Introduction
Skin wound repair often results in the formation of
scar tissue (1). Despite efforts,
scars still cannot be avoided in clinical practice. Compared with
skin, oral mucosa has the characteristics of quick healing and less
scar tissue formation (2).
Therefore, it is of great significance to study the factors of scar
formation and the biological indicators in the process of skin
wound repair. Wound repair is a complex process of interaction
between cells, growth factors and extracellular matrices (3). Epidermal growth factor (EGF) is a
heat-resistant single-chain polypeptide that can bind to cell
membrane receptors and exert various biological effects (4). EGF, a potent mitogenic factor, promotes
epithelial cell proliferation and division, improves collagen
construction and regulates protein synthesis, thereby accelerating
wound healing. EGF has chemotactic effects on vascular endothelial
cells, fibroblasts, inflammatory cells and epidermal cells
(5). Vascular endothelial growth
factor C (VEGF-C) is a member separated from the vascular
endothelial growth factor (VEGF)/platelet-derived factor (PDGF)
family and is the fourth member of the VEGF subfamily. VEGF-C gene
includes seven exons and is the largest VEGF gene currently
described (6). Previous research has
shown that high expression of VEGF-C plays an important role in the
angiogenesis of limb ischemia in adult animals (7).
The moisture of oral mucosa is different from that
of skin. Some scholars believe that the oral mucosa can be repaired
with little or no scar because of the moisture and salivary
protease (8,9). In order to further clarify the factors
in the process of scar formation and skin wound repair, the rat
oral mucosa was transplanted to the right abdomen in this study. By
comparing the differences in the expression of EGF and VEGF-C
during skin healing, the role of the two factors in scar formation
and skin wound repair was revealed.
Materials and methods
Experimental animals
Sixty-four inbred strain SPF SD rats (150–190 g, 4–8
weeks) were purchased from Kaixue Biological Science and Technology
(Shanghai) Co., Ltd. [SCXK (Shanghai) 2009–0037; Shanghai, China].
Rats were kept in a clean environment with good ventilation. The
indoor humidity was 48–59%, and the temperature was 21–26°C. Rats
were fed with SPF experimental rat food provided by Jiangsu Synergy
Pharmaceutical Bioengineering Co., Ltd. (Jiangsu, China). Rats had
free access to food and water.
The investigation was approved by the Ethics
Committee of Jinan Maternity and Child Care Hospital (Jinan, China)
and the experimental procedures were in compliance with the Guiding
Principles for the Protection and Use of Experimental Animals
(10). Patients who participated in
this research had complete clinical data. Signed informed consents
were obtained from the patients or the guardians.
Animal model preparation
The 64 inbred strain SPF SD rats were randomly
separated into group A, B, C and D (16 rats in each group)
according to the principle of similar weight. Ten percent chloral
hydrate were used for intraperitoneal injection anesthesia at a
dose of 350 mg/kg. The abdomen of the rat faced up, and the limbs
were immobilized. The right abdomen of the rat was first
transplanted with oral mucosa. The full-thickness skin with a
diameter of 1 cm of the right abdomen of the rat was cut. The skin
was transplanted to the skin of the right abdomen and then sutured
and bandaged. After that, rats were placed in a squirrel cage with
free access to water and food. When the wound of the oral mucosa
was healed for 14 days, intraperitoneal injection anesthesia was
performed with the same dose of chloral hydrate, and then a
full-thickness skin incision was made on the right abdomen of the
rat with a round blade. The incision was deep to fascia layfer and
was 1 cm in diameter. The same cut was made in the left abdomen of
the rats and the wound diameter was 1 cm. Rats were allowed to
reach food and water freely for a week. Rats in group A, B, C and D
were anesthetized with chloral hydrate (350 mg/kg) and sacrificed
by cervical dislocation 1, 3, 5 and 7 days after injury. Skin
tissue with a diameter of about 1.5 cm around the center of the
wound was collected. Skin tissue of the right abdomen was the study
group, and the left abdomen skin tissue was the control group,
being cryopreserved after collection.
