Introduction
The therapeutic management of a patient with
psoriasis and infection with the hepatitis B virus (HBV) is a
challenge as the classical systemic treatment [methotrexate (MTX),
acitretin, cyclosporine] shows a high risk of immunosuppression
and/or hepatic toxicity and the biological therapy is endangered by
the possibility of HBV reactivation. We wish to emphasize that the
use of etanercept in a patient with psoriasis and hepatitis B is a
successful therapeutic alternative which may be safely used
concomitantly with entecavir, with regular monitoring of viral load
and hepatic function tests.
Case report
A 38-year old patient diagnosed with psoriasis,
presented with moderate-severe psoriasis vulgaris, lesions
aggravating in the past few years. The patient followed long-term
local treatment with keratolytics, emollients and potent
dermocorticoids which led to the occurrence of abdominal stretch
marks, but also systemic treatment with MTX and photochemotherapy
(PUVA) with unsatisfactory therapeutic effect.
The patient received MTX in a dose of 15 mg/week
from March 2004 to June 2004 and 20 mg/week from February 2008 to
May 2008 and 20 treatments/month of PUVA therapy between October
1998 and December 1998, both treatments with no therapeutic
effect.
The study was approved by the local Ethics Committee
of ‘Carol Davila’ University of Medicine and Pharmacy (Bucharest,
Romania), and a signed informed consent was obtained from the
patient included in this study.
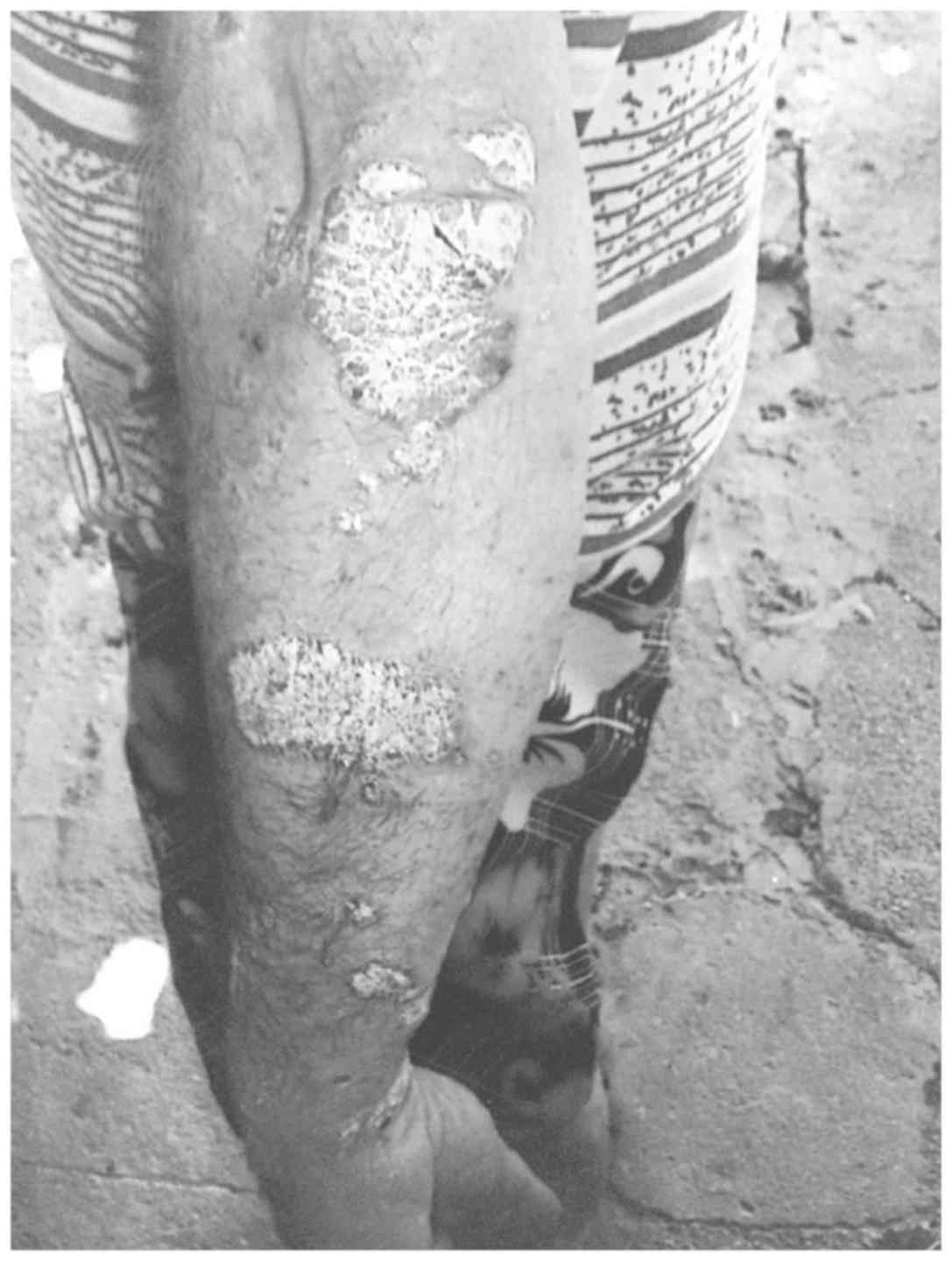
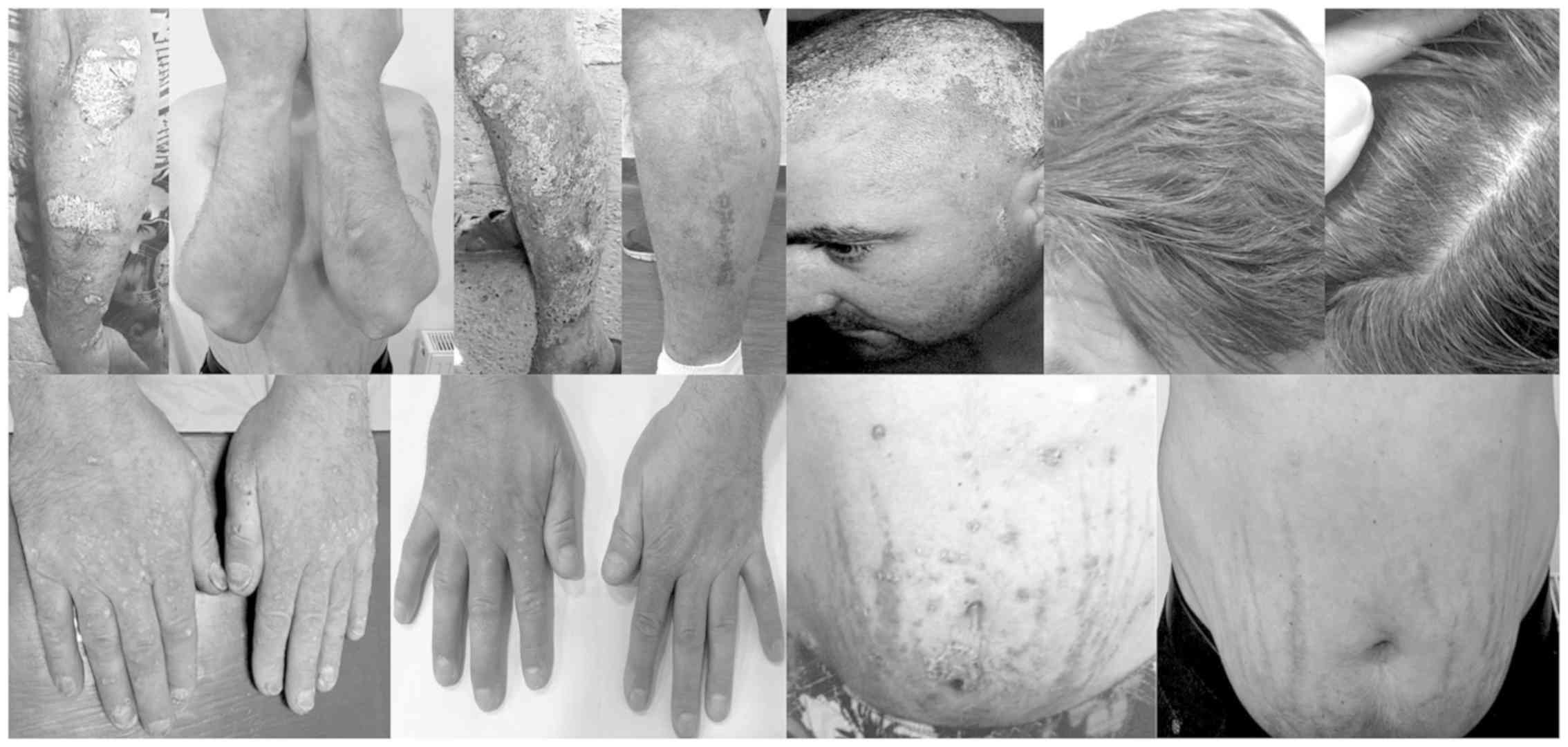
The dermatological examination upon hospitalization
shows the presence of relatively large erythematous-squamous
plaques and patches with clearly outlined borders (5–10 cm
diameter) and tendency to coalescence. The lesions are infiltrated,
covered with thick, whitish, easily detachable flakes and are
located at the level of the extension areas (elbows, forearms, the
back of the hands, knees, before the tibia, lumbosacral) and in the
