Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection, involving the whole body, with the lungs being
one of the first organs affected. Acute lung injury (ALI) caused by
sepsis is a serious disease that can further develop into
life-threating acute respiratory distress syndrome. Current
treatment measures include protective mechanical ventilation,
surfactant replacement therapy, glucocorticoids and nitric oxide
inhalation; however, they do not significantly improve the
prognosis of ALI, and the mortality rate is high at 30–40%
(1).
Due to the air-blood membrane barrier function in
normal lung tissue, the protein-rich liquid cannot leak freely into
the alveolar space or pulmonary interstitium (2). In sepsis-induced ALI, the lung
microvascular endothelial cells and alveolar epithelial cells are
impaired. This increases the permeability of the alveolar air-blood
barrier leading to edema of alveolar space and the pulmonary
interstitium, pulmonary hemorrhage and the infiltration of a large
number of neutrophils and alveolar macrophages, which exacerbates
injury. The increased permeability of the alveolar air-blood
barrier is a crucial component of ALI with the alveolar epithelial
barrier function regarded as more important than the microvascular
endothelial barrier. Gorin et al (3) determined that the barrier function of
the alveolar epithelium was stronger than the vascular endothelium.
Even under normal conditions, injury to the barrier function of
alveolar epithelium can lead to the occurrence of pulmonary edema.
Matthay et al (4)
demonstrated that the alveolar epithelial barrier function is the
most crucial in the pathogenesis of ALI; the damage degree of
alveolar epithelial barrier determined the condition of the ALI
patients, and the recovery of epithelial barrier function
determined the prognosis of patients.
A previous study demonstrated that the permeability
of the alveolar membrane barrier largely depends on the
intercellular connections in the paracellular pathway (5). Intercellular connections include three
major junction complexes: adherence junction, tight junction and
gap junction (GJ). A GJ is a special membrane channel structure
that exists between two adjacent tissue cells and consists of two
mirror-symmetric connexons (Cn). The lung tissue epithelial cells
mainly express Cx43, Cx37, and Cx40, of which Cx43 is the major
connexin in ATII cells (6). The GJ
consisting of connexin Cx43 forms a gap junction channel (GJC)
between cells. Substances with a size of ~1,000 Da, such as direct
dispersion of hydrophilic ions, molecules, metabolites or signal
transduction molecules, can pass through; thereby GJCs serve a
gating role, and regulate the transport and distribution of ions,
currents, and low molecular weight metabolites. This connection
between ATII cells ensures the integrity of the alveolar air-blood
barrier. When the expression of Cx43 is upregulated, the channel
and communication function of GJs is greatly changed, so that the
macromolecular substances that could not initially pass through can
now smoothly cross into the alveolar cavity and pulmonary
interstitium affecting the permeability of the alveolar air-blood
barrier. A study reported that post-traumatic cerebral edema is
associated with Cx43 expression and that blocking Cx43 reduces the
number of gap junctions formed between astrocyte, which in turn
reduces glutamate release and alleviates brain edema (7). Previous research on intercellular GPs
have focused on the development and metastasis of tumors,
cardiovascular diseases and organ development but the relationship
between Cx43 protein and lung injury is less studied. Therefore,
exploring the relationship between Cx43 and alveolar air-blood
barrier permeability has important theoretical significance for the
prevention and treatment of sepsis-induced ALI.
microRNA (miRNA) is a small non-coding gene
expression regulator that mediates gene silencing following
transcription. miRNA regulates mRNA expression via two regulatory
mechanisms. One mechanism occurs when the miRNA is completely
complementary to the target mRNA and protein expression is reduced
via degradation of the target mRNA. The other mechanism involves
non-complementary miRNA and target mRNA, where mRNA translation is
inhibited, reducing the protein expression of the target protein
but mRNA expression is not affected. miRNA-206 (miR-206) is a
multifunctional miRNA, that is widely involved in various
pathological and physiological processes in different tissues. For
example, miR-206 was involved in the development of bronchoalveolar
dysplasia by down-regulating fibronectin 1 in premature infants
with the disease (8). It also
downregulates brain-derived neurotrophic factor expression leading
to neurological dysfunction of airway smooth muscle, which in turn
causes lung inflammatory disease (9). Zhang et al (10) determined that miR-206 regulates
vesicle-associated membrane protein expression by direct inhibition
of the target gene and affects the secretion of surfactants by ATII
cells. Therefore miR-206 is widely involved in the regulation of
lung function.
It has been demonstrated that miR-206 inhibits
breast cancer cell proliferation and metastasis by targeting the
Cx43 gene 3′untranslated region (UTR) 478–484 and 1609–1615
sequences, blocking Cx43 expression (11). Anderson et al (12) identified that miR-206 was involved in
skeletal muscle differentiation by downregulating Cx43 expression
at the post-transcription level. Kin et al (13) demonstrated that miR-206 regulates
Cx43 expression and participates in the induction of C2C12 myoblast
differentiation. However, further investigation is required to
determine whether the ATII cell barrier function is regulated by
miR-206 via targeting the Cx43 gene in sepsis-induced ALI.
The present study utilized the miRNA target gene
prediction software TargetScan (http://www.targetscan.org/) to identify that the 3′UTR
of the Cx43 gene had complementary sequences to miR-206 at sites
466–473 and 1580–1587. Therefore, dual luciferase reporter gene
assay was used to determine the effects of Cx43 on the regulation
of the ATII barrier in sepsis-induced ALI in vivo and in
vitro. It was hypothesized that miR-206 targeted Cx43 mRNA to
regulate the permeability of the ATII barrier.
Materials and methods
Animals and cells
A total of 32 male C57BL/6 mice (4–6 weeks; 18–22 g
weight) were provided by the Physical Laboratory Animal Center at
the Shanxi Medical University [animal license: SCXK (jin)
2015–0001] and raised in a specific-pathogen-free animal
laboratory. The mice were placed on clean bedding in a room with
laminar airflow, the indoor temperature was controlled at 18–22°C,
the humidity was 50–60% and the mice were given access to food and
water ad libitum. Rat ATII cells RLE-6TN were purchased from
Shanghai Baili Biotechnology Co., Ltd., and 293T human embryonic
kidney cells were purchased from the Type Culture Collection of the
Chinese Academy of Sciences.
