Introduction
In 2006, Melles et al (1) presented Descemet's membrane endothelial
keratoplasty (DMEK), a technique, which requires that the
DM-endothelium complex is fabricated prior to the operation. The
postoperative anatomical structure of DMEK conforms to the
physiological state of the cornea (1), however, a worldwide shortage of donor
cornea has limited its application. In vitro corneal
endothelial cell (CEC) culture is expected to solve this
problem.
In 1979, Gospodarowicz et al (2) seeded in vitro-cultured bovine
CECs onto decellularized bovine corneas, a study which inspired
several fabricated implant studies, including the seeding of CECs
onto gelatine film (3), amniotic
membrane (4), gelatine hydrogel
(5) and a thin corneal stromal plate
(6). Despite these efforts,
maintaining the normal morphology and density of CECs in
vivo remains a problem.
The corneal endothelium originates from the neural
crest and lines the innermost layer of the cornea (7). Normal CECs are a hexagonal monolayer of
flat cells, which arrange in a cobblestone-like morphology that
form a physical barrier between the aqueous humour and the corneal
stroma (8). Normal human CECs
(HCECs) do not proliferate in vivo, due to arrest at the
G1 phase (9). HCEC injury
is primarily repaired by the expansion and migration of nearby
cells that fill the damaged area (10). Proliferation of functional HCECs is
difficult to achieve using standard cell culture techniques
(11). However, HCECs have been
successfully cultured in vitro with epidermal growth factor,
platelet-derived growth factor, bovine pituitary extract and foetal
bovine serum (10). However, after
multiple passages, HCEC proliferation decreases significantly and
changes in cell morphology occur (11).
Rho-associated protein kinases (ROCKs) are involved
in a variety of cellular activities, which include cell adhesion,
proliferation, metabolism, apoptosis and cell cycle regulation
(12). Y-27632 is a selective ROCK
inhibitor, which can be used to inhibit the Rho signalling pathway
(13). In the current study, Y-27632
was added to the culture medium to enhance the proliferation of
functional in vitro-cultured rabbit CECs (RCECs). A
heterologous implant was constructed using the in
vitro-cultured RCECs and a porcine DM carrier. To prevent
immunological rejection and transplant failure, an antigen-free
technique was used to improve histocompatibility. To the best of
our knowledge, the method used to construct this heterologous
implant in the present study has not been previously reported.
Materials and methods
RCEC isolation and cell culture
A total of 3 New Zealand white rabbits (female, n=2;
male, n=1; mean body weight, 2.5 kg) were provided by the
Experimental Animal Center of the Tongji University School of
Medicine. Rabbits were maintained under controlled conditions
(temperature, 22±2°C; humidity, 55±5%; 12-h light/dark cycles) and
were allowed free access to food and water. Rabbits were sacrificed
by an injection of sodium pentobarbital solution (100 mg/kg; Bayer)
in the ear vein and their eyeballs were removed. Cornea were
dissected and placed on a petri dish, endothelium side up. The
DM-endothelium complex was isolated from the cornea under an
anatomic microscope, tissue was minced thoroughly and cells were
detached following treatment with 0.25% trypsin for 5 min. Isolated
cells were collected and centrifuged at 252 × g for 5 min at room
temperature. The supernatant was removed and RCECs were cultured in
Dulbecco's modified Eagle medium (DMEM)/F12 medium (HyClone; GE
Healthcare Life Sciences) supplemented with 10% FBS (HyClone; GE
Healthcare Life Sciences), 1% penicillin-streptomycin solution
(Beyotime Institute of Biotechnology) and 0.1% Y-27632 (10 µM;
Sigma-Aldrich; Merck KGaA) (14),
and maintained at 37°C in a 5% CO2-humidified incubator.
The culture medium was changed every 2 days. RCECs in the control
group were cultured in DMEM/F12 without Y-27632. RCECs were
passaged when cells reached 80–90% confluence. The current study
was approved by the Ethics Committee of Tongji Hospital Affiliated
with Tongji University School of Medicine (permit no.
42501068531010711A1001).