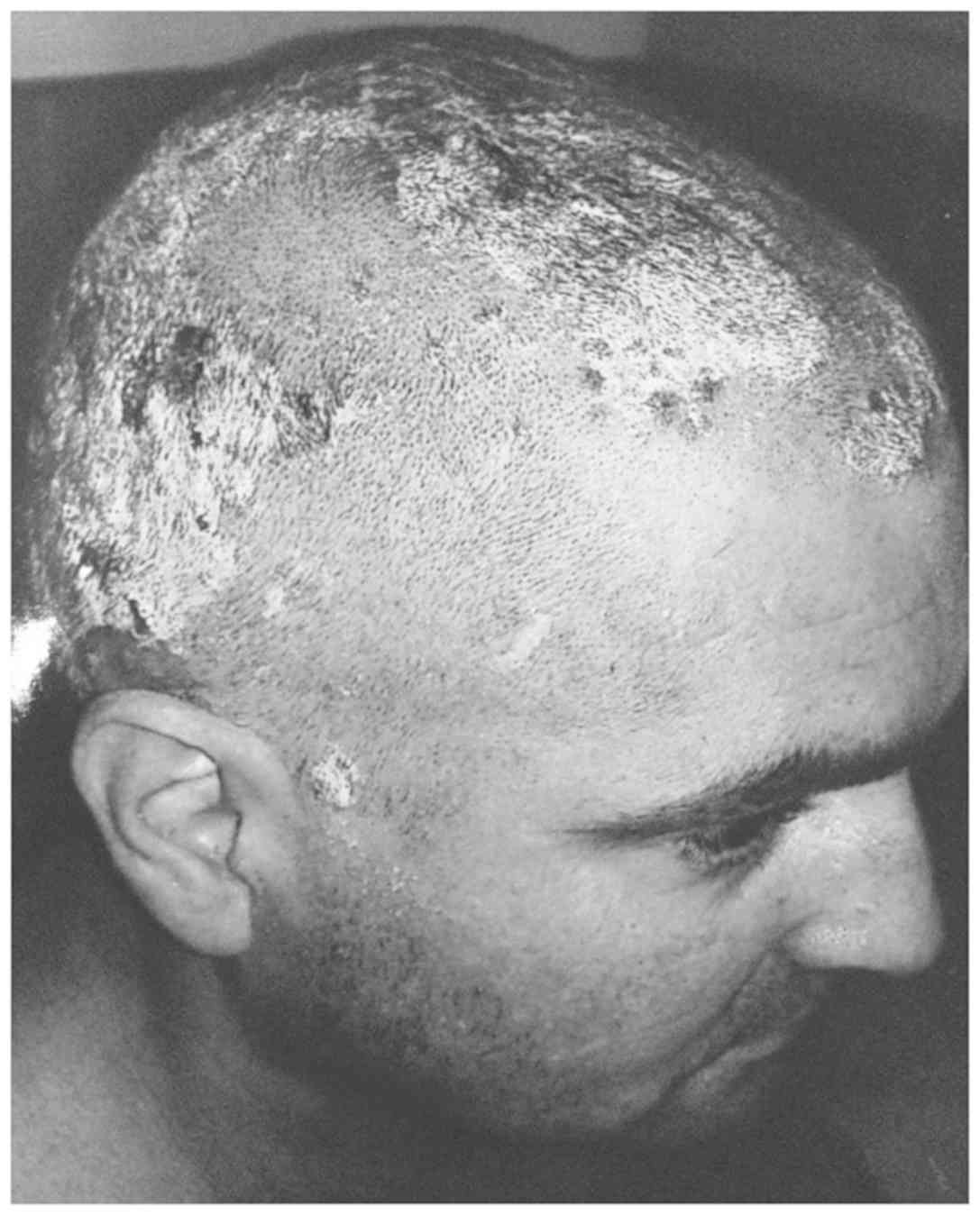
abdominal region (Fig. 1). The scalp
is 70% affected, the circumscribed erythematous-squamous plaques
being limited by the hairline and accompanied by moderate itching
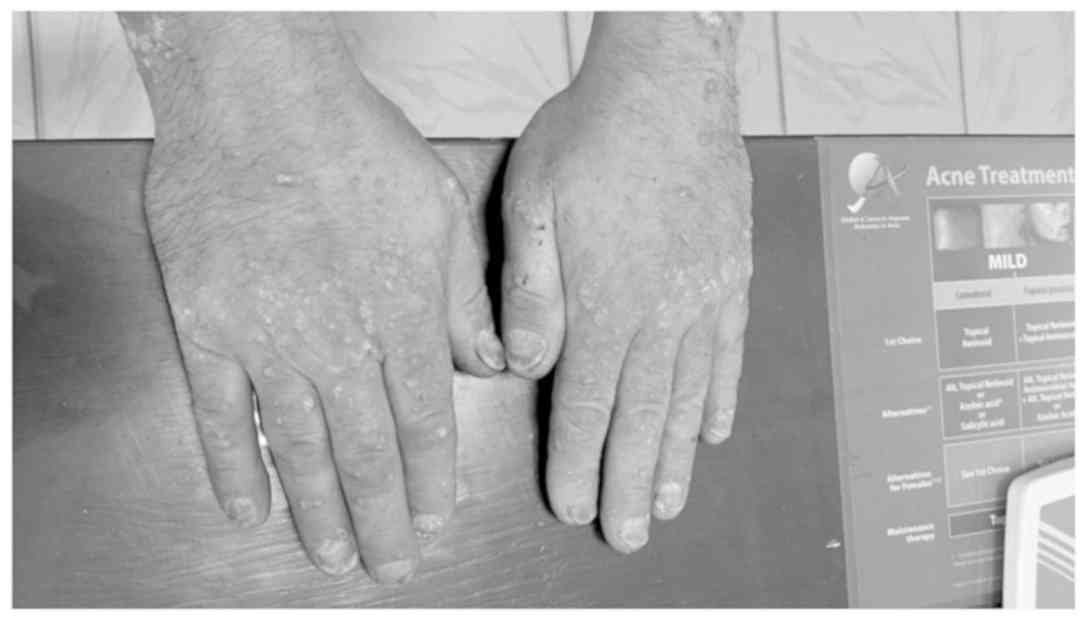
(Fig. 2). Besides, there are also
alterations of the finger and toe nails, with subungual
hyperkeratosis, distal onycholysis, pitting, ‘oil spot’ yellow
discoloration and multiple cross lines (Fig. 3). Furthermore, the patient suffers
from arthralgia and morning joint stiffness of the hands, elbows
and knees.
Results
Paraclinical investigations included complete blood
count, tests to assess hepatic and kidney function, protein
electrophoresis, total cholesterol, urine exam, viral markers for
hepatitis B, hepatitis C and HIV, QuantiFERON-TB Gold test
(Cellestis Limited, Carnegie, Australia), EKG, BMI, radiography of
the hand joint and pulmonary radiography. The results have shown
borderline hypercholesterolaemia (210 mg/dl), positive Ag HBs,
positive Ac HBs, positive Ag HBe, positive QuantiFERON-TB Gold
test, BMI=29.4 kg/m2, elevated levels of ESR erythrocyte
sedimentation rate (ESR) and C-reactive protein (CRP) and normal
transaminases.
The radiographies showed marginal bone erosions in
both hands and knees, bone proliferation and right wrist fusion.
After pneumology examination the chemical prophylaxis was initiated
for tuberculosis with isoniazid 300 mg/day for 9 months, but after
1 month of chemotherapy, the therapy for psoriasis was started.
Additionally, the patient was diagnosed with chronic HBV hepatitis
with positive Ag HBe (positive Ag HBs, positive Ag HBe, negative
IgG anti HVD, PCR ADN-VHB=2341650). Fibromax assessment of fibrosis
and inflammatory activity revealed minimum fibrosis at FibroTest
(score 0.31) and minimum steatosis at SteatoTest (score 0.30) (both
from BioPredictive, Paris, France). Subsequently, we initiated
treatment with entecavir 0.5 mg/day.