Reagents
Transwell plates were purchased from Corning, Inc.
fluorescein-labeled dextran (FITC-dextran; cat. no. FD40S) and
lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (Merck
KGaA). Fetal bovine serum (FBS) was purchased from Cellmax. Cx43
mRNA inhibitors, an siRNA, (antogamir;
5′-CUCUCGCUCUGAAUAUCAUTTAUGAUAUUCAGAGCGAGAGTT-3′) and miR-206
mimics (agomir; 5′-UGGAAUGUAAGGAAGUGUGUGGACACACUUCCUUACAUUCCAUU-3′)
were purchased from Shanghai Gemma Pharmaceutical Technology Co.
Ltd. Cx43 antibody was purchased from Proteintech, Inc. and GAPDH
antibody was purchased from Bioworld Technology, Inc. Dulbecco's
modified Eagle's medium (DMEM) containing sodium pyruvate, sheep
anti-rabbit horseradish peroxidase-conjugated immunoglobulin G
antibodies, bovine serum albumin (BSA), coomassie brilliant blue
G-250 and bicinchoninic acid BCA protein quantitative kits were all
purchased from Wuhan Boster Biological Technology, Ltd. TRIzol
total RNA extraction kit, Fast Quant RT kit with gDNA and SuperReal
fluorescence Quantitative Premix were purchased from Tiangen
Biotech Co., Ltd. M5 Pre-stained Protein Ladder was purchased from
PCM Biotechnology Co., Ltd. Histostain™-Plus kit (cat. no. SP-0022)
was purchased from Beijing Boaosen Biotechnology Co., Ltd. All the
primers were purchased from the Sangon Biotech Co., Ltd.
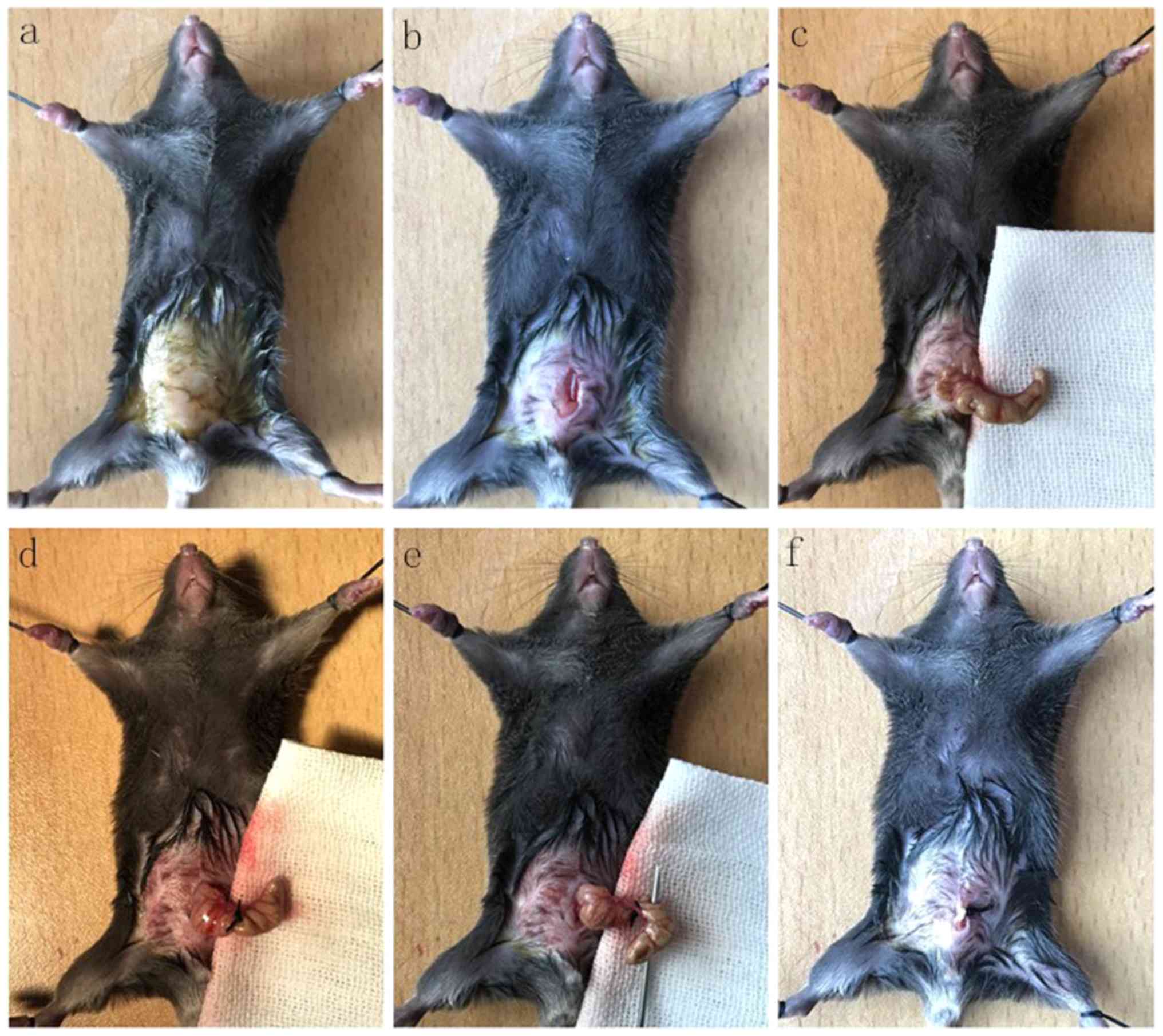
Mouse sepsis model
Mice were randomly divided into 4 groups: Sham,
caecum ligation and perforation (CLP), CLP+Cx43 inhibitors
(Cx43-In) and CLP+miR-206 mimics (miR-206-Mi) groups. A modified
experimental paradigm of CLP was used to induce ALI based on
methods described in a previous study (14). In brief, mice were anesthetized with
intraperitoneal injection of 3.5% chloral hydrate (10 ml/kg). The
caecum was exposed by midline incision then the proximal caecum was
ligated with silk thread. The distal caecum was punctured with a 20
ml syringe needle, squeezing out any intestinal contents, then
placed back into the abdominal cavity. The abdominal cavity was
closed by suturing the wound (Fig.
1). In the sham group, the caecum was only turned by median
incision, without ligation and perforation.
Animal treatments
Mice in the Cx43-In group were injected in the tail
vein with 125 µl Cx43 antagomir (16.5 µg/µl) and the mice in the
miR-206-Mi group were injected with 125 µl miR-206 agomir (16.5
µg/µl). The mice in the sham and CLP groups were injected with 125
µl normal saline. The sepsis model was established after 24 h of
intervention.
