Introduction
Colorectal lesions are found in symptomatic and
asymptomatic patient populations during colonoscopy procedures
(1). The majority of sporadic
colorectal cancers are hypothesised to develop from adenomatous
polyps and villous adenomas, which accumulate genetic alterations
over a period of years via environmental influences (2).
The colonoscopic removal of polyps has been a
mainstay in colorectal cancer prevention following a 67–76%
reduction in the incidence of colorectal cancer (3,4).
Therefore, an improvement in detection rates of adenomas or polyps
would aid in decision making regarding endoscopic treatment,
including resection strategy and appropriate surveillance intervals
following colonoscopies (5).
High-quality colonoscopies are mandatory to prevent
adenoma recurrence and colorectal cancer (3). In the past few years, technical
advances have been developed with the purpose of improving
detection rates of colorectal lesions, including adenoma, polyps
and flat lesions. Image-enhanced endoscopy (IEE) has been
demonstrated to facilitate the detection and characterization of
polyps and especially nonpolypoid colorectal neoplasms. Indigo
carmine is the most frequently used dye in colonoscopies as it
deposits in depressed lesions (4,5). Virtual
chromoendoscopies have emerged as an effective contrast enhancement
technology without the limitation of preparing dyes and applying
them through the colonoscope working channel (2,5).
However, colonic lesions are often subtle in
appearance and difficult to identify with conventional optical
colonoscopy (6). Enhancing mucosal
contrast, either by dye use or advanced optical imaging, may
improve the detection of adenomatous lesions, but the question as
to which endoscopic technique is preferable for detecting lesions
remains controversial (6). Previous
conventional meta-analyses either could not clearly determine the
efficacy of different endoscopic techniques to detect adenomas or
other kinds of lesions, or failed to integrate all the evidence
(7,8). The aim of the current study was to
compare the standard-definition white-light endoscopy (SDWL),
high-definition white-light endoscopy (HDWL), chromoendoscopy
(CHRO), narrow-band imaging (NBI), autofluorescence imaging (AFI),
Fuji Intelligent Color Enhancement (FICE) colonoscopy and i-SCAN
colonoscopy to determine the modalities that yielded the highest
number of colorectal lesions identified per subject and subjects
with colorectal lesions using a network meta-analysis.
Additionally, the current study sought to provide a systematic
review about endoscopic modalities and new-generation
image-enhanced endoscopy facilitates, including blue laser imaging
(BLI) and linked color imaging (LCI).
Materials and methods
Search strategy
A literature search of PubMed (www.ncbi.nlm.nih.gov/pubmed), Embase (www.embase.com) and the Cochrane Library (www.cochranelibrary.com) database was conducted
for all studies comparing the detection of colon adenomas/polyps
using SDWL, HDWL, AFI, FICE, NBI, i-SCAN, BLI, LCI and CHRO in
patients undergoing colonoscopies. The reference lists of included
studies, relevant reviews and meta-analyses selected from the
electronic database search were also manually searched to avoid
missing relevant studies. In cases where studies presented
duplicate data, the most recent study was used in the current
analysis.
Study selection
Studies were eligible for the current meta-analysis
if they met the following criteria: i) Randomised controlled trials
or prospective cohort studies; ii) assessed for the detection rate
of adenomas/polyps using SDWL, HDWL, AFI, FICE, NBI, BLI, LCI,
i-SCAN and CHRO regardless of indication (i.e., screening,
surveillance or symptoms). Studies were excluded for the following
reasons: i) Included participants with inflammatory bowel diseases
or hereditary nonpolyposis colorectal cancer; ii) data was
inadequate (the content of literature research was not related to
polyps/adenoma) for extraction; or iii) designed as a review,
editorial, comment or meta-analysis. Two reviewers (SL and LL)
independently reviewed the studies derived from the searches to
determine whether they were eligible for inclusion and obtained the
full articles of the included studies.
Data extraction
The following data were extracted from each eligible
study: i) Study characteristics, including the start and end date
of the study, location, study design, and publication status of the
study; ii) participants' characteristics, including the total
number of patients, age, inclusion and exclusion criteria and
indication for colonoscopy; iii) type of colonoscopy; iv) the
outcome of the study, including the total number of lesions and
adenomas or polyps, the number of participants with adenomas or
polyps, the size and type of colorectal lesions.
Study quality assessment and
statistical methods
Two reviewers (LF and OH) independently assessed the
quality and risk of bias of the included studies with Quality
Assessment of Diagnostic Accuracy Studies (QUADAS) (9). The tool is based on a 14-item
questionnaire, with each item having the response of ‘yes’, ‘no’ or
‘unclear’. Any discrepancies in interpretation were resolved by
discussion. Network meta-analyses combine direct and indirect
evidence for all relative treatment effects and provide estimates
with maximum power (10). The
R2WinBUGS Package (version 2.1–21; http://cran.r-project.org/) was used to conduct a
Bayesian analysis that combined data from multiple randomised
control trials (11). Network
meta-analyses are better at integrating different types of evidence
compared with conventional pairwise meta-analyses, however this
type of analysis leads to inevitable heterogeneity (10). Thus, pairwise meta-analyses were also
conducted for SDWL and HDWL endoscopy, comparing them with other
types of techniques to supplement the network meta-analysis. RevMan
5.3 software (The Nordic Cochrane Centre, The Cochrane
Collaboration, Copenhagen, Denmark) was used to conduct pairwise
meta-analyses. For continuous outcomes, the relative effect sizes
were calculated as standardised mean differences (SMDs), as
previously described (10). For
binary outcomes, relative effect sizes were calculated as odds
ratios (ORs). The two types of effect sizes were reported with
their 95% confidence intervals (CIs). The I2 statistic
is a test used to quantify heterogeneity and it was used to
calculate the proportion of variation due to heterogeneity rather
than by chance, between the included studies. Values of
I2 >50% indicated that heterogeneity existed. When
statistical heterogeneity was identified, individual study
characteristics were examined and a sensitivity analysis was
performed on the primary outcomes. In the sensitivity analysis,
after excluding a relatively low-quality study, the combined effect
was re-estimated to evaluate the source of heterogeneity (10).