In this context, the patient was diagnosed with
moderately-severe psoriasis vulgaris [Psoriasis Area and Severity
Index (PASI)=24.3; Dermatology Life Quality Index (DLQI)=25],
chronic HBV hepatitis with positive Ag HBe and overweight
(BMI=29.4). The therapeutic algorithm included initiation of
anti-TNF therapy with etanercept (2×50 mg/week) combined with
entecavir, an antiviral treatment administered continuously since
the diagnosis of the HBV hepatitis, with hepatic function and viral
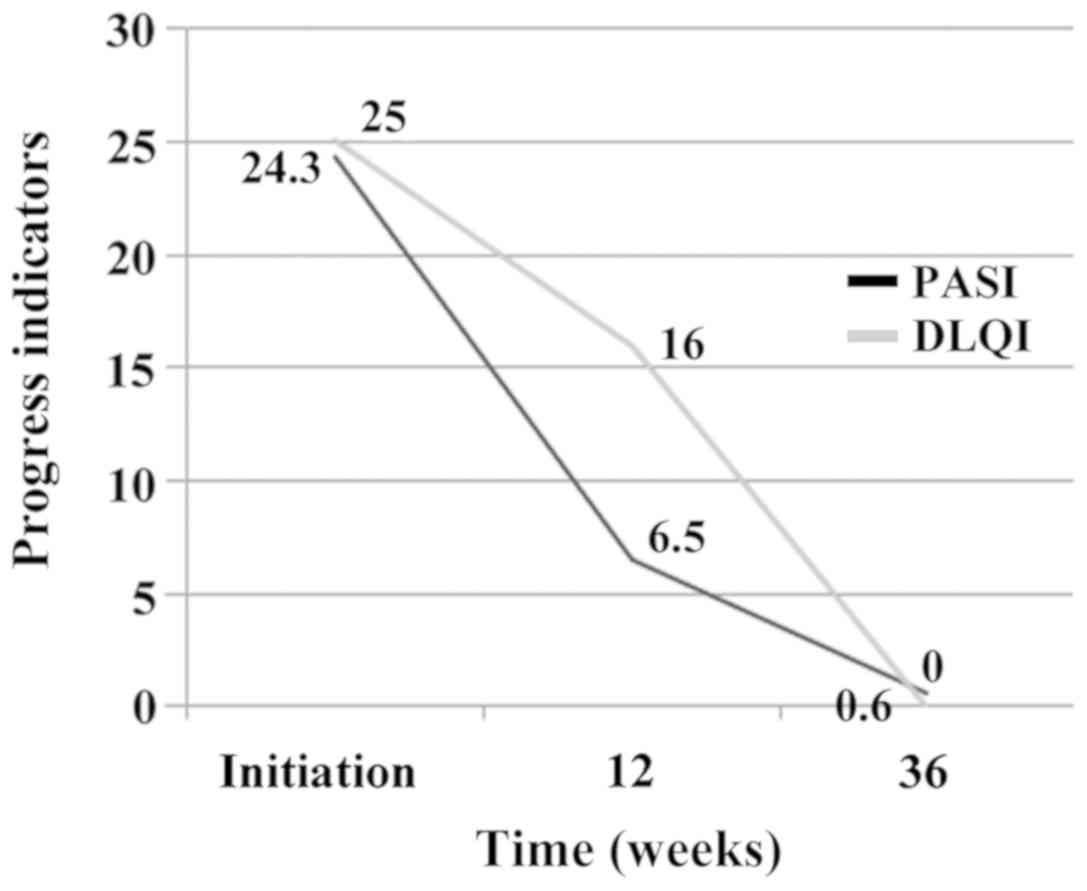
load monitoring. Patient reached PASI 75 at week 12 and registered
significant improvement of DLQI (Fig.
4) and decrease of viral load (below the detection limit of 20
copies/ml), however, without changes in viral markers. After 3
months of therapy with etanercept the patient was given a dose of
etanercept of 50 mg/week combined with entecavir 0.5 mg/day which
he continued until week 36 when psoriatic lesions had cleared
(PASI=0.6; DLQI=0). The patient had normal levels of ESR, CRP,
including transaminases after the cure with MTX.
A previous interesting study indicated that an
increase in malondialdehyde (MDA) level and a decrease in
antioxidants levels are features of psoriasis and that MTX seems to
have protective antioxidative properties during the treatment of
psoriasis (1).
No adverse effects were registered and there was no
evidence of HBV viral replication or changes in viral markers. The
patient is continuing the combined therapy at the present time with
very good results and the evolution can be observed in Fig. 5. All tests above are performed
regularly. They are in normal ranges and the HBV DNA is
undetectable.
Discussion
Our patient was diagnosed clinically in 1998 and
histopathologically in 2014. A cutaneous punch-biopsy was performed
and the histopathology results revealed hyperkeratosis and
parakeratosis with neutrophils inclusions between keratin blades,
wide areas of agranulocytosis, moderate acanthosis with relatively
uniformly elongated and thickened epidermal cristae, increased
mitotic keratinocyte activity and moderate perivascular
inflammatory infiltrate around dilated capillaries. The
histopathological aspect confirmed the clinical diagnosis of
psoriasis vulgaris.
There are specific dermoscopic features suggestive
for psoriasis and reflectance confocal microscopy (RCM) enables
identification of most of the histological features of psoriatic
lesions and both dermoscopy and RCM allow a real-time non-invasive
examination of skin lesions offering the possibility to perform
serial determinations (2).