Indicator detection
Expression of EGF mRNA and VEGF-C mRNA in skin
tissue were detected by RT-qPCR. Appropriate amount of tissue was
washed twice with PBS, and the total RNA extraction of skin tissue
was carried out in strict accordance with the instructions of
TRIzol extraction kit (Shanghai Yusheng Biotechnology Co., Ltd.,
Shanghai, China). Absorbance of the RNA was measured using an
ultraviolet-visible spectrophotometer (Mettler-Toledo International
Trading Co., Ltd., Shanghai, China), and the concentration of RNA
sample was calculated. One micrograms of RNA was
reverse-transcribed into cDNA, and cDNA was prepared according to
the instructions of M-MLV reverse transcription kit (Hanheng
Biotechnology Co., Ltd., Shanghai, China). Reverse transcription
reaction conditions: 42°C for 60 min and 85°C for 5 min,
termination reaction at 4°C. Synthesized cDNA was stored at −20°C.
Ten microliters of reaction system was prepared according to the
instruction of Takara Real-Time PCR (Beijing Borma Biotechnology
Co., Ltd., Beijing, China). Ten microliters of reaction system
included 50 ng of total RNA, PCR Premix, double distilled water,
ROX Dye and 200 nM primers. β-actin was used as an internal
reference. Primer sequences are shown in Table I. Reaction procedure included 40
cycles of 95°C for 30 sec and 60°C for 33 sec. The experiment was
repeated 3 times. Amplification data were analyzed by vendor
software and the expression of EGF mRNA and VEGF-C mRNA was
calculated by 2−ΔCq method (11).
 | Table I.Primers of EGF mRNA, VEGF-C mRNA and
β-actin. |
Table I.
Primers of EGF mRNA, VEGF-C mRNA and
β-actin.
| Gene | Forward primers | Reverse primers |
|---|
| EGF |
5′-TGCCAACTGGGGGTGCACAG-3′ |
5′-CTGCCCGTGGCCAGCGTGGC-3′ |
| VEGF-C |
5′-TTCCATTATTAGACGTTCCCTG-3′ |
5′-GTGTTTTCATCAAATTCTCGGT-3′ |
| β-actin |
5′-AAATCGTGCGTGACATTAA-3′ |
5′-CTCGTCATACTCCTGCTTG-3′ |
Expressions of EGF protein and VEGF-C protein in
skin tissue were detected by ELISA. Appropriate amount of skin
tissue was taken and washed in pre-cooled PBS (0.02 mol/l, pH
7.0–7.2) to remove blood, then crushed to prepare homogenate. The
homogenate was centrifuged at 5,000 × g for 5 min at 4°C, and the
supernatant was saved. EGF and VEGF-C ELISA assay kit (cat. nos.
DEG00 and DVEC00; R&D Systems, Minneapolis, MN, USA) was used
for the detection in accordance with the instructions. The sample
and the kit was taken to room temperature 45 min in advance from
the refrigerator, and the sample well, standard well and blank well
were set. No reagents were added to the blank well. The sample well
and the standard well were added with 50 µl of the sample to be
tested and standard diluted in different multiples, respectively,
with 50 µl of biotin-labeled antibody. The membrane was covered,
and incubated at 37°C for 1 h. Liquid in each well was discarded,
dried, and washed 3 times. Affinity streptavidin (80 µl) was added
to each well, mixed and incubated at 37°C for 30 min. Liquid was
discarded in each well, dried, and washed 3 times. Substrate A and
B at 50 µl each, were added to the wells, mixed and incubated at
37°C for 10 min. A color change was produced in the dark at room
temperature. Fifty microliters of the stop solution was added to
each well, and the OD value of each well was measured at a
wavelength of 450 nm using a 680 automatic microplate reader
(Bio-Rad, Hercules, CA, USA) to calculate the expression levels of
EGF and VEGF-C proteins.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA). Measurement data were expressed as
mean ± standard deviation (mean ± SD). Chi-square test was used for
the comparisons of inter-group count data. One-way ANOVA was used
for the comparison of means among multiple groups. LSD-t-test was
used for multiple comparisons between groups with LSD test as post
hoc test. P<0.05 indicates the difference is statistically
significant.