Results
Study description and quality
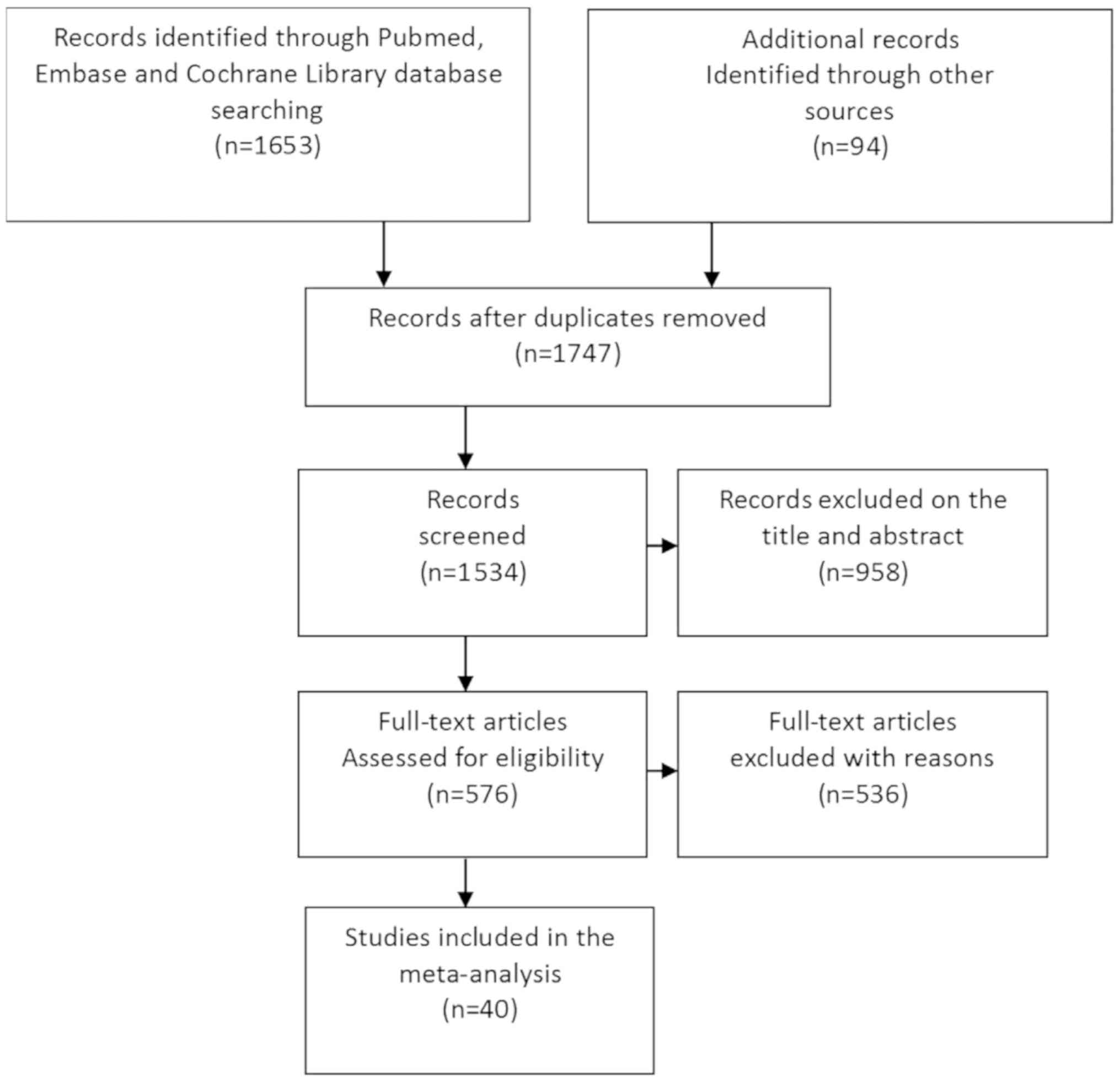
From the initial search, a total of 1,747
potentially relevant studies were identified between December 1949
and December 2017. A total of 958 studies were excluded based on
the title and abstract, as they were not relevant to the
meta-analysis conducted in the current study, and the full text of
the rest of the initial search studies were obtained for further
assessment (Fig. 1). A total of 54
studies were excluded as they were review articles, meta-analyses
or case reports; 146 studies that were not randomised controlled
trials were excluded; and 335 studies that did not have enough data
to be extracted were excluded. Only one study about BLI met the
inclusion criteria, however an effective meta-analysis could not be
conducted and was excluded due to insufficient data. Finally, 40
studies with a total of 14,109 participants were included, which
provided enough data for the analyses conducted in the current
study (1,12–50). All
studies were reported in English and more than one endoscopic
technique was assessed in several studies.
Of the 40 studies included, 37 studies were
described in full-paper articles and 3 studies were published as
abstracts. A total of 12 studies provided data about SDWL, 32 about
HDWL, 14 about NBI, nine about CHRO, five about AFI, six about FICE
and two about i-SCAN. A total of 23 studies focused on the
differentiation of diminutive lesions or flat adenomas. A total of
27 studies compared the number of detected adenomas, 19 compared
the number of adenomas detected per participant, 12 compared the
number of polyps detected per participant, 29 compared the
proportion of patients with at least one adenoma detected and 20
compared the proportion of patients with at least one polyp
detected.
The score of the included studies was assessed by
the QUADAS tool. Of the 40 studies, 36 were considered to be high
quality whilst the remaining four studies were considered to be
poor quality and were subsequently removed from the meta-analysis
(data not shown). Three of the four relatively poor-quality reports
were only published as an abstract.
Network meta-analysis of adenoma
detection
A Bayesian network analysis was conducted on the
data of subjects with adenoma and for adenoma number detection,
which comprised of sufficient controlled studies to avoid excessive
heterogeneity (Table I). A total of
29 studies featuring 10,805 patients were included in the analysed
group of subjects with adenoma. NBI (OR, 1.29; 95% CI, 1.04–1.58),
FICE (OR, 1.39; 95% CI, 1.11–1.77), CHRO (OR, 1.53; 95% CI,
1.22–1.93) and AFI (OR, 1.81; 95% CI, 1.07–2.89) were significantly
more efficient at identifying patients with adenoma compared with
SDWL, and CHRO (OR, 1.30; 95% CI, 1.06–1.60) was also significantly
better at identifying patients with adenoma compared with HDWL.