RCM can also successfully evaluate dilated and
tortuous dermal capillaries, which represent a histological feature
of psoriasis vulgaris. Compared with the skin of healthy subjects,
capillaries examined in lesions of psoriasis have higher mean
values of micromorphological parameters (area, perimeter, Feret's
diameter) and that for each papilla, the number of capillary
sections is higher (3).
As for the treatment, recent studies argue that in
moderately-severe unstable psoriasis, with low DLQI or therapeutic
failure of other systemic treatments, the use of anti-TNF-α agents
in patients with an HBV infection could be an option, but it
requires a multidisciplinary approach and especially a good
collaboration with the enterologist (4–7).
At present, antiviral treatment of adult patients
with chronic viral hepatitis B in Romania includes entecavir,
adefovir, lamivudine or pegylated interferon α-2a (8). Entecavir is a guanosine nucleoside
analogue active against HBV polymerase, from the class of
nucleoside and nucleotide reverse transcriptase inhibitors, and
through its antiviral action, it inhibits the synthesis of AND HBV.
Entecavir, the first line of monotherapy treatment and long-term
use in chronic HBV hepatitis, may be administered in doses of 0.5
or 1 mg/day with regular 6 months assessments; the treatment may be
continued depending on the biochemical and virologic response until
the occurrence of Ac HBs (8).
Recent studies underline the association of anti-TNF
agents with increased risk of viral reactivation in patients with
inactive chronical HBV infection (9,10). Data
from the literature indicate a higher incidence of infections and
lymphoma in patients treated with infliximab and adalimumab as
compared to etanercept. Also, the general safety profile of
etanercept when it comes to the risk of infection and
hepatotoxicity in patients with psoriasis, psoriatic arthritis and
rheumatoid arthritis is higher than that of the other anti-TNF-α
agents (11).
Biosimilars could be a treatment option, but
unfortunately, at present there is rather limited evidence provided
by the clinical trials, which would help a dermatologist feel more
comfortable about prescribing biosimilars. After the studies ended,
we can conclude that there is no specific safety concern today that
was raised and no specific lack of efficacy either. There are also
ongoing centered postmarketing studies regarding biosimilar
immunogenicity which is the main concern for biosimilars (12).
The maximum estimated risk of reactivation of HBV in
patients with history of hepatitis B who did not receive any
biological therapy for psoriasis for an average follow-up period of
30 months was of 2.7% according to a 2015 study (13). In a different study carried out on a
group of 468 patients with negative Ag HBs and positive Ac HBc
treated with anti-TNF agents for rheumatoid conditions, HBV
reactivation was reported for 1.7% of patients and viral markers
were monitored during anti-TNF treatment (14). In the case of our patient, no HBV
reactivation was recorded at 9 months after initiation of treatment
with etanercept and entecavir.
Treatment of patients with psoriasis and chronic HBV
hepatitis with etanercept administered concomitantly with
lamivudine or entecavir has proven to be safe and it reduces the
risk of reactivation of HBV hepatitis (15).
Studies having shown reactivation of HBV after
anti-TNF-α therapy in patients with inactive hepatitis B (negative
Ag HBs) support the prophylactic use of antiviral agents for HBV
carriers even if hepatic function is within normal limits or viral
load is undetectable (16).
During the first 12 weeks after the initiation of
therapy, etanercept may be administered in different doses, either
50 mg twice a week or 50 mg once a week - both therapeutic schemes
being efficient. Nevertheless, the rate of therapeutic response at
week 24 is higher if etanercept is initially administered with 50
mg doses twice a week; after week 24 the treatment may be
administered either continuously or intermittently, both cases
showing long and mid-term efficiency (17,18). In
our case, we followed the treatment protocol approved for Romania
with an initial dose of 2×50 mg/week followed by a usual dose of 50
mg/week combined with entecavir 0.5 mg/day with good results, as
shown by the dynamics of PASI and DLQI and hepatic function.
Recent studies show good adherence to etanercept
treatment in patients with psoriasis, higher in the case of
intermittent treatment than in continuous treatment, which suggests
that the intermittent therapy with etanercept could be the best
therapeutic option both clinically and in terms of adherence
(19,20).
The Medical Council of the National Psoriasis
Foundation of America recommends triple serology (Ag HBs, Ac HBs,
Ac HBc) for hepatitis B screening before starting therapy with
anti-TNF agents, but also with other immunosuppressive drugs such
as ustekinumab, cyclosporine or MTX (21).
Ustekinumab can be an effective alternative therapy
for moderate to severe psoriasis because the benefit-risk profile
in the studied group was favorable, consistent with the global
studies of ustekinumab, but further additional studies are needed
to evaluate long-term administration and the safety profile
(22).