Results
General condition of rats
One day after injury, wounds in the two groups began
to heal. Wounds in the study group were smaller than those in the
control group, and there were a small amount of granulation tissue
on the wound surface. Three days after injury, wounds of the study
group were significantly smaller than those of the control group,
and the irregular granulation tissue was observed at the edge of
the wound. Five days after injury, wounds of the two groups began
to heal, and blood stasis was formed. The granulation tissue had
shrunk, and the wound surface was closed. Seven days after injury,
wound healing of the study group was better than that of the
control group. Scar of the wound in the study group was better than
that in the control group, and the wound surface was flatter
compared with the control group. There were no differences in sex,
age, length, blood glucose (Glu), body mass before and after
modeling, indoor temperature and indoor humidity among group A, B,
C and D (Table II).
 | Table II.General condition of rats [n (%)]
(mean ± SD). |
Table II.
General condition of rats [n (%)]
(mean ± SD).
| Items | A (n=16) | B (n=16) | C (n=16) | D (n=16) | F/χ2 | P-value |
|---|
| Sex |
|
|
|
| 4.726 | 0.193 |
| Male | 11 (68.75) | 9 (56.25) | 14 (87.50) | 13 (81.25) |
|
|
|
Female | 5
(31.25) | 7 (43.75) | 2
(12.50) | 3
(18.75) |
|
|
| Age (weeks) | 8.41±0.27 | 8.32±0.41 | 8.59±0.49 | 8.26±0.38 | 2.118 | 0.107 |
| Length (cm) | 18.45±1.42 | 19.41±0.82 | 18.91±1.57 | 18.45±1.19 | 2.043 | 0.117 |
| Glu (mmol/l) | 73.48±4.27 | 70.78±4.53 | 69.24±5.76 | 71.13±5.34 | 1.957 | 0.130 |
| Body mass before
modeling (g) | 221.54±12.35 | 227.12±14.24 | 218.36±9.87 | 216.63±11.58 | 2.319 | 0.085 |
| Body mass after
modeling (g) | 212.63±7.57 | 208.53±7.41 | 206.47±7.82 | 209.47±8.63 | 1.697 | 0.177 |
| Indoor temperature
(°C) | 24.05±1.24 | 23.78±1.08 | 24.25±0.93 | 24.16±0.57 | 0.682 | 0.566 |
| Indoor humidity
(%) | 51.52±2.63 | 50.74±1.93 | 50.71±1.35 | 49.93±1.07 | 1.982 | 0.126 |
Expressions of EGF mRNA and protein at
different time-points in rats
Expressions of EGF mRNA and protein in the skin
tissue of the study group were not significantly different from
that of the control group at 1 and 3 days (P>0.05). At 3 days,
expression levels of EGF mRNA and protein in skin tissue of the two
groups were significantly higher than those at 1 day (P<0.05).
At 5 days, expression levels of EGF mRNA and protein in skin tissue
of the two groups were significantly higher than those at 3 days
(P<0.05), and the expression levels of EGF mRNA and protein in
skin tissue of the study group were significantly higher than those
of the control group (P<0.05). At 7 days, expression levels of
EGF mRNA and protein in skin tissue of the two groups were
significantly lower than those at 5 days (P<0.05) and the
expression levels of EGF mRNA and protein in skin tissue of the
study group were significantly lower than those of the control
group (P<0.05) (Tables III and
IV and Fig. 1).
