly exceeded 120 bpm due to repeated
episodes of infection and seizures, reaching 170 bpm at one point.
Decompensated AHF with an EF of 28% occurred within 2 weeks. It
remains elusive whether IRD patients may have a high incidence of
TCM during a state of inflammation, but sinus tachycardia was
frequent in those patients.
Marked and rapid improvement of LVEF after rate
control in the patients of the current study supports the present
results on TCM. Quick recovery from systolic AHF in patients was
mainly attributed to rapid HR control but not the effect of
glucocorticoids or other immunosuppressant drugs. In most patients,
a HR reduction was achieved after 2 days of treatment, which is
consistent with a previous study on ivabradine treatment in AHF
patients (22). Relief of HF
symptoms was reported by or observed in most patients, and this
alleviation was consistent with the decreased HR shortly after
ivabradine treatment. Case no. 3 exhibited a marked decrease of HR
and improvement of EF within hours. This patient was successfully
extubated 5 days later, and 16 days later, LV systolic function
returned to normal with an EF of 60% on the echocardiogram.
It is worth noting that most patients presented with
a marked change in HR (19.0–48.1%) after treatment with ivabradine
at a relatively low dosage (2.5 mg twice daily). In comparison, the
HR was reduced by only 10.7–13.0% in previous large clinical trials
where ivabradine was given at 5 mg twice daily (10,23–25). Two
factors may be accountable for this difference. First, the average
HR in previous studies, whose patients had already received
maximum-tolerated doses of β-blockers, was between 70 and 90 bpm,
while the initial HR of the patients in the present study was
>100 bpm. Second, previous studies included patients with stable
coronary artery disease and/or CHF (10,23–25);
however, only patients with acute decompensated HF were included in
the present study. HR reduction by ivabradine led to improvement of
AHF, which consequently reduced HR. Given the obvious reversibility
of TCM, the HR tended to spontaneously decrease after AHF and
tachycardia was successfully controlled.
The usage and dosages of β-blockers were not limited
in the present study. The mean duration of ivabradine treatment was
short-term (~18.1 days). During this period, most patients had no
or only one increase in the dose of β-blockers. The daily dose of
β-blocker did not exceed 50 mg of metoprolol daily, which is far
less than the maximally tolerated dose, except for case no. 8, who
was maintained on a higher dose of carvedilol (25 mg twice daily)
according to a previous long-term prescription. Accordingly, it may
be considered that the drop in HR within 48 h was directly linked
to the use of ivabradine rather than β-blockers. However, the
present study was not a prospectively controlled study. Recently, a
randomized controlled study compared the efficacy of ivabradine
plus β-blockers versus β-blockers alone in patients with AHF
(11). It suggested that early
combined treatment with ivabradine and β-blockers in congestive HF
is feasible and safe. The distinguishing feature of the present
study was that all patients were complicated with IRD and had no
previous history of HF. The patients in the present study exhibited
exacerbated AHF symptoms, and 33.3% of them underwent mechanical
ventilation and did not require 48 h for stabilization in
accordance with data of a previous study (11).
Ivabradine also exhibited sufficient clinical safety
in the AHF patients of the present study. No hemodynamic
deterioration or impairment of liver or renal function was observed
in any of the patients. To the best of our knowledge, no previous
study has reported on the application of ivabradine in patients
with a creatinine clearance of <15 ml/min. In the present study,
case no. 8 had lupus nephritis and started to receive hemodialysis,
with an estimated glomerular filtration rate of 9.7 ml/min, due to
AHF and reduced urine output. After careful addition of ivabradine,
the patient's HF was alleviated and renal perfusion was improved.
The patient was void of the requirement for hemodialysis for three
months by the end of this study. In addition, according to the
manufacturer's guidelines for ivabradine, its use in children and
adolescents (age, <18 years) is not recommended due to lack of
evidence. However, both cases 3 and 4 of the present study were
slightly younger than 18 years. After careful communication with
their guardians, ivabradine treatment was initiated. The two
patients achieved acceptable short-term therapeutic effects using a
small dose of ivabradine (2.5 mg twice daily) without exhibiting
any adverse effects. However, the long-term efficacy and safety of
the drug in these patients remains to be determined.