Flow cytometry
Logarithmic growth phase cells (80,000 cells) were
isolated and centrifuged at 252 × g for 5 min at 37°C. Cells were
resuspended in 195 ml of Annexin V-FITC binding buffer (Beyotime
Institute of Biotechnology) and subsequently stained with 2 ml of
Annexin V-FITC. The solution was mixed and incubated in the dark
for 10 min at room temperature. The cell suspension was centrifuged
at 252 × g for 5 min at 37°C, the supernatant was removed and cells
were resuspended in 190 ml of Annexin V-FITC binding buffer. Cells
were subsequently incubated with 10 ml of propidium iodide and
incubated in the dark using an ice bath method, as previously
described (15). Cells were filtered
through a nylon mesh prior to analysis. Stained cell suspensions
were detected using an AccuriC6 flow cytometer (BD Biosciences) and
analyzed using FlowJo software (version 7.6.1; Tree Star,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from RCECs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reverse transcribed into cDNA using ExScript RT reagent kit
(Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was subsequently
performed using SYBR Green PCR Master mix (Invitrogen; Thermo
Fisher Scientific, Inc.), with a final volume of 20 ml that
included 10 µl SYBR-green mix, 0.4 µl 50X ROX reference Dye II, 2.5
µl reverse primer (1 µM), 2.5 µl forward primer (1 µM), 1 ml
diluted cDNA (10 ng/ml) and 3.6 ml of ddH2O. The target
gene primer sequences were designed with PrimerPremier5 software
(Premier Biosoft) and aligned in BLAST. The primers used are
summarised in Table I. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95°C for 10 min; followed by 39 cycles of 98°C for 30 sec, 58°C
for 30 sec and 74°C for 30 sec. Relative mRNA expression levels
were quantified using the 2−ΔΔCq method (16) and normalized to the internal
reference gene GAPDH.
 | Table I.Quantitative PCR primer
sequences. |
Table I.
Quantitative PCR primer
sequences.
| Gene | Primer sequence
(5′-3′) | Product length
(bp) |
|---|
|
Na+-K+-ATPase | F:
TCTGTAACAGGGCGGTATT | 176 |
|
| R:
GGTGGAGTTGAAGGGTATCT |
|
| GAPDH | F:
CAGTCTTCTGGGTGGCAGTG | 217 |
|
| R:
AGCCAAACGGGTCATCATCTC |
|
| Collagen type IV
α2 | F:
GGCTGGCGGAGTCTGTGGAT | 137 |
|
| R:
ACTCGATGAAGGGCGTGGC |
|
| Collagen type VIII
α1 | F:
GCAGACGGGCATCTTCAC | 172 |
|
| R:
CATCACGGGCTCGTTGTT |
|
| AQP1 | F:
GCTCACCCACAACTTCAACAA | 152 |
|
| R:
ATCGCCGTCCAGGTCATAC |
|
| Keratin-12 | F:
TCTCCAAACCGCAGACAC | 162 |
|
| R:
GACCACTTCGCCATTCAC |
|
|
| F:
GAGAAAGUAUGUGAAGAUUTT |
|
| VDAC2 | R:
AAUCUUCACAUACUUUCUGTT | 158 |
|
| F:
GACUCUUGAUACCAUAUUUTT |
|
| VDAC3 | R:
AAAUAUGGUAUCAAGAGUCTT | 174 |
Immunocytochemistry
Third-generation CECs were seeded onto six-well
plates at a density of 1×103 cells/well and cultured for
up to 4 days. CECs were fixed in 95% ethanol for 15 min at room
temperature. Cells were incubated with 0.3%
H2O2 for 10 min at 37°C to inhibit endogenous
peroxidase activity. Subsequently cells were incubated with 2% goat
serum albumin (Invitrogen; Thermo Fisher Scientific, Inc.) for 10
min at 37°C. Cells were incubated with primary antibody directed
against connexin (Cx)43 (1:400; cat. no. 13-8300; Invitrogen;
Thermo Fisher Scientific, Inc.) overnight at 4°C. Following primary
incubation, cells were incubated with biotin-labeled goat
anti-rabbit secondary antibodies (1:400; cat. no. 65-6120;
Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min at room
temperature. After incubating with catalase-labeled streptomyces
antibiotic proteins for 10 min, cells were subsequently stained
with 3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA) and observed
under a phase-contrast microscope (magnification, ×40). In the
control group, cells were incubated with PBS instead of primary
antibody.
Immunofluorescence
Third-generation CECs were seeded onto six-well
plates at a density of 1×103 cells/well and cultured for
up to 4 days. CECs were fixed in 4% formaldehyde for 10 min at room
temperature. Cells were incubated with 0.2% Triton X-100 for
membrane permeabilization, prior to being blocked with 2% goat
serum albumin for 30 min at room temperature. Cells were incubated
with primary antibody directed against Cx43 (1:400) overnight at
4°C. Cells were washed three times in PBS. Following this, cells
were incubated with FITC goat anti-rabbit IgG secondary antibody
(1:100; cat. no. 65-6120; Invitrogen; Thermo Fisher Scientific,
Inc.) in the dark for 1.5 h at 37°C. Cell nuclei were
counterstained with DAPI for 5 min at room temperature and
fluorescence was observed under a fluorescence microscope
(magnification, ×40). In the control group, cells were incubated
with PBS instead of primary antibody.