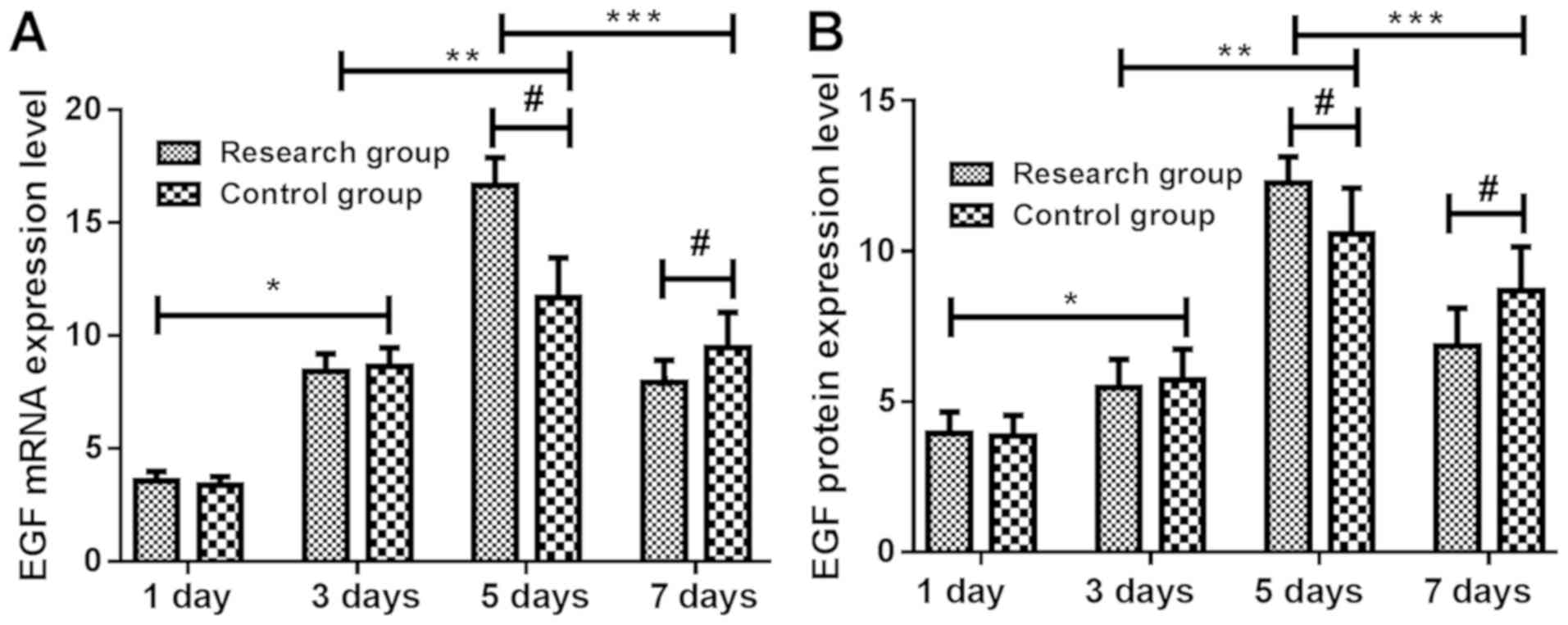 | Figure 1.Changes in the expression levels of
EGF mRNA and protein at different time-points in rats. The results
of RT-qPCR and ELISA showed that the expression of EGF (A) mRNA and
(B) protein in the skin of the study group were not significantly
different from those of the control group at 1 and 3 days
(P>0.05). At 3 days, expression levels of EGF mRNA and protein
in skin tissue of study group and control group were significantly
higher than those at 1 day (P<0.05). At 5 days, expression
levels of EGF mRNA and protein in skin tissue of the two groups
were significantly higher than those at 3 days (P<0.05), and the
expression levels of EGF mRNA and protein in skin tissue of the
study group were significantly higher than those of the control
group (P<0.05). At 7 days, expression levels of EGF mRNA and
protein in skin tissue of the two groups were significantly lower
than those at 5 days (P<0.05) and the expression levels of EGF
mRNA and protein in skin tissue of the study group were
significantly lower than those of the control group (P<0.05).
*P<0.05, 1 vs. 3 days; **P<0.05, 3 vs. 5 days; ***P<0.05,
5 vs. 7 days; #P<0.05, study vs. control group. EGF, epidermal
growth factor; RT-qPCR, reverse transcription-quantitative PCR;
ELISA, enzyme-linked immunosorbent assay. |
 | Table III.Expression of EGF mRNA at different
time-points in rats (mean ± SD). |
Table III.
Expression of EGF mRNA at different
time-points in rats (mean ± SD).
| Groups | 1 day (n=16) | 3 days (n=16) | 5 days (n=16) | 7 days (n=16) | F-value | P-value |
|---|
| Study | 3.56±0.43 |
8.43±0.76a |
16.63±1.25b |
7.93±0.97c | 582.700 | <0.001 |
| Control | 3.37±0.38 |
8.63±0.83a |
11.67±1.77b |
9.46±1.56c | 123.700 | <0.001 |
| t-value | 0.489 | 0.515 | 12.760 | 3.937 |
|
|
| P-value | 0.626 | 0.608 | <0.001 | <0.001 |
|
|
 | Table IV.Expression of EGF protein at
different time-points in rats (ng/ml) (mean ± SD). |
Table IV.