Protein content in bronchoalveolar
lavage fluid (BALF)
Mice were sacrificed via cervical dislocation 6 h
following modeling. The chest was immediately opened, the right
main bronchus ligated and endotracheal intubation was performed
with intravenous indwelling needle to tracheal bifurcation. Then
sterile and cooled PBS (0.3 ml) was used to lavage the left lung,
pumping 3 times, and then BALF was gently drawn. The process was
repeated 3 times and the recycled liquid stored was in a centrifuge
tube in ice water temporarily. The recovery rate was ~75%. The BALF
was centrifuged at 25,000 × g for 10 min at 4°C, then the
supernatant was stored at −20°C prior to protein content analysis
using coomassie brilliant blue stain.
Hematoxylin and eosin staining
The middle lobe of the right lung was fixed in 4%
paraformaldehyde at room temperature for 24 h, dehydrated with
alcohol, embedded in paraffin, cut into 0.5–0.8 µm sections then
dewaxed. The sections were stained with hematoxylin for 10 min at
room temperature. Then they were placed in eosin for 5 min at room
temperature. The sections were observed under a fluorescent
microscope at magnifications of ×100 and ×400.
Wet to dry weight ratio (W/D)
The lower lobe of the right lung tissue was blotted
with filter paper to remove any surface moisture then weighed to
get wet weight (W). Samples were dried at 50°C in an oven for 48 h
to a constant weight, and then weighed to get the dry weight (D)
The W/D was then calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue and cells using
the ultra-pure RNA extraction kit according to the manufacturer's
instructions. RNA was then quantified using the NanoDrop 1000
(Thermo Fisher Scientific, Inc.). Reverse transcription was carried
out with FastQuant cDNA First Chain Synthesis kit from 2 µg of
total RNA according to the manufacturer's instructions. qPCR was
performed using SYBR Green and measured with ABI 7500 Real-Time PCR
System. The primer sequences are listed in Table I. Thermocycling conditions were as
follows: 95°C for 15 min, and then 40 cycles of 95°C for 10 sec,
65°C for 32 sec. mRNA levels were quantified using the
2−ΔΔCq method (15) and
normalized to GAPDH.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| Mouse Cx43 |
ACAGGAGAGTGCCTTGGTAGTGAC |
GTTGCTGGACTTGCTGGACCTTC |
| Mouse miR-206 |
GCTCGTGGAATGTAAGGAAGT |
AGTGCAGGGTCCGAGGTATT |
| Mouse GAPDH |
AACGGGAAGCCCATCACC |
CAGCCTTGGCAGCACCAG |
| Rat Cx43 |
GGAAATCGAACGGCTGGGCGT |
TCGCGTGAAGGGAAGAAGCGAT |
| Rat miR-206 |
GCGCGTGGAATGTAAGGAAGT |
AGTGCAGGGTCCGAGGTATT |
| Rat GAPDH |
TGATTCTACCCACGGCAAGTT |
TGATGGGTTTCCCATTGATGA |
Immunohistochemistry
The middle lobe of the right lung of each mouse was
fixed in 4% paraformaldehyde at room temperature for 24 h. Samples
were embedded in paraffin then cut into 0.5–0.8 µm sections, then
deparaffinized using xylene. Samples underwent a gradient ethanol
hydration then were blocked in 10% normal goat serum (Beijing
Solarbio Biotechnology Co., Ltd.) at room temperature for 30 min.
Tissue sections were incubated with primary antibody against Cx43
(1:100; cat. no 15386-1-AP) at 37°C for 2 h. Samples were rinsed
with PBS three times then incubated with horseradish
peroxidase-labeled secondary antibody (1:50; cat. no. ZDR5306;
OriGene Technologies, Inc.) at room temperature for 45 min. Samples
were rinsed with PBS three times, 3,3′-diaminobenzidine staining
was performed then samples were sealed with neutral gum. A
fluorescence inverted microscope (IX51; Olympus Corporation) was
used to observe samples.
Western blot analysis
The upper lobes of right lung were removed from the
mice, then they were placed separately in a mortar and the total
proteins were extracted with radioimmunoprecipitation assay lysate
solution (cat. no. AR0102; Wuhan Boster Biotechnology Co., Ltd.).
The total protein concentration was quantified using the BCA
quantitative kit (cat. no. AR0146; Wuhan Boster Biotechnology Co.,
Ltd.). A total of 30 µg sample protein was loaded per lane and
separated using SDS-PAGE on a 20% gel, then transferred to a
polyvinylidene difluoride membrane. The membrane was blocked in 5%
milk for 2 h at room temperature. Membranes were incubated with
primary antibody against Cx43 (1:500) overnight at 4°C. Membranes
were rinsed with Tris-buffered saline and Polysorbate 20 three
times then incubated with horseradish peroxidase-labeled secondary
antibody GAPDH (1:5,000; cat. no. BSAP0063; Bioworld Technology,
Inc.) for 2 h at room temperature. Samples were rinsed five times.
Then protein bands were visualized using super-sensitive enhanced
chemiluminescent solution (cat. no. AR1111; Wuhan Boster
Biotechnology Co., Ltd.) and analyzed using the Molecular
Imager® Gel Doc™ XR+ system (Bio-Rad Laboratories,
Inc.).
Monolayer cell culture
Cell culture was performed as previously described
(16). One day prior to
transfection, 2×105 cells were seeded in the upper
chamber on a Transwell plate to form a monolayer. Samples were
randomly divided into six groups: sham, CLP, Cx43-In, Cx43-NC
(5′-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3′), miR-206-Mi and
miR-206-NC groups. Cx43 inhibitor (20 µmol/l) and 2 µl of
Lipofectamine 2000 transfection reagent were diluted with 50 µl
serum-free DMEM and gently mixed to form 100 µl of a siRNA and
Lipofectamine 2000 complex at room temperature for 20 min, then the
complex was added to the cells in the Cx43-In group. miR-206 mimic
(20 µmol/l; 5 µl) and 2 µl of Lipofectamine 2000 transfection
reagent were diluted in 50 µl serum-free DMEM, then gently mixed at
room temperature for 20 min to make 100 µl of the mimic and
Lipofectamine 2000 complex; the complex was then added to cells in
the miR-206-Mi group. The sham group and CLP group were incubated
in 100 µl DMEM at 37°C for 24 h. Transfection success rate was
demonstrated by measuring miR-206 and Cx43 mRNA expression. After
24 h, all groups were supplemented with DMEM containing LPS (100
µg/l) for 6 h except for the sham group which was supplemented with
DMEM only.
