Introduction
Tissue engineering techniques are important for
maxillofacial surgery. Current techniques involve combinations of
cellular components, carrier/scaffold, and bioactive components.
Platelets contain high intracellular concentrations of cytokines
and growth factors, including platelet-derived growth factors
(PDGF-AA, PDGF-BB and PDGF-AB), transforming growth factor-β
(TGF-β1 and TGF-β2) and vascular endothelial growth factors
(VEGFs), all of which can stimulate cell proliferation, matrix
remodeling and angiogenesis (1).
Following platelet aggregation, these molecules are released from
intracellular pools and act to repair injured tissue. Platelets not
only secrete cytokines from their cytoplasm but continue to
synthesize cytokines using their mRNA reserves for at least another
7 days (2). Therefore, platelet
concentrates, which are regarded as autologous alternatives to
fibrin glue without anti-coagulants, contain fibrin glue rich in
cytokines and are widely utilized for tissue regeneration following
surgical treatment.
Platelet concentrates were originally utilized to
treat haemorrhage-based severe thrombocytopenia, which is caused by
medullar aplasia or acute leukaemia. Platelet concentrates, called
platelet-rich plasma (PRP), have been used successfully for bone
grafting in patients undergoing maxillofacial surgery (3) and for regeneration of periodontal
tissue (4–6). PRP preparations are needed by subjects
administered thrombin as an anti-coagulant. Some alternative
preparations without anti-coagulant have been described, including
platelet rich fibrin (PRF) (7) and
concentrated growth factors (CGF) using a centrifuge (Medifuge)
designated only for these preparations (8,9).
Regardless of their methods of preparation, the resulting platelet
concentrates are rich in the above-mentioned growth factors
(10). Therefore, platelet
concentrates, which act as a source of growth factors as well as
containing a cellular scaffold, are thought to promote tissue
regeneration.
Bone remodeling is maintained through a balance
between bone formation and resorption (11). TGF-β and bone morphogenetic protein-2
(BMP-2) promote bone forming activity, by activating Smad1/2 and by
increasing the production of type I collagen, alkaline phosphatase
(ALP), and osteocalcin. The Wnt protein family also contributes to
bone formation, either alone or combined with TGF-β/BMP-2 receptor
signalling (12–15). Wnt signaling is initiated by its
binding to its receptor, Frizzled. To date, two types of Wnt
signaling pathways have been identified, the β-catenin-dependent
‘canonical’ pathway, involving, for example, Wnt3a; and the
β-catenin-independent, ‘non-canonical’ pathway, involving, for
example, Wnt5a (16–18). Phosphorylation of β-catenin by
glycogen synthase kinase (GSK)-3β causes the former to become
unstable, leading to its degradation under non-stimulating
conditions. Inactivation of GSK-3β by Wnt signaling, however,
stabilizes β-catenin and induces its binding to the transcription
factor Lef1/Tcf, leading to osteoblastic differentiation (19).
In contrast, the differentiation and activation of
osteoclasts are physiologically stimulated by osteoblasts through
receptor activator of nuclear factor-κB (RANK) and its ligand RANKL
(20). Because RANKL, a type II
homotrimeric transmembrane protein, is expressed on the outer
membrane of osteoblasts, osteoblasts play a central role in both
the osteogenic and osteolytic activities of bone. Bone resorption
is an important process, not only for bone remodeling but for
calcium release from bone, which acts as a calcium store to
maintain blood calcium concentrations. Calcium release is
controlled by the RANK-RANKL system, which can be activated by an
external signaling molecule such as parathyroid hormone (PTH) or
1α,25(OH)2D3, a stimulation that
down-regulates the expression of osteoprotegerin (OPG). OPG is a
decoy receptor for RANKL, inhibiting osteoclast differentiation and
preventing excess bone resorption. Both Wnt/β-catenin and BMP-2
signaling activate Lef1/Tcf for transcription of Tnfrsf11b,
the gene that encodes OPG (21).
Treatment of human osteogenic sarcoma U2OS cells with PRF induced
the production of OPG protein within one day, with production
maintained for five 5 days, but rapidly decreasing at day 7
(22). The OPG/RANKL ratio has been
reported to reflect osteoblastic differentiation status, with a
high OPG/RANKL ratio resulting in a switch to osteoblast maturation
(23). To date, however, the effect
of PRF on OPG/RANKL ratio has not been widely accepted as a
standard effect of PRF. The present study was designed to confirm
that a PRF-induced increase in OPG/RANKL ratio resulted in
osteoblastic differentiation of the mouse osteoblast MC3T3-E1 cell
line and that PRF was involved in the healing process in a rat bone
defect model. These findings confirm that PRF may play a role in
bone defect/fracture healing.
Materials and methods
Cells and cell culture
The MC3T3-E1 cell line, a clonal pre-osteoblastic
cell line derived from newborn mouse calvaria, was grown in
α-minimum essential medium (α-MEM; ICN Pharmaceuticals, Inc.)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific), 50 µg/ml ascorbate 2-phosphate, 10 mM
β-glycerophosphate, and 40 mM HEPES (pH 7.4), as described
(24). Mouse NIH3T3 fibroblasts were
purchased from Riken (Tsukuba) and maintained in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific)
supplemented with FBS. All cells were cultured at 37°C in a
humidified atmosphere containing 5% CO2 and 95% air.
Animals
Male Wistar rats, aged 8–10 weeks and weighing
400–450 g, were obtained from Clea Japan Inc., Tokyo, Japan). The
rats were housed individually in a barrier facility for laboratory
animals with a 12 h light-dark cycle and allowed food and water
ad libitum. All surgical procedures were performed under
general anesthesia with sevoflurane (Mylan; 4% for the induction
and 3% for the maintenance), with local anaesthesia provided by 2%
lidocaine (250 µg/kg) if necessary. Rats were sacrificed by
intraperitoneal injection of over dose (120 mg/kg) of sodium
pentobarbital (Kyoritsu) under general anaesthesia with
sevoflurane.
All animal experiments were approved by the animal
ethics committee of Ohu University (Koriyama, Japan) and done in
the Animal Facility where animals were cared by the Animal Care
Staff according to compliance by the ARRIVE guidelines (no.
2017-14). Number of rats used for preparation of PRF were as
follows: One donor rat for a set of in vitro experiment and
one donor rat for two recipient rats to treat defect. One in
vivo experiment used eight rats as the recipient, which were
divided into two groups: One group was used as PRF-grafted group
and the other one was as the control. Thus, we minimized number of
rats and used total 23 rats including repetition.
Preparation of PRF
PRF was prepared as described (7,25,26),
with slight modifications. Briefly, rats were anaesthetized with
sevoflurane. Whole blood (6 ml) was collected from the apex of the
heart using a 23-gauge needle and BD Vacutainer® Blood
Collection Tubes (Becton-Dickinson) without anti-coagulants.
Immediately after collection, the blood was centrifuged at 890 ×
g at room temperature for 13 min. The intermediate layer was
defined as PRF to be subjected to in vitro and in
vivo experiments (27).