Preparation of porcine DM as a
carrier
A total of 3 pigs (; female, n=2; male, n=1; mean
body weight, 280 kg) were provided by Shanghai Qinnong Animal
Husbandry Technology Co., Ltd. (license no. 310230000455889). Pigs
were maintained under controlled conditions (temperature, 22±2°C;
humidity, 55±5%; 12-h light/dark cycles) and were allowed free
access to food and water. Pigs were sacrificed by an injection of
sodium pentobarbital solution (100 mg/kg) in the ear vein and their
eyeballs were removed. Porcine corneas were dissected and placed on
a petri dish, endothelium side up. A drop of Trypan blue solution
(Sigma-Aldrich; Merck KGaA) was added to each cornea. The
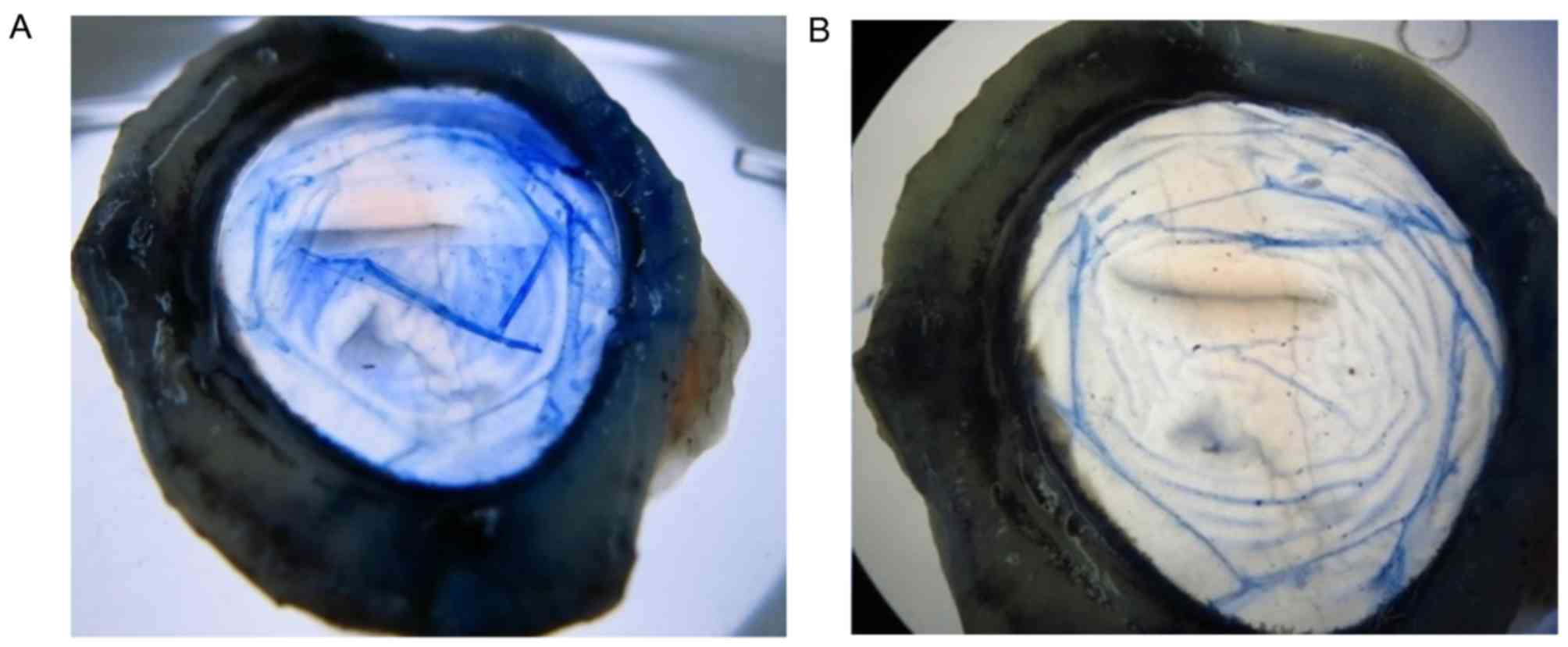
DM-endothelium complexes were isolated, and a 9 mm trephine was
used to make a circular shape (Fig.
1). CECs were subsequently removed by gently wiping with a
cotton swab under a microscope. After washing with 0.9% NaCl, the
porcine DM carriers were preserved in liquid nitrogen for 48 h,
followed by storage in sterile glycerol at 4°C for three
months.
Porcine DM antigenicity
A total of 6 wild-type female Swiss albino mice
(age, 6–8 weeks; weight, 22 g) were provided by the Experimental
Animal Center of the Tongji University School of Medicine
(Shanghai, China). Mice were maintained under controlled conditions
(temperature, 20–23°C; humidity, 40–60%; 12-h light/dark cycles)
and were given access to water and food ad libitum. The porcine DM
carriers were washed three times with PBS. The porcine DM carriers
were implanted into mice enterocoelia (n=6) and rabbit
paravertebral muscle (n=4), in rabbits following CEC isolation,
after general anaesthesia with sodium pentobarbital (50 and 30
mg/kg, respectively). In the control groups, mice enterocoelia
(n=3) and the rabbit contralateral muscle (n=2) were incised
without porcine DM carrier implantation. Close postoperative
observation was performed to monitor vital signs, postoperative
eating and activity behaviour. After 2 weeks, mice enterocoelia and
rabbit paravertebral muscle was incised and observed. To examine
the effect of porcine DM carrier implantation on splanchnic tissue
and muscle, haematoxylin and eosin (H&E) staining was
performed, as previously described (17). Briefly, tissue samples were fixed in
10% formalin overnight at 4°C and embedded in paraffin.
