Introduction
Moringa oleifera Lam. (MO) is one of the most
well-known, widely distributed and cultivated species of the
Moringaceae family (1), which is
also known as a miracle tree.
MO-based preparations are scientifically documented
as being anti-inflammatory, antihypertensive, antimicrobial,
antioxidant, and antidiabetic (2–4). Other
works have reported that MO improves hepatic and renal functions
and regulates thyroid hormones, protecting against oxidative
stress, inflammation, hepatic fibrosis, liver damage,
hypercholesterolaemia and cancer (5,6).
The majority of human populations have long used
medicinal plants as their primary source of health care. Many of
these medicinal plants may have the scientific evidences to be
considered in general practice (7).
Recently, particular attention has been provided to
study the effects on human health of natural substances present in
Mediterranean diet and their bioactive compounds demonstrating
anticancer, antioxidant, antimicrobial and antiviral properties of
olive oil correlated with the activity of phenolic and polyphenolic
compounds present in it (8–12).
Recently, scientific studies have demonstrated the
existence of a so-called ‘cross-kingdom interaction’, which is
mediated by exogenous miRNAs that are derived from plants: These,
inside the host cell, serve to regulate the gene expression
machinery (12–16).
Concerning the latter, MO dried leaf extracts have
exhibited anti-proliferative and antineoplastic activities in human
tumour cell lines through apoptotic pathways (17–19).
Moreover, in vivo and in vitro studies conducted in
rats and human peripheral blood mononuclear cells, respectively,
have demonstrated that MO-extracts are not toxic (6). Since MO derivatives are used worldwide,
both as food and as an alimentary supplement, various studies have
been performed to assess the scientific bases underlying the
biological effects of this plant species. In particular, MO
bioactivities were mainly associated with the secondary metabolites
of the plant, such as phenols, terpenes, and alkaloids (6,20).
For centuries, MO has been widely used in African
traditional medicine, in the form of aqueous infusions, to promote
health and cure diseases (21). In
several African communities, different parts of this plant, such as
the leaves, fruits, flowers and seeds, are also used to fight
malnutrition, especially among children and nursing mothers
(22,23). The general aim of this work was to
study in depth the effects of MO extracts and their bioactive
components on apoptosis processes.
According to this evidence, the main purpose of the
present work was the evaluation of the effects of MO aqueous
extracts, obtained as suggested by African recipes, on monocytoid
(THP1) and lymphoid (Jurkat) tumour cell lines and peripheral blood
mononuclear cells (PBMCs) from healthy donors.
In particular, the ability of MO to inhibit tumour
cell growth was investigated through the study of different
apoptotic cellular mechanisms such as the repression of B-cell
lymphoma 2 (BCL2) mRNA translation. BCL2 is a crucial
anti-apoptotic protein that is involved in the intrinsic pathway
and the block of NAD-dependent deacetylase sirtuin-1 (SIRT1), which
induces apoptosis via regulating p53 activity (12,24–26).
Recently it has been shown how vegetal miRNAs
derived from olive drupes are able to regulate the expression of
BCL2 and SIRT1 in human tumor cells (12), a characterization of the boiled
aqueous extract from MO to evaluate the presence of miRNAs and
their possible involvement in the regulation of pro-apoptotic
mechanisms has been done.
Materials and methods
Plant material
Moringa oleifera Lam. leaves and seeds were
collected from a Cameroonian plantation and sampled by a
‘traditional healer’. Leaves (L) and seeds (S), dried in the sun,
were collected from the same trees, and they were used in
traditional African medicine preparations. Moreover, a
non-conventional preparation was prepared using fresh seeds
(FS).
Extract preparation
M. oleifera extracts (MOE) were prepared
according to African traditional methods: L, S and FS were ground
with a mortar and pestle and boiled for 15 min in bidistilled
water. Other types of extracts were obtained from the same MO plant
parts, but they were powdered, resuspended in bidistilled water and
frozen for 24 h at −80°C. All extracts were centrifuged, and the
supernatants were recovered, filtered (0.22 µm) and stored at
−20°C. The extracts obtained from the boiling and freezing
procedures are, from now on, referred to as boiled and frozen
extracts, respectively.
Cell cultures
Human Jurkat E6-1 lymphoid and THP1 monocytoid cell
lines (American Type Culture Collection, Manassas, VA, USA) were
grown in a suspension culture at a density of 7×105
cells/ml.
Human PBMCs were obtained from 17 healthy blood
donors attending the local Blood Transfusion Unit of Policlinico
‘Tor Vergata’ in Rome. PBMCs were separated by density gradient
from the buffy coat according to the Ficoll-Hypaque standard
technique (Lonza, Morristown, NJ, USA) and were cultured at a
density of 106 cells/ml. Tumour cell lines, Jurkat and
THP1, and PBMCs from healthy donors were cultured in Roswell Park
Memorial Institute (RPMI) 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 2
mM glutamine (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA), 50 U/ml penicillin and 50 U/ml streptomycin (HyClone; GE
Healthcare Life Sciences). All cell lines and PBMCs were cultured
at 37°C in a 5% CO2 humidified atmosphere in the
presence or absence of the various MO extracts (MOE) at different
concentrations, which varied between 0.01 and 10 mg of fresh plant
weight equivalent (FW) per ml of culture medium, for 72 h. The
ethical approval for the collection and use of human samples was
obtained in 2014, from ethical board of ‘Tor Vergata’ hospital,
protocol number 15/14 (D.M.08.02.2013-D.G.R.146/2013; D.D.G.467 del
25.07.2013), all patients provided written informed consent.
Cell death/viability and proliferation
assays
Amounts of viable and dead cells were assessed by
the trypan blue (Euroclone S.p.A., Milan, Italy) exclusion test,
and a negative (water) proliferation control was used. After 72 h,
viable and dead cells, treated and untreated, were calculated as a
fold change with respect to untreated cells at Time 0.
Calculation of EC50 and LD50
Cumulative results from at least three different
measurements were used to calculate the MOE concentration required
to reduce cell proliferation by 50% (EC50) or to induce death in
50% of cells (LD50). EC50 and LD50 were evaluated in all cell lines
and in PBMCs using sigmoidal dose-response regression curves, using
GRAPH PAD PRISM software.
Intracellular BCL2 and SIRT1
staining
BCL2 and SIRT1 intracellular expression was
evaluated by flow cytometry analysis. After 72 h, transfected cells
were harvested, fixed and permeabilized with 70% ethanol and
incubated with PE-conjugated anti-human BCL2 (BD Biosciences,
Franklin Lakes, NJ, USA), rabbit anti-human SIRT1 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-rabbit IgG-PE
antibodies (Calbiochem; Merck KGaA, Darmstadt, Germany). Stained
cells were analysed using a CytoFLEX flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA) and CytExpert 1.2 software (Beckman
Coulter, Inc.).
Apoptosis assays
After 72 h of incubation with MOE, apoptosis was
evaluated by flow cytometry (CytoFLEX; Beckman Coulter, Inc.)
assessment of hypodiploid events. Cells were harvested, washed
twice in PBS and incubated for 20 min in 70% EtOH at −20°C. After
incubation, cells were washed and stained in propidium iodide 1.25
µg/ml. Events were gated on forward vs. orthogonal scatter in such
a way that degraded DNA from cell debris or from doublets was
excluded and nuclei from viable, apoptotic and necrotic cells were
assayed. Data acquisition and analyses were performed for 150,000
events for each sample and analysed using CytExpert 2.0 (Beckman
Coulter, Inc.).
Bromodeoxyuridine proliferation
assay
The percentage of DNA synthesis in the cells was
detected by the bromodeoxyuridine (BrdU) In Situ Cell
Proliferation kit (FLUOS, Roche), according to the manufacturer's
instructions, and analysed using CytoFLEX (Beckman Coulter,
Inc.).
HPLC-DAD analysis
M. oleifera extracts were analysed by a
Shimadzu HPLC (Shimadzu Corporation, Kyoto, Japan) equipped with a
diode array detector (DAD) SPD-M20A (Shimadzu Corporation).
Analytes were separated using a Kynetex C18 column 2.6 µm × 75×2.1
mm (Phenomenex, Torrance, CA, USA). Data acquisition and peak
integration were performed with a LabSolutions software (Shimadzu
Corporation). The mobile phases used were 1% trifluoroacetic acid
at pH 2.5 (solvent A) and acetonitrile (solvent B). The system was
run with the following gradient elution program: 0 min, 85% solvent
A; 2.5 min 65% solvent A; 7 min, 25% solvent A; 9 min, 25% solvent
A; 13.5 min, 85% solvent A; and 25 min, 85% solvent A. The flow
rate was kept constant throughout the analysis at 1
ml/min−1, and the injection volume was 20 µl.
Chromatographic profiles were obtained at 254 nm.
Total simple phenol and flavonoid
content
The amount of total simple phenols and flavonoids in
the plant samples was measured using a method that has been widely
reported (27,28). Briefly, for total simple phenols
quantitation, the equivalent of 400 µg of plant extract was mixed
with 100 µl of Folin-Ciocalteu reagent and 80 µl of 0.7 M
Na2CO3, incubated for 1 h in the dark and
spectrophotometrically analysed at 760 nm. For the measurement of
flavonoid levels, the equivalent of 400 µg of plant extract was
adjusted with ddH2O to reach 250 µl of final volume,
which was added to 30 µl of pure methanol, 2 µl of 10%
AlCl3 and 2 µl of 1 M CH3CO2K. The
sample was left in the dark for 15 min and absorbance was read at
415 nm. The concentration of total phenols and flavonoids was
obtained by comparing the sample absorbance values with calibration
curves properly obtained using adequate amounts of pure gallic acid
(GA) and quercetin (Q) as standards. Consequently, the results were
reported as µg of GA or Q equivalents per 100 mg of sample fresh
weight (µg GAE or QE/100 mg SFW).