Osteoblastic differentiation and PRF
treatment
We used preosteoblastic cell line MC3T3-E1 cells,
which was well established model for osteoblastic differentiation,
to analyze the effects of PRF on osteoblastic differentiation
according to Ogino et al (27) with slight modifications. MC3T3-E1
cells that reached 70% confluence in 6-well culture plates were
induced to undergo osteoblastic differentiation by the addition of
50 µg/ml ascorbate 2-phosphate and 10 mM β-glycerophosphate, as
described (24), with the culture
medium renewed every 2–3 days. PRF (21 µg/cm2) was
placed at the center of a cell monolayer sheet and the treatment
was started at the same time.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from samples with acid
guanidinium thiocyanate-phenol-chloroform (AGPC) and
reverse-transcribed to cDNA with a High-Capacity cDNA Reverse
Transcription Kit (Thermo Fisher Scientific). The resulting cDNA
was PCR amplified using a GoTaq® Real-Time qPCR Kit
(Bio-Rad) with specific primers (Table
I) in a Thermal Cycler Dice Real Time System (TP-870; Takara).
Results were normalized relative to Actb (β-actin) mRNA
expression levels in the same samples.
 | Table I.Primer sets. |
Table I.
Primer sets.
| Genes
(products) | Primer sequences
(5′-3′) |
|---|
| Alpl
(ALP) |
|
|
Forward |
GCAGTATGAATTGAATCGGAACAAC |
|
Reverse |
ATGGCCTGGTCCATCTCCAC |
| Runx2
(Cbfa1/Runx2) |
|
|
Forward |
ACTCCAGGCATACTGTACAACT |
|
Reverse |
AGGCTGTTTGACGCCATAGT |
| Bmp2
(Bmp-2) |
|
|
Forward |
TGACTGGATCGTGGCACCTC |
|
Reverse |
CAGAGTCTGCACTATGGCATGGTTA |
| Bmp4
(Bmp-4) |
|
|
Forward |
AGCCGAGCCAACACTGTGAG |
|
Reverse |
TCACTGGTCCCTGGGATGTTC |
| Csf1
(M-CSF) |
|
|
Forward |
AGTGCTCTAGCCGAGATGTG |
|
Reverse |
CTGCTAGGGGTGGCTTTAGG |
| Tnfsf11
(RANKL) |
|
|
Forward |
AGCGCAGATGGATCCTAACA |
|
Reverse |
CCAGAGTCGAGTCCTGCAAAT |
| Tnfrsf11b
(OPG) |
|
|
Forward |
AGTGTGAGGAAGGGCGTTAC |
|
Reverse |
AATGTGCTGCAGTTCGTGTG |
| Wnt3a
(Wnt3a) |
|
|
Forward |
CTACCCGATCTGGTGGTCCT |
|
Reverse |
ACAGAGAATGGGCTGAGTGC |
| Wnt5a
(Wnt5a) |
|
|
Forward |
AAAGGGAACGAATCCACGCT |
|
Reverse |
CAGCACGTCTTGAGGCTACA |
| Actb
(β-actin) |
|
|
Forward |
CATCCGTAAAGACCTCTATGCCAAC |
|
Reverse |
ATGGAGCCACCGATCCACA |
TCF-4 binding activity
The TCF-4 binding motif was cloned by PCR and
inserted into the pGL3-OT vector (Addgene #16558). Following
transfection of this vector into MC3T3-E1 cells using Xfect
Transfection Reagent (Takara), the cells were stimulated with PRF
(0.2 g/well; 21 µg/cm2) in 6-well plates for 24 h. TCF-4
binding activity was evaluated using Dual-Luciferase Reporter Assay
System (Promega). Cells were co-transfected with the pGL4.75 [hRluc
(Renilla reniformis)/CMV] vector to control for transfection
efficiency.
Alizarin red S (AR-S)
Mineralized matrix in culture plates was stained
with AR-S as described (24).
Briefly, cells were fixed in 70% ethanol for 1 h at room
temperature and stained with 40 mM AR-S at pH 4.2 for 10 min at
room temperature. After washing with deionized water and
Ca2+- and Mg2+-free phosphate buffered saline
[PBS(−)], AR-S-positivity was quantified using Molecular Imager
(Bio-Rad).
Alkaline phosphatase (ALP)
activity
Cells were washed twice with PBS(−) and sonicated in
ice-cold 0.1 M Tris-HCl (pH 7.2) containing 0.1% Triton X-100. ALP
activity was determined using a Lab Assay ALP kit (Wako), according
to the Bessey-Lowry method.
Western blotting
Proteins in cell lysates (20 µg), prepared in RIPA
buffer, and those in conditioned medium (CM, 10 µg), which were
concentrated by acetone precipitation, were separated by sodium
dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE)
and transferred onto polyvinylidene difluoride (PVDF) membranes.
The membranes were sequentially incubated with primary antibody,
biotinylated anti-rabbit IgG or anti-mouse IgG (Jackson
ImmunoResearch Laboratories) as secondary antibody, and horseradish
peroxidase (HRP)-conjugated streptavidin (Bio-Rad). Signals were
detected using Luminate™ Forte Western HRP Substrate
(Merck Millipore). The primary antibodies used in this study
included anti-RANKL antibody (BioLegend), anti-M-CSF antibody
(Abcam), anti-OPG antibodies, and anti-β-actin antibodies (both
from GeneTex).
Bone defect-healing model
A bone defect-healing model in calvaria was adapted
to the mental foramen bone to assess the effects of PRF on bone
regeneration (1,28–31).
Briefly, following anesthetisation with 4% sevoflurane, both sides
of the mental foramen bone were treated with 100 µg of lidocaine
and each side was surgically drilled to make holes 2.0 mm in
diameter while avoiding injury to the roots of the teeth. The bone
defect was filled with PRF (doses equivalent to 0.3 g/defect area)
on one side (PRF-grafted group, n=4) and without PRF on the other
side (non-grafted or control group, n=4). Four days after surgery,
rats were sacrificed by over dose of pentobarbital subject to
histological analysis.
Histological analysis
Samples of mandibular tissue were obtained from rats
under sodium pentobarbital anaesthesia and fixed in
phosphate-buffered 4% paraformaldehyde (pH 7.2) at 4°C for 24 h.
The samples were decalcified with 10% EDTA (pH 7.0), which was
changed every 2–3 days, at 4°C for 4 weeks. The tissue samples were
embedded in paraffin and sectioned. The sections were stained with
haematoxylin and eosin (H&E) and then tartrate-resistant acid
phosphatase (TRAP) using a commercial TRAP staining kit (Wako),
according to the manufacturer's protocol.