Expression of EGF protein at
different time-points in rats (ng/ml) (mean ± SD).
| Groups | 1 day (n=16) | 3 days (n=16) | 5 days (n=16) | 7 days (n=16) | F-value | P-value |
|---|
| Study | 3.95±0.71 |
5.46±0.94a |
12.26±1.07b |
6.85±1.26c | 203.500 | <0.001 |
| Control | 3.85±0.69 |
5.71±1.03a |
10.57±1.53b |
8.69±1.46c |
95.920 | <0.001 |
| t-value | 0.251 | 0.628 | 4.248 | 4.625 |
|
|
| P-value | 0.802 | 0.531 | <0.001 | <0.001 |
|
|
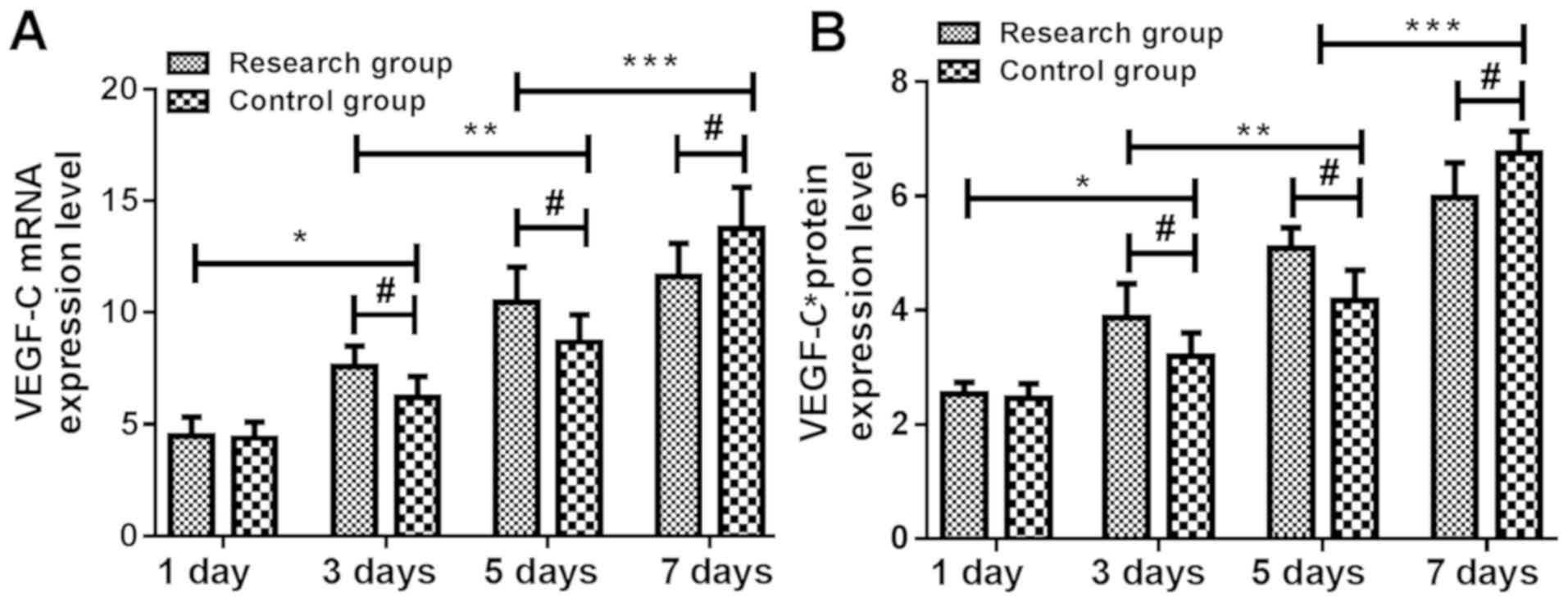
Expression of VEGF-C mRNA and protein
at different time-points in rats
Expression levels of VEGF-C mRNA and protein in skin
tissue of the study group were not significantly different from
those of the control group at 1 day (P>0.05). At 3 and 5 days,
expression levels of VEGF-C mRNA and protein in the skin tissue of
the study group were significantly higher than those of the control
group. At 3 days, expression levels of VEGF-C mRNA and protein in
skin tissue of the two groups were significantly higher than those
at 1 day (P<0.05). At 5 days, expression levels of VEGF-C mRNA
and protein in skin tissue of the two groups were significantly
higher than those at 3 days (P<0.05). At 7 days, expression
levels of VEGF-C mRNA and protein in skin tissue of the two groups
were significantly higher than those at 5 days (P<0.05) and the
expression levels of VEGF-C mRNA and protein in skin tissue of the
study group were significantly lower than those of the control
group (P<0.05) (Tables V and
VI and Fig. 2).