Plant small RNA pool extraction
The total small RNA pool of MO
(mol-small RNA pool) was extracted from 50 mg FW of MOE
seeds using NucleoSpin miRNA in accordance with the manufacturer's
instructions (NucleoSpin miRNA experiments protocols®;
MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany).
Reverse transcription-quantitative
polymerase chain reaction analysis (RT-qPCR) analysis
The presence of microRNA purified, as reported
above, from the aqueous extract of MOE-S (mol-miR)
was evaluated by RT-qPCR, as has been widely reported (29,30). In
brief, cDNA was synthesized using a specific reverse transcription
kit for microRNA (miRCURY LNA Universal RT microRNA PCR, Synthesis
kit II; EXIQON; Qiagen GmbH, Hilden, Germany), according to the
manufacturer's guidelines. To verify the absence of nucleases in
the reaction and evaluate the efficiency of retro-transcription and
qPCR amplification, 108 copies of a synthetic spike-in
control miRNA (UniSp6; EXIQON; Qiagen GmbH) were added to each RNA
sample before conversion to cDNA. qPCR was carried out in a 10 µl
reaction volume that included 20 ng cDNA, 50% SYBR green (ExiLENT
SYBR® Green master mix; EXIQON; Qiagen GmbH) and 1 µl of
a mixture containing pre-designed PCR primers specific for microRNA
amplification (microRNA LNA PCR primer sets; EXIQON; Qiagen GmbH).
The qPCR assay was performed using a Bio-Rad (IQ5) thermal cycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Amplification
parameters were set as recommended by the instruction manual of the
EXIQON pre-designed primers (Table
I).
 | Table I.miRNA sequences primers for
Moringa oleifera miR pool. |
Table I.
miRNA sequences primers for
Moringa oleifera miR pool.
| mol miRs | 5′-3′
sequences |
|---|
| miR-156a |
CUGACAGAAGAGAGUGAGCAC |
| miR-159a |
UUUGGAUUGAAGGGAGCUCUA |
| miR-159c |
UUUGGAUUGAAGGGAGCUCCU |
| miR-160h |
UGCCUGGCUCCCUGUAUGCCAUU |
| miR-162a |
UCGAUAAACCUCUGCAUCCA |
| miR-166i |
UCGGACCAGGCUUCAUUCCCCC |
| miR-167-5p |
UGAAGCUGCCAGCAUGAUCUU |
| miR-168a |
UCGCUUGGUGCAGGUCGGGAA |
| miR-171d |
UGAUUGAGCCGUGCCAAUAU |
| miR-393a |
CAUCCAAAGGGAUCGCAUUGA |
| miR-395a |
CUGAAGUGUUUGGGGGAACUC |
| miR-396a |
UUCCACAGCUUUCUUGAACAG |
| miR-396c |
UUCCACAGCUUUCUUGAACGU |
| miR-397-5p |
UCAUUGAGUGCAGCGUUGAUG |
| miR-398b |
GGGUUGAUUUGAGAACAUAUG |
| miR-482b |
UCUUUCCUAUCCCUCCCAUUCC |
| miR-858a |
UUCGUUGUCUGUUCGACCUUG |
| miR-858b |
UUCGUUGUCUGUUCGACCUUG |
| miR-2118a |
CUACCGAUGCCACUAAGUCCCA |
Quantification of mol-miRs was performed
using the threshold cycle (Ct) comparative method according to the
MIQE guidelines (29) comparing the
mol-miRs expression respect to the lower expressed miR
(mol-miR858b). 5S rRNA was used as a housekeeping gene.
Statistical analysis
All data were presented as the mean ± SD of at least
three independent experiments performed on THP1 and Jurkat cells
and PMBCs from 10 healthy donors. Data analyses were performed
using the SPSS statistical software system (v.17.0; SPSS, Inc.,
Chicago, IL, USA).
Comparison between treated vs. untreated
cells for trypan blue assay, apoptosis assay, BCL2 and SIRT1
intracellular protein expression were all conducted using t-test. A
comparison of trypan blue apoptosis and BrdU assays results, in
response to the different concentration of extracts, was carried
out using an ANOVA and a Bonferroni significant difference test as
a multiple comparison test. *P<0.05, **P<0.01 and
***P<0.001 were considered to indicate a statistically
significant difference. For non-parametric correlations, a Pearson
correlation coefficient was calculated.
Results
Effects of different preparations of
M. oleifera leaves and seeds on THP1 cells, Jurkat cells and PBMCs
from healthy donors
To obtain information about MO's effect on cell
viability, Jurkat cells, THP1 cells and PBMCs were treated with
extracts obtained from the leaves (L), seeds (S) and fresh seeds
(FS) of MO, after being frozen (MOE-f) or boiled in water
(MOE-b).
THP1 and Jurkat cells and PBMCs from healthy donors
were treated with frozen or boiled MOE in a concentration ranging
from 0 to 10 mg/ml.
Seventy-two hours after treatment, cell viability
was analysed using a trypan blue assay.
For each type of preparation, the MOE concentration
that was able to reduce cell proliferation by 50% (EC50) or induce
death in 50% of cells (LD50), with respect to the number of cells