OPG was detected by immunohistochemistry, as
previously described (32–34). Specimens were blocked with 5% skim
milk and incubated sequentially with anti-OPG antibodies (GeneTex),
biotinylated anti-rabbit IgG (Jackson ImmunoResearch Laboratories,
West Grove, PA, USA), and fluorescein isothiocyanate
(FITC)-conjugated streptavidin. Signals were observed with a
fluorescence microscope (Axio Observer, Carl Zeiss Microscopy
GmbH).
Protein assay
Protein concentration was determined by the Bradford
method, using a Bio-Rad protein assay kit, with bovine serum
albumin (BSA) as the standard.
Statistical analysis
Representative results from three independent
experiments (unless otherwise noted) were shown and statistical
significance between two groups was evaluated by Student's
t-tests. Simple regression analysis was used to compare two
slopes obtained by the least squares method. A P-value <0.05 was
considered statistically significant.
Results
PRF induced expression of OPG-encoding
mRNA in osteoblastic cells
MC3T3-E1 cells were treated with PRF to evaluate OPG
production during osteoblastic differentiation. PRF treatment
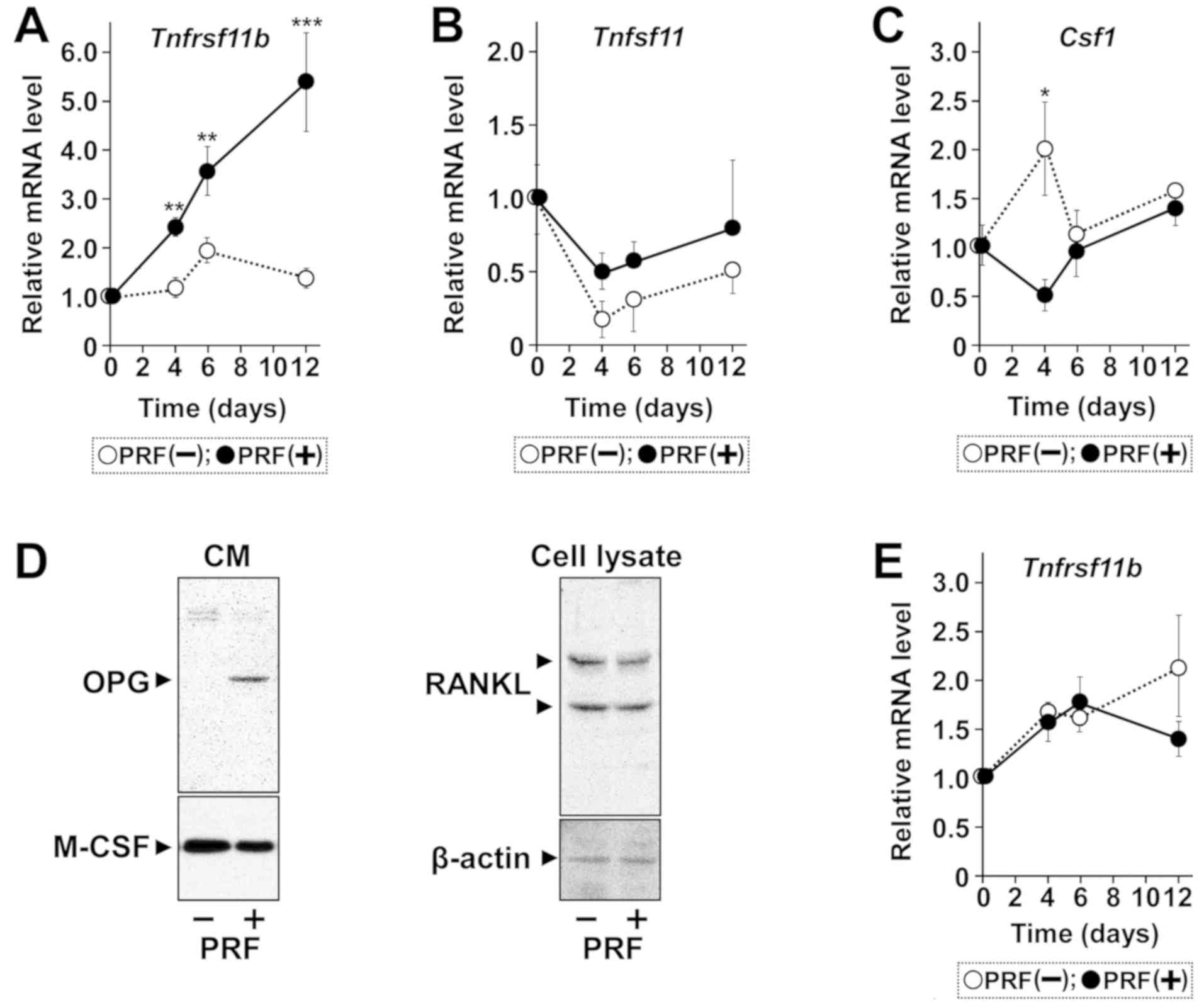
gradually but significantly increased expression of
Tnfrsf11b mRNA, encoding OPG, for 12 days (Fig. 1A-C). In contrast, Tnfsf11
mRNA, encoding RANKL, was constitutively expressed but not affected
by treatment of cells with PRF. Expression of Csf1 mRNA,
encoding M-CSF was transiently lowered in PRF treated cells on day
4, with no significant differences at later times. Western blot
analysis showed that PRF strongly upregulated the expression of
OPG, but not of RANKL or M-CSF, by these cells (Fig. 1D). PRF, however, did not induce
expression of Tnfrsf11b mRNA in non-osteoblastic NIH3T3
fibroblasts (Fig. 1E), although
non-osteoblastic cells, such as gingival fibroblasts (35), adipocytes (36), and endothelial cells (37), can produce OPG. PRF also increased
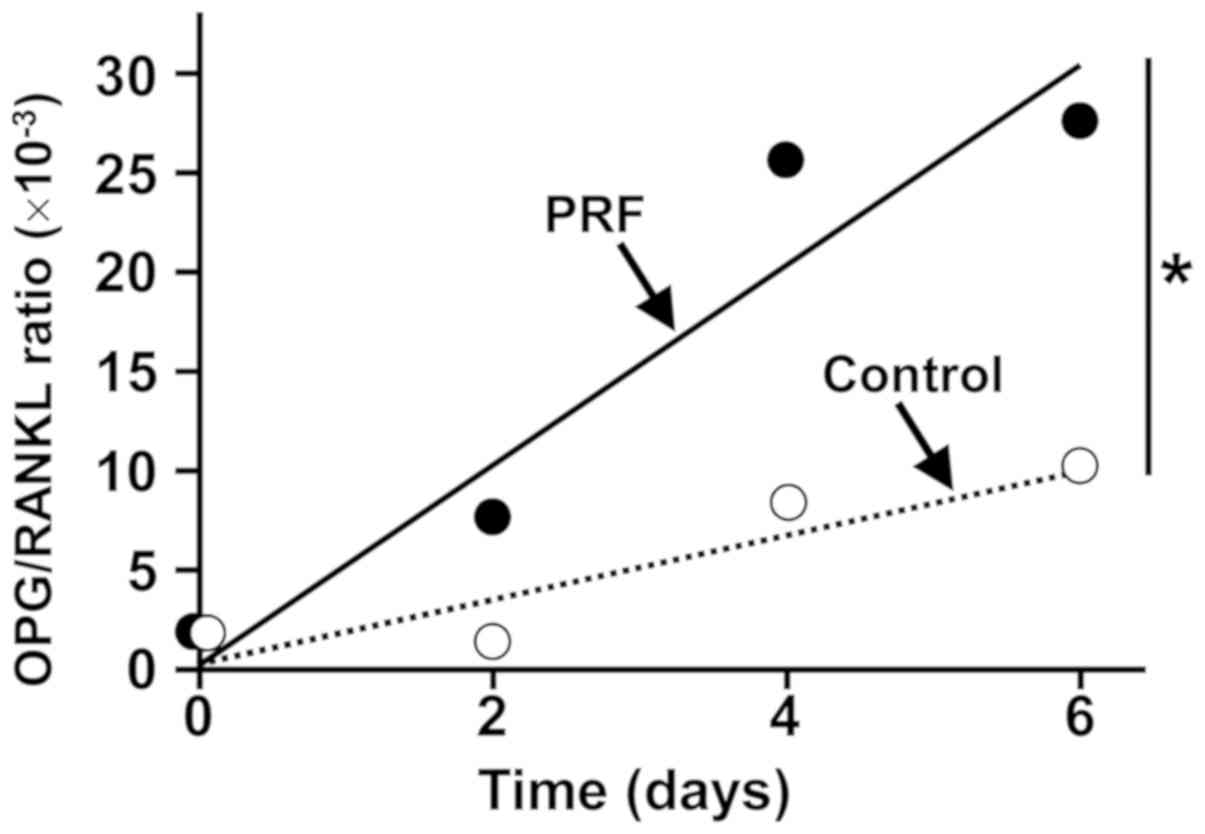
the ratio of OPG-encoding mRNA to RANKL-encoding mRNA in these
cells (Fig. 2).