Paraffin-embedded tissue samples were cut into 3-µm-thick sections.
Tissue sections were subsequently deparaffinized and rehydrated.
Deparaffinized sections were stained with hematoxylin-imidine Red
(HE) at room temperature for 3 min. Any pathological changes were
observed under a light microscope (magnification, ×40).
Preparation of the porcine DM-RCEC
complex
After the glycerol was removed, the porcine DM
carriers were washed three times with 50 mg/ml vitriolic gentamicin
(Bayer) and PBS buffer. RCECs that had been passaged twice in
vitro were resuspended (1×106 cells/ml). The porcine
DM carriers (n=8) were placed in a six-well plate and the RCECs
were seeded on top of the porcine DM carriers. The DM-RCEC mixture
was cultured in DMEM/F12 at 37°C in a 5% CO2-humidified
incubator. Once cell adherence was observed, more culture medium
was added to the plate. The complex was incubated until cell
density reached 2,000–2,500 cells/mm2. The culture
medium was changed once every 3 days.
Alizarin red-trypan blue staining
The porcine DM-RCEC complexes (n=2) were transferred
onto a glass slide with the endothelium side up. Cells were stained
with 0.25% Trypan blue (Sigma-Aldrich; Merck KGaA) for 90 sec at
room temperature. Cells were washed with PBS and excess liquid was
removed using filter paper. Cells were subsequently stained with
0.2% alizarin red (pH 4.2; Sigma-Aldrich; Merck KGaA) for 90 sec
and rinsed twice with saline. The porcine DM-RCEC complexes were
fixed with 2% glutaraldehyde (Beyotime Institute of Biotechnology)
for 10 min at room temperature and observed under a microscope
(magnification, ×40).
Cell membrane potential
measurement
RCECs obtained from the porcine DM-RCEC complexes
were used as the experimental group (n=4), whereas RCECs from fresh
rabbit eyeballs were used as the control group (n=4). A total of 4
New Zealand white rabbits (female, n=2; male, n=2; mean body
weight, 2.5 kg) were provided by the Experimental Animal Center of
the Tongji University School of Medicine. Rabbits were maintained
under controlled conditions (temperature, 22±2°C; humidity, 55±5%;
12-h light/dark cycles) and were allowed free access to food and
water. Rabbits were sacrificed by an injection of sodium
pentobarbital solution (100 mg/kg; Bayer) in the ear vein and their
eyeballs were removed. RCECs in both groups were prepared as a cell
suspension (1×106 cells/ml), transferred onto a glass
slide and placed in a recording bath. Measurements were made in
well-differentiated cells, which were observed using an immersion
objective lens in the perfusate. A tight-seal, whole-cell recording
patch-clamp technique was used to record the membrane potential
(18). Briefly, the patch-clamp
amplifier in voltage-clamp mode was used to seal the connection,
while the microelectrode was used to generate a high-resistance up
to 1 GW. After generating resistance, action potentials were
recorded once the patch-clamp amplifier was in current-clamp mode.
Data were analysed using PCLAMP 6.0 software (Molecular Devices,
LLC).