at time 0, was estimated.
Effects of M. oleifera extracts
obtained by boiling on cell viability
After 72 h, the treatment of THP1 (Fig. 1A, D and G) and Jurkat (Fig. 1B, E and H) cells with boiled extracts
of the three different parts of the MO plant induced a significant
reduction of proliferation, with respect to untreated cells, in a
dose dependent manner, starting from 0.01 mg/ml (P<0001 for each
concentration vs. untreated cells; Fig. 1).
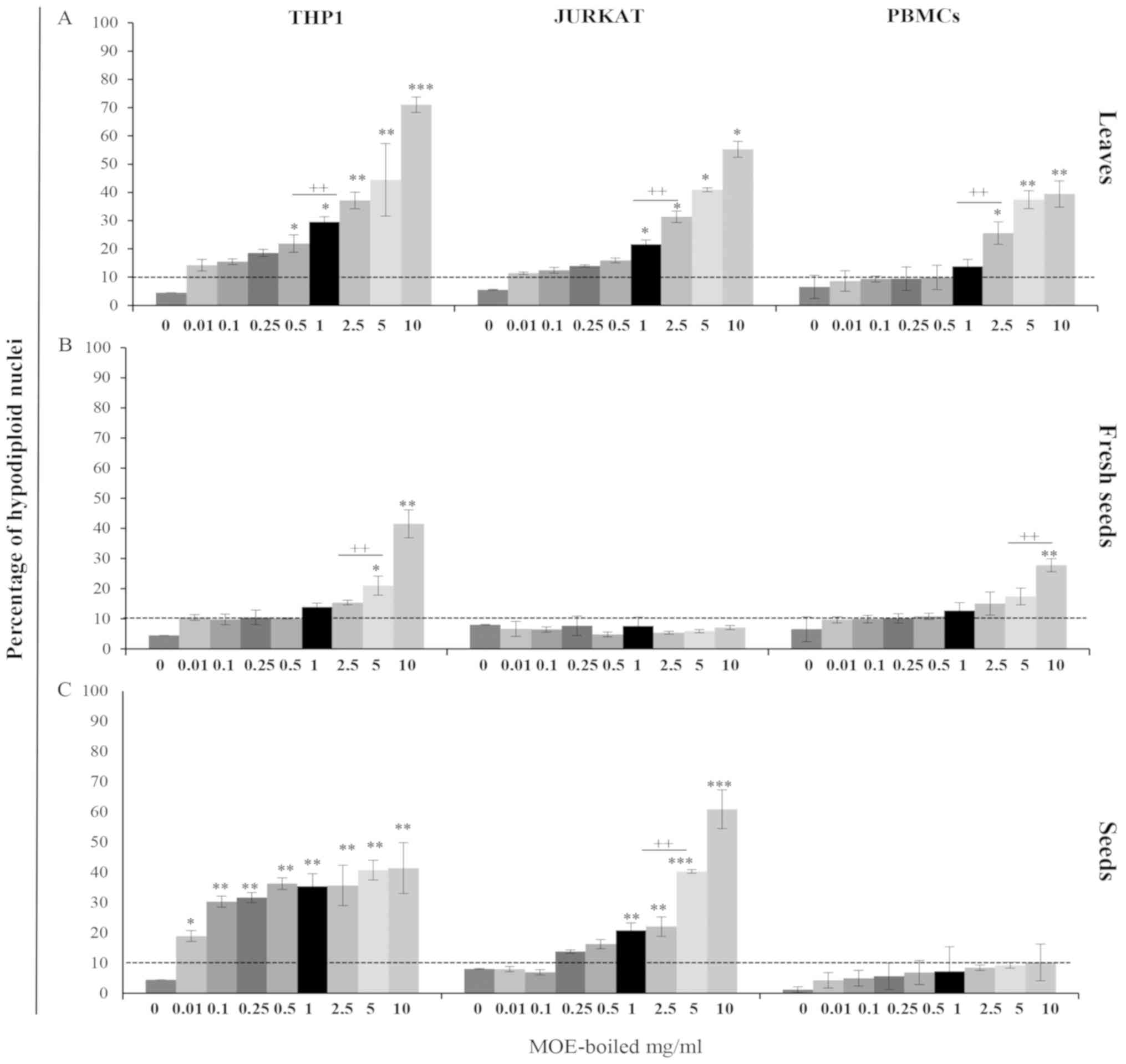
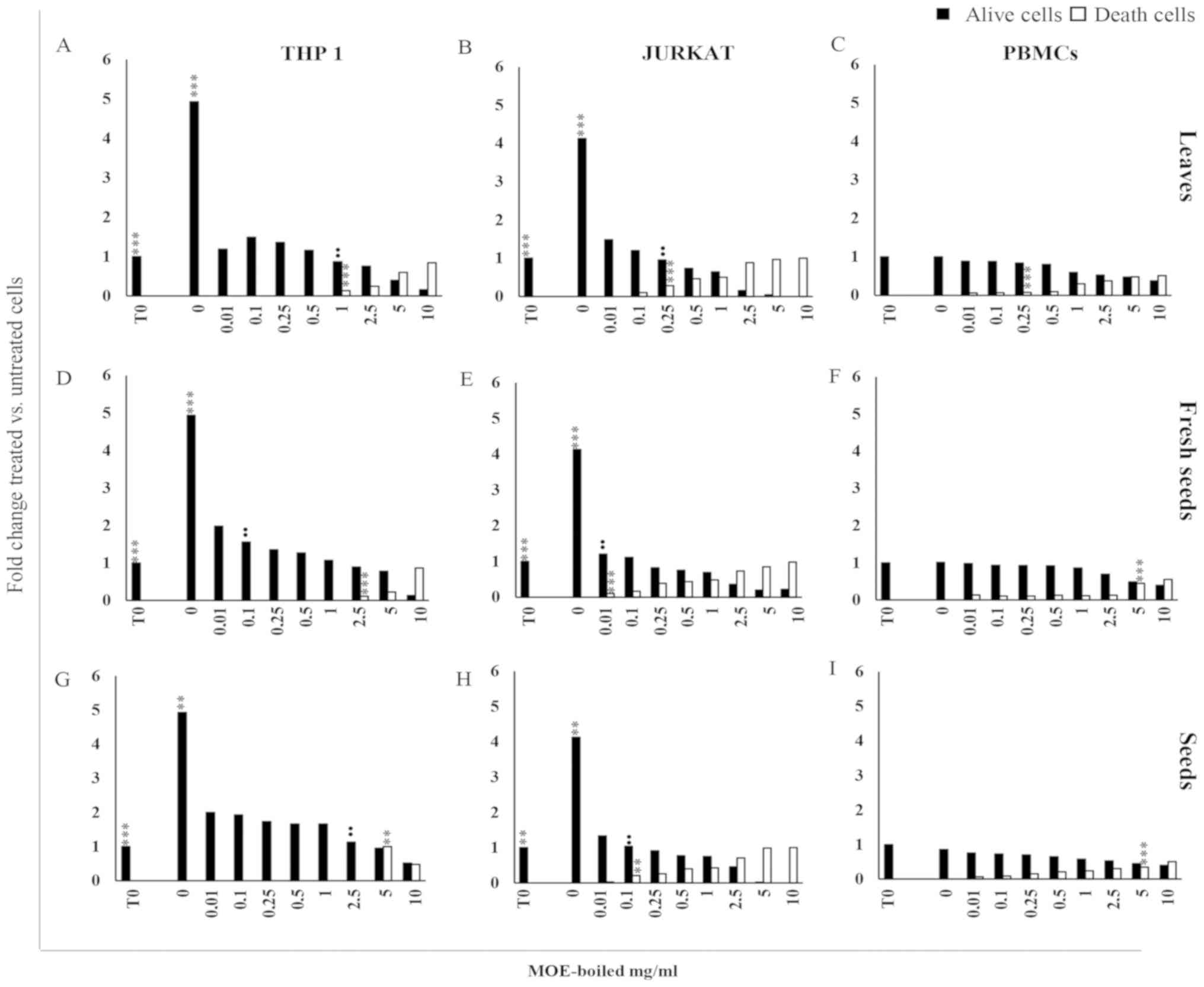 | Figure 1.(A-H) Anti-proliferative effect of MO
boiled extracts. Cell viability analysed by trypan blue exclusion
assay in cells. (A, D, G) THP1, (B, E, H) Jurkat cells and (C, F,
I) PBMCs from healthy donors treated with MOE boiled leaves, fresh
seeds and seeds with a concentration ranging from 0 to 10 mg/ml for
72 h. Control cells (0 mg/ml) were incubated for the same amount of
time with an equivalent volume of water. The results are expressed
as fold change histograms of trypan blue positive (white square) or
negative cells (black square) with respect to cells at time 0. Data
are reported as the mean of three different experiments and of 17
healthy donors' PBMCs ± SD. Symbols indicate significant
differences: **P<0.01, ***P<0.001 all treatment vs.
untreated cells. ••P<0.01 represent the lowest
concentration able to significantly reduce or increase cell
viability or death, respectively. |
Jurkat and THP1 cells showed a different
susceptibility to MOE treatments: Although MOE-b reduced cell
growth in both tumour cells at a low concentration, it showed a
lower toxicity in THP1 cells with respect to Jurkat cells. Indeed,
THP1 cells demonstrated a significant increase in trypan blue
positive cells at a concentration of 1 mg/ml for leaves (Fig. 1A), 2.5 mg/ml for fresh seeds
(Fig. 1D) and 5 mg/ml for seeds
(Fig. 1G), whereas a cytotoxic
effect was evident in Jurkat cells starting from 0.25 mg/ml of
leaves (Fig. 1B), 0.01 mg/ml of
fresh seeds (Fig. 1E), and 0.1 mg/ml
of seeds (Fig. 1H).
MOE treatments at a low concentration preserved
viability in PBMCs from healthy donors; a decrease of cell
proliferation associated with a significant increase of trypan blue
positive cells was detectable at the concentration of 0.25 mg/ml
for leaves (Fig. 1C) and 5 mg/ml for
all seed preparations (Fig.
1F-I).
Based on these results, EC50 at 72 h of MOE
treatment was calculated compared to control cells.
As shown in Table
II, THP1 cells were the most sensitive to treatments with the
different boiled parts of M. oleifera. In fact, for all
boiled preparations, a concentration between 0.014 and 0.020 mg/ml
was enough to reduce proliferation of THP1 cells by 50%, whereas
for Jurkat cells, concentrations of 0.129, 0.121 and 0.035 mg/ml
for leaves, seeds and fresh seeds, respectively, were necessary to
reduce proliferation by 50%. PBMCs appeared to be less sensitive to
the treatments: Concentrations of 1.94 mg/ml of leaves and of more
than 10 mg/ml of all seed extracts were required to reach the EC50
value.
 | Table II.EC50 of boiled extracts of Moringa
oleifera (mg/ml). |
Table II.
EC50 of boiled extracts of Moringa
oleifera (mg/ml).
|
| THP1 | JURKAT | PBMC |
|---|
| Leaves | 0.015±0.001 | 0.129±0.050 | 1.94±0.50 |
| Fresh seeds | 0.014±0.010 | 0.035±0.050 | >10 |
| Seeds | 0.020±0.001 | 0.121±0.020 | >10 |
The different MO tissue boiled extracts induced the
death of 50% of cells (LD50) more effectively in Jurkat cells with
respect to THP1 cells and PBMCs. In detail, LD50 in THP1 cells and
PBMCs was obtained for an MOE concentration of higher than 10
mg/ml, while a concentration between 2–4 mg/ml was sufficient in
Jurkat cells (Table III).
 | Table III.LD50 of boiled extracts of Moringa
oleifera (mg/ml). |
Table III.
LD50 of boiled extracts of Moringa
oleifera (mg/ml).
|
| THP1 | JURKAT | PBMC |
|---|
| Leaves | >10 | 2.790±1.550 | >10 |
| Fresh seeds | >10 | 2.010±1.180 | >10 |
| Seeds | >10 | 3.860±2.680 | >10 |
Effects of M. oleifera extracts
obtained by freezing on cell proliferation
The treatment of THP1 and Jurkat cells with frozen
extracts of the three different parts of the MO plant induced a
significant reduction of proliferation, in a dose dependent manner,
starting from 0.01 mg/ml (P<0001 for each concentration
vs. untreated cells; Fig. 2).