 | Table V.Expression levels of VEGF-C mRNA at
different time-points in rats (mean ± SD). |
Table V.
Expression levels of VEGF-C mRNA at
different time-points in rats (mean ± SD).
| Groups | 1 day (n=16) | 3 days (n=16) | 5 days (n=16) | 7 days (n=16) | F-value | P-value |
|---|
| Study | 4.47±0.86 |
7.58±0.93a |
10.47±1.57b |
11.63±1.47c | 105.300 | <0.001 |
| Control | 4.38±0.72 |
6.19±0.95a |
8.67±1.24b |
13.76±1.85c | 166.400 | <0.001 |
| t-value | 0.203 | 3.131 | 4.055 | 4.798 |
|
|
| P-value | 0.840 | 0.002 | <0.001 | <0.001 |
|
|
 | Table VI.Expression levels of VEGF-C protein
(ng/ml) at different time-points in rats (mean ± SD). |
Table VI.
Expression levels of VEGF-C protein
(ng/ml) at different time-points in rats (mean ± SD).
| Groups | 1 day (n=16) | 3 days (n=16) | 5 days (n=16) | 7 days (n=16) | F-value | P-value |
|---|
| Study | 2.53±0.21 |
3.87±0.59a |
5.08±0.47b |
5.97±0.61c | 145.100 | <0.001 |
| Control | 2.46±0.26 |
3.19±0.41a |
4.17±0.53b |
6.75±0.64c | 242.700 | <0.001 |
| t-value | 0.405 | 3.935 | 5.266 | 4.513 |
|
|
| P-value | 0.686 | <0.001 | <0.001 | <0.001 |
|
|
Discussion
Scar formed after wound healing is a clinical
problem. The requirements for the prognosis of wounds are getting
higher, and scar-free wound repair is expected. Therefore, the scar
healing after wound healing has become a research hotspot for
scholars (12,13). Healing process of oral mucosa is
rapid, with few scars. Therefore, oral mucosal healing is of
importance in the deeper understanding of skin wound repair
(14,15). This study transplanted the rat tongue
mucosa to the right abdomen skin, made a full-thickness skin wound
and observed biological indicators that may become factors of scar
formation, providing a theoretical basis for scar wound
healing.
When the body is damaged, cells and tissues at the
wound site will secrete a large number of wound healing growth
factors. Growth factors are cytokines that stimulate cell growth
activity and play an important role in the body wound repair
process (16). Cytokines can attract
fibroblasts and inflammatory cells into the wound, which promotes
vascularization and cell proliferation of the wound (17). EGF is a novel type of polypeptide
factor found in the purification of mouse submandibular gland nerve
growth factor. It is a biologically active substance that
stimulates cell proliferation and is widely distributed in tissues
and body fluids (18,19). EGF may be one of the most
characterized growth factors in the process of skin wound healing.