Tension detection
RCECs obtained from the porcine DM-RCEC complexes
were used as the experimental group (n=2), whereas fresh porcine
DM-endothelium complex were used as the control group (n=2). Both
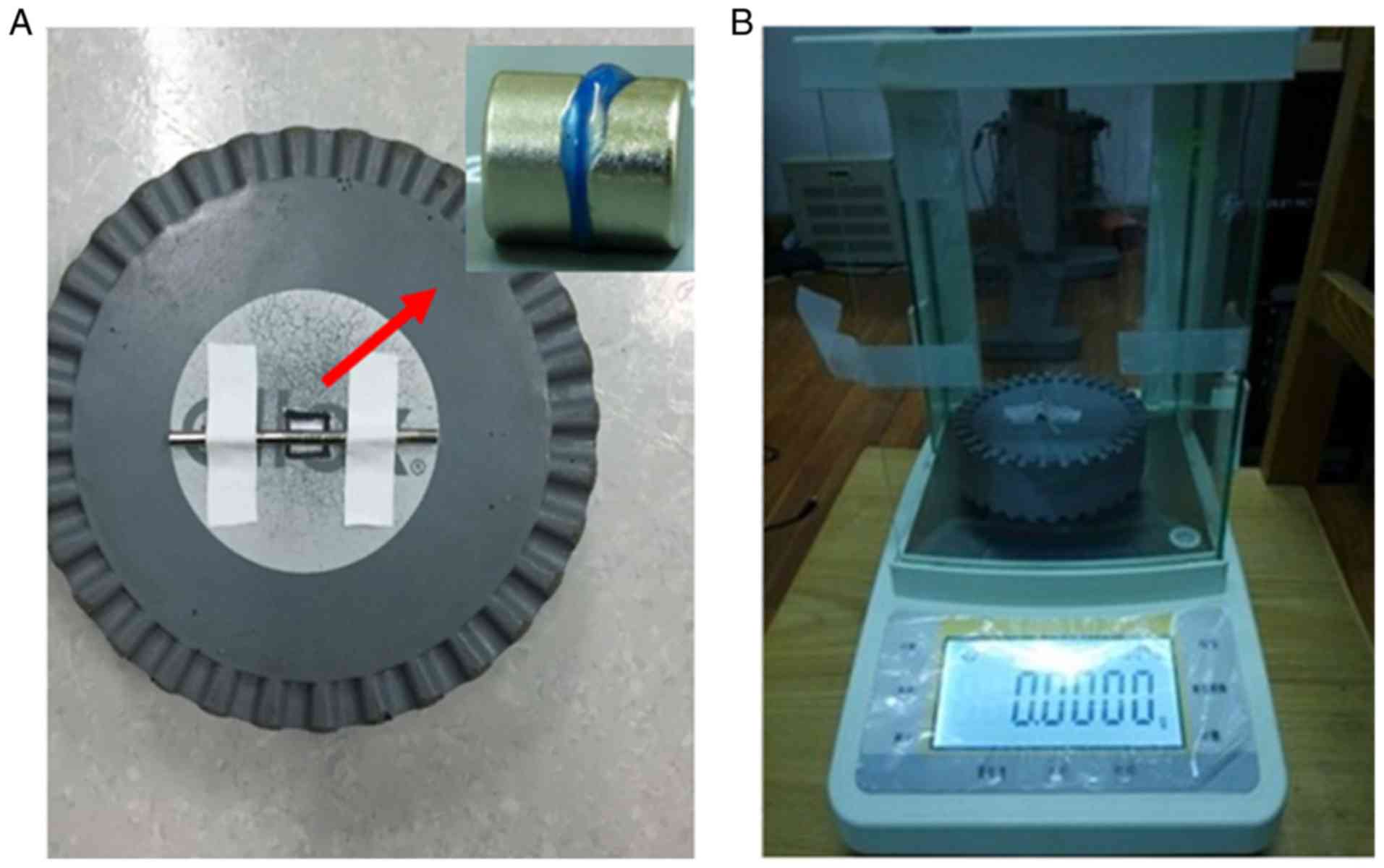
groups comprised 10 circular samples, each 9 mm in diameter. An
electronic balance was preheated for 30 min and circular foam
padding was used to isolate the magnetic field (Fig. 2A). Each sample was flattened between
two circular magnets (8×5 mm), which were immobilized at the centre
of the foam padding (Fig. 2B). After
peeling, the sample was strongly pulled in a vertical direction
using antimagnetic microforceps. The value on the electronic
balance was recorded when the sample broke, and the absolute value
was taken as the sample's tension value.
Statistical analysis
Data presented as the mean ± standard deviation. All
statistical analyses were performed using SPSS software (version
11.0; SPSS, Inc.). Group means were analyzed using the
χ2 test and Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
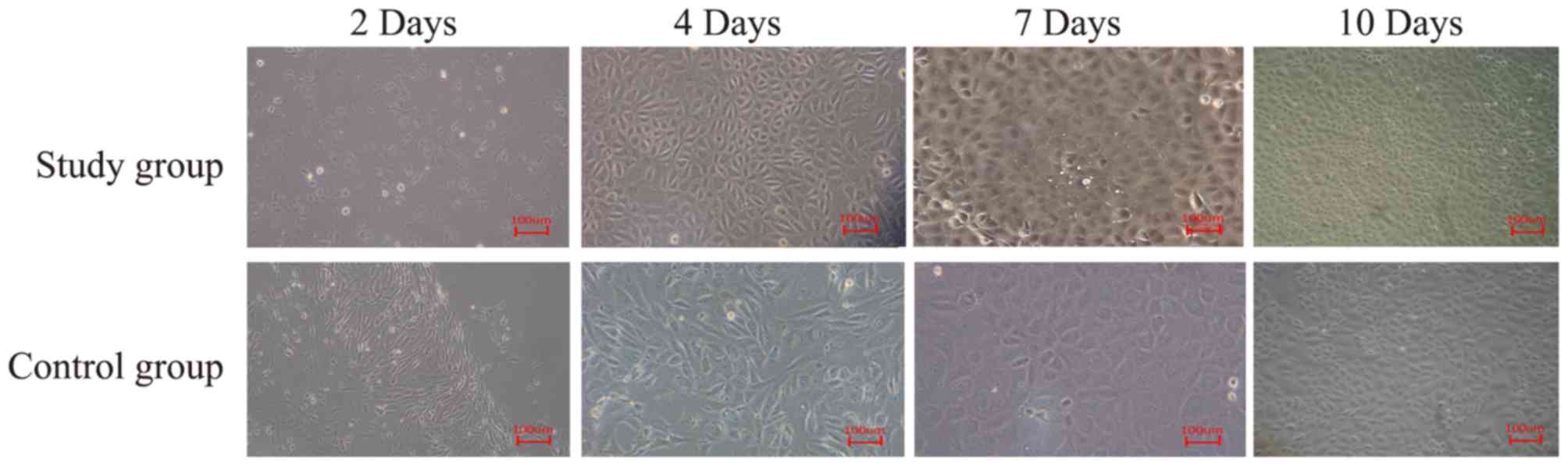
RCEC cultures
Primary cultured RCECs were cultivated in a
monolayer and cells displayed polygonal and cobblestone-like
morphology. In addition, cells demonstrated strong proliferation
ability and reached 80–90% confluence within 2 weeks. Following
24-h culture, there were more adherent RCECs in the experimental