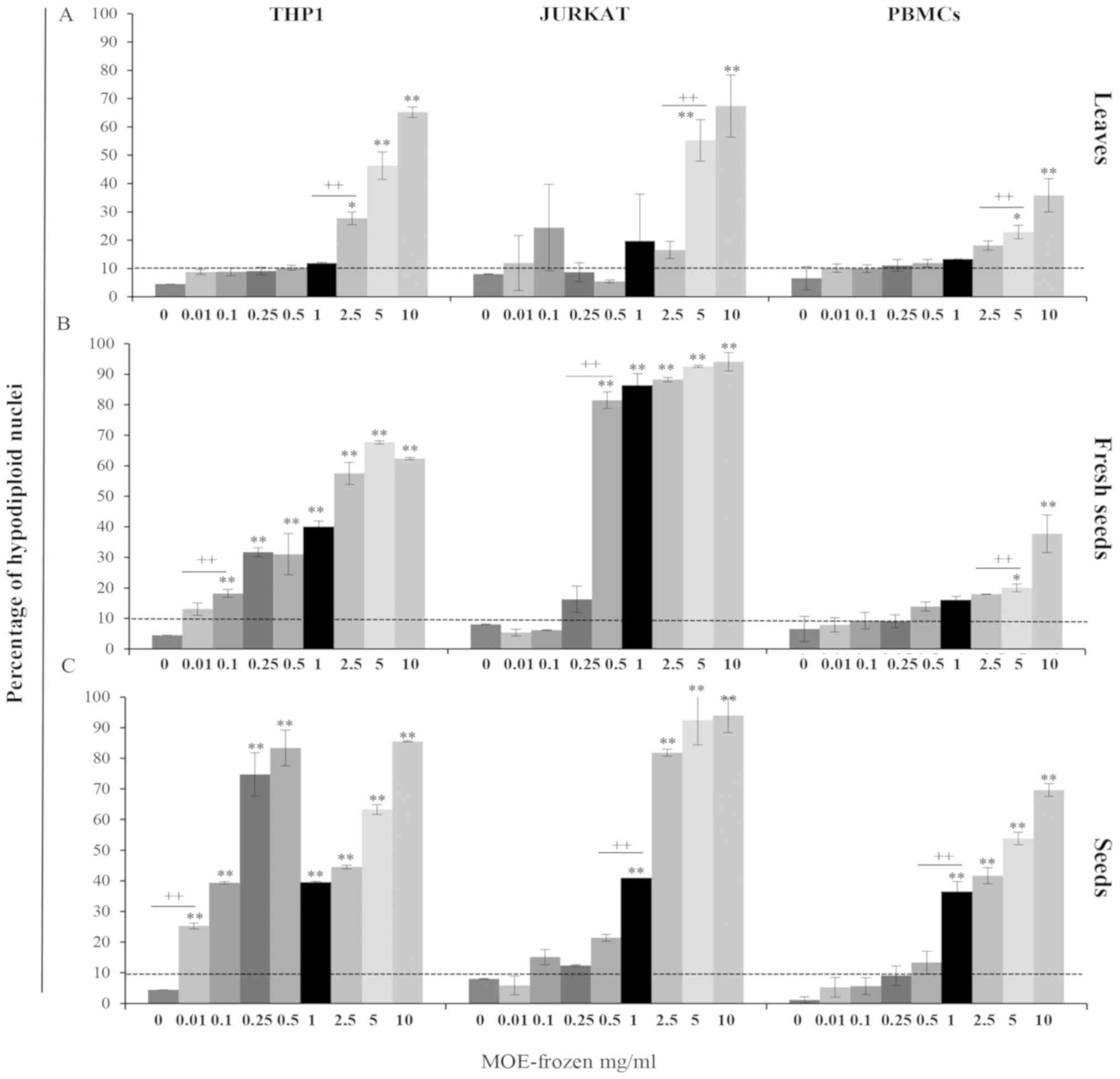
The treatment became cytotoxic with a different intensity in THP1
cells with respect to Jurkat cells: For THP1 cells starting from 5
mg/ml for leaves, 2.5 mg/ml for seed samples (Fig. 2A and G), and from 1 mg/ml for fresh
seed preparations (Fig. 2D) and in
Jurkat cells starting at 0.1 mg/ml for all preparations (Fig. 2B, E, H).
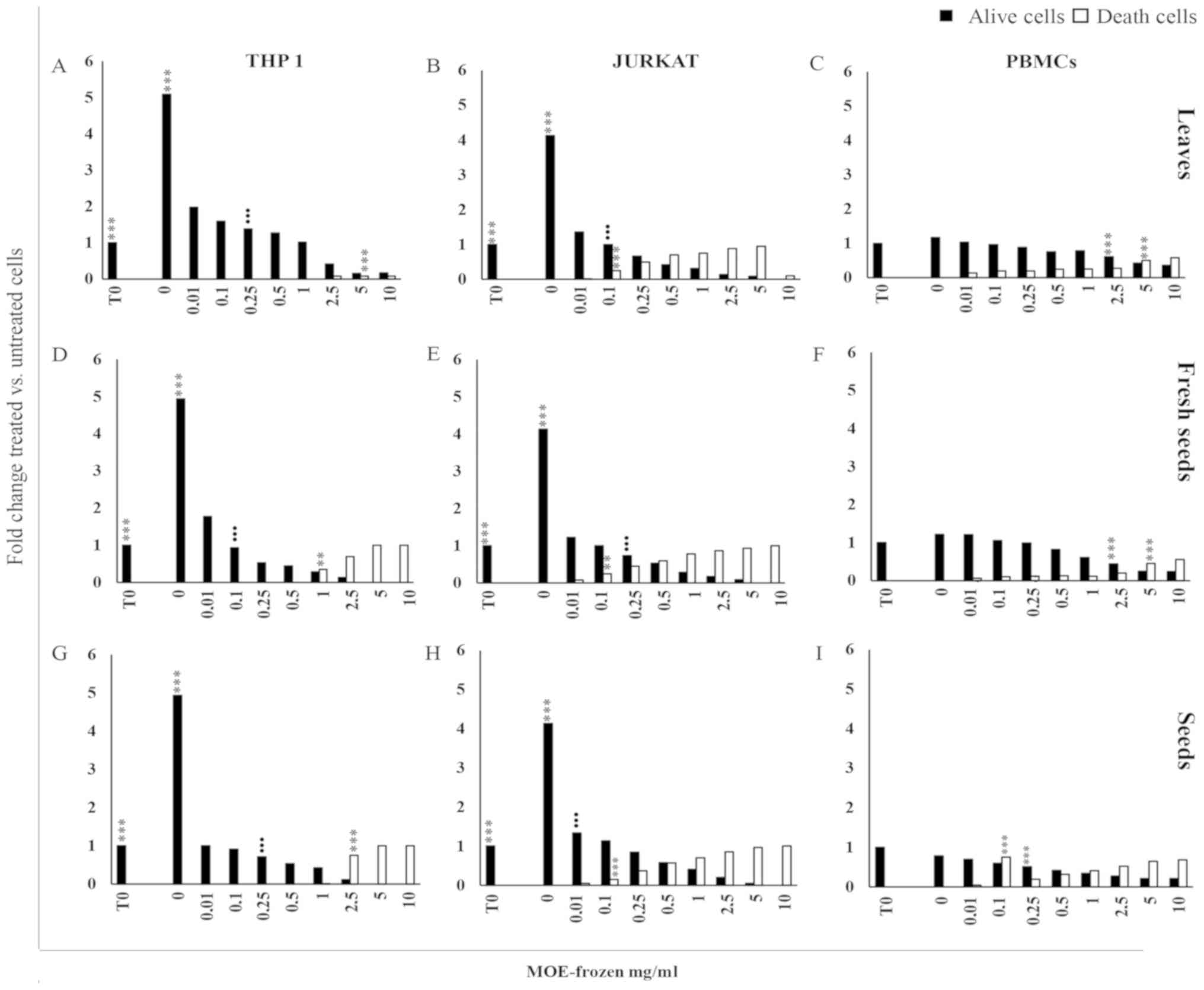 | Figure 2.Anti-proliferative effect of MO
frozen extracts. Cell viability. (A, D, G) THP1, (B, E, H) Jurkat
cells and (C, F, I) PBMCs from healthy donors after treatment with
MOE frozen leaves, fresh seeds and seeds, at a concentration of 0
to 10 mg/ml for 72 h and analysed by trypan blue exclusion test.
Control cells (0 mg/ml) were incubated for the same time with an
equivalent volume of water. The results are expressed as a fold
change of trypan blue positive (black square) or negative cells
(white square) with respect to cells at time 0. Data are reported
as the mean of three different experiments and of 17 healthy
donors' PBMCs ± SD. Symbols indicate significant differences:
**P<0.01, ***P<0.001 all treatment vs. untreated
cells. •••P<0.001 represent the lowest concentration
able to significantly reduce or increase cell viability or death,
respectively. |
A reduction of viable PBMCs was observed when they
were exposed to 2.5–10 mg/ml of leaves and fresh seeds (Fig. 2C and F) and 0.25–10 mg/ml of the seed
preparation (Fig. 2I). In PBMCs, the
cytotoxic effect of frozen MOE appeared at high concentration (5–10
mg/ml) for the treatment with L and FS (Fig. 2C and F), while S extract promoted the
development of trypan blue positive cells starting from 0.1 mg/ml
(Fig. 2I).
EC50 values were also measured for the treatments
using frozen preparations of leaves, fresh seeds and seeds of MOE
(Table IV).
 | Table IV.EC50 of frozen extracts Moringa
oleifera (mg/ml). |
Table IV.
EC50 of frozen extracts Moringa
oleifera (mg/ml).
|
| THP1 | JURKAT | PBMCs |
|---|
| Leaves | 0.019±0.001 | 0001±0.003 | 5.195±0.890 |
| Fresh seeds | 0.010±0.01 | 0.010±0.001 | 2.895±1.300 |
| Seeds | 0.010±0.003 | 0.011±0.010 | 1.065±1.840 |
EC50 of frozen MOE in THP1 and Jurkat cells is
within a concentration range of 0.001–0.019 mg/ml. As well as for
boiled extracts, PBMCs were less sensitive to the treatments.
Indeed, for these cells, higher concentrations of extracts, with
respect to tumour cell lines, were necessary to reduce cell
proliferation by 50% (in detail, 5.195 mg/ml for L, 2.895 mg/ml for
FS and 1.065 mg/ml for S).