Psoriasis has mast cell activation as a key
mechanism. The important role of mast cells in stress-induced
activation of skin inflammation is suggested by their strategic
location near cutaneous nerve structures and blood vessels and
their complex interconnections with nerve fibers, immune cells and
keratinocytes. An interesting study shows that in the hairy skin
acute stress induces an increased mast cell degranulation that
persists even after prolonged exposure to stress, while in glabrous
skin a short-term stress exposure has a strong stimulating effect
of mast cell degranulation that subsides in intensity as exposure
to stress persists (23).
For treatment, we can use acitretin in combination
with biologic therapies because it is not immunosuppressive. A
large number of observations indicate that psoriasis is linked to
plasma membrane alterations in different types of cells. Some
studies showed that acitretin, even at low concentrations, is
capable of inducing the reduction of red cell deformability
(24).
For the patients with psoriatic arthritis, the
heterogeneity and complexity of psoriatic arthritis pathogenesis
requests complex methodological approaches. Going deeper in the
pathogenesis of this disease, the associated genetic predisposition
can lead to new immune biomarkers whether from the proteomic or
genomic area and identify new therapeutic approaches (25).
Many systemic therapies available for the management
of psoriasis patients who cannot be treated with more conservative
options, such as topical agents and/or phototherapy, can worsen or
reactivate a chronic infection. Therefore, before administering
immunosuppressive therapies it is mandatory to screen patients for
some infections, including hepatitis B virus.
For our patient, etanercept was chosen due to its
high efficiency and a very good safety profile with low
immunogenicity and intermittent administration in selected
cases.
Biologic therapy did not cause HBV reactivation.
However, there are potentially serious complications associated
with HBV reactivation and it is important to measure viral load in
patients with a history of HBV infection prior to initiation of
biologic therapy to rule out occult carriage. These patients should
also be monitored regularly together with a hepatologist (26).
In conclusion, by presenting this case we wish to
emphasize that the use of etanercept in a patient with psoriasis
and hepatitis B is a successful therapeutic alternative which may
be safely used concomitantly with entecavir, with regular
monitoring of viral load and hepatic function tests.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MMC contributed to the design and conception of the
study, and revised it carefully for important intellectual content.
SB, CC, TC, NM were responsible for acquiring the data by screening
the papers identified on Pubmed. SB revised the study critically
for important intellectual content. CC, TC, NM were involved in
drafting the study. IEN analyzed and interpreted the data. AR
contributed to the conception and design of the study, she revised
the language and contributed to drafting the study. All authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the local Ethics
Committee of ‘Carol Davila’ University of Medicine and Pharmacy
(Bucharest, Romania), and a signed informed consent was obtained
from the patient included in this study.
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boda D, Negrei C, Nicolescu F and Balalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
2
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl):1191–1196.
2014.PubMed/NCBI
|
|
3
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|
|
4
|
Nosotti L, Francesconi F, Izzi S,
Berardesca E, Morrone A and Bonifati C: Safety of antitumour
necrosis factor-α therapy in psoriatic patients with hepatitis B
virus infection. Br J Dermatol. 162:1408–1410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fotiadou C, Lazaridou E and Ioannides D:
Safety of anti-tumour necrosis factor-α agents in psoriasis
patients who were chronic hepatitis B carriers: A retrospective
report of seven patients and brief review of the literature. J Eur
Acad Dermatol Venereol. 25:471–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cassano N, Mastrandrea V, Principi M,
Loconsole F, De Tullio N, Di Leo A and Vena GA: Anti-tumor necrosis
factor treatment in occult hepatitis B virus infection: A
retrospective analysis of 62 patients with psoriatic disease. J
Biol Regul Homeost Agents. 25:285–289. 2011.PubMed/NCBI
|
|
7
|
Prignano F, Ricceri F, Pescitelli L,
Zanieri F and Lotti T: Tumour necrosis factor-α antagonists in
patients with concurrent psoriasis and hepatitis B or hepatitis C:
A retrospective analysis of 17 patients. Br J Dermatol.
164:645–647. 2011.PubMed/NCBI
|
|
8
|
Romanian Therapeutic Guidelines: Chronic
Hepatitis. http://www.ms.ro/wp-content/uploads/2018/08/Anexa_Ordin_protocoale_29_august_2018.pdf
|
|
9
|
Zoulim F and Durantel D: Antiviral
therapies and prospects for a cure of chronic hepatitis B. Cold
Spring Harb Perspect Med. 5(a021501)2015.doi:
10.1101/cshperspect.a021501. PubMed/NCBI
|
|
10
|
Seto WK: Hepatitis B virus reactivation
during immunosuppressive therapy: Appropriate risk stratification.