In acute trauma, EGF is mainly secreted by platelets, macrophages
and fibroblasts, and is upregulated in a short time after injury
(20,21). Release of EGF stimulates migration
and proliferation of epithelial cells, thereby promoting
re-epithelialization (22). Kim
et al (23) found that EGF
can reduce the expression of TGF-β, reduce skin scars, and mediate
collagen formation by inhibiting inflammatory response. During the
process of lymphangiogenesis, it can be stimulated by various
cytokines, including VEGF-C. VEGF-C is the first identified ligand
of growth factor receptor 3 (Flt4) and is a member of the
polypeptide growth factor family (24,25). It
has been shown that overexpressed VEGF-C cDNA in the skin of
transgenic mice can induce lymphatic endothelial cell proliferation
and lymphangiogenesis, recombinant VEGF-C and can specifically
stimulate lymphangiogenesis in the chorioallantoic membrane
(26). Results of this study showed
that expression levels of EGF and VEGF-C mRNA and protein in skin
tissue of the two groups were significantly higher at 3 days than
at 1 day; expression levels of EGF and VEGF-C mRNA and protein in
skin tissue of the two groups were significantly higher at 5 days
than at 3 days; expression levels of EGF mRNA and protein in skin
tissue of the two groups were significantly lower at 7 days than at
5 days, while expression levels of VEGF-C mRNA and protein were
significantly higher at 7 days than at 5 days. It is suggested that
EGF and VEGF-C may be involved in scar formation. Irregular
granulation tissue appeared on the 3rd day after injury, and blood
stasis formed on the 5th day. Hyperplasia of rat skin repair tissue
cells and wound healing began from this stage. In this study,
expression levels of VEGF-C mRNA and protein in skin tissue of the
study group were significantly higher than those of the control
group at 3 days. Expression levels of EGF and VEGF-C mRNA and
protein in skin tissue of the study group were significantly higher
than those of the control group at 5 days. At 7 days, expression
levels of EGF and VEGF-C mRNA and protein in skin tissue of the
study group were significantly lower than those of the control
group. It is suggested that oral mucosal transplantation has the
characteristics of quick repair and good effect in skin wound
repair. EGF and VEGF-C may play an important role in skin wound
repair.
Previous studies have shown that exogenous growth
factors can promote scar-free healing of wounds. However, the
separation of growth factors remains to be studied, because the
level of growth factor is low in the body. Determination of
appropriate concentration of growth factor and the regulation of it
to interfere with cell proliferation is of great significance for
wound healing to reduce scar formation, which will be the focus of
our future study.
In summary, EGF and VEGF-C may be involved in scar
formation and play an important role in the process of skin wound
repair.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SQ wrote the manuscript, interpreted the data and
drafted the manuscript. CY assisted with the construction of the
animal model. MZ and HC performed PCR and ELISA. All the authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The investigation was approved by the Ethics
Committee of Jinan Maternity and Child Care Hospital (Jinan, China)
and the experimental procedures were in compliance with the Guiding
Principles for the Protection and Use of Experimental Animals
(10). Patients who participated in
this research had complete clinical data. Signed informed consents
were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu J, Wang MY, Tai HC and Cheng NC: Cell
sheet composed of adipose-derived stem cells demonstrates enhanced
skin wound healing with reduced scar formation. Acta Biomater.
77:191–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asatiani E, Huang WX, Wang A, Rodriguez
Ortner E, Cavalli LR, Haddad BR and Gelmann EP: Deletion,
methylation, and expression of the NKX3.1 suppressor gene in
primary human prostate cancer. Cancer Res. 65:1164–1173. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tracy LE, Minasian RA and Caterson EJ:
Extracellular matrix and dermal fibroblast function in the healing
wound. Adv Wound Care (New Rochelle). 5:119–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purba ER, Saita EI and Maruyama IN:
Activation of the EGF receptor by ligand binding and oncogenic
mutations: The ‘Rotation Model’. Cells. 6:62017. View Article : Google Scholar
|
|
5
|
Basso FG, Soares DG, Pansani TN, Cardoso
LM, Scheffel DL, de Souza Costa CA and Hebling J: Proliferation,
migration, and expression of oral-mucosal-healing-related genes by
oral fibroblasts receiving low-level laser therapy after
inflammatory cytokines challenge. Lasers Surg Med. 48:1006–1014.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hominick D, Silva A, Khurana N, Liu Y,
Dechow PC, Feng JQ, Pytowski B, Rutkowski JM, Alitalo K and
Dellinger MT: VEGF-C promotes the development of lymphatics in bone
and bone loss. eLife. 7:e343232018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chilov D, Kukk E, Taira S, Jeltsch M,
Kaukonen J, Palotie A, Joukov V and Alitalo K: Genomic organization
of human and mouse genes for vascular endothelial growth factor C.
J Biol Chem. 272:25176–25183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kosten IJ, van de Ven R, Thon M, Gibbs S
and de Gruijl TD: Comparative phenotypic and functional analysis of
migratory dendritic cell subsets from human oral mucosa and skin.