Frozen MOE also showed a higher LD50 value in Jurkat
cells than in THP1 cells and PBMCs (Table V).
 | Table V.LD50 Moringa oleifera Frozen
extract (mg/ml). |
Table V.
LD50 Moringa oleifera Frozen
extract (mg/ml).
|
| THP1 | JURKAT | PBMC |
|---|
| Leaves | >10 | 0.145±0.230 | >10 |
| Fresh seeds | >10 | 0.275±0.140 | >10 |
| Seeds | >10 | 0.785±0.500 | >10 |
In THP1 cells and PBMCs, a concentration higher than
10 mg/ml was necessary to reach LD50, while in Jurkat cells, a dose
between 0.1–0.8 mg/ml was sufficient to induce death in 50% of
cells.
Taken together, these data suggest that Jurkat and
THP1 cells are more sensitive than PBMCs to treatment with MO
aqueous extracts obtained by boiling, as well as freezing, in terms
of cell proliferation.
Moreover, Jurkat cells were the most sensitive in
terms of cytotoxic effect, compared to THP1 cells and PBMCs. In
particular, in Jurkat cells, MOE-f was more toxic than MOE-b;
indeed, a 10-fold lower concentration of all types of plant parts,
in MOE-f with respect to MOE-b, was sufficient to reach the LD50
value.
Effects of different preparations of
M. oleifera leaves and seeds on the apoptosis of THP1 cells, Jurkat
cells and PBMCs from healthy donors
To evaluate the effects of M. oleifera
aqueous extracts on apoptosis, PBMCs from healthy donors and THP1
and Jurkat cells were treated with boiled and frozen aqueous
preparations of different plant parts in a concentration ranging
from 0 to 10 mg/ml. After 72 h, the apoptotic level was measured by
assessing the percentage of hypodiploid nuclei, through propidium
iodide (PI) staining and flow cytometry analysis.
Effects of M. oleifera boiled extracts
on apoptosis
The results obtained after cells were treated with
MO boiled extracts are shown in Fig.
3. Among MOE-b, treatment with the L preparation induced a high
level of apoptosis in both cell lines and PBMCs. The treatment
increased the hypodiploid nuclei in THP1 cells by approximately 20%
starting at a concentration of 0.5 mg/ml, while in Jurkat cells, a
concentration twice as high (1 mg/ml) was necessary to have the
same apoptotic level.
The PBMCs were less sensitive to MOE-b; 2.5 mg/ml of
extracts were required to induce apoptosis of approximately 25% in
treated cells (Fig. 3A).
The fresh seed preparation induced significant
apoptotic levels only in THP1 cells, while it did not affect Jurkat
cells or PBMCs, in which only higher concentrations induced a
significant effect (Fig. 3B).
The effects of treatment with S boiled extracts were
highly variable, depending on cell type. In THP1 cells, a
significant increase in apoptotic level (20% of hypodiploid nuclei)
from a concentration of 0.01 mg/ml was observed, while Jurkat cells
needed 1 mg/ml of Fresh Weight (FW) to be affected by this
treatment.
Interestingly, the boiled S preparation showed a
specific pro-apoptotic effect on tumour cells, compared to the
PBMCs of healthy donors, which were particularly resistant to the
treatment. In fact, in PBMCs, the apoptotic level never exceeded
10%, even at the highest concentration used (10 mg/ml) (Fig. 3C).
Taken together, these results emphasized the
non-significant (P>0,05) pro-apoptotic effect of the boiled
mature seed preparation on lymphocytes from healthy donors, in
contrast to the significant effect observed in the two tumour cell
lines analysed.
In particular, we have also demonstrated that THP1
cells were more sensitive to the treatments in a dose dependent
manner, while Jurkat cells appeared to be more resistant.
Effects of M. oleifera frozen extracts
on apoptosis
The evaluation of apoptosis after 72 h of treatment
with MOE-f preparations underlined the high toxic effect of all
these extracts on both cell lines and lymphocytes from healthy
donors (Fig. 4).
Treatment with the frozen preparation of leaves
induced a high level of apoptosis in all analysed cells. In
particular, it increased the hypodiploid nuclei by approximately
25% with respect to the untreated cells; in THP1 cells, this
increase occurred starting at a concentration of 2.5 mg/ml and in
Jurkat cells, 5 mg/ml of FW induced approximately 50% of
apoptosis.
The PBMCs were less sensitive; 5 mg/ml of MO
was required to reach a 20% level of apoptosis (Fig. 4A).
The fresh seed preparation demonstrated a
significant increase in apoptosis in THP1 and Jurkat cells at a low
concentration. In detail, in THP1 cells, a significant increase of
30% in the apoptosis level, starting at a concentration of 0.25
mg/ml, was observed, while in Jurkat cells, we observed an 80%
increase in apoptosis at a concentration of 0.5 mg/ml.
Interestingly, PBMCs from healthy donors were
particularly resistant to the treatment.
In fact, the apoptosis level in these cells never
exceeded 10%, even at the highest concentration (5–10 mg/ml;
Fig. 4B).
In THP1 cells, treatment with seed preparation
induced a significant increase in apoptosis (25% of hypodiploid
nuclei) starting at a concentration of 0.01 mg/ml; in Jurkat cells
and PBMCs, 1 mg/ml of FW was necessary to reach this level of
apoptosis (Fig. 4C).
These results confirm the results obtained on cell
proliferation and cytotoxicity: MOE-f preparations were more toxic
than MOE-b preparations in all cells analysed.
Characterization of the
anti-proliferative and pro-apoptotic effects of MOE-S-b
Considering the inclusion of MO seeds in the List of
Plant and Vegetable Integrators, in respect to the European
Pharmaceutical Plant Legislation, and their low cytotoxic effects
(Tables II–III), the anti-proliferative and
pro-apoptotic activities of MO seeds on Jurkat cells were
characterized in detail.
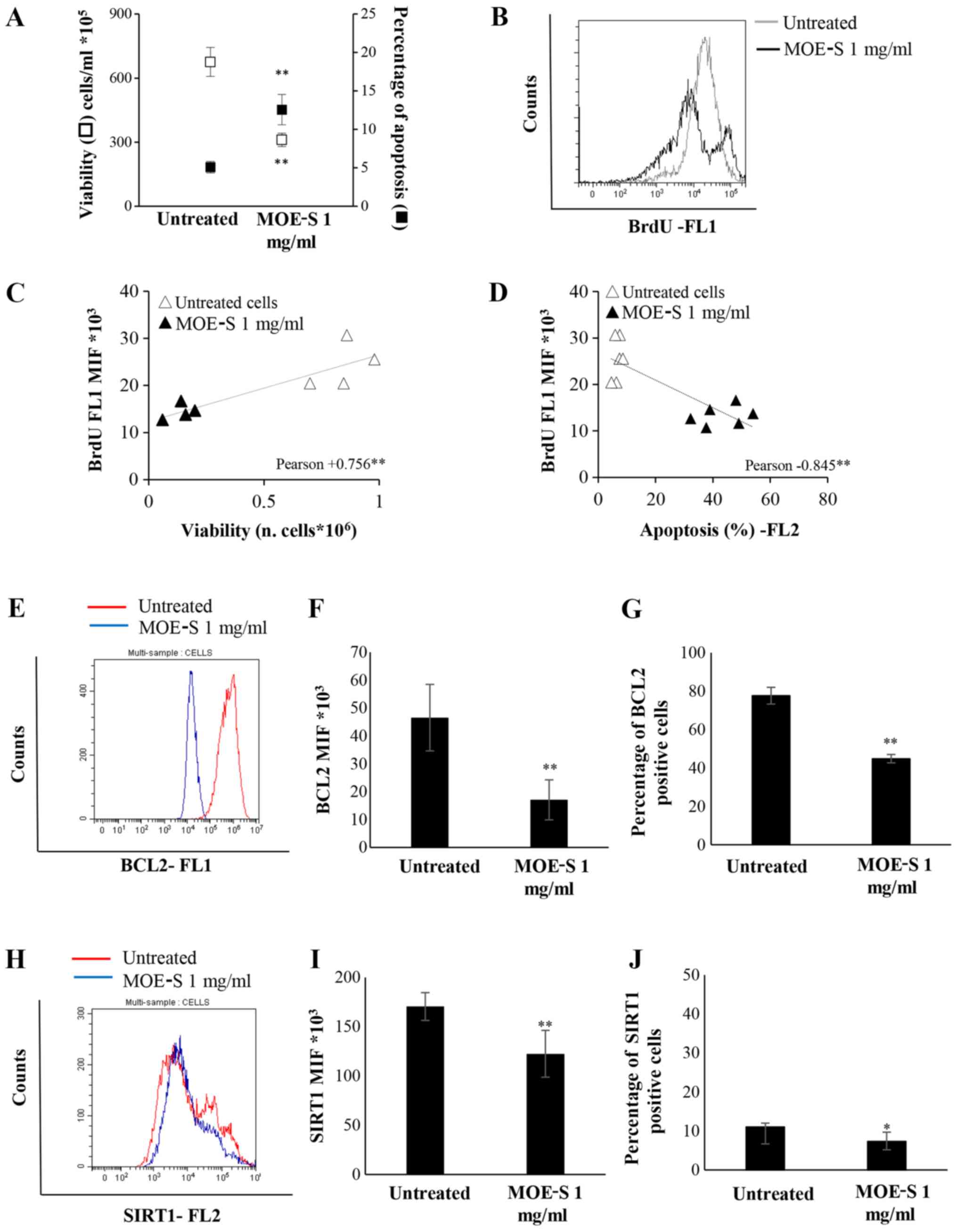
After 72 h of treatment, a significant reduction in
viability and an increase in apoptosis level were observed in
Jurkat cells (Fig. 5A). Moreover,
MOE-S-b induces a significant reduction in DNA synthesis compared
to untreated cells, as demonstrated by the BrdU assay (Fig. 5B).