PRF did not affect the expression of
other osteoblastic marker encoding genes and mineralization in
vitro
Treatment of MC3T3-E1 cells with PRF did not induce
formation of nodules resulting from mineral deposition (Fig. 3A). PRF reduced ALP mRNA level
on day 4, with low ALP mRNA level maintained through day 8,
although ALP mRNA level was higher on day 8 than on day 4
(Fig. 3B). ALP activity was also
lower in the presence than in the absence of PRF on day 8 (Fig. 3C), ALP activity in PRF-treated cells
was higher on day 8 than on day 4, whereas ALP activity in control
cells was higher on day 4 than on day 8, suggesting that PRF
regulates bone regeneration by delaying the peak of osteoblast
differentiation. The expression of other osteoblastic marker genes,
encoding Runx2, BMP2, and BMP4, were not affected by PRF treatment
(Fig. 3D-F).
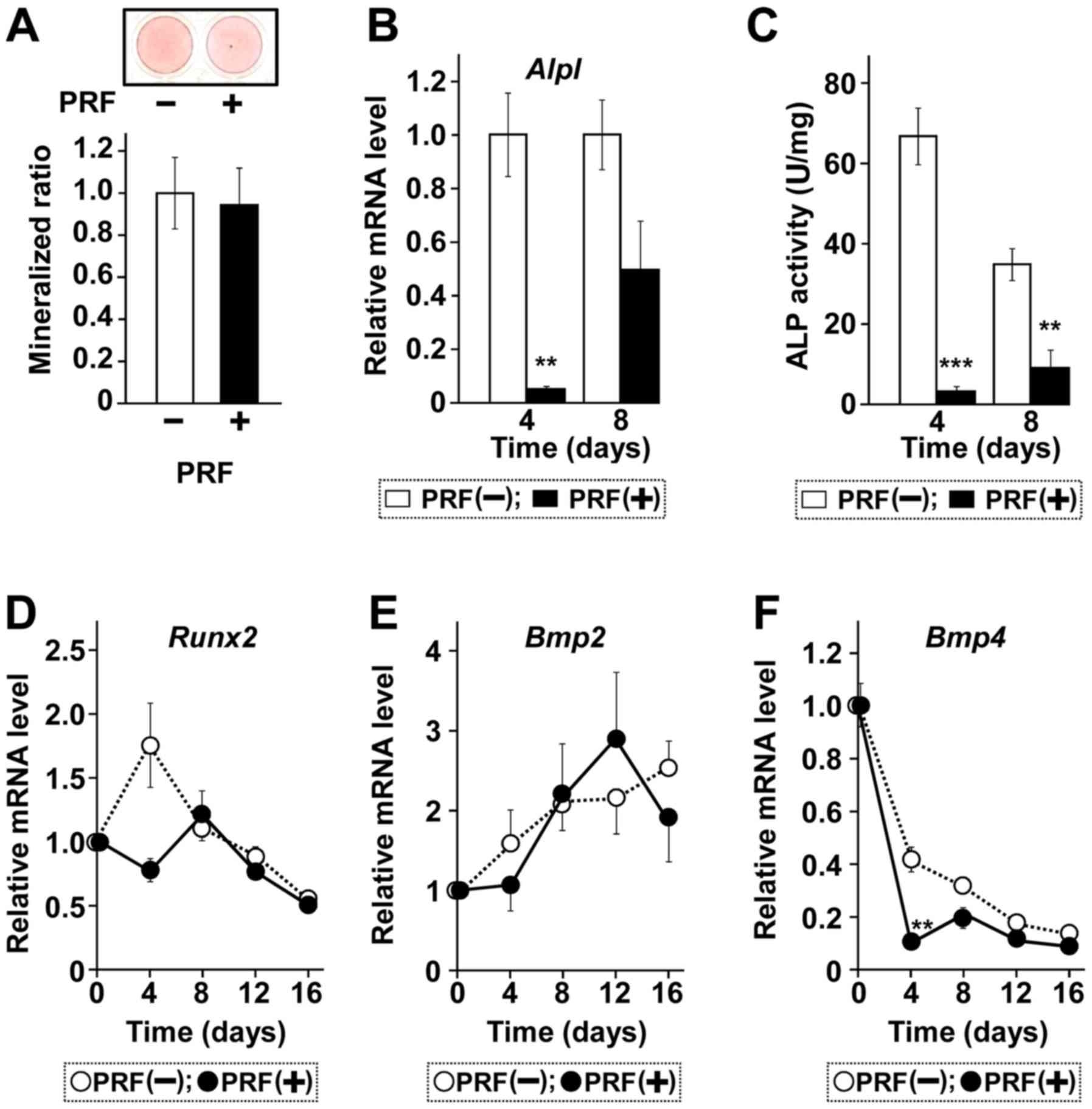 | Figure 3.PRF does not affect in vitro
mineralization or osteoblastic differentiation for mineralization.