Permeability of monolayer cells
After 6 h of LPS stimulation and three PBS washes,
100 µl FITC-dextran (1 µg/ml) was added into the upper chamber and
600 µl sterile PBS was added into the lower chamber at 37°C for 1
h. Cytation3 multi-function detection system (BioTek Instruments,
Inc.) was used to test the absorbance value of FITC in the lower
chamber. Results were displayed as a ratio of the experimental
group to the control group.
Dual-luciferase reporter assay
miR-206 mimics (5′-UGGAAUGUAAGGAAGUGUGUGG-3′) and
miRNA-206-negative controls (NC; 5′-UUCUCCGAACGUGUCACGUTT-3′) were
synthesized, and the target sequence of the 3′UTR of the Cx43 gene
was cloned into the on pmirGLO vector (Wuhan GeneCreate Biological
Engineering Co., Ltd.). pmirGLO vector contained Firefly luciferase
and Renilla luciferase as a control. The final transfected
concentration was 0.97 µg/µl for the Cx43-wild-type (WT) plasmid,
and 1.33 µg/µl for the Cx43-mutant (MT) plasmid. Cells (293T) were
cultured in DMEM+FBS (10%) the day before transfection, so that the
cell density at transfection was 70–80%. Firstly, 2 µl
Lipofectamine 2000 transfection reagent was diluted in 50 µl
serum-free DMEM; then plasmids and mimics were diluted in 50 µl
serum-free DMEM (plasmid 1 µg; mimics 20 pmol). Lipofectamine 2000,
and the plasmid and mimics solution (both 50 µl) were mixed at room
temperature for 20 min, then 100 µl serum-free DMEM was added to
form 200 µl transfection complex. Finally, the transfection complex
was incubated with the cells at 37°C for 5 h, then DMEM containing
the transfection complex was removed. A total of 500 µl complete
DMEM including 10% FBS without the transfection reagent was added
and the cells were cultured at 37°C for 48 h. Transfection was
repeated for five times.
Detection of reporter gene expression
intensity
Cell lysis solution (200 µl; cat. no. AR0102; Wuhan
Boster Biotechnology Co., Ltd.) was added into each well of 293T
cells and incubated at room temperature for 10 min. Following cell
lysis, the solution was collected then centrifuged for 5 min at
10,000 × g/min (Avanti™J-30I; Beckman Coulter, Inc.) and the
supernatant collected.
Firstly, firefly luciferase test reagent and
Renilla luciferase test buffer from Luciferase Reporter
Assay Kit (BioVision, Inc.) were defrosted at room temperature, and
Renilla luciferase detection substrate (×100) was placed on
in an ice box. In accordance with the manusfacturer's protocol, the
chemiluminescence meter (BK-L96C; Beijing Binsino Biotechnology
Co., Ltd.) was opened and 100 µl of the supernatant was added into
the 96-well luminescence plate. The firefly luciferase detection
working fluid (100 µl) was added, and the mixture was mixed.
Finally, the luminescence value was measured on the computer; the
integral time was 5 sec. The working fluid of 100 µl Renilla
luciferase was added, and the luminescence value was measured on
the computer after the mixture was mixed. The integral time was 5
sec. The relative light unit (RLU) value determined by
Renilla luciferase was divided by the RLU value determined
by firefly luciferase. The authors compared the activation degree
of reporter gene between different samples according to the
obtained ratio.
Statistical analysis
Data was processed using SPSS v19.0 (IBM Corp.). The
data in this study were presented as mean ± standard error.
Statistical significance was assessed using one-way analysis of
variance followed by Fisher's least significant difference method
for uniform variance and Dunnett's test for non-uniform variance.
All experiments were repeated at least five times. P<0.05 was
considered to indicate statistical significance.
Results
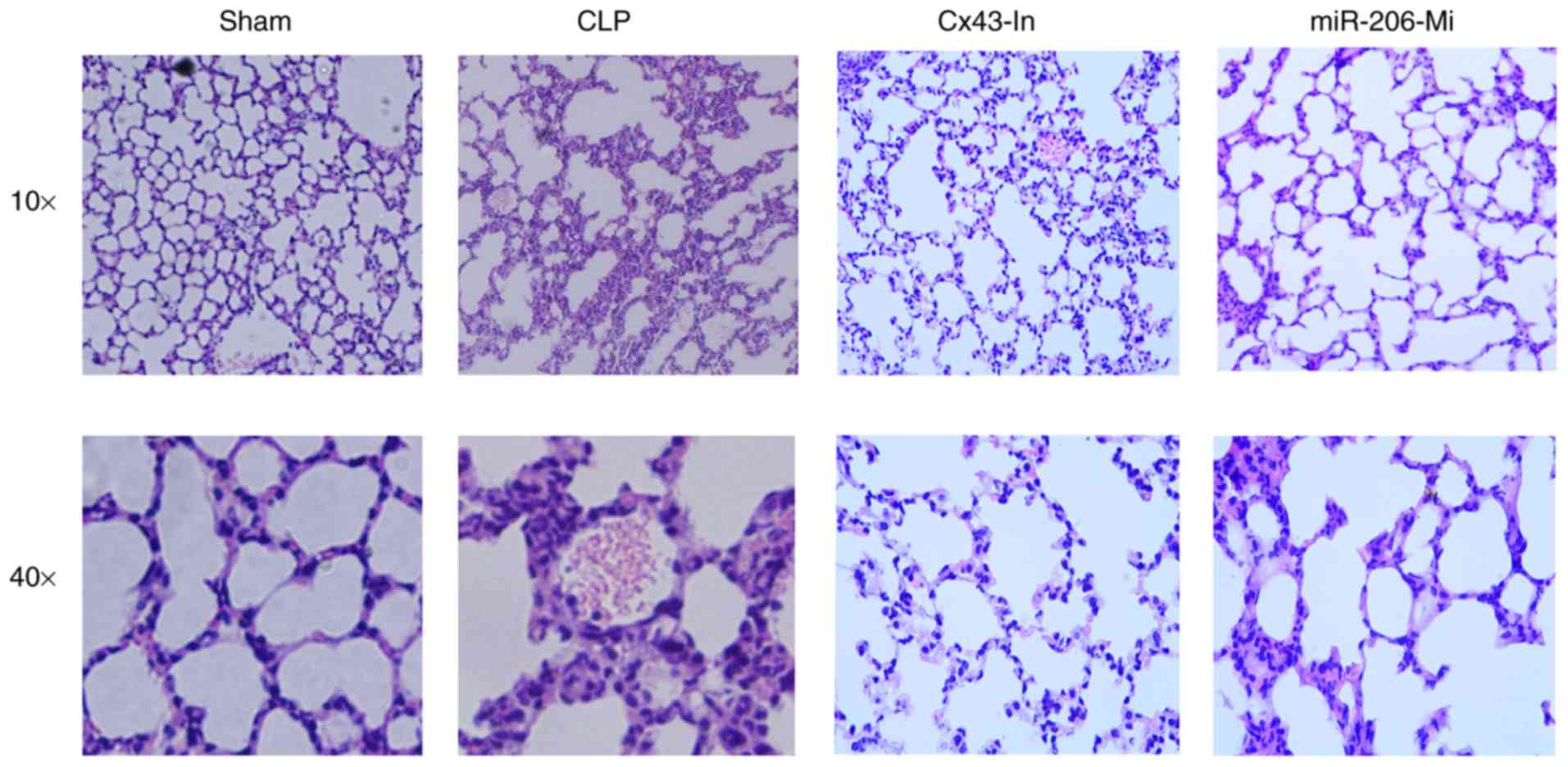
CLP induces a strong inflammatory
response in mouse lung
The degree of damage to the alveolar structure was
dependent on the treatment group, with the CLP group demonstrating
the most significant changes (Fig.