group compared with the control group (data not shown). At day 4,
confluence occurred and cells demonstrated logarithmic growth. At
day 7, cells demonstrated confluence and regular cellular
morphology. At day 10, the second passage of RCECs was uniformly
distributed and maintained cobblestone-like morphology. In
addition, RCECs in the experimental group had reached 70%
confluence compared with the control group, which had reached 55%
confluence (Fig. 3). The second
passage was established at day 12 and day 15 in the experimental
and control groups, respectively (data not shown). RCECs gradually
changed in morphology, with enlarged cell bodies in subsequent
passages. At similar passage states, the RCECs in the experimental
group had fewer morphological changes, fewer cytoplasmic bubbles
and particles, and more active proliferation compared with the
control group (data not shown). Based on these results, the second
passage of RCECs from the experimental group was used to prepare
the implant.
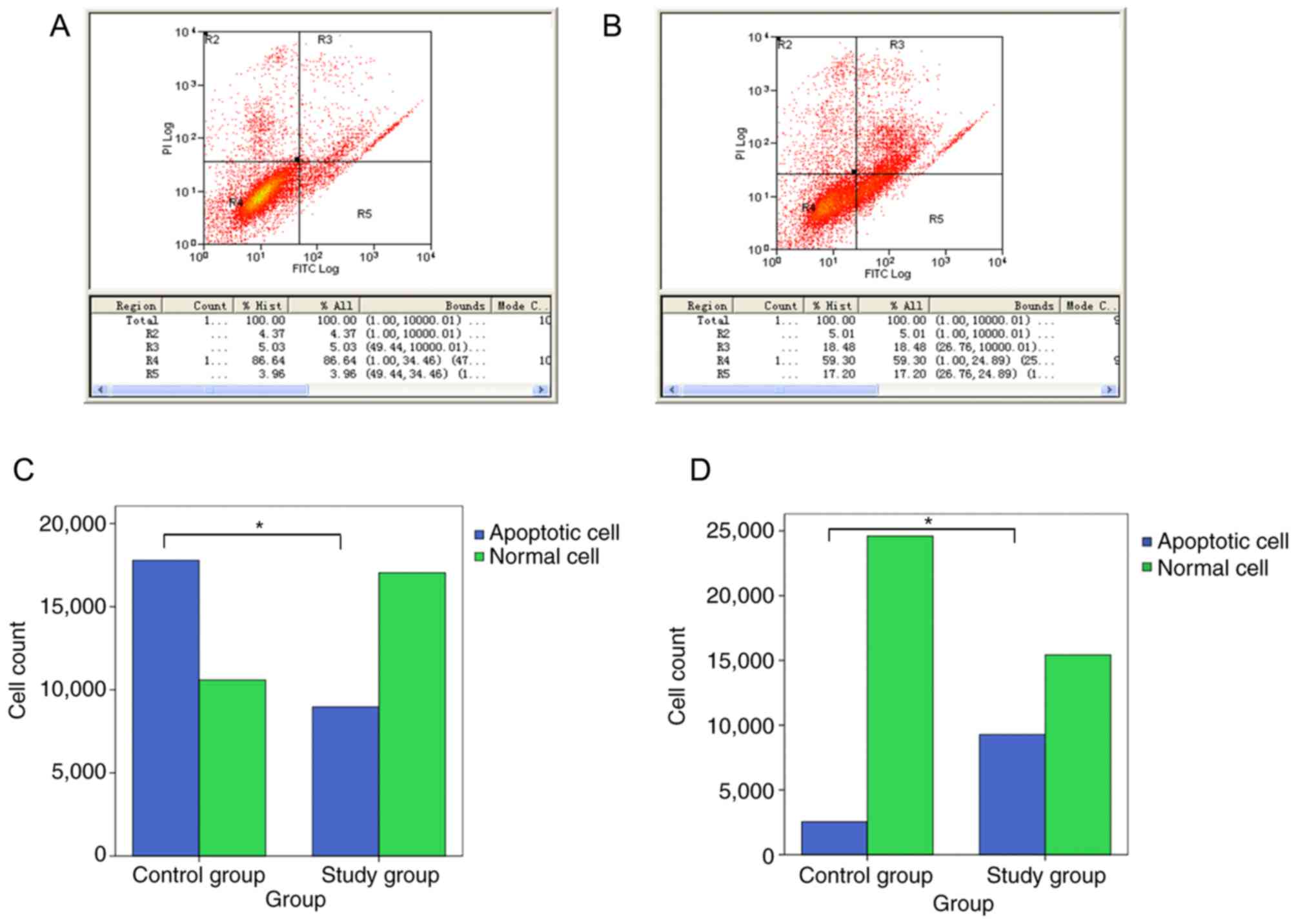
RCEC growth and apoptosis
The ratio of normal cells to total cells in the
experimental and control groups were 86.64 and 59.30%, respectively
(Fig. 4A and B). The cell apoptosis
rates of the two groups were 8.99 and 35.68%, respectively
(P<0.05; Fig. 4A and B). The
R2/R1 value, an indicator of the RCEC proliferation rate, was
significantly increased in the experimental group compared with the
control group.