However, other endoscopic techniques were not better than HDWL as
all 95% CI included 1.00. A total of 27 studies featuring 10,094
patients were included in the group in which the number of adenoma
detected was analysed. However, no significant differences were
identified among these seven targeted endoscopic techniques in this
subgroup; all 95% CI included 1.00.
 | Table I.Network meta-analyses of adenoma
detection. |
Table I.
Network meta-analyses of adenoma
detection.
|
| Odds ratio (95%
confidence interval) |
|---|
|
|
|
|---|
| Endoscopic
modalities | Patients with
adenoma | Number of
adenoma/subject detected |
|---|
| SDWL |
|
HDWL | 1.18
(0.98–1.39) | 1.17
(0.86–1.55) |
|
NBI | 1.29
(1.04–1.58)a | 1.10
(0.74–1.62) |
|
FICE | 1.39
(1.11–1.77)a | 1.09
(0.71–1.63) |
|
CHRO | 1.53
(1.22–1.93)a | 1.10
(0.73–1.58) |
|
i-SCAN | 1.52
(0.81–2.61) | 1.66
(0.74–3.23) |
|
AFI | 1.81
(1.07–2.87)a | 1.64
(0.83–2.95) |
| HDWL |
|
NBI | 1.09
(0.95–1.25) | 0.95
(0.72–1.25) |
|
FICE | 1.17
(0.98–1.40) | 0.94
(0.66–1.28) |
|
CHRO | 1.30
(1.06–1.60)a | 0.95
(0.64–1.38) |
|
i-SCAN | 1.28
(0.70–2.14) | 1.43
(0.68–2.61) |
|
AFI | 1.54
(0.89–2.47) | 1.42
(0.68–2.47) |
| NBI |
|
FICE | 1.08
(0.88–1.32) | 1.01
(0.66–1.44) |
|
CHRO | 1.20
(0.94–1.50) | 1.03
(0.63–1.54) |
|
i-SCAN | 1.18
(0.62–2.05) | 1.54
(0.67–3.05) |
|
AFI | 1.42
(0.82–2.32) | 1.53
(0.69–2.83) |
| FICE |
|
CHRO | 1.11
(0.88–1.38) | 1.04
(0.64–1.60) |
|
i-SCAN | 1.10
(0.59–1.88) | 1.57
(0.66–3.27) |
|
AFI | 1.32
(0.74–2.11) | 1.55
(0.69–2.78) |
| CHRO |
|
i-SCAN | 1.00
(0.51–1.73) | 1.56
(0.68–3.25) |
|
AFI | 1.19
(0.68–1.93) | 1.54
(0.70–3.00) |
| i-SCAN |
|
AFI | 1.30
(0.56–2.57) | 1.13
(0.40–2.46) |
Pairwise meta-analysis of colorectal
lesions detection
To explain the heterogeneity and to supply
comprehensive comparisons of the network analysis, pairwise studies
were also performed. In the analysis group comparing the number of
adenomas per subject, 19 studies featuring 7,727 subjects were
included. Two studies (1,45) compared HDWL with CHRO and revealed
that CHRO was significantly better than HDWL (MD, −0.44; P=0.0004).
Additionally, the number of adenomas per subject was no more likely
to be identified using NBI (P=0.78), FICE (P=0.77), i-SCAN (P=0.54)
and AFI (P=0.98) compared with HDWL, with MD ranging from −0.09 to
0. In these analysis groups, significant statistical heterogeneity
was observed as P≤0.05, however I2 for HDWL-CHRO was 78
(P=0.03), which indicates that there are differences between these
studies.
A total of 12 studies featuring 4,409 subjects used
number of polyps per patient as the outcome. In these studies, CHRO
with a specific dye was significantly superior to SDWL (MD, −0.73;
95% CI, −1.13, −0.33) and HDWL (MD, −1.11; 95% CI, −1.46, −0.76) at
detecting the number of polyps per patient. CHRO with a specific
dye was also significantly superior at determining the number of
flat lesions per subject compared with SDWL (MD, −0.15; 95% CI,
−0.30, −0.00) and HDWL (MD, −0.30; 95% CI, −0.49, −0.10), without
significant heterogeneity (1,33,44,45).
CHRO detected more subjects with polyps compared
with SDWL (MD, 0.43; 95% CI, 0.32–0.57) and HDWL (MD, 0.29; 95% CI,
0.11–0.78), and FICE detected more subjects with polyps compared
with HDWL (MD, 0.78; 95% CI, 0.64–0.96). HDWL was significantly
more efficient at identifying subjects with flat lesions compared
with NBI (OR, 0.77; 95% CI, 0.60–0.99). Studies involving subjects
with >3 adenomas were also analysed, however no significant
differences were identified between SDWL, HDWL, FICE, NBI and
CHRO.
HDWL proved to be a more effective at detecting
diminutive lesions compared with SDWL (OR, 0.51; 95% CI,
0.39–0.68). However, HDWL was less effective at detecting flat
lesions compared with SDWL (OR, 1.54; 95% CI, 1.09–2.17).
Traditional CHRO seemed to be slightly better at detecting
diminutive lesions compared with HDWL (OR, 0.34; 95% CI, 0.19–0.63)
and FICE (OR, 0.52; 95% CI, 0.35–0.75), however sufficient evidence
was lacking (Table II) (32,38).
 | Table II.Pairwise meta-analyses of colorectal
lesions in different endoscopic modalities. |
Table II.