2). The CLP, Cx43-In and miR-206-Mi groups demonstrated
alveolar wall thickening, pulmonary interstitium hyperemia and
hemorrhage, inflammatory cells infiltration, pulmonary interstitium
and alveolar space edema, alveolar cavities neutrophils and reddish
edema fluid exudate (Fig. 2). There
was no significant change in lung tissue in the sham group
(Fig. 2). Compared with the CLP
group, the inflammatory response was alleviated in the Cx43-In and
miR-206-Mi groups.
miR-206 significantly decreases
alveolar air-blood barrier permeability following sepsis-induced
ALI
W/D of lung tissue reflected the degree of pulmonary
edema and the protein content of BALF reflected the permeability of
the alveolar air-blood barrier. At 6 h following modeling, the lung
tissue water content and the concentration of protein in the BALF
were significantly increased compared with the sham group
(P<0.05; Table II). The water
content of lung tissue and the protein concentration of BALF in the
Cx43-In group and miR-206-Mi groups were significantly decreased
compared to the CLP group (P<0.05; Table II).
 | Table II.Protein concentration in BALF and lung
W/D ratio. |
Table II.
Protein concentration in BALF and lung
W/D ratio.
| Group | W/D | Protein in BALF
(mg/l) |
|---|
| Sham | 5.0±0.3 | 4.1±0.9 |
| CLP | 11.7±0.5a | 11.9±3.9a |
| Cx43-In | 5.9±0.3b | 6.3±2.0b |
| miR-206-Mi | 6.4±0.6b | 8.5±0.9b |
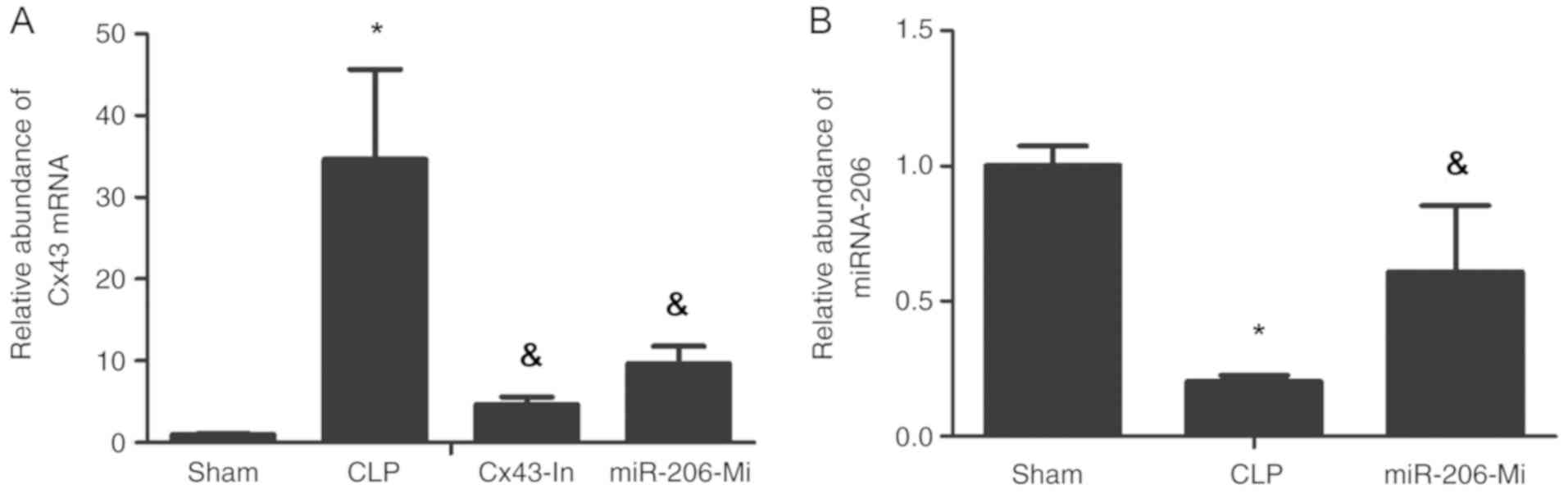
miR-206 significantly decreases Cx43
mRNA expression following sepsis-induced ALI
Cx43 mRNA expression in the lung tissue of the CLP
group was significantly increased compared with the sham group
(P<0.05; Fig. 3A). Cx43 mRNA
expression for the Cx43-In group and the miR-206-Mi group was
decreased compared with the CLP group (P<0.05; Fig. 3A). The expression of miR-206 was
significantly decreased in the CLP group compared with the sham
group (P<0.05; Fig. 3B). The
expression of miR-206 was increased in the miR-206-Mi group
compared with the CLP group (P<0.05; Fig. 3B).
miR-206 decreases Cx43 protein
expression following sepsis-induced ALI
Immunohistochemistry analysis demonstrated that Cx43
protein expression in the CLP group was significantly enhanced
compared with the sham group (Fig.
4). Cx43 protein expression was decreased in the Cx43-In group
and the miR-206-Mi group compared with the CLP group (Fig. 4).