Cells were treated with (+) or without (−) PRF for 24 days. (A)
Calcification determined by AR-S staining. (B) Total RNA was
extracted, reverse-transcribed and amplified by qPCR with primer
sets for Alpl mRNA. (C) Samples from whole-cell extracts
were assayed using an ALP kit. Time course of the effect of PRF on
expression of (D) Runx2, (E) Bmp2 and (F) Bmp4
mRNAs, as determined by reverse transcription-quantitative PCR.
Cells were treated with or without PRF for the time periods
indicated. Representative results were from two independent
experiments. Data are presented as the mean ± standard error (n=3).
**P<0.01 and ***P<0.001 vs. samples incubated in the absence
of PRF at each time point. PRF, platelet rich fibrin; AR-S,
alizarin red S; ALP, alkaline phosphatase; CM, conditioned medium;
OPG, osteoprotegerin; M-CSF, macrophage colony-stimulating factor;
RANKL, receptor activator of NF-κB ligand. |
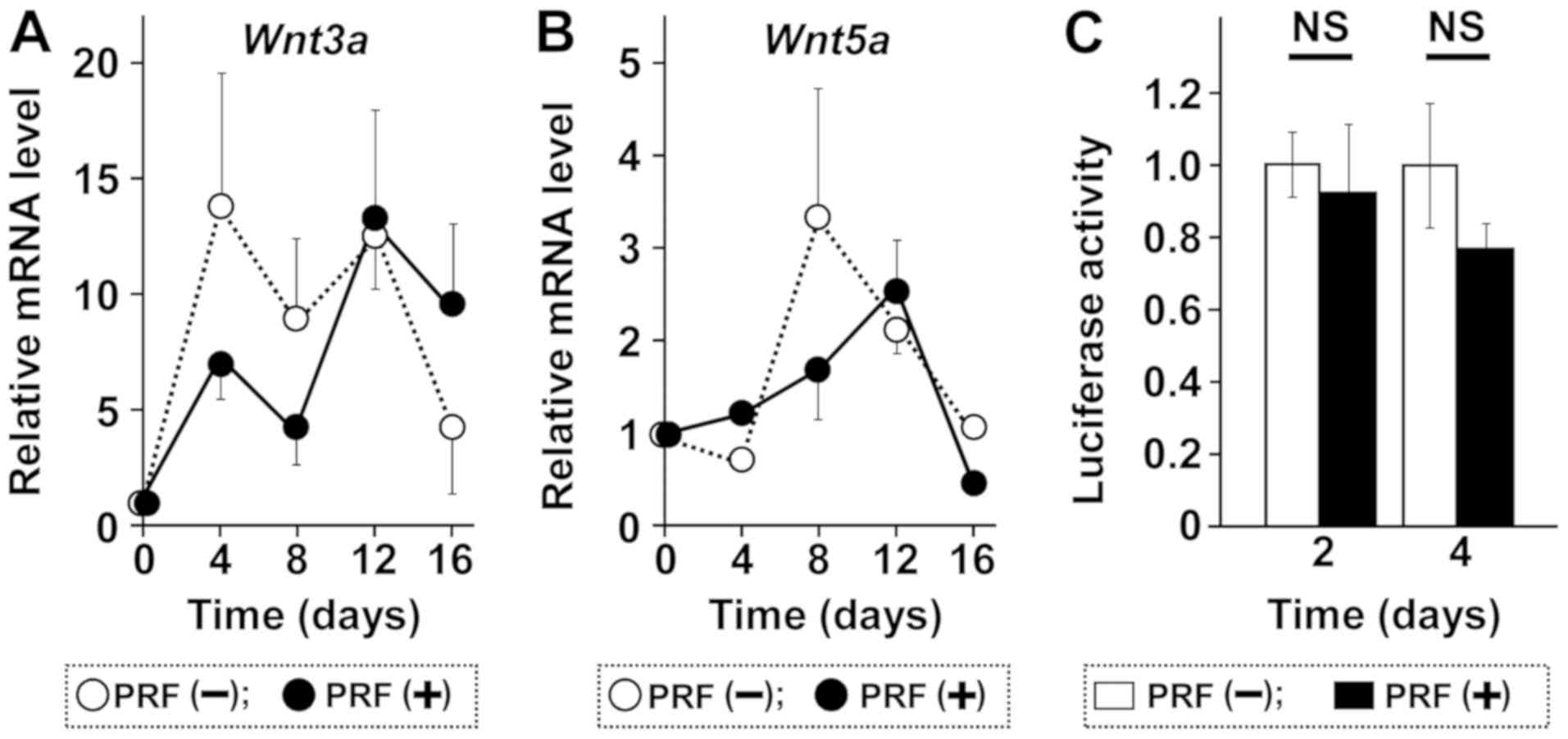
In assessing the effect of PRF on Wnt signaling
which plays a role in OPG expression, we found that PRF had no
effect on the expression of Wnt3a and Wnt5a (Fig. 4A and B). In addition, PRF did not
significantly activate the transcription of β-catenin from a TCF-4
binding motif containing a reporter vector (Fig. 4C). These findings suggested that the
Wnt pathway is not involved in the induction of Tnfrsf11b
mRNA in MC3T3-E1 cells.
PRF increases the number of OPG
producing cells but reduces the number of active osteoclasts
To determine whether PRF mediates OPG induction and
osteoclastogenesis in vivo, a bone defect-healing model in
calvaria was adapted for use in mental foramen bone. A bone defect
area 2.7 mm in diameter treated with a low-level laser was reported
filled with calcium (~180 mg/g-tissue) and phosphorus (~115
mg/g-tissue) within 2 weeks (28).