Pairwise meta-analyses of colorectal
lesions in different endoscopic modalities.
|
|
|
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|
|---|
| Endoscopic
modalities | Number of
studies | Number of
subjects | Mean
Difference/Odds Ratio (95% CI) | P-value | I2
(%) | P-value |
|---|
| Adenomas per
subject |
|
SD-HD | 3 | 1,140 | −0.06a (−0.19–0.08) | 0.4 | 55 | 0.11 |
|
SD-CHRO | 2 | 248 | −0.21a (−0.44–0.01) | 0.07 | 59 | 0.12 |
|
HD-CHRO | 2 | 960 | −0.44a (−0.68–0.20) | 0.0004 | 78 | 0.03 |
|
HD-FICE | 3 | 1,587 | −0.01a (−0.09–0.07) | 0.77 | 0 | 0.58 |
|
HD-AFI | 2 | 194 | 0.00a (−0.12–0.13) | 0.98 | 0 | 0.44 |
|
HD-i-SCAN | 1 | 237 | −0.09a (−0.38–0.20) | 0.54 | N/A | N/A |
|
HD-NBI | 6 | 3,137 | −0.01a (−0.07–0.06) | 0.78 | 0 | 0.86 |
| Polyps per
subject |
|
SD-HD | 3 | 1,140 | −0.11a (−0.30–0.08) | 0.26 | 48 | 0.14 |
|
SD-CHRO | 2 | 248 | −0.73a (−1.13–0.33) | 0.0003 | 0 | 0.46 |
|
HD-CHRO | 2 | 960 | −1.11a (−1.46–0.76) | <0.00001 | 0 | 0.55 |
|
HD-NBI | 2 | 696 | 0.09a (−0.06–0.23) | 0.25 | 0 | 0.32 |
|
HD-FICE | 1 | 359 | −0.21a (−0.54–0.12) | 0.21 | N/A | N/A |
|
HD-i-SCAN | 1 | 237 | −0.21a (−0.54–0.12) | 0.89 | N/A | N/A |
| Flat lesions per
subject |
|
SD-CHRO | 1 | 198 | −0.15a (−0.30–0.00) | 0.04 | N/A | N/A |
|
HD-CHRO | 2 | 960 | −0.30a (−0.49–0.10) | 0.003 | 62 | 0.1 |
|
HD-NBI | 3 | 859 | 0.46a (−0.30–1.23) | 0.24 | 96 | <0.00001 |
| Subjects with
polyp |
|
SD-HD | 4 | 1,374 | 0.86b (0.69–1.07) | 0.17 | 0 | 0.46 |
|
SD-CHRO | 4 | 767 | 0.43b (0.32–0.57) | <0.00001 | 0 | 0.86 |
|
HD-FICE | 3 | 1,805 | 0.78b (0.64–0.96) | 0.02 | 0 | 0.41 |
|
HD-CHRO | 2 | 600 | 0.29b (0.11–0.78) | 0.01 | 81 | 0.02 |
|
HD-NBI | 5 | 3,477 | 0.86b (0.63–1.18) | 0.34 | 76 | 0.002 |
|
HD-i-SCAN | 1 | 234 | 0.66b (0.40–1.11) | 0.12 | N/A | N/A |
| Subjects with flat
lesion |
|
HD-NBI | 3 | 1,413 | 0.77b (0.60–0.99) | 0.05 | 43 | 0.17 |
| Subjects with more
than three adenomas |
|
SD-HD | 2 | 750 | 0.93b (0.31–2.81) | 0.9 | 73 | 0.06 |
|
SD-CHRO | 2 | 458 | 0.47b (0.22–1.03) | 0.06 | 47 | 0.17 |
|
HD-FICE | 2 | 1,459 | 0.90b (0.54–1.50) | 0.68 | 15 | 0.28 |
|
HD-NBI | 3 | 2,096 | 1.22b (0.87–1.72) | 0.25 | 0 | 0.45 |
| Flat-lesion
number |
|
SD-HD | 3 | 1,022 | 1.54b (1.09–2.17) | 0.01 | 0 | 0.72 |
|
SD-CHRO | 2 | 192 | 0.76b (0.41–1.40) | 0.37 | 36 | 0.21 |
|
SD-AFI | 1 | 152 | 0.64b (0.21–1.98) | 0.44 | N/A | N/A |
|
HD-FICE | 4 | 1,023 | 0.96b (0.65–1.42) | 0.85 | 0 | 0.41 |
|
HD-NBI | 9 | 2,862 | 0.89b (0.62–1.26) | 0.5 | 68 | 0.002 |
|
HD-CHRO | 1 | 1,680 | 0.54b (0.44–0.66) | <0.00001 | N/A | N/A |
|
HD-AFI | 2 | 246 | 0.65b (0.36–1.15) | 0.14 | 9 | 0.29 |
| Diminutive lesion
number |
|
SD-HD | 3 | 1,024 | 0.51b (0.39–0.68) | <0.00001 | 0 | 0.86 |
|
HD-FICE | 3 | 938 | 0.94b (0.69–1.28) | 0.69 | 0 | 0.64 |
|
HD-NBI | 6 | 2,372 | 0.94b (0.78–1.14) | 0.53 | 0 | 0.43 |
|
HD-CHRO | 1 | 202 | 0.34b (0.19–0.63) | 0.0006 | N/A | N/A |
|
HD-AFI | 1 | 173 | 0.50b (0.24–1.06) | 0.07 | N/A | N/A |
|
FICE-CHRO | 1 | 507 | 0.52b (0.35–0.75) | 0.0006 | N/A | N/A |
Discussion
Early detection of cancers and adenomas of the colon
is the key to reducing the incidence rate of colorectal carcinoma.
According to several reports, 10–15% of lesions, especially small
and flat lesions, remained undiagnosed following conventional
colonoscopy, even by experienced colonoscopists (26,41).
Therefore, image-enhanced endoscopic techniques, including CHRO,
FICE, NBI, AFI and i-SCAN, have come into practice to increase the
detection rate of colorectal lesions, and the new-generation
image-enhanced techniques have been demonstrated to be superior to
conventional techniques in polyp visibility (51,52).
Based on the results of the current study, newly developed
techniques could greatly enhance the early detection of lesions
during a colonoscopy. CHRO could be useful for the detection of
colorectal adenomas, especially in the flat and diminutive lesions
compared with the other analysed techniques.