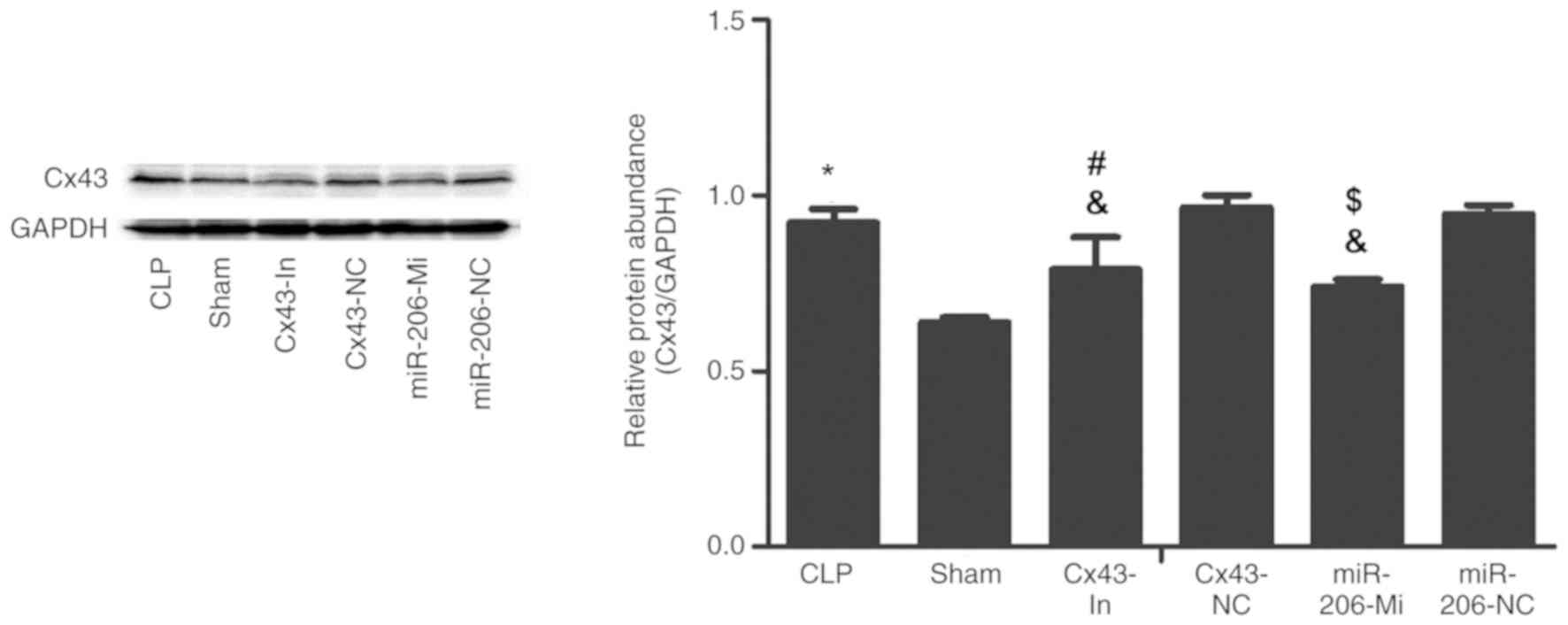
These findings were further confirmed by western
blot analysis. A presented in Fig.
5, Cx43 protein expression in the CLP group was significantly
increased compared with the sham group (P<0.05). Compared with
the CLP group, Cx43 protein expression in the Cx43-In group and the
miR-206-Mi group was significantly decreased (P<0.05), but was
still higher than the sham group (P<0.05; Fig 5).
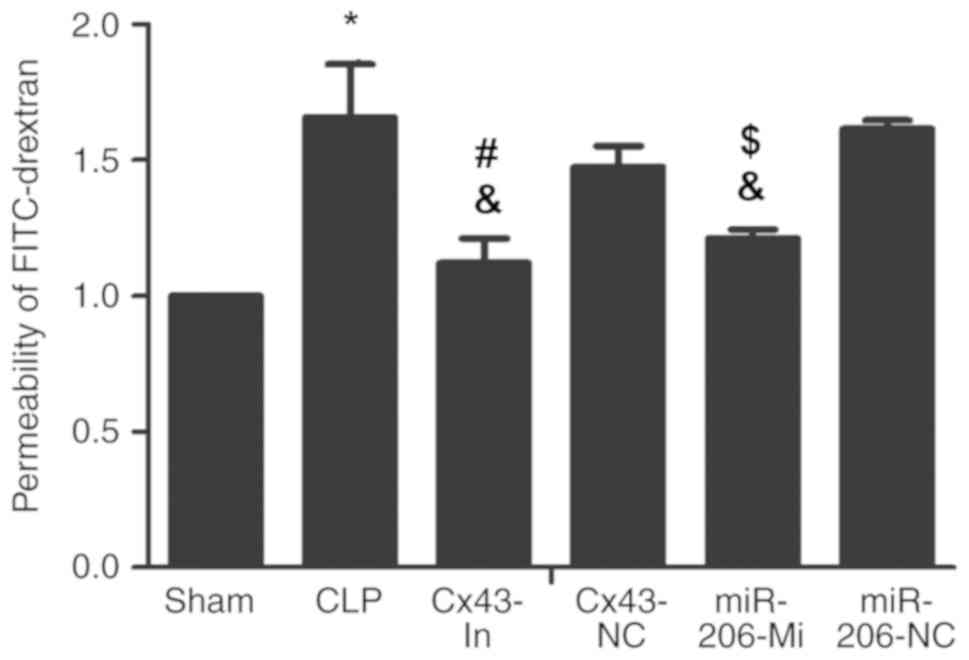
miR-206 decreases the permeability of
ATII monolayer following ALI induction
The permeability of the ATII monolayer in the CLP
group was increased compared with the sham group (P<0.05;
Fig. 6). Compared with the CLP
group, the permeability of the ATII monolayer in the Cx43-In group
and the miR-206-Mi group were significantly decreased (P<0.05;
Fig. 6).
Successful transfection confirmed by
Cx43 and miR-206 expression
Transfection success rate was demonstrated by
RT-qPCR analysis for miR-206 and Cx43 mRNA expression. Cx43 mRNA
expression in the CLP group was significantly increased compared
with the sham group (P<0.05; Fig
7A). Cx43 mRNA expression in the Cx43-In group was
significantly decreased compared with the CLP group (P<0.05;
Fig 7A). Of note, the Cx43 mRNA
expression was not significantly decreased in the miR-206-Mi group
compared with the miR-206-NC (P>0.05; Fig 7A). Cx43 mRNA expression decreased in
the Cx43-In group relative to the Cx43-NC group (P<0.05;
Fig. 7A).