Both values were 1.4 times higher than in control, non-laser
treated, bone. After an additional 2 weeks, these increases in
calcium and phosphorus deposition were 1.3- and 1.2-fold higher
than in control, respectively, suggesting that early stage bone
repair process, within 2 weeks after surgery, was extremely
important. Therefore, we created a small defect (2.0 mm in
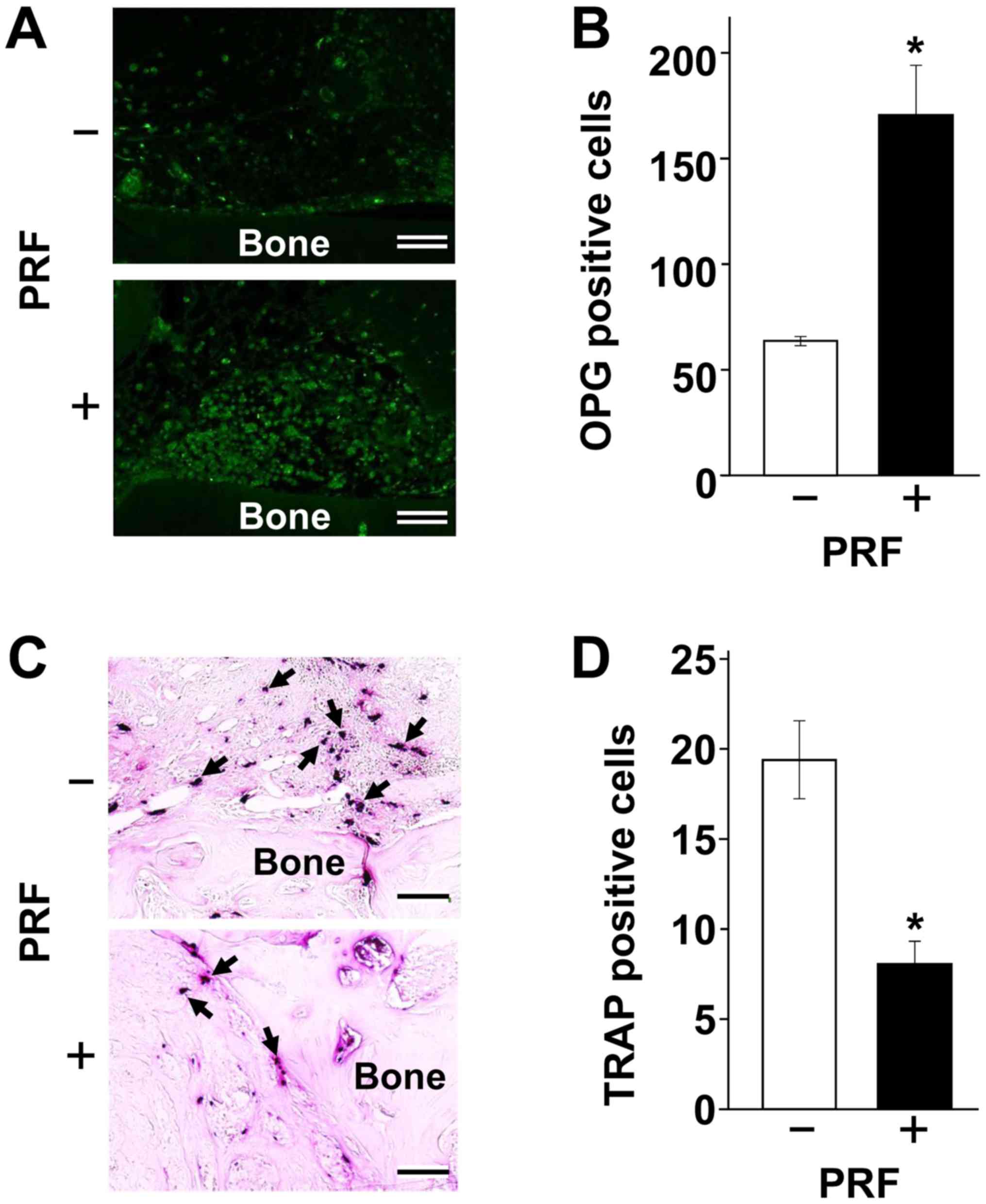
diameter) and observed early stage regeneration. After 4 days,
OPG-positive cells were 2.7-fold more abundant in the PRF-engrafted
than in the control region (Fig.
5A). Conversely, PRF grafting decreased TRAP positivity to
41.7% of that observed in the control region (Fig. 5B). Taken together, these findings
indicate that an elevation in OPG/RANKL ratio may be critical
during early stage osteoblastic differentiation.
Discussion
Fibrin acts as a scaffold for cells during wound
repair (38,39). Insertion of PRF as the sole filling
material was found to promote bone regeneration and subsequent
sinus elevation (40). Moreover, the
combination of PRF and a demineralized freeze-dried bone allograft
(DFDBA) accelerated bone formation; although DFDBA-induced
maturation required about 8 months, the addition of PRF shortened
bone formation to 4 months (41).
PRF has been reported to accelerate bone healing both in
vivo and in vitro (8,42,43). The
ability of platelet concentrate clots to gradually release PDGFs,
TGF-β, IGF-1, and FGF-2 (44)
enables them to assist in bone healing in the defect/fracture
region, similar to the activity of PRF.
More preclinical and clinical studies have evaluated
PRP than PRF, with most studies of PRP showing its benefits for
bone repair, although several have reported that PRP has low or
limited impact on osteogenesis in bone defect repair (45,46).
These discrepancies may be explained by differences among PRP
preparations in components and/or their ratios, including
differences not only in growth factors but platelets and leukocytes
(47,48). Even studies reporting that PRP does
not affect bone healing activity did not find that PRP had negative
effects on bone healing. Using MC3T3-E1 cell line, which has been
widely used as a model for osteoblastic differentiation (14,49,50),
here we showed that ALP expression/activity was lower in
PRF-treated than in control cells, in which medium contained
ascorbate 2-phosphate and β-glycerophosphate, but no other
osteogenic inducers. Because ALP activity is high in mature
osteoblasts, PRF did not inhibit osteoblastic differentiation, but
rather may have optimized osteoblastic differentiation. Thus, the
low ALP mRNA/activity in response to PRF treatment suggests that
PRF regulated ALP expression by delaying it, thereby optimizing
bone remodeling to an osteogenic state during the early stages of
osteoblastic differentiation in our culture system. Indeed, ALP
expression/activity gradually increased during the time in
culture.
The present study found that OPG was the only
molecule induced by PRF stimulation. Many studies have focused on
OPG/RANKL ratio and bone resorption, but few have linked these
findings to osteoblastic differentiation (51). OPG/RANKL ratio has been reported: Low
in immature osteoblasts and high in mature osteoblasts (23,52),
suggesting that the PRF-induced increase in OPG/RANKL ratio is
associated with bone formation. Our results also showed that PRF
affected OPG production, leading to a high OPG/RANKL ratio,
suggesting that autologous platelet concentrates may be suitable to
balance bone formation (53–55). PRF-induced OPG production by an
osteosarcoma cell line was found to peak within 1 day, remaining
high up to day 3 and decreasing to baseline level by day 5, while
RANKL production remained steady (22). Although our OPG induction peak
differed slightly, the two studies, taken together, indicate that
PRF-induced increases in OPG/RANKL ratio occur during early stage
osteoblastic differentiation, similar to the effects of genestein
on early stage osteoblastic differentiation (51).
Clinically, PRF, combined with other materials, has
been used in surgical repairs (47,56).
Because we found that PRF did not induce markers of osteogenic
differentiation, such as Runx2, Bmp2, or Bmp4 mRNAs,
in vitro, PRF may play a role during the early stages in
vivo to optimize osteoblastic differentiation towards an
osteogenic state by inducing OPG production. Similarly, the
OPG/RANKL ratio in osteoblastic differentiation was reported to be
associated with bone formation during the remodeling process
(57).
This study could not determine which factors in PRF
are responsible for the induction of OPG. Since OPG expression in
osteoblasts was reported to be induced by BMP-2, TGF-β, and PDGF
(57,58), it is reasonable to assume that these
factors, either alone or in combination in PRF, induce OPG
expression. Reverse signaling of RANKL stimulated by RANK was
recently reported (59). Although
osteoblasts in OPG-deficient mice did not show reduced osteogenic
activity (60), studies are needed
to elucidate the role of OPG in RANKL-induced reverse signaling
during the bone healing process in bone defect/fracture models.