Chromoscopy with indigo carmine has been
demonstrated to be effective in detecting neoplastic and
non-neoplastic lesions, with accuracy ranging from 84–97% (53). Individuals with a history or a family
history of colorectal cancer, or who have more than three
colorectal adenomas, have a high risk of developing cancer
(3). Therefore, the aim of
colonoscopies is to accurately detect the number of adenomas in
patients. Two studies suggested that CHRO was good at detecting
adenomas in high-risk populations (25,44).
However, the studies' design of tandem colonoscopy, which is
usually performed by only one colonoscopist, might induce
investigator-dependent bias and thus influence the outcome
analysis. That the same colonoscopist performed both of the
targeted colonoscopic techniques, and the same region of the colon
was again investigated, may influence the CHRO results. Le Rhun
et al (33) suggested that
the high adenoma detection rates may be due to HDWL used in
combination with CHRO. The current study revealed that CHRO was
superior to HDWL in the detection of colorectal lesions and that
high definition should be one of several factors leading to an
improved detection rate.
The duration of withdrawal time, a potential
contributory factor, demonstrated a positive association with the
adenoma detection rate (ADR) (54).
CHRO is known to be a time-consuming technique (6). It is theoretically possible that the
increased withdrawal time could result in enhanced adenoma
detection. Flat and diminutive lesions are easily missed if
adequate time and attention are not devoted to their detection. As
flat adenomas present a more likely increased risk for malignant
progression compared with polyploid adenomas, it is especially
imperative to detect them (52). The
Lau and Sung study (55)
demonstrated that CHRO improved the detection of flat lesions
comparing with HDWL with magnification.
Despite being expensive and time-consuming, CHRO
remains irreplaceable, especially in the screening of high-risk
patients (55). With more new image
enhancement techniques, including BLI, LCI and new NBI (51,52,56), it
was demonstrated that the detection rate of colon lesions may be
improved without sacrificing image sharpness and brightness.
The efficiency of FICE for the ADR was controversial
as well (12,16,17). The
data of the current analysis demonstrated that FICE improved the
detection of polyps in (P=0.02), however it did not improve the
detection of total adenomas and flat lesions. However, in
medium-risk individuals presenting for screening or diagnostic
colonoscopy, the ADR of FICE was superior to SDWL colonoscopy and
equivalent to conventional CHRO (38). When including high-risk individuals
undergoing postoperative (sigmoidectomy or rectal anterior
resection) follow-up colonoscopy, FICE missed significantly fewer
lesions compared with HDWL (24 vs. 46%) (30). Considering the shorter duration of
inspection and the outstanding efficiency of FICE for
distinguishing between neoplastic and non-neoplastic lesions
(crucial for determining therapeutic strategies), and the lower
miss rate of FICE in high-risk individuals, the authors of the
current study hypothesise that it may be appropriate to perform
FICE in the screening or diagnostic colonoscopy of high-risk
individuals.
Aminalai et al (12) revealed that SDWL imaging using high
image resolution technology is just as accurate as techniques that
improve contrast enhanced colonoscopies, such as FICE; however the
outcome may also be influenced by experience (16). The majority of the studies included
in the current analysis were performed by experienced endoscopists
and no significant differences in the detection of adenomas were
identified between FICE and other endoscopic techniques. Additional
studies will be required to identify whether FICE could improve
detection rates when performed by less experienced
endoscopists.
Novel colonoscopic technologies were developed to
improve adenoma detection and to decrease miss rates. Ikematsu
et al (51) reported the mean
number of adenomas per patient was significantly higher in the BLI
group compared with that in the HDWL group, however the ADR was not
significantly higher. Thus, the authors of the current study
hypothesise that BLI and LCI is a promising modality for the
detection of diminutive lesions, especially polypoid and flat
lesions, however further studies are required.
NBI has been studied extensively with the ADR
results reported in much more detail; positive and negative results
have been reported (21,27,49). In
the current network analysis, no significant differences in the ADR
were identified between NBI and other endoscopic techniques,
however NBI seemed to be of value in identifying patients with flat
lesions (P=0.05). NBI may be beneficial in colonoscopies, but
several factors may contribute to its reduced effectiveness in
randomised controlled trials focusing on NBI (19,21,27).
As mentioned, a colonoscopist's experience may have
a considerable impact on the detection rate, thus this may also be
true with NBI. The efficiency of NBI in the detection of adenomas
may not be evident if the colonoscopist does not have sufficient
training with an NBI system. Combining data from multiple operators
may potentially conceal colonoscopists who have high ADRs using
novel technologies or those who perform worse (19). Similar to other endoscopic
technologies, NBI also has poorer brightness and resolution
compared with HDWL. However, recently these factors were improved
by the new-generation video processor system (EVIS LUCERA ELITE)
when compared with the previous NBI system (56). Ogiso et al (56) used the polyp visibility scores to
evaluate the detection efficiency and demonstrated that NBI with
the ELITE system were significantly higher compared with those of
HDWL.
The learning effect itself may also be a reason to
evaluate the efficiency of NBI. In a UK study, the ADR of the NBI
group was consistently 25%; however, the ADR of the HDWL group rose
continuously, from 8–26.5% in each consecutive 100 patients
(50). The increasing ADR with HDWL
may have been as a result of some form of learning effect following
the use of the NBI contrast-enhancement technique. The elements of
NBI and novel NBI techniques emphasise the contrast of mucosal
microvessels, which potentially obscure surface detail to influence
the detection of adenomas with NBI (56). Therefore, it suggested that NBI may
be helpful under optimal conditions and in right-sided colons
(19,40,50).
As yet, few studies have investigated the efficiency
of i-SCAN in the colon. Hong et al (24) suggested that i-SCAN failed to prevent
missed polyps or to improve adenoma detection compared with HDWL,
but further research is required to support this view.
In the current analysis, only two studies that
compared the efficiency between i-SCAN and WL met the criteria for
inclusion of the current study. With the resulting dearth of
subjects, the sample size may be too small to meaningfully compare
the ADR efficiency between i-SCAN and WL. Additionally, the optimal
settings for the modes of image enhancement of i-SCAN, including
contrast, surface enhancement and tone enhancement, remain unclear.
This limitation may be a reason to conduct further studies into the
efficiency of the ADR of i-SCAN.