The current study has several limitations. It is a
small-sample, single center study without a control group (patients
who did not receive ivabradine treatment). Further randomized
control studies are therefore required to better testify the
efficacy of ivabradine in AHF. Additionally, ivabradine was only
evaluated in AHF patients with IRD. It is therefore not clear
whether the same treatment could be applied to wider spectrum of
patients with AHF.
In conclusion, the present study evaluated early
short-term ivabradine treatment in new-onset AHF patients with IRD.
The observations suggest that early ivabradine treatment in this
type of patients was safe. A significant reduction in HR was
observed, and none of the patients developed cardiac shock.
Successful control of HR may contribute to hemodynamic and/or heart
function improvement, particularly in patients with suspected TCM.
Further randomized controlled trials are required to better
demonstrate the efficacy and evaluate the long-term prognosis for
AHF patients treated with ivabradine.
Acknowledgements
The authors are grateful for the efforts of Dr Li
Zhang, Dr Wei Bai (Department of Rheumatology) and Dr Ke Zheng
(Department of Nephrology) from Peking Union Medical College
Hospital (Beijing, China) for their special care of the
patients.
Funding
The present study was supported by The National Key
Research and Development Program of China (grant nos.
2016YFC0905102 and 2016YFC0901501) and the Chinese Academy of
Medical Sciences Innovation Fund for Medical Sciences (grant no.
2017-I2M-2-002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
Conception and design: WW and ZS; data collection
and/or processing: WW, LZ and YG; materials: JZ, J-JL, DS, JY, YL
and J-ZL; analysis and/or interpretation: YG and ZS; literature
search: WW; writing: WW, LZ and ZS; critical review: ZS. All
authors confirmed the accuracy of this manuscript.
Ethics approval and consent to
participate
All patients or their legal guardians provided
written informed consent. This study complied with the ethical
guidelines of the 1964 Declaration of Helsinki, as revised in 2002.
The study protocol was reviewed and approved by the ethics
committee of Peking Union Medical College Hospital (Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hollan I, Meroni PL, Ahearn JM, Cohen
Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M,
Shields K and Wasko MC: Cardiovascular disease in autoimmune
rheumatic diseases. Autoimmun Rev. 12:1004–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mason JC and Libby P: Cardiovascular
disease in patients with chronic inflammation: Mechanisms
underlying premature cardiovascular events in rheumatologic
conditions. Eur Heart J. 36:482–489c. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sarzi-Puttini P, Atzeni F, Gerli R,
Bartoloni E, Doria A, Barskova T, Matucci-Cerinic M, Sitia S,
Tomasoni L and Turiel M: Cardiac involvement in systemic rheumatic
diseases: An update. Autoimmun Rev. 9:849–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seferovic PM, Ristic AD, Maksimovic R,
Simeunovic DS, Ristic GG, Radovanovic G, Seferović D, Maisch B and
Matucci-Cerinic M: Cardiac arrhythmias and conduction disturbances
in autoimmune rheumatic diseases. Rheumatology (Oxford). 4 (Suppl
45):iv39–iv42. 2006. View Article : Google Scholar
|
|
5
|
Mueller KAL, Heinzmann D, Klingel K,
Fallier-Becker P, Kandolf R, Kilias A, Walker-Allgaier B, Borst O,
Kumbrink J, Kirchner T, et al: Histopathological and immunological
characteristics of tachycardia-induced cardiomyopathy. J Am Coll
Cardiol. 69:2160–2172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta S and Figueredo VM: Tachycardia
mediated cardiomyopathy: Pathophysiology, mechanisms, clinical
features and management. Int J Cardiol. 172:40–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong YH, Choi KJ, Song JM, Hwang ES, Park
KM, Nam GB, Kim JJ and Kim YH: Diagnostic approach and treatment
strategy cin tachycardia-induced cardiomyopathy. Clin Cardiol.
31:172–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thollon C, Cambarrat C, Vian J, Prost JF,
Peglion JL and Vilaine JP: Electrophysiological effects of S 16257,
a novel sino-atrial node modulator, on rabbit and guinea-pig
cardiac preparations: Comparison with UL-FS 49. Br J Pharmacol.