In conclusion, our results showed that PRF increased
the OPG/RANKL ratio by inducing OPG production, suggesting that PRF
assists in early stage osteogenesis by optimizing osteoblastic
differentiation. Because PRF aids in earlier and better wound
healing (61). PRF has advantages in
patients undergoing oral surgery, such as sinus lift.
Acknowledgements
The authors would like to thank Dr Keiko Kasai
(Jusendo Hospital, Koriyama, Japan) for technical assistance and Dr
Satoshi Takada, Dr Hirosi Ito and Dr Yoko Sakurai (Ohu University,
Koriyama, Japan) for their critical comments. This work was based
on a PhD thesis (Ryuta Sumida), which was published by Ohu
University Press (Koriyama, Japan) in 2018.
Funding
This work was supported by JSPS KAKENHI (grant no.
JP16K11517).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YK conceived the study and planned the experiments.
RS performed all experiments. IK and JY assisted in the animal
experiments, and reviewed the manuscript critically for important
intellectual content. RS, TM and YK analysed and interpreted the
data, and wrote the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Ohu University (Koriyama, Japan; no.
2017-14).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part II: Platelet-related
biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e45–e50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Senzel L, Gnatenko DV and Bahou WF: The
platelet proteome. Curr Opin Hematol. 16:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitman DH, Berry RL and Green DM:
Platelet gel: An autologous alternative to fibrin glue with
applications in oral and maxillofacial surgery. J Oral Maxillofac
Surg. 55:1294–1299. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaishnavi C, Mohan B and Narayanan LL:
Treatment of endodontically induced periapical lesions using
hydroxyapatite, platelet-rich plasma, and a combination of both: An
in vivo study. J Conserv Dent. 14:140–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta C, Mehrotra D, Mohammad S, Khanna V,
Kumar Singh G, Singh G, Chellappa AA and Passi D: Alveolar bone
graft with platelet rich plasma in cleft alveolus. J Oral Biol
Craniofac Res. 3:3–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawase T: Platelet-rich plasma and its
derivatives as promising bioactive materials for regenerative
medicine: Basic principles and concepts underlying recent advances.
Odontology. 103:126–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part I: Technological
concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e37–e44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodella LF, Favero G, Boninsegna R,
Buffoli B, Labanca M, Scari G, Sacco L, Batani T and Rezzani R:
Growth factors, CD34 positive cells, and fibrin network analysis in
concentrated growth factors fraction. Microsc Res Tech. 74:772–777.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durmuşlar MC, Balli U, Dede FÖ, Misir AF,
Bariş E, Kürkçü M and Kahraman SA: Histological evaluation of the
effect of concentrated growth factor on bone healing. J Craniofac
Surg. 27:1494–1497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su CY, Kuo YP, Tseng YH, Su CH and Burnouf
T: In vitro release of growth factors from platelet-rich fibrin
(PRF): A proposal to optimize the clinical applications of PRF.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 108:56–61. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raggatt LJ and Partridge NC: Cellular and
molecular mechanisms of bone remodeling. J Biol Chem.
285:25103–25108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franceschi RT and Iyer BS: Relationship
between collagen synthesis and expression of the osteoblast
phenotype in MC3T3-E1 cells. J Bone Miner Res. 7:235–246. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spelsberg TC, Subramaniam M, Riggs BL and
Khosla S: The actions and interactions of sex steroids and growth
factors/cytokines on the skeleton. Mol Endocrinol. 13:819–828.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maeda T, Matsunuma A, Kawane T and
Horiuchi N: Simvastatin promotes osteoblast differentiation and
mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun.
280:874–877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sparks AB, Morin PJ, Vogelstein B and
Kinzler KW: Mutational analysis of the APC/beta-catenin/Tcf pathway
in colorectal cancer. Cancer Res. 58:1130–1134. 1998.PubMed/NCBI
|
|
17
|
Dejmek J, Säfholm A, Kamp Nielsen C,
Andersson T and Leandersson K: Wnt-5a/Ca2+-induced NFAT
activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha
signaling in human mammary epithelial cells. Mol Cell Biol.
26:6024–6036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin SS, Ueng SW, Niu CC, Yuan LJ, Yang CY,
Chen WJ, Lee MS and Chen JK: Hyperbaric oxygen promotes osteogenic
differentiation of bone marrow stromal cells by regulating
Wnt3a/β-catenin signaling-an in vitro and in vivo study. Stem Cell
Res. 12:260–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krause U, Harris S, Green A, Ylostalo J,
Zeitouni S, Lee N and Gregory CA: Pharmaceutical modulation of
canonical Wnt signaling in multipotent stromal cells for improved
osteoinductive therapy. Proc Natl Acad Sci USA. 107:4147–4152.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderson DM, Maraskovsky E, Billingsley
WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D
and Galibert L: A homologue of the TNF receptor and its ligand
enhance T-cell growth and dendritic-cell function. Nature.
390:175–179. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato MM, Nakashima A, Nashimoto M, Yawaka
Y and Tamura M: Bone morphogenetic protein-2 enhances
Wnt/beta-catenin signaling-induced osteoprotegerin expression.
Genes Cells. 14:141–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang IC, Tsai CH and Chang YC:
Platelet-rich fibrin modulates the expression of extracellular
signal-regulated protein kinase and osteoprotegerin in human
osteoblasts. J Biomed Mater Res A. 95:327–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rogers A, Saleh G, Hannon RA, Greenfield D
and Eastell R: Circulating estradiol and osteoprotegerin as
determinants of bone turnover and bone density in postmenopausal
women. J Clin Endocrinol Metab. 87:4470–4475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda T, Suzuki A, Yuzawa S, Baba Y,
Kimura Y and Kato Y: Mineral trioxide aggregate induces
osteoblastogenesis via Atf6. Bone Rep. 2:36–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim TH, Kim SH, Sándor GK and Kim YD:
Comparison of platelet-rich plasma (PRP), platelet-rich fibrin
(PRF), and concentrated growth factor (CGF) in rabbit-skull defect
healing. Arch Oral Biol. 59:550–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukui N, Ueno T, Ito Y, Takahashi Y,
Kimura Y, Nakajima Y, Kasuya S, Kanou M, Takubo K, Yamamoto K, et
al: Quantification of growth factors in platelet-rich Fibrin: A
preliminary study. J Hard Tissue Biol. 24:231–234. 2015. View Article : Google Scholar
|
|
27
|
Ogino Y, Ayukawa Y, Kukita T, Atsuta I and
Koyano K: Platelet-rich plasma suppresses osteoclastogenesis by
promoting the secretion of osteoprotegerin. J Periodontal Res.