Matsuda et al (34), who examined the left and right sides
of the colon separately, demonstrated a higher detection rate of
adenomas and flat adenomas with AFI compared with WL. In addition,
a study demonstrated a significant improvement in the detection of
adenomas in a high-risk group of patients (39). However, the results of the current
analysis revealed that AFI was no better at detection compared with
other endoscopic techniques with the exception of SDWL, regardless
of the lesion's shape. The duration and difficulty of operation may
limit the use of AFI in detecting colonic lesions (34,35).
The current meta-analysis has several limitations.
Marked heterogeneity in some of the analysis groups decreased the
power of the findings, with respect to patients' details and
endoscopic factors. The current analysis included average-risk
individuals undergoing screening endoscopy, high-risk individuals
with positive faecal occult blood tests and patients with adenomas
on previous endoscopies. The endoscopic factors were the
differences in withdrawal time among the studies and the bias
generated by a non-blinded design where the same endoscopist
performed the two techniques being compared in certain studies, as
was predictable from the nature of the intervention. Studies
investigating i-SCAN and AFI were scarce, leading to less
meaningful results about these novel endoscopic techniques.
Additionally, lesion location, morphology and pathology are all
important aspects of colonoscopies, however the data were not
clearly described in the majority of individual studies.
In conclusion, the current meta-analyses of 40
studies demonstrated that NBI, FICE and AFI were significantly
better compared with SDWL in identifying patients with adenomas.
Furthermore, CHRO, as a time-consuming technique requiring
endoscopy and the spraying of dye, was superior to SDWL and HDWL
colonoscopies in the detection of adenomas, polyps and flat
lesions. NBI appeared effective in detecting subjects with flat
lesions, whereas FICE effective at detecting polyps. The authors of
the current study would not suggest applying CHRO in the screening
of average-risk individuals, but it may be the first choice to
apply in high-risk individuals as it efficiently detected
colorectal lesions. Digital colonoscopy methods, including NBI or
FICE, could be used to improve neoplasm diagnosis rates and be
potentially efficacious at detecting colorectal lesions, including
polyps and flat, diminutive adenomas. Additionally, novel image
enhanced system, including BLI, LCI and the novel NBI system, were
also revealed to have great potential in the detection of
colorectal lesions.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Beijing Medical
and Health Foundation (grant no. YWJKJJHKYJJ-B17303-Z06).
Availability of data and materials
The analyzed data sets generated during the study
are available in the published article.
Authors' contributions
LL designed the study and prepared the manuscript.
YO and HS collected the data and performed the majority of
analyses. SL performed the pairwise analysis. FH, QP and PC
provided technical support. HY and SD revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kahi CJ, Anderson JC, Waxman I, Kessler
WR, Imperiale TF, Li X and Rex DK: High-definition
chromocolonoscopy vs. high-definition white light colonoscopy for
average-risk colorectal cancer screening. Am J Gastroenterol.
105:1301–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muto T, Bussey HJ and Morson BC: The
evolution of cancer of the colon and rectum. Cancer. 36:2251–2270.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winawer SJ, Zauber AG, Ho MN, O'Brien MJ,
Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF,
et al: Prevention of colorectal cancer by colonoscopic polypectomy.
The National Polyp Study Workgroup. N Engl J Med. 329:1977–1981.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kahi CJ, Imperiale TF, Juliar BE and Rex
DK: Effect of screening colonoscopy on colorectal cancer incidence
and mortality. Clin Gastroenterol Hepatol. 7:770–775; quiz 711.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zauber AG, Winawer SJ, O'Brien MJ,
Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH,
Schapiro M, Panish JF, et al: Colonoscopic polypectomy and
long-term prevention of colorectal-cancer deaths. N Engl J Med.
366:687–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuda T, Ono A, Sekiguchi M, Fujii T and
Saito Y: Advances in image enhancement in colonoscopy for detection
of adenomas. Nat Rev Gastroenterol Hepatol. 14:305–314. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin XF, Chai TH, Shi JW, Yang XC and Sun
QY: Meta-analysis for evaluating the accuracy of endoscopy with
narrow band imaging in detecting colorectal adenomas. J
Gastroenterol Hepatol. 27:882–887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Omata F, Ohde S, Deshpande GA, Kobayashi
D, Masuda K and Fukui T: Image-enhanced, chromo, and cap-assisted
colonoscopy for improving adenoma/neoplasia detection rate: A
systematic review and meta-analysis. Scand J Gastroenterol.
49:222–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whiting P, Rutjes AW, Reitsma JB, Bossuyt
PM and Kleijnen J: The development of QUADAS: A tool for the
quality assessment of studies of diagnostic accuracy included in
systematic reviews. BMC Med Res Methodol. 3:252003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dias S, Welton NJ, Sutton AJ and Ades AE:
A generalised linear modelling framework for pairwise and network
meta-analysis of randomised controlled trials. NICE DSU Technical
Support Document. 2:2011.
|
|
11
|
Sturtz S, Ligges U and Gelman A:
R2WinBUGS: A package for running WinBUGS from R. J Stat Softw.
12:1–16. 2005. View Article : Google Scholar
|
|
12
|
Aminalai A, Rösch T, Aschenbeck J, Mayr M,
Drossel R, Schröder A, Scheel M, Treytnar D, Gauger U, Stange G, et
al: Live image processing does not increase adenoma detection rate
during colonoscopy: A randomized comparison between FICE and
conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am J
Gastroenterol. 105:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bisschops R, Tejpar S, Willekens H, De
Hertogh G and Van Cutsem E: Su1432 I-SCAN detects more polyps in
lynch syndrome (HNPCC) patients: A prospective controlled
randomized back-to-back study. Gastrointest Endosc. 75:AB3302012.