World J Hepatol. 7:825–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Domm S, Cinatl J and Mrowietz U: The
impact of treatment with tumour necrosis factor-alpha antagonists
on the course of chronic viral infections: A review of the
literature. Br J Dermatol. 159:1217–1228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Girolomoni G, Altomare G, Ayala F,
Berardesca E, Calzavara-Pinton P, Chimenti S, Peserico A, Puglisi
Guerra A and Vena GA: Safety of anti-TNFα agents in the treatment
of psoriasis and psoriatic arthritis. Immunopharmacol
Immunotoxicol. 34:548–560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olteanu R, Zota A and Constantin MM:
Biosimilars: An update on clinical trials (review of published and
ongoing studies). Acta Dermatovenerol Croat. 25:57–66.
2017.PubMed/NCBI
|
|
14
|
Sanz-Bueno J, Vanaclocha F, García-Doval
I, Torrado R, Carretero G, Daudén E, Patricia Ruiz-Genao D,
Alsina-Gibert MM, Pérez-Zafrilla B, Pérez-Rial G, et al members of
the BIOBADADERM group, : Risk of reactivation of hepatitis B virus
infection in psoriasis patients treated with biologics: A
retrospective analysis of 20 cases from the BIOBADADERM Database.
Actas Dermosifiliogr. 106:477–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YH, Bae SC and Song GG: Hepatitis B
virus (HBV) reactivation in rheumatic patients with hepatitis core
antigen (HBV occult carriers) undergoing anti-tumor necrosis factor
therapy. Clin Exp Rheumatol. 31:118–121. 2013.PubMed/NCBI
|
|
16
|
Kuroda T, Wada Y, Kobayashi D, Sato H,
Murakami S, Nakano M and Narita I: Effect of etanercept and
entecavil in a patient with rheumatoid arthritis who is a hepatitis
B carrier: A review of the literature. Rheumatol Int. 32:1059–1063.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung SJ, Kim JK, Park MC, Park YB and Lee
SK: Reactivation of hepatitis B viral infection in inactive HBsAg
carriers following anti-tumor necrosis factor-alpha therapy. J
Rheumatol. 36:2416–2420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strohal R, Chimenti S, Vena GA and
Girolomoni G: Etanercept provides an effective, safe and flexible
short- and long-term treatment regimen for moderate-to-severe
psoriasis: A systematic review of current evidence. J Dermatolog
Treat. 24:199–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strohal R, Puig L, Chouela E, Tsai TF,
Melin J, Freundlich B, Molta CT, Fuiman J, Pedersen R and Robertson
D: The efficacy and safety of etanercept when used with as-needed
adjunctive topical therapy in a randomised, double-blind study in
subjects with moderate-to-severe psoriasis (the PRISTINE trial). J
Dermatolog Treat. 24:169–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esposito M, Gisondi P, Cassano N, Babino
G, Cannizzaro MV, Ferrucci G, Chimenti S and Giunta A: Treatment
adherence to different etanercept regimens, continuous vs.
intermittent, in patients affected by plaque-type psoriasis. Drug
Dev Res. 75 (Suppl 1):S31–S34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babino G, Esposito M, Mazzotta A, Chimenti
S and Giunta A: Entecavir and intermittent etanercept therapy in a
patient with concurrent hepatitis B virus infection and psoriasis.
Acta Derm Venereol. 93:373–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Olteanu R, Constantin MM, Zota A,
Dorobantu DM, Constantin T, Șerban ED, Bălănescu P, Mihele D and
Gheucă Solovăstru L: Original clinical experience and approach to
treatment study with interleukin 12/23 inhibitor in
moderate-to-severe psoriasis patients. Farmacia. 64:918–921.
2016.
|
|
23
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1059502014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Negrei C, Arsene AL, Toderescu CD, Boda D
and Ilie M: Acitretin treatment in psoriasis may influence the cell
membrane fluidity. Farmacia. 60:767–771. 2012.
|
|
25
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
26
|
Motaparthi K, Stanisic V, Van Voorhees AS,
Lebwohl MG and Hsu S; Medical Board of the National Psoriasis
Foundation, : From the Medical Board of the National Psoriasis
Foundation: Recommendations for screening for hepatitis B infection
prior to initiating anti-tumor necrosis factor-alfa inhibitors or
other immunosuppressive agents in patients with psoriasis. J Am
Acad Dermatol. 70:178–186. 2014. View Article : Google Scholar : PubMed/NCBI
|