Pearson's correlation analysis of all these data
show that the reduction of DNA synthesis correlated with the
decreased number of viable cells (Fig.
5C) and the increase of apoptotic cells (Fig. 5D) treated with MOE-S.
The pro-apoptotic effect, induced by 1 mg/ml FW of
MOE-S in Jurkat cell lines, was further investigated, evaluating
the expression of SIRT1 and BCL2 proteins through flow cytometry
analysis. The treatment of Jurkat cells with MOE-S-b induced a
significant decrease of alive cells that express BCL2 and SIRT1
anti apoptotic proteins. This significant reduction was observed
analysing the median intensity fluorescence (MIF) and the
percentage of BCL2 (Fig. 5E-G) and
SIRT1 protein expression (Fig.
5H-J).
HPLC analysis: Total simple phenols
and flavonoid content
To better understand and explain the previously
observed biological activity of MOE-S on human cells, we analysed
the HPLC-DAD chemical profiles of MOE-S and measured their total
concentrations of simple phenols and flavonoids by
spectrophotometric assays.
HPLC-DAD analysis demonstrated that L (green line),
FS (pink line) and S (black line) samples presented very different
chemical profiles (Fig. 6). In
particular, L extracts appeared richer in secondary metabolites
than FS and S preparations, which only showed little
chromatographic peaks. In detail, all L extracts showed several
secondary metabolites with retention times of between 9 and 12.5
min, which were not present in MO seed extracts. Likely, these
peaks could be flavonoids, due to their higher affinity with the
apolar B solvent used during the analysis. Generally, all profiles
were richer in the initial part of the chromatogram with respect to
the second part, indicating that polar compounds, including sugars,
were abundant in all extracts. Surprisingly, we also observed that
the metabolic profiles of each sample (L, FS and S) varied greatly
according to the extraction method (boiled or frozen; Fig. 6A and B, respectively), in qualitative
and quantitative terms. The boiling method appeared, on average, to
better preserve the MO secondary metabolites in the extracts than
the freezing procedure.
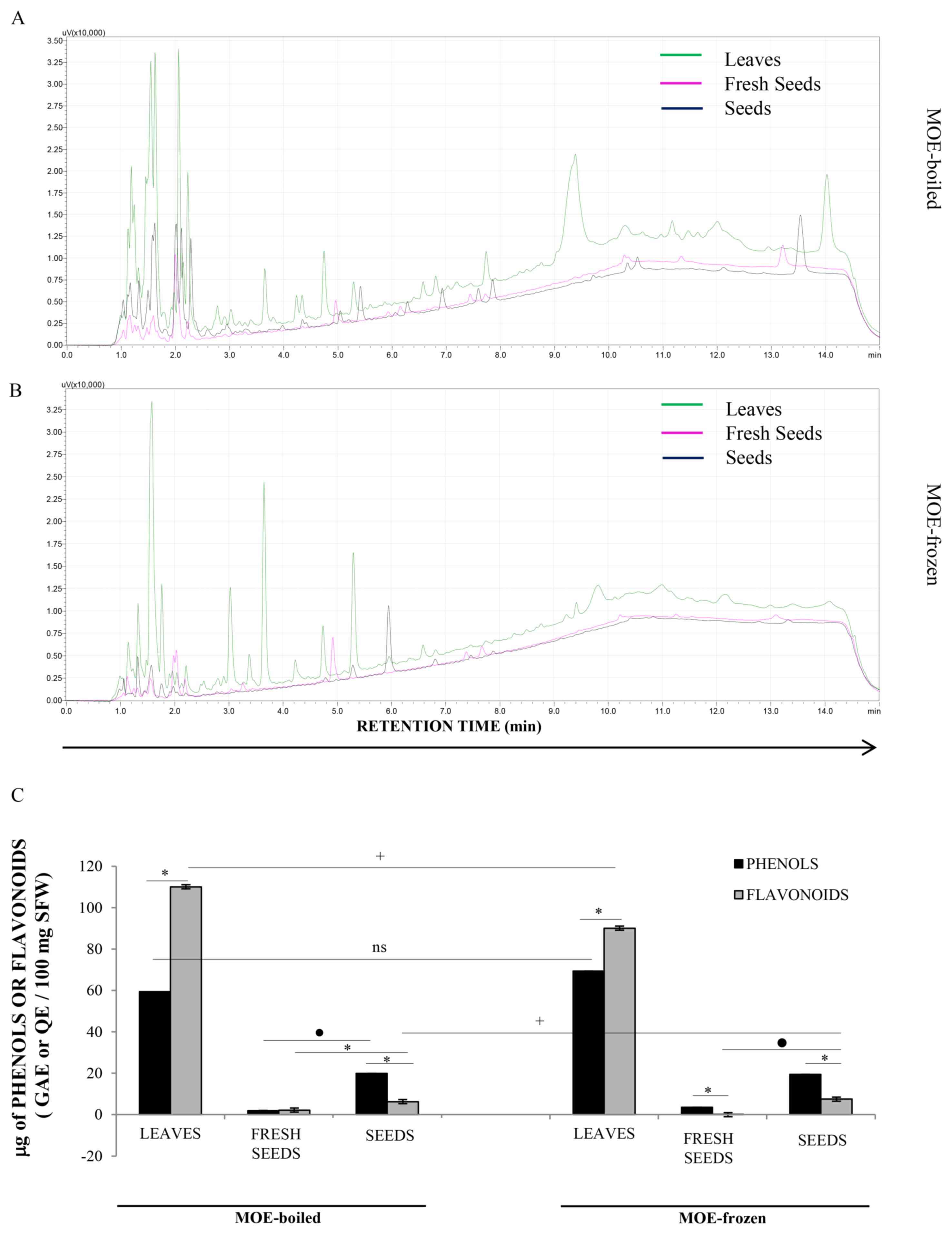 | Figure 6.Analysis of the HPLC profile of
leaves and seeds. HPLC-DAD chromatograms, observed at 254 nm, of
(A) boiled MOE of seeds (black line), fresh seeds (pink line) and
leaves (green line). HPLC chromatograms, observed at 254 nm, of (B)
frozen MOE of seeds (black line), fresh seeds (pink line) and
leaves (green line). The results obtained by spectrophotometric
analyses were reported; in particular, (C) the total simple phenol
and flavonoid concentrations measured in leaves, seeds and fresh
seeds of MOE-b and MOE-f were shown. These results were expressed
as µg of gallic acid (GA) and quercetin equivalents (Q) for total
phenol and flavonoid quantitation, respectively, per 100 mg of
sample fresh weight (µg GAE or QE/100 mg SFW). Each value
represents the mean of three independent determinations ± SD.
*P<0.05, represent the differences between phenol and flavonoid
amount in the different parts of plant. +P<0.05
represent the differences of phenol and flavonoid amount in leaves,
fresh seeds and seeds. •P<0.05 represent the
differences of phenol or flavonoid amount between fresh seeds and
seeds in MOE-boiled as well as in MOE frozen. |
Spectrophotometric analysis measured the total
amount of phenols and flavonoids in MOE (Fig. 6C). L preparations had more phenols
and flavonoids than FS and S ones, both in boiled and in frozen
extracts, confirming the previous HPLC-DAD observation. In
particular, the amount of flavonoids was higher than simple phenols
in L samples, supporting the existence of peaks at a high retention
time in the chromatographic analysis of these extracts. In S and FS
samples, simple phenols were more concentrated than flavonoids.
However, S samples were generally richer in both of these two
classes of plant compounds than the FS extract.
Results also demonstrated that the frozen
preparation protocol degraded flavonoids but not simple phenols in
L extracts, with respect to the boiling procedure, while it did not
influence the concentration of secondary metabolites in FS and
S.
microRNA expression profile of M.
oleifera seed aqueous extract
A recent publication characterised the natural
Olea europea small RNAs profile in olive drupes highlighting
the presence of the most conserved plant miRNAs. The study
demonstrated the effects of this small RNAs pool on apoptosis and
proliferation in tumors cells (12).
Based on this result and on our recent paper
reporting the miRNome of MO seeds (31,32), the
mol-small RNA pool was extracted from MOE-b seeds and the
presence of plant mol-miR was evaluated.