View Article : Google Scholar
|
|
14
|
Boparai KS, van den Broek FJ, van Eeden S,
Fockens P and Dekker E: Increased polyp detection using narrow band
imaging compared with high resolution endoscopy in patients with
hyperplastic polyposis syndrome. Endoscopy. 43:676–682. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brooker JC, Saunders BP, Shah SG, Thapar
CJ, Thomas HJ, Atkin WS, Cardwell CR and Williams CB: Total colonic
dye-spray increases the detection of diminutive adenomas during
routine colonoscopy: A randomized controlled trial. Gastrointest
Endosc. 56:333–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha JM, Lee JI, Joo KR, Jung SW and Shin
HP: A prospective randomized study on computed virtual
chromoendoscopy versus conventional colonoscopy for the detection
of small colorectal adenomas. Dig Dis Sci. 55:2357–2364. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung SJ, Kim D, Song JH, Kang HY, Chung
GE, Choi J, Kim YS, Park MJ and Kim JS: Comparison of detection and
miss rates of narrow band imaging, flexible spectral imaging
chromoendoscopy and white light at screening colonoscopy: A
randomised controlled back-to-back study. Gut. 63:785–791. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung SJ, Kim D, Song JH, Park MJ, Kim YS,
Kim JS, Jung HC and Song IS: Efficacy of computed virtual
chromoendoscopy on colorectal cancer screening: A prospective,
randomized, back-to-back trial of Fuji Intelligent Color
Enhancement versus conventional colonoscopy to compare adenoma miss
rates. Gastrointest Endosc. 72:136–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
East JE, Ignjatovic A, Suzuki N, Guenther
T, Bassett P, Tekkis PP and Saunders BP: A randomized, controlled
trial of narrow-band imaging vs. high-definition white light for
adenoma detection in patients at high risk of adenomas. Colorectal
Dis. 14:e771–e778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
East JE, Stavrindis M, Thomas-Gibson S,
Guenther T, Tekkis PP and Saunders BP: A comparative study of
standard vs. high definition colonoscopy for adenoma and
hyperplastic polyp detection with optimized withdrawal technique.
Aliment Pharmacol Ther. 28:768–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Glenn T, Hoffman BJ, Romagnuolo J and
Hawes RH: Does narrow band imaging (NBI) enhance colon polyp
detection? Gastrointest Endosc. 61:AB2772005. View Article : Google Scholar
|
|
22
|
Gross SA, Buchner AM, Crook JE, Cangemi
JR, Picco MF, Wolfsen HC, DeVault KR, Loeb DS, Raimondo M, Woodward
TA and Wallace MB: A comparison of high definition-image enhanced
colonoscopy and standard white-light colonoscopy for colorectal
polyp detection. Endoscopy. 43:1045–1051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Helbig CD and Rex DK: Narrow band imaging
(NBI) versus white-light (WL) for colon polyp detection using high
definition (HD) colonoscopes. Gastrointest Endosc. 63:AB2132006.
View Article : Google Scholar
|
|
24
|
Hong SN, Choe WH, Lee JH, Kim SI, Kim JH,
Lee TY, Kim JH, Lee SY, Cheon YK, Sung IK, et al: Prospective,
randomized, back-to-back trial evaluating the usefulness of i-SCAN
in screening colonoscopy. Gastrointest Endosc. 75:1011–1021.e2.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hüneburg R, Lammert F, Rabe C, Rahner N,
Kahl P, Büttner R, Propping P, Sauerbruch T and Lamberti C:
Chromocolonoscopy detects more adenomas than white light
colonoscopy or narrow band imaging colonoscopy in hereditary
nonpolyposis colorectal cancer screening. Endoscopy. 41:316–322.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hurlstone DP, Cross SS, Slater R, Sanders
DS and Brown S: Detecting diminutive colorectal lesions at
colonoscopy: A randomised controlled trial of pan-colonic versus
targeted chromoscopy. Gut. 53:376–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikematsu H, Saito Y, Tanaka S, Uraoka T,
Sano Y, Horimatsu T, Matsuda T, Oka S, Higashi R, Ishikawa H and
Kaneko K: The impact of narrow band imaging for colon polyp
detection: A multicenter randomized controlled trial by tandem
colonoscopy. J Gastroenterol. 47:1099–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inoue T, Murano M, Murano N, Kuramoto T,
Kawakami K, Abe Y, Morita E, Toshina K, Hoshiro H, Egashira Y, et
al: Comparative study of conventional colonoscopy and pan-colonic
narrow-band imaging system in the detection of neoplastic colonic
polyps: A randomized, controlled trial. J Gastroenterol. 43:45–50.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaltenbach T, Friedland S and Soetikno R:
A randomised tandem colonoscopy trial of narrow band imaging versus
white light examination to compare neoplasia miss rates. Gut.
57:1406–1412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiriyama S, Matsuda T, Nakajima T,
Sakamoto T, Saito Y and Kuwano H: Detectability of colon polyp
using computed virtual chromoendoscopy with flexible spectral
imaging color enhancement. Diagn Ther Endosc. 2012:5963032012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuiper T, van den Broek FJ, Naber AH, Van
Soest EJ, Scholten P, Mallant-Hent RCh, van den Brande J, Jansen
JM, van Oijen AH, Marsman WA, et al: Endoscopic trimodal imaging
detects colonic neoplasia as well as standard video endoscopy.
Gastroenterology. 140:1887–1894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lapalus MG, Helbert T, Napoleon B, Rey JF,
Houcke P and Ponchon T; Société Française d'Endoscopie Digestive, :
Does chromoendoscopy with structure enhancement improve the
colonoscopic adenoma detection rate? Endoscopy. 38:444–448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le Rhun M, Coron E, Parlier D, Nguyen JM,
Canard JM, Alamdari A, Sautereau D, Chaussade S and Galmiche JP:
High resolution colonoscopy with chromoscopy versus standard
colonoscopy for the detection of colonic neoplasia: A randomized
study. Clin Gastroenterol Hepatol. 4:349–354. 2006. View Article : Google Scholar
|
|
34
|
Matsuda T, Saito Y, Fu KI, Uraoka T,
Kobayashi N, Nakajima T, Ikehara H, Mashimo Y, Shimoda T, Murakami
Y, et al: Does autofluorescence imaging videoendoscopy system
improve the colonoscopic polyp detection rate?-A pilot study. Am J
Gastroenterol. 103:1926–1932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moriichi K, Fujiya M, Sato R, Watari J,
Nomura Y, Nata T, Ueno N, Maeda S, Kashima S, Itabashi K, et al:
Back-to-back comparison of auto-fluorescence imaging (AFI) versus
high resolution white light colonoscopy for adenoma detection. BMC
Gastroenterol. 12:752012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paggi S, Radaelli F, Amato A, Meucci G,
Mandelli G, Imperiali G, Spinzi G, Terreni N, Lenoci N and Terruzzi
V: The impact of narrow band imaging in screening colonoscopy: A
randomized controlled trial. Clin Gastroenterol Hepatol.