112:37–42. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bucchi A, Baruscotti M and DiFrancesco D:
Current-dependent block of rabbit sino-atrial node I(f) channels by
ivabradine. J Gen Physiol. 120:1–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swedberg K, Komajda M, Bohm M, Borer JS,
Ford I, Dubost-Brama A, Lerebours G and Tavazzi L; SHIFT
Investigators, : Ivabradine and outcomes in chronic heart failure
(SHIFT): A randomised placebo-controlled study. Lancet.
376:875–885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hidalgo FJ, Anguita M, Castillo JC,
Rodríguez S, Pardo L, Durán E, Sánchez JJ, Ferreiro C, Pan M, Mesa
D, et al: Effect of early treatment with ivabradine combined with
beta-blockers versus beta-blockers alone in patients hospitalised
with heart failure and reduced left ventricular ejection fraction
(ETHIC-AHF): A randomised study. Int J Cardiol. 217:7–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim CH, Al-Kindi SG, Jandali B, Askari AD,
Zacharias M and Oliveira GH: Incidence and risk of heart failure in
systemic lupus erythematosus. Heart. 103:227–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhakal BP, Kim CH, Al-Kindi SG and
Oliveira GH: Heart failure in systemic lupus erythematosus. Trends
Cardiovasc Med. 28:187–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolfe F and Michaud K: Heart failure in
rheumatoid arthritis: Rates, predictors, and the effect of
anti-tumor necrosis factor therapy. Am J Med. 116:305–311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Myasoedova E, Crowson CS, Nicola PJ,
Maradit-Kremers H, Davis JM III, Roger VL, Therneau TM and Gabriel
SE: The influence of rheumatoid arthritis disease characteristics
on heart failure. J Rheumatol. 38:1601–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantel A, Holmqvist M, Andersson DC, Lund
LH and Askling J: Association between rheumatoid arthritis and risk
of ischemic and nonischemic heart failure. J Am Coll Cardiol.
69:1275–1285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kyaw H, Misra D, Mani MM, Park WJ and
Shinnar M: Unusual cardiac involvement in granulomatosis with
polyangiitis manifesting as acute congestive heart failure. Anatol
J Cardiol. 18:158–160. 2017.PubMed/NCBI
|
|
18
|
Lundberg IE: The heart in dermatomyositis
and polymyositis. Rheumatology (Oxford). 4 (Suppl 45):iv18–iv21.
2006. View Article : Google Scholar
|
|
19
|
Kahan A and Allanore Y: Primary myocardial
involvement in systemic sclerosis. Rheumatology (Oxford). 4 (Suppl
45):iv14–iv17. 2006. View Article : Google Scholar
|
|
20
|
Chen J, Tang Y, Zhu M and Xu A: Heart
involvement in systemic lupus erythematosus: A systemic review and
meta-analysis. Clin Rheumatol. 35:2437–2448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang CR, Tsai YS and Li WT: Lupus
myocarditis receiving the rituximab therapy-a monocentric
retrospective study. Clin Rheumatol. 37:1701–1707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pascual Izco M, Alonso Salinas GL,
Sanmartin Fernandez M, Del Castillo Carnevalli H, Jimenez Mena M,
Camino Lopez A and Zamorano Gómez JL: Clinical experience with
ivabradine in acute heart failure. Cardiology. 134:372–374. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fox K, Ford I, Steg PG, Tendera M,
Robertson M and Ferrari R; BEAUTIFUL Investigators, : Relationship
between ivabradine treatment and cardiovascular outcomes in
patients with stable coronary artery disease and left ventricular
systolic dysfunction with limiting angina: A subgroup analysis of
the randomized, controlled BEAUTIFUL trial. Eur Heart J.
30:2337–2345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fox K, Ford I, Steg PG, Tardif JC, Tendera
M and Ferrari R; SIGNIFY Investigators, : Ivabradine in stable
coronary artery disease without clinical heart failure. N Engl J
Med. 371:1091–1099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tardif JC, Ponikowski P and Kahan T;
ASSOCIATE Investigators, : Effects of ivabradine in patients with
stable angina receiving beta-blockers according to baseline heart
rate: An analysis of the ASSOCIATE study. Int J Cardiol.
168:789–794. 2013. View Article : Google Scholar : PubMed/NCBI
|