The expression of miR-206 was significantly
decreased in the CLP group compared with the sham group (P<0.05;
Fig. 7B). miR-206 expression was
increased in the miR-206-Mi group compared with the CLP group
(P<0.05; Fig. 7B). The expression
of miR-206 in the miR-206-Mi group was increased relative to the
miR-206-NC group (P<0.05; Fig.
7B).
miR-206 significantly decreases Cx63
protein expression in vitro
Cx43 protein expression in the CLP group was
significantly increased compared with the sham group (P<0.05;
Fig. 8). Cx43 protein expression in
the Cx43-In group and the miR-206-Mi group was significantly lower
compared with the CLP group (P<0.05; Fig. 8) but higher compared with the sham
group (P<0.05; Fig. 8). Cx43
expression was lower in the Cx43-In group compared with the Cx43-NC
group (P<0.05; Fig. 8). Cx43
expression in the miR-206-Mi group was lower compared with the
miR-206-NC group (P<0.05; Fig.
8).
miR-206 interacts with the 3′UTR of
Cx43
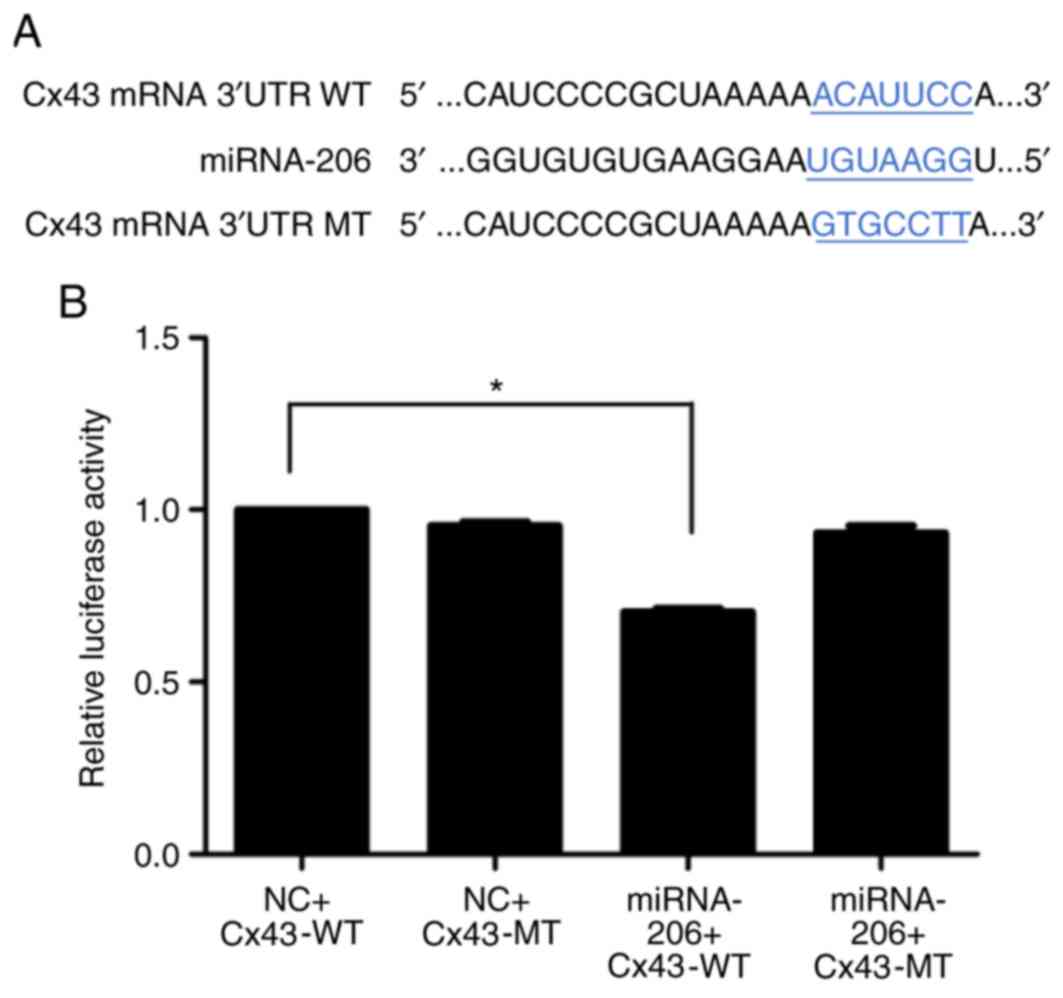
miRNA target gene prediction software TargetScan
indicated that the 3′UTR of the Cx43 gene had 466–473 and 1580–1587
sequences complementary to miR-206 (Fig.
9A). Fluorescence intensity was significantly downregulated
compared with the control group following miR-206 and Cx43-WT
transfection (P<0.05, Fig. 9B)
which suggested that miRNA-206 interacted directly with the 3′UTR
of Cx43.
Discussion
The present study determined that the alveolar
air-blood barrier permeability was increased in sepsis-induced ALI
mice, permeability of monolayer ATII cells was increased by LPS and
Cx43 downregulation due to Cx43 mRNA inhibitors (antagomir)
decreased permeability of ATII barrier. It was also demonstrated
that Cx43 expression decreased following the application of
miRNA-206 analogs (agomir) and the dual-luciferase report gene
assay demonstrated that miR-206 targeted Cx43 mRNA 3′UTR to
influence Cx43 mRNA translation.
HE staining, W/D and BALF protein content confirmed
the establishment of a successful sepsis-induced ALI mouse model.
HE staining revealed that the CLF method induced alveolar structure
destruction, alveolar wall thickening, pulmonary interstitium
hyperemia, interstitium hemorrhage, inflammatory cell infiltration,
alveolar edema, reddish edema in the alveolar cavity, and
neutrophils exudation, which is consistent with the previous
literature (17). The W/D and BALF
protein concentration in sepsis-induced ALI mice were also
increased, further indicating successful modeling. Experiments were
performed 6 h following modeling due to the inflammatory response
peaking at this time point (18).
To explore the role of Cx43 in alveolar air-blood
barrier permeability in vitro, ATII cells were cultured in
monolayer. Following LPS stimulation, the monolayer permeability
increased significantly which is consistent with previous studies
(4,5). This experiment demonstrated the
important role of the ATII barrier in alveolar air-blood barrier
permeability. Cx43 serves various roles in the development of
different diseases. It has been reported that inhibition of miR-20,
upregulates Cx43 expression which attenuates alkali burn-induced
keratitis (19). Anderson et
al (12) determined that miR-206
downregulates Cx43 expression which serves an important role in
skeletal myoblast formation and differentiation. The present study
demonstrated that the severity of sepsis-induced ALI was attenuated
and the permeability of monolayer cells was decreased by using
miR-206 mimics and Cx43 mRNA inhibitors. The important role of Cx43
in the alveolar air-blood barrier and the regulation of Cx43
expression by miRNAs were verified in vivo and in
vitro. Finally, using dual luciferase reporter gene assay, it
was determined that miR-206 affected the translation of Cx43 mRNA
and downregulated Cx43 expression by targeting the 3′UTR of Cx43
mRNA.