Confirmation of primary RCECs
The solubility-curve analysis was unimodal and
demonstrated the specificity of the result (data not shown). The
high cycle threshold (Ct) values (>30) and 2−∆∆Cq
values (=0) indicated low amounts of target sequence. As shown in
Table II, the Ct and
2−∆∆Cq values of the epithelial-associated markers,
Na+-K+-ATPase, collagen α2 (IV), collagen α1
(VIII), aquaporin (AQP)1 and keratin-12 indicated that the primary
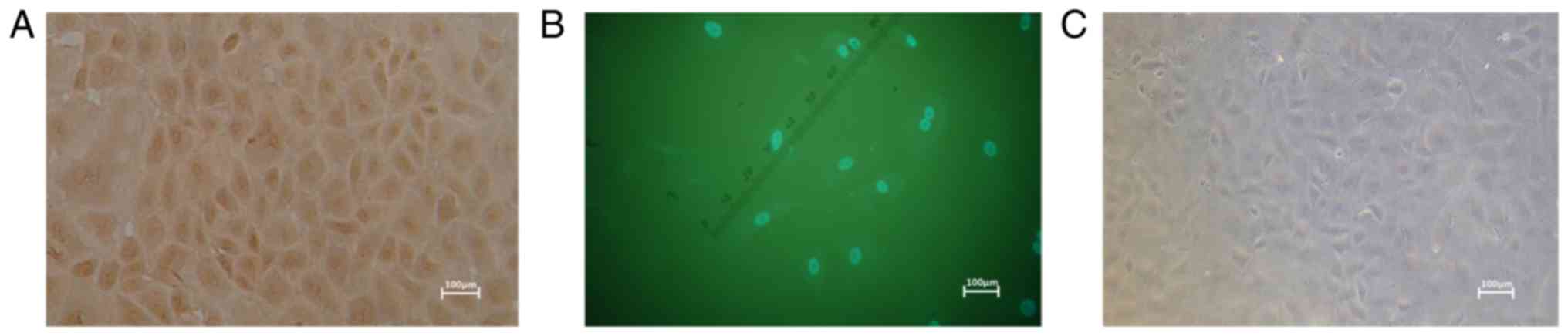
RCECs were endothelial cells. Immunostaining demonstrated that Cx43
expression was well preserved in the cytoplasm and cell membrane of
in vitro CECs compared with the control (Fig. 5). The labeling rate of positively
stained CECs was ~95%, which suggests that CECs have increased
activity and high expression levels of intercellular junction
protein Cx43 in vitro (data not shown). These results
suggest that the signal transduction, substance exchange and cell
metabolism may be enhanced between CECs.
 | Table II.Ct and 2−ΔΔCq values of
endothelial-associated markers. |
Table II.
Ct and 2−ΔΔCq values of
endothelial-associated markers.