PLoS One. 12:e01803332017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song IB, Gu H, Han HJ, Lee NY, Cha JY, Son
YK and Kwon J: Effects of 7-MEGATM 500 on oxidative stress,
inflammation, and skin regeneration in
H2O2-treated skin cells. Toxicol Res.
34:103–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sikes RS; Animal Care and Use Committee of
the American Society of Mammalogists, : 2016 Guidelines of the
American Society of Mammalogists for the use of wild mammals in
research and education. J Mammal. 97:663–688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 [-Delta Delta C(T)] method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramos-Lewis W, LaFever KS and Page-McCaw
A: A scar-like lesion is apparent in basement membrane after wound
repair in vivo. Matrix Biol. 74:101–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Occleston NL, Metcalfe AD, Boanas A,
Burgoyne NJ, Nield K, O'Kane S and Ferguson MW: Therapeutic
improvement of scarring: mechanisms of scarless and scar-forming
healing and approaches to the discovery of new treatments. Dermatol
Res Pract. 2010:4052622010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DiPietro LA: Angiogenesis and wound
repair: When enough is enough. J Leukoc Biol. 100:979–984. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iglesias-Bartolome R, Uchiyama A, Molinolo
AA, Abusleme L, Brooks SR, Callejas-Valera JL, Edwards D, Doci C,
Asselin-Labat ML, Onaitis MW, et al: Transcriptional signature
primes human oral mucosa for rapid wound healing. Sci Transl Med.
10:eaap87982018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han J, Jin W, Ho NA, Hong J, Kim YJ, Shin
Y, Lee H and Suh JW: Decursin and decursinol angelate improve wound
healing by upregulating transcription of genes encoding
extracellular matrix remodeling proteins, inflammatory cytokines,
and growth factors in human keratinocytes. Biochem Biophys Res
Commun. 499:979–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Velasquez LS, Sutherland LB, Liu Z,
Grinnell F, Kamm KE, Schneider JW, Olson EN and Small EM:
Activation of MRTF-A- dependent gene expression with a small
molecule promotes myofibroblast differentiation and wound healing.
Proc Natl Acad Sci USA. 110:16850–16855. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, et al: Efficacy of gefitinib, an inhibitor of the
epidermal growth factor receptor tyrosine kinase, in symptomatic
patients with non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stoddard MA, Herrmann J, Moy L and Moy R:
Improvement of atrophic acne scars in skin of color using topical
synthetic epidermal growth factor (EGF) serum: A pilot study. J
Drugs Dermatol. 16:322–326. 2017.PubMed/NCBI
|
|
20
|
Shiraha H, Glading A, Gupta K and Wells A:
IP-10 inhibits epidermal growth factor-induced motility by
decreasing epidermal growth factor receptor-mediated calpain
activity. J Cell Biol. 146:243–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schultz G, Rotatori DS and Clark W: EGF
and TGF-alpha in wound healing and repair. J Cell Biochem.
45:346–352. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haase I, Evans R, Pofahl R and Watt FM:
Regulation of keratinocyte shape, migration and wound
epithelialization by IGF-1- and EGF-dependent signalling pathways.
J Cell Sci. 116:3227–3238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim YS, Lew DH, Tark KC, Rah DK and Hong
JP: Effect of recombinant human epidermal growth factor against
cutaneous scar formation in murine full-thickness wound healing. J
Korean Med Sci. 25:589–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leak LV and Jones M: Lymphangiogenesis
in vitro: Formation of lymphatic capillary-like channels
from confluent monolayers of lymphatic endothelial cells. In Vitro
Cell Dev Biol Anim. 30:512–518. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh SJ, Jeltsch MM, Birkenhäger R, McCarthy
JE, Weich HA, Christ B, Alitalo K and Wilting J: VEGF and VEGF-C:
Specific induction of angiogenesis and lymphangiogenesis in the
differentiated avian chorioallantoic membrane. Dev Biol.
188:96–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeltsch M, Kaipainen A, Joukov V, Meng X,
Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK and Alitalo K:
Hyperplasia of lymphatic vessels in VEGF-C transgenic mice.
Science. 276:1423–1425. 1997. View Article : Google Scholar : PubMed/NCBI
|