44:217–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khadra M, Kasem N, Haanaes HR, Ellingsen
JE and Lyngstadaas SP: Enhancement of bone formation in rat
calvarial bone defects using low-level laser therapy. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 97:693–700. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choukroun J, Diss A, Simonpieri A, Girard
MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J and Dohan DM:
Platelet-rich fibrin (PRF): A second-generation platelet
concentrate. Part IV: Clinical effects on tissue healing. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 101:e56–e60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pripatnanont P, Nuntanaranont T,
Vongvatcharanon S and Phurisat K: The primacy of platelet-rich
fibrin on bone regeneration of various grafts in rabbit's calvarial
defects. J Craniomaxillofac Surg. 41:e191–e200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamazaki T, Tetsuya K and Yokose S:
Histological demonstration of bone healing in rat tibiae influenced
by diode laser irradiation. J Japan Soc Laser Surg Med. 37:80–86.
2016.
|
|
32
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
et al: Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang X, Wang Y, Han X, Shu R, Chen T, Zeng
H, Xu X, Huang L, Ren A, Song J, et al: Effects of TGF-β1 on
OPG/RANKL expression of cementoblasts and osteoblasts are similar
without stress but different with mechanical compressive stress.
Scientific World Journal. 2015:7181802015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baek KJ, Choi Y and Ji S: Gingival
fibroblasts from periodontitis patients exhibit inflammatory
characteristics in vitro. Arch Oral Biol. 58:1282–1292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gautam J, Khedgikar V, Choudhary D,
Kushwaha P, Dixit P, Singh D, Maurya R and Trivedi R: An isoflavone
cladrin prevents high-fat diet-induced bone loss and inhibits the
expression of adipogenic gene regulators in 3T3-L1 adipocyte. J
Pharm Pharmacol. 68:1051–1063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Songia P, Branchetti E, Parolari A,
Myasoedova V, Ferrari G, Alamanni F, Tremoli E and Poggio P: Mitral
valve endothelial cells secrete osteoprotegerin during endothelial
mesenchymal transition. J Mol Cell Cardiol. 98:48–57. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sahni A and Francis CW: Vascular
endothelial growth factor binds to fibrinogen and fibrin and
stimulates endothelial cell proliferation. Blood. 96:3772–3778.
2000.PubMed/NCBI
|
|
39
|
van Hinsbergh VW, Collen A and Koolwijk P:
Role of fibrin matrix in angiogenesis. Ann NY Acad Sci.
936:426–437. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tajima N, Ohba S, Sawase T and Asahina I:
Evaluation of sinus floor augmentation with simultaneous implant
placement using platelet-rich fibrin as sole grafting material. Int
J Oral Maxillofac Implants. 28:77–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choukroun J, Diss A, Simonpieri A, Girard
MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J and Dohan DM:
Platelet-rich fibrin (PRF): A second-generation platelet
concentrate. Part V: Histologic evaluations of PRF effects on bone
allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 101:299–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takeda Y, Katsutoshi K, Matsuzaka K and
Inoue T: The effect of concentrated growth factor on rat bone
marrow cells in vitro and on calvarial bone healing in vivo. Int J
Oral Maxillofac Implants. 30:1187–1196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Litvinov RI and Weisel JW: What is the
biological and clinical relevance of fibrin? Semin Thromb Hemost.
42:333–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marx RE, Carlson ER, Eichstaedt RM,
Schimmele SR, Strauss JE and Georgeff KR: Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:638–646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Plachokova AS, Nikolidakis D, Mulder J,
Jansen JA and Creugers NH: Effect of platelet-rich plasma on bone
regeneration in dentistry: A systematic review. Clin Oral Implants
Res. 19:539–545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Roffi A, Di Matteo B, Krishnakumar GS, Kon
E and Filardo G: Platelet-rich plasma for the treatment of bone
defects: From pre-clinical rational to evidence in the clinical
practice. A systematic review. Int Orthop. 41:221–237. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Broggini N, Hofstetter W, Hunziker E,
Bosshardt DD, Bornstein MM, Seto I, Weibrich G and Buser D: The
influence of PRP on early bone formation in membrane protected
defects. A histological and histomorphometric study in the rabbit
calvaria. Clin Implant Dent Relat Res. 13:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Simonpieri A, Del Corso M, Vervelle A,
Jimbo R, Inchingolo F, Sammartino G and Dohan Ehrenfest DM: Current
knowledge and perspectives for the use of platelet-rich plasma
(PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial
surgery part 2: Bone graft, implant and reconstructive surgery.
Curr Pharm Biotechnol. 13:1231–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: An in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li F, Yang Y, Zhu P, Chen W, Qi D, Shi X,
Zhang C, Yang Z and Li P: Echinacoside promotes bone regeneration
by increasing OPG/RANKL ratio in MC3T3-E1 cells. Fitoterapia.
83:1443–1450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen X, Garner SC, Quarles LD and Anderson
JJ: Effects of genistein on expression of bone markers during
MC3T3-E1 osteoblastic cell differentiation. J Nutr Biochem.
14:342–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gori F, Hofbauer LC, Dunstan CR, Spelsberg
TC, Khosla S and Riggs BL: The expression of osteoprotegerin and
RANK ligand and the support of osteoclast formation by
stromal-osteoblast lineage cells is developmentally regulated.
Endocrinology. 141:4768–4776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Clark RA: Fibrin and wound healing. Ann NY
Acad Sci. 936:355–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Intini G: The use of platelet-rich plasma
in bone reconstruction therapy. Biomaterials. 30:4956–4966. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Anitua E, Andia I, Ardanza B, Nurden P and
Nurden AT: Autologous platelets as a source of proteins for healing
and tissue regeneration. Thromb Haemost. 91:4–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Marques LF, Stessuk T, Camargo IC, Sabeh
Junior N, dos Santos L and Ribeiro-Paes JT: Platelet-rich plasma
(PRP): Methodological aspects and clinical applications. Platelets.
26:101–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Silva I and Branco JC: Rank/Rankl/opg:
Literature review. Acta Reumatol Port. 36:209–218. 2011.PubMed/NCBI
|
|
58
|
McCarthy HS, Williams JH, Davie MW and
Marshall MJ: Platelet-derived growth factor stimulates
osteoprotegerin production in osteoblastic cells. J Cell Physiol.
218:350–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ikebuchi Y, Aoki S, Honma M, Hayashi M,
Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, et
al: Coupling of bone resorption and formation by RANKL reverse
signalling. Nature. 561:195–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fei Q, Guo C, Xu X, Gao J, Zhang J, Chen T
and Cui D: Osteogenic growth peptide enhances the proliferation of
bone marrow mesenchymal stem cells from osteoprotegerin-deficient
mice by CDK2/cyclin A. Acta Biochim Biophys Sin (Shanghai).
42:801–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kapse S, Surana S, Satish M, Hussain SE,
Vyas S and Thakur D: Autologous platelet-rich fibrin: Can it secure
a better healing? Oral Surg Oral Med Oral Pathol Oral Radiol.
127:8–18. 2019. View Article : Google Scholar : PubMed/NCBI
|