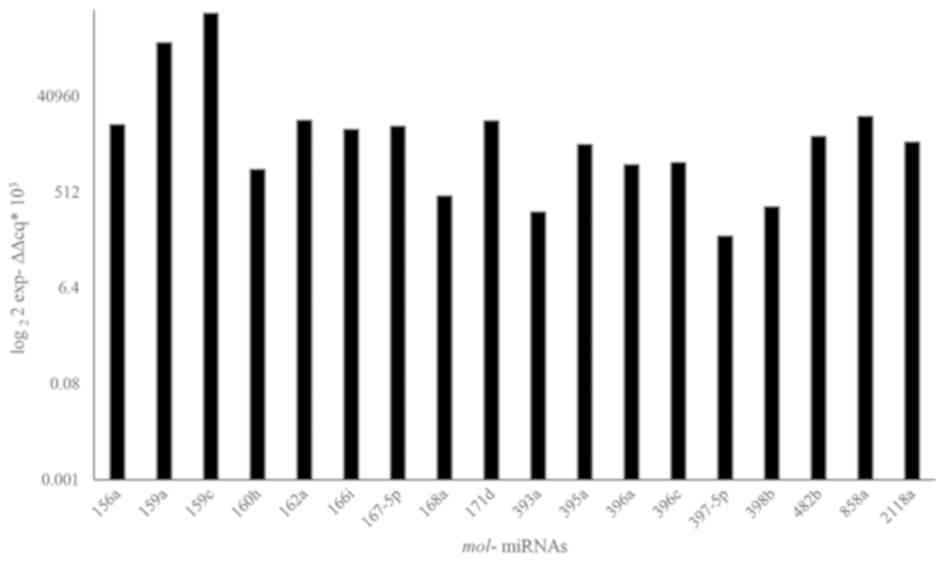
In this extract the qRT-PCR analysis revealed the
presence of plant miRNAs (Fig. 7)
belonging to 40 conserved families of plant microRNAs (32) involved in different important plant
cellular pathways such as the response to abiotic stress, growth,
differentiation and cell proliferation (32–34)
(http://www.mirbase.org/search). This
result suggests their possible involvement in apoptosis and
proliferation, previously observed with Olea europea small
RNAs.
Discussion
In the last years, different studies have been
performed to confirm the beneficial effects of MO on humans
(6,35,36).
MO is used for medicinal purposes, as well as for
human nutrition, since this plant is rich in antioxidants and other
nutrients, which are commonly deficient in people living in
undeveloped countries (22,23,37).
Moreover, MO showed chemo-preventive properties, being able to
inhibit the growth of several human cancer cells (38). Indeed, different studies have
recognized the anti-proliferative and pro-apoptotic effects of
M. oleifera extracts (17,38,39), but
they have not explained the mechanisms underlying the
phenomena.
According to this evidence, the aim of the present
study was the evaluation of the biological effects of MO on human
tumour cell lines and PBMCs from healthy donors.
Several studies have shown that the use of various
organic and inorganic solvents allow for the isolation of several
active components from plant tissues (7,40). For
this reason, we investigated the bioactivity of different types of
MO extracts on proliferation and apoptosis mechanisms. Plant
extracts were produced from MO leaves, seeds and fresh seeds, by
both simulating the traditional method used in African traditional
medicine (hot water maceration) and frozen extraction (obtained by
freezing the plant material in water).
MO extracts obtained by boiling the plant material
demonstrated a significant decrease of cell proliferation of Jurkat
and THP1 cell lines, in a dose dependent manner, while MO
preparations produced by freezing plant material, demonstrated a
significant decrease in cell proliferation in both tumour cell
lines at a low concentration.
The cytotoxic effect of the MO extracts was
dependent on the cell type. Jurkat cells were more susceptible than
THP1 cells to all MOE-b preparations; indeed, 2–4 mg/ml of these
extracts were necessary to induce 50% cell death, while for THP1
cells and PBMCs, 10 mg/ml was necessary to reach the same
effect.
Similarly, all MOE-f preparations were more toxic in
the Jurkat cell line than in THP1 cells and PBMCs from healthy
donors.
In particular, MOE-b revealed a specific
anti-proliferative activity on tumour cells but not on PBMCs, which
were not affected by this treatment.
The information obtained on cell viability prompted
us to analyse the main cause of the proliferation decrease induced
by MO extracts. Therefore, we evaluated the apoptosis in cell lines
treated with MOE-b and MOE-f, analysing the percentage of
hypodiploid nuclei.
Our studies showed that, generally, MOE-b
preparations were less toxic than MOE-f preparations. Jurkat and
THP1 cell lines and PBMCs treated with boiled extracts showed a
significant dose-dependent increase of apoptosis in all analysed
cells.
As boiled extracts were the least toxic, we decided
to select them for further investigation. Interestingly, MOE-b
seeds showed, with respect to the other parts of plant (leaves and
fresh seeds), a more specific pro-apoptotic effect on tumour cell
lines compared to PBMCs from healthy donors, which were
particularly resistant to this treatment.
Considering the inclusion of MO seeds in the List of
Plant and Vegetable Integrators, in respect to the European
Pharmaceutical Plant Legislation, and their low cytotoxic effects
previously described, our studies were focused on the
characterization of the boiled aqueous extract of Moringa
oleifera seeds. The experiments conducted with MOE-S showed
that 1 mg/ml FW of MOE-S induces a decrease of BCL2 and SIRT1
protein expression associated with the enhancement of apoptosis and
the anti-proliferative effect mediated by the downregulation of DNA
synthesis.
To obtain qualitative and quantitative data about
MOE biochemical compounds, we conducted HPLC-DAD analyses on MO
preparations. These investigations allowed us to reveal the
chemical profiles of boiled and frozen extracts, demonstrating
that, in both cases, seeds and fresh seed samples had a different
biochemical profile compared to the leaves, although the
biochemical profiles were similar between seeds and fresh seeds. In
particular, L chromatograms appeared richer in peaks than seed
chromatograms.
These results were confirmed by spectrophotometric
measurements; indeed, all seed preparations seemed to be richer in
total simple phenols than in flavonoids, with respect to leaves, in
which flavonoids were more abundant compared to phenols. Several
studies have demonstrated that the biological effects of MO may be
associated with its secondary metabolites, such as flavonoids and
simple phenols (20,41,42).
Boiling, in general, seemed to be the best method to
quantitatively and qualitatively extract the largest number of
secondary metabolites from the plant material. In particular,
leaves appeared to be the richest plant tissue in the plant
compounds, confirming the results of a previous study (27) in which the authors demonstrated that
the high concentration of metabolites in MO leaf preparations was
able to induce cytotoxicity (28).
Zhang and other researchers demonstrated the
cross-kingdom interaction concept, in which miRNA present in plant
extracts introduced by the diet were able to control gene
expression in human cells (16,43,44).
More recently, we have sequenced the miRnome of Moringa
oleifera, and miRNA homologous to human miRNA was identified
(12,31). For this reason, in the present work,
we have detected the presence of the most conserved plant microRNA,
which could be the bioactive plant compounds involved in MO
activity.
In conclusion, this paper described the effects of
the African traditional preparation of MO seeds, highlighting its
anti-proliferative and pro-apoptotic activities that may be
responsible for the well-known curative properties of the plant
known as the miracle tree. Moreover, we have demonstrated that
small RNAs purified from Moringa oleifera seeds aqueous
extract may be considered new important micronutrient elements.
Therefore, vegetal smallRNAs may be considered a new
class of micronutrients responsible for the medical properties of
plants and, the cross kingdom hypothesis may be though as a modern
reinterpretation of the Hippocrates sentence ‘Let food be thy
medicine and medicine be thy food’.
Acknowledgements
Not applicable.
Funding
The present study was supported by the STARBIOS2
European Union's Horizon 2020 research and innovation programme
under grant agreement No. 709517 oriented to promote Responsible
Research and Innovation in biosciences.
Availability of data and materials
The materials used during the present study are
available from the corresponding author on reasonable request.
Authors' contribution
CM, VC, AM and MP conceived and designed the present
study. LC and AG performed HPLC analysis, and AG primarily
identified and quantified the relevant vegetal small RNAs. AM, MP
and VR performed cells treatment and Flow Cytometry analysis. FM
analyzed the cytotoxic data. AM, MP and SG performed the ex
vivo lymphocytes experiments. CM, AM, MP, AG, AC and VC
critically assessed the results and wrote the paper. AM and MP
contributed equally the experiments performed.
Ethics approval and consent to
particpate
For the present study was obtained a written
statement of 17 healthy donors consent to participate in the study
as specified in the Declaration of Helsinki. The ethical approval
for the collection and use of human samples was obtained in 2014,
from ethical board of ‘Tor Vergata’ hospital, protocol number 15/14
(D.M.08.02.2013-D.G.R.146/2013; D.D.G.467 del 25.07.2013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MOE
|
Moringa oleifera aqueous
extracts
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
MOE-S
|
Moringa oleifera aqueous
extract seeds
|
|
BCL2
|
B-cell lymphoma 2
|
|
SIRT1
|
sirtuin-1
|
|
MO
|
Moringa oleifera Lam
|
|
L
|
Leaves
|
|
S
|
seeds
|
|
FS
|
fresh seeds
|
|
FW
|
fresh weight
|
|
EC50
|
50% effective concentration 50;
lethal dose
|
|
BrdU
|
Bromodeoxyuridine
|
|
mol- small RNA
|
Moringa oleifera small RNA
|
|
mol-miRs
|
Moringa oleifera seeds
microRNAs
|
|
MOE-f
|
Moringa oleifera extract,
frozen
|
|
MOE-b
|
Moringa oleifera extract
boiled
|
|
PI
|
Propidium Iodide
|
References
|
1
|
Padayachee B and Baijnath H: An overview
of the medicinal importance of Moringaceae. J Med Plants Res.
6:5831–5839. 2012.
|
|
2
|
Goyal BR, Agrawal BB, Goyal RK and Mehta
AA: Phyto-pharmacology of Moringa oleifera Lam?? an overview. Nat
Prod Radiance. 6:347–353. 2007.
|
|
3
|
Ojewole JA: Antinociceptive,
anti-inflammatory and antidiabetic properties of Hypoxis
hemerocallidea Fisch. & C.A. Mey. (Hypoxidaceae) corm [‘African
Potato’] aqueous extract in mice and rats. J Ethnopharmacol.