7:1049–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pellisé M, Fernández-Esparrach G, Cárdenas
A, Sendino O, Ricart E, Vaquero E, Gimeno-García AZ, de Miguel CR,
Zabalza M, Ginès A, et al: Impact of wide-angle, high-definition
endoscopy in the diagnosis of colorectal neoplasia: A randomized
controlled trial. Gastroenterology. 135:1062–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pohl J, Lotterer E, Balzer C, Sackmann M,
Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G, et
al: Computed virtual chromoendoscopy versus standard colonoscopy
with targeted indigocarmine chromoscopy: A randomised multicentre
trial. Gut. 58:73–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramsoekh D, Haringsma J, Poley JW, van
Putten P, van Dekken H, Steyerberg EW, van Leerdam ME and Kuipers
EJ: A back-to-back comparison of white light video endoscopy with
autofluorescence endoscopy for adenoma detection in high-risk
subjects. Gut. 59:785–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rastogi A, Early DS, Gupta N, Bansal A,
Singh V, Ansstas M, Jonnalagadda SS, Hovis CE, Gaddam S, Wani SB,
et al: Randomized, controlled trial of standard-definition
white-light, high-definition white-light, and narrow-band imaging
colonoscopy for the detection of colon polyps and prediction of
polyp histology. Gastrointest Endosc. 74:593–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rex DK and Helbig CC: High yields of small
and flat adenomas with high-definition colonoscopes using either
white light or narrow band imaging. Gastroenterology. 133:42–47.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rotondano G, Bianco MA, Sansone S, Prisco
A, Meucci C, Garofano ML and Cipolletta L: Trimodal endoscopic
imaging for the detection and differentiation of colorectal
adenomas: A prospective single-centre clinical evaluation. Int J
Colorectal Dis. 27:331–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sabbagh LC, Reveiz L, Aponte D and De
Aguiar S: Narrow-band imaging does not improve detection of
colorectal polyps when compared to conventional colonoscopy: A
randomized controlled trial and meta-analysis of published studies.
BMC Gastroenterol. 11:1002011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stoffel EM, Turgeon DK, Stockwell DH,
Normolle DP, Tuck MK, Marcon NE, Baron JA, Bresalier RS, Arber N,
Ruffin MT, et al: Chromoendoscopy detects more adenomas than
colonoscopy using intensive inspection without dye spraying. Cancer
Prev Res (Phila). 1:507–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Togashi K, Hewett DG, Radford-Smith GL,
Francis L, Leggett BA and Appleyard MN: The use of indigocarmine
spray increases the colonoscopic detection rate of adenomas. J
Gastroenterol. 44:826–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tribonias G, Theodoropoulou A,
Konstantinidis K, Vardas E, Karmiris K, Chroniaris N, Chlouverakis
G and Paspatis GA: Comparison of standard vs high-definition,
wide-angle colonoscopy for polyp detection: A randomized controlled
trial. Colorectal Dis 12 (10 Online). e260–e266. 2010. View Article : Google Scholar
|
|
47
|
Van den Broek FJ, Fockens P, Van Eeden S,
Kara MA, Hardwick JC, Reitsma JB and Dekker E: Clinical evaluation
of endoscopic trimodal imaging for the detection and
differentiation of colonic polyps. Clin Gastroenterol Hepatol.
7:288–295. 2009. View Article : Google Scholar
|
|
48
|
Park SY, Lee SK, Kim BC, Han J, Kim JH,
Cheon JH, Kim TI and Kim WH: Efficacy of chromoendoscopy with
indigocarmine for the detection of ascending colon and cecum
lesions. Scand J Gastroenterol. 43:878–885. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Adler A, Aschenbeck J, Yenerim T, Mayr M,
Aminalai A, Drossel R, Schröder A, Scheel M, Wiedenmann B and Rösch
T: Narrow-band versus white-light high definition television
endoscopic imaging for screening colonoscopy: A prospective
randomized trial. Gastroenterology. 136:410–416.e1; quiz 715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adler A, Pohl H, Papanikolaou IS,
Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC,
Setka E, Koch M, Wiedenmann B and Rösch T: A prospective randomised
study on narrow-band imaging versus conventional colonoscopy for
adenoma detection: Does narrow-band imaging induce a learning
effect? Gut. 57:59–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ikematsu H, Sakamoto T, Togashi K, Yoshida
N, Hisabe T, Kiriyama S, Matsuda K, Hayashi Y, Matsuda T, Osera S,
et al: Detectability of colorectal neoplastic lesions using a novel
endoscopic system with blue laser imaging: A multicenter randomized
controlled trial. Gastrointest Endosc. 86:386–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suzuki T, Hara T, Kitagawa Y, Takashiro H,
Nankinzan R, Sugita O and Yamaguchi T: Linked-color imaging
improves endoscopic visibility of colorectal nongranular flat
lesions. Gastrointest Endosc. 86:692–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dos Santos CE, Malaman D, Lopes CV,
Pereira-Lima JC and Parada AA: Digital chromoendoscopy for
diagnosis of diminutive colorectal lesions. Diagn Ther Endosc.
2012:2795212012. View Article : Google Scholar
|
|
54
|
Leung FW: PDR or ADR as a quality
indicator for colonoscopy. Am J Gastroenterol. 108:1000–1002. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lau PC and Sung JJ: Flat adenoma in colon:
Two decades of debate. J Dig Dis. 11:201–207. 2010.PubMed/NCBI
|
|
56
|
Ogiso K, Yoshida N, Siah KT, Kitae H,
Murakami T, Hirose R, Inada Y, Dohi O, Okayama T, Kamada K, et al:
New-generation narrow band imaging improves visibility of polyps: A
colonoscopy video evaluation study. J Gastroenterol. 51:883–890.
2016. View Article : Google Scholar : PubMed/NCBI
|