The present study demonstrated the important role of
ATII cells in the alveolar barrier permeability of sepsis-induced
ALI; however, it has several limitations. The effects of pulmonary
microvascular endothelial permeability on alveolar epithelial
production were not studied in vitro or in vivo as
the focus of this study was investigation into the role of ATII
cells in alveolar air-blood barrier permeability. The mechanism of
how siRNA reached the alveolar epithelium through the air-blood
barrier before modeling was also not explored although it was
hypothesized that it may be related to the molecular weight and
homology of the siRNA. Future study will include labeling siRNAs
with fluorescent dyes to dynamically observe the migration process
of the siRNAs. Also the regulation of Cx43 mRNA by miR-206 in human
ATII cells by dual luciferase reporter gene assay and the
relationship between Cx43 and alveolar air-blood barrier
permeability in human A549 cells will be investigated.
In conclusion, the present study demonstrated that
the permeability of the alveolar air-blood barrier in
sepsis-induced ALI was positively correlated with Cx43 expressed by
ATII cells. miR-206 was involved in the regulation of alveolar
air-blood barrier permeability by regulating Cx43 expression. The
present findings provide a potential novel approach for the
treatment of sepsis-induced ALI.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Scientific Research Funding Project for Returnees in Shanxi
Province of China (grant no. 2011–105) and the Taiyuan Science and
Technology Project of Shanxi Province of China (grant no.
12016905).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KL cultured the cells. YMF prepared the animal
models. LYH performed the immunohistochemistry, immunoblotting and
reverse transcription-quantitative polymerase chain reaction
analysis. JWZ and WKZ designed the experiments, anlaysed the data
and wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of the Second Hospital of Shanxi Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Minamino T and Komuro I: Regeneration of
the endothelium as a novel therapeutic strategy for acute lung
injury. J Clin Invest. 116:2316–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berger G, Klorin G, Ismael-Badarneh R,
Guetta J and Azzam ZS: The cellular mechanisms of lung edema
clearance: Does the alveolar epithelium play a role? Harefuah.
156:663–665. 2017.PubMed/NCBI
|
|
3
|
Gorin AB and Stewart PA: Differential
permeability of endothelial and epithelial barriers to albumin
flux. J Appl Physiol. 47:1315–1324. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matthay MA, Cleriei C and Saumon G:
Invited review: Active fluid clearance from the distal air spaces
of the lung. J Appl Physilo. 93:1533–1541. 2002. View Article : Google Scholar
|
|
5
|
He ZG, Xiao N, Liu R, Tian KL, Diao YF and
Fan XQ: Effects of endotoxin on the permeability of rat
lung/intestinal microvesselS and cultured endothelial cells in
vitro and the role apoptosis in the altered permeability. J
Chin Med Res. 5:1214–1216. 2015.(In Chinese).
|
|
6
|
Johnson LN and Koval M: Cross-talk between
pulmonary injury, oxidant stress, and gap junctional communication.
Antioxid Redox Sign. 11:355–367. 2009. View Article : Google Scholar
|
|
7
|
Wu ZL, Liao CH and Ren N: Study on the
correlation between connexin 43 and brain edema in experimental
brain injury. Chin J Neurosurgery Dis Res. 7:201–204. 2012.(In
Chinese).
|
|
8
|
Duan J, Zhang X, Zhang S, Hua S and Feng
Z: MiR-206 inhibits FN1 expression and proliferation and promotes
apoptosis of rat type II alveolar epithelial cells. Exp Ther Med.
13:3203–3208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radzikinas K, Aven L, Jiang Z, Tran T,
Paez-Cortez J, Boppidi K, Lu J, Fine A and Ai X: A Shh/miR-206/BDNF
cascade coordinates innervation and formation of airway smooth
muscle. J Neurosci. 31:15407–15415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Guo Y, Mishra A, Gou D,
Chintagari NR and Liu L: MicroRNA-206 regulates surfactant
secretion by targeting VAMP-2. FEBS Lett. 589:172–176. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin ZJ, Ming J, Yang L, Du JZ, Wang N and
Luo HJ: Mechanism of regulatory effect of microRNA-206 on connexin
43 in distant metastasis of breast cancer. Chin Med J. 129:424–434.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anderson C, Catoe H and Werner R: MiR-206
regulates connexin 43 expression during skeletal muscle
development. Nucleic Acids Res. 34:5863–5871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HK, Lee YS, Sivaprasad U, Malhotra A
and Dutta A: Muscle-specific microRNA miR-206 promotes muscle
differentiation. J Cell Biol. 174:677–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zahar JR, Timsit JF, Garrouste-Orgeas M,
Francais A, Vesin A, Descorps-Declere A, Dubois Y, Souweine B,
Haouache H, Goldgran-Toledano D, et al: Outcomes in severe sepsis
and patients with septic shock: Pathogen species and infection
sites are not associated with mortality. Crit Care Med.
39:1886–1895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) methods. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Fan Y, Wu DZ, Gong YQ, Zhou JY and Hu ZB:
Effects of calycosin on the impairment of barrier function induced
by hypoxia in human umbilical vein endothelial cells. Eur J
Pharmacol. 481:33–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang JF, Zhang ZQ, Luo XT, Hou LY, Jiang
Q, Lv JP and Zhang WK: Bone marrow mesenchymal stem cells regulate
nuclear factor kappaB expression in alveolar macrophages of acute
lung injury rats with sepsis. Chin J Tissue Engineering Res.
19:1556–1561. 2015.(In Chinese).
|
|
18
|
Zhang F, Li ZL, Shi Y, Zhao M, Xin XF and
Qian GS: Nuclear factor κB in LPS stimulated rat alveolar
macrophages promotes TNF-Α secretion. Chin J Pathophysiol.
23:1412–1414. 2007.
|
|
19
|
Li XY, Zhou HF, Tang WQ, Guo Q and Zhang
Y: Transient downregulation of microRNA-206 protects alkali burn
injury in mouse cornea by regulating connexin 43. Int J Clin Exp
Pathol. 8:2719–2727. 2015.PubMed/NCBI
|