103:126–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anwar F, Latif S, Ashraf M and Gilani AH:
Moringa oleifera: A food plant with multiple medicinal uses.
Phyther Res. 21:17–25. 2007. View Article : Google Scholar
|
|
5
|
Almatrafi MM, Vergara-Jimenez M, Murillo
AG, Norris GH, Blesso CN and Fernandez ML: Moringa leaves prevent
hepatic lipid accumulation and inflammation in guinea pigs by
reducing the expression of genes involved in lipid metabolism. Int
J Mol Sci. 18(pii): E13302017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stohs SJ and Hartman MJ: Review of the
safety and efficacy of moringa oleifera. Phytother Res. 29:796–804.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sagnia B, Fedeli D, Casetti R, Montesano
C, Falcioni G and Colizzi V: Antioxidant and anti-inflammatory
activities of extracts from Cassia alata, Eleusine indica,
Eremomastax speciosa, carica papaya and Polyscias fulva medicinal
plants collected in Cameroon. PLoS One. 9:e1039992014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Musumeci G, Maria Trovato F, Imbesi R and
Castrogiovanni P: Effects of dietary extra-virgin olive oil on
oxidative stress resulting from exhaustive exercise in rat skeletal
muscle: A morphological study. Acta Histochem. 116:61–69. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szychlinska MA, Castrogiovanni P, Trovato
FM, Nsir H, Zarrouk M, Lo Furno D, Di Rosa M, Imbesi R and Musumeci
G: Physical activity and Mediterranean diet based on olive tree
phenolic compounds from two different geographical areas have
protective effects on early osteoarthritis, muscle atrophy and
hepatic steatosis. Eur J Nutr. Feb 15–2018.(Epub ahead of print).
PubMed/NCBI
|
|
10
|
Estruch R, Ros E, Salas-Salvadó J, Covas
MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M,
Lapetra J, et al: Primary prevention of cardiovascular disease with
a Mediterranean diet. N Engl J Med. 368:1279–1290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gorzynik-Debicka M, Przychodzen P,
Cappello F, Kuban-Jankowska A, Marino Gammazza A, Knap N, Wozniak M
and Gorska-Ponikowska M: Potential health benefits of olive oil and
plant polyphenols. Int J Mol Sci. 19(pii): E6862018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minutolo A, Potestà M, Gismondi A, Pirrò
S, Cirilli M, Gattabria F, Galgani A, Sessa L, Mattei M, Canini A,
et al: Olea europaea small RNA with functional homology to human
miR34a in cross-kingdom interaction of anti-tumoral response. Sci
Rep. 8:124132018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lukasik A and Zielenkiewicz P: Plant
MicroRNAs-novel players in natural medicine? Int J Mol Sci.
18(pii): E92016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Z, Li X, Liu J, Dong L, Chen Q, Liu
J, Kong H, Zhang Q, Qi X, Hou D, et al: Honeysuckle-encoded
atypical microRNA2911 directly targets influenza A viruses. Cell
Res. 25:39–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang G, Zhu Y, Sun B, Shao Y, Jing A,
Wang J and Xiao Z: Assessing the survival of exogenous plant
microRNA in mice. Food Sci Nutr. 2:380–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang
Y, Li J, Bian Z, Liang X, Cai X, et al: Exogenous plant MIR168a
specifically targets mammalian LDLRAP1: Evidence of cross-kingdom
regulation by microRNA. Cell Res. 22:107–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sreelatha S, Jeyachitra A and Padma PR:
Antiproliferation and induction of apoptosis by Moringa oleifera
leaf extract on human cancer cells. Food Chem Toxicol.
49:1270–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung IL: Soluble extract from Moringa
oleifera leaves with a new anticancer activity. PLoS One.
9:e954922014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tiloke C, Phulukdaree A and Chuturgoon AA:
The antiproliferative effect of Moringa oleifera crude aqueous leaf
extract on cancerous human alveolar epithelial cells. BMC
Complement Altern Med. 13:2262013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moyo B, Oyedemi S, Masika PJ and Muchenje
V: Polyphenolic content and antioxidant properties of Moringa
oleifera leaf extracts and enzymatic activity of liver from goats
supplemented with Moringa oleifera leaves/sunflower seed cake. Meat
Sci. 91:441–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soladoye MO, Amusa NA, Raji-Esan SO,
Chukwuma E and Taiwo AA: Ethnobotanical survey of anti-cancer
plants in ogun state, nigeria. Ann Biol Res. 1:261–273. 2010.
|
|
22
|
Fuglie LJ: Combating malnutrition with
Moringa. Engineering. 3:1999–2002. 2001.
|
|
23
|
Mahmood KT, Mugal T and Haq IU: Moringa
oleifera: A natural gift-a review. J Pharm Sci Res. 2:775–781.
2010.
|
|
24
|
Brenner D and Mak TW: Mitochondrial cell
death effectors. Curr Opin Cell Biol. 21:871–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L, Yuan L, Luo J, Gao J, Guo J and Xie
X: MiR-34a inhibits proliferation and migration of breast cancer
through down-regulation of Bcl-2 and SIRT1. Clin Exp Med.
13:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Musumeci G, Castrogiovanni P, Loreto C,
Castorina S, Pichler K and Weinberg AM: Post-traumatic caspase-3
expression in the adjacent areas of growth plate injury site: A
morphological study. Int J Mol Sci. 14:15767–15784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gismondi A, Canuti L, Impei S, Di Marco G,
Kenzo M, Colizzi V and Canini A: Antioxidant extracts of African
medicinal plants induce cell cycle arrest and differentiation in
B16F10 melanoma cells. Int J Oncol. 43:956–964. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gismondi A, Reina G, Orlanducci S, Mizzoni
F, Gay S, Terranova ML and Canini A: Nanodiamonds coupled with
plant bioactive metabolites: A nanotech approach for cancer
therapy. Biomaterials. 38:22–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gismondi A, Di Marco G and Canini A:
Detection of plant microRNAs in honey. PLoS One. 12:e01729812017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pirrò S, Minutolo A, Galgani A, Potestà M,
Colizzi V and Montesano C: Bioinformatics prediction and
experimental validation of MicroRNAs involved in cross-kingdom
interaction. J Comput Biol. 23:976–989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pirrò S, Zanella L, Kenzo M, Montesano C,
Minutolo A, Potestà M, Sobze MS, Canini A, Cirilli M, Muleo R, et
al: MicroRNA from Moringa oleifera: Identification by high
throughput sequencing and their potential contribution to plant
medicinal value. PLoS One. 11:e01494952016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xia R, Zhu H, An QY, Beers EP and Liu Z:
Apple miRNAs and tasiRNAs with novel regulatory networks. Genome
Biol. 13:R472012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang BH, Pan XP, Wang QL, Cobb GP and
Anderson TA: Identification and characterization of new plant
microRNAs using EST analysis. Cell Res. 15:336–360. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saini RK, Sivanesan I and Keum YS:
Phytochemicals of Moringa oleifera: A review of their nutritional,
therapeutic and industrial significance. 3 Biotech. 6:2032016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Razis AFA, Ibrahim MD and Kntayya SB:
Health benefits of Moringa oleifera. Asian Pac J Cancer Prev.
15:8571–8576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kou X, Li B, Olayanju JB, Drake JM and
Chen N: Nutraceutical or pharmacological potential of Moringa
oleifera lam. Nutrients. 10(pii): E3432018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karim NA, Ibrahim MD, Kntayya SB, Rukayadi
Y, Hamid HA and Razis AF: Moringa oleifera Lam: Targeting
chemoprevention. Asian Pacific J Cancer Prev. 17:3675–3686.
2016.
|
|
39
|
Suphachai C: Antioxidant and anticancer
activities of Moringa oleifera leaves. J Med Plants Res. 8:318–325.
2014. View Article : Google Scholar
|
|
40
|
Sasidharan S, Chen Y, Saravanan D, Sundram
KM and Yoga Latha L: Extraction, isolation and characterization of
bioactive compounds from plants' extracts. Afr J Tradit Complement
Altern Med. 8:1–10. 2011.PubMed/NCBI
|
|
41
|
Fard MT, Arulselvan P, Karthivashan G,
Adam SK and Fakurazi S: Bioactive extract from moringa oleifera
inhibits the pro-inflammatory mediators in lipopolysaccharide
stimulated macrophages. Pharmacogn Mag. 11 (Suppl 4):S556–S563.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Coppin JP, Xu Y, Chen H, Pan MH, Ho CT,
Juliani R, Simon JE and Wu Q: Determination of flavonoids by LC/MS
and anti-inflammatory activity in Moringa oleifera. J Funct Foods.
5:1892–1899. 2013. View Article : Google Scholar
|
|
43
|
Hou D, He F, Ma L, Cao M, Zhou Z, Wei Z,
Xue Y, Sang X, Chong H, Tian C, et al: The potential
atheroprotective role of plant MIR156a as a repressor of monocyte
recruitment on inflamed human endothelial cells. J Nutr Biochem.
57:197–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chin AR, Fong MY, Somlo G, Wu J, Swiderski
P, Wu X and Wang SE: Cross-kingdom inhibition of breast cancer
growth by plant miR159. Cell Res. 26:217–228. 2016. View Article : Google Scholar : PubMed/NCBI
|