| Gene | Average Ct | SD |
2−ΔΔCq |
|---|
| GAPDH | 18.555 | 0.142606 |
|
|
Na+-K+-ATPase | 26.82833 | 0.740632 | 0.003 |
| Collagen
α2(IV) | 24.21967 | 0.052539 | 0.018 |
| Collagen
α1(VIII) | 24.90067 | 0.325694 | 0.011 |
| AQP1 | 22.25867 | 0.143420 | 0.068 |
| Keratin-12 | 30.6200 | 0.132556 | 0.000 |
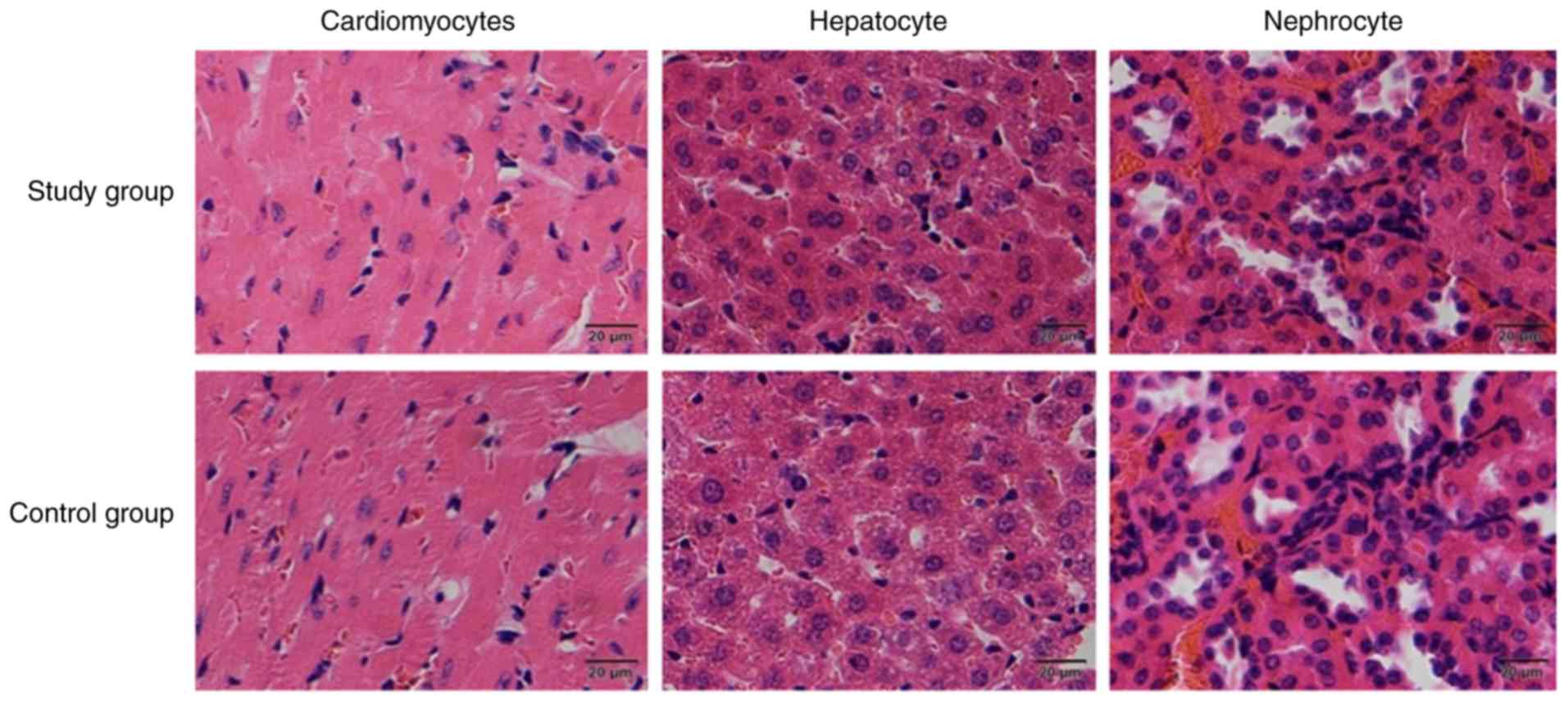
Porcine DM carrier antigenicity
During the 2-week porcine DM antigenicity test, mice
and rabbits maintained normal activity, without demonstrating any
psychological or limb disorders and no mortality was observed. All
incisions healed well without infection or transudation. H&E
staining was performed two weeks following implanation. H&E
staining revealed that following implantation, normal
cardiomyocyte, hepatocyte and nephrocyte morphology without any
obvious abnormalities following implantation of the porcine DM
carrier in mice enterocoelia in both the experimental and control
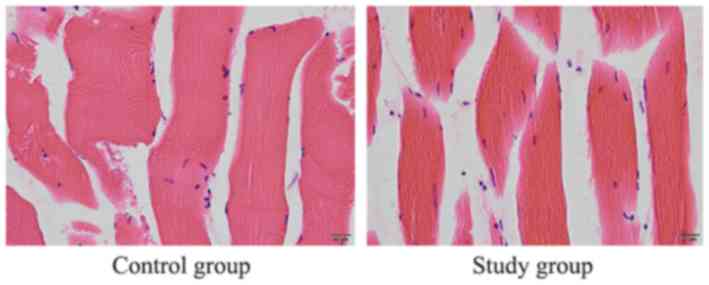
groups (Fig. 6). In addition,
H&E staining demonstrated myofiber with clear microstructure
and without signs of inflammation, necrosis or degeneration in
rabbit paravertebral muscle following implantation of the porcine
DM carrier in the experimental and control groups (Fig. 7).
Confirmation of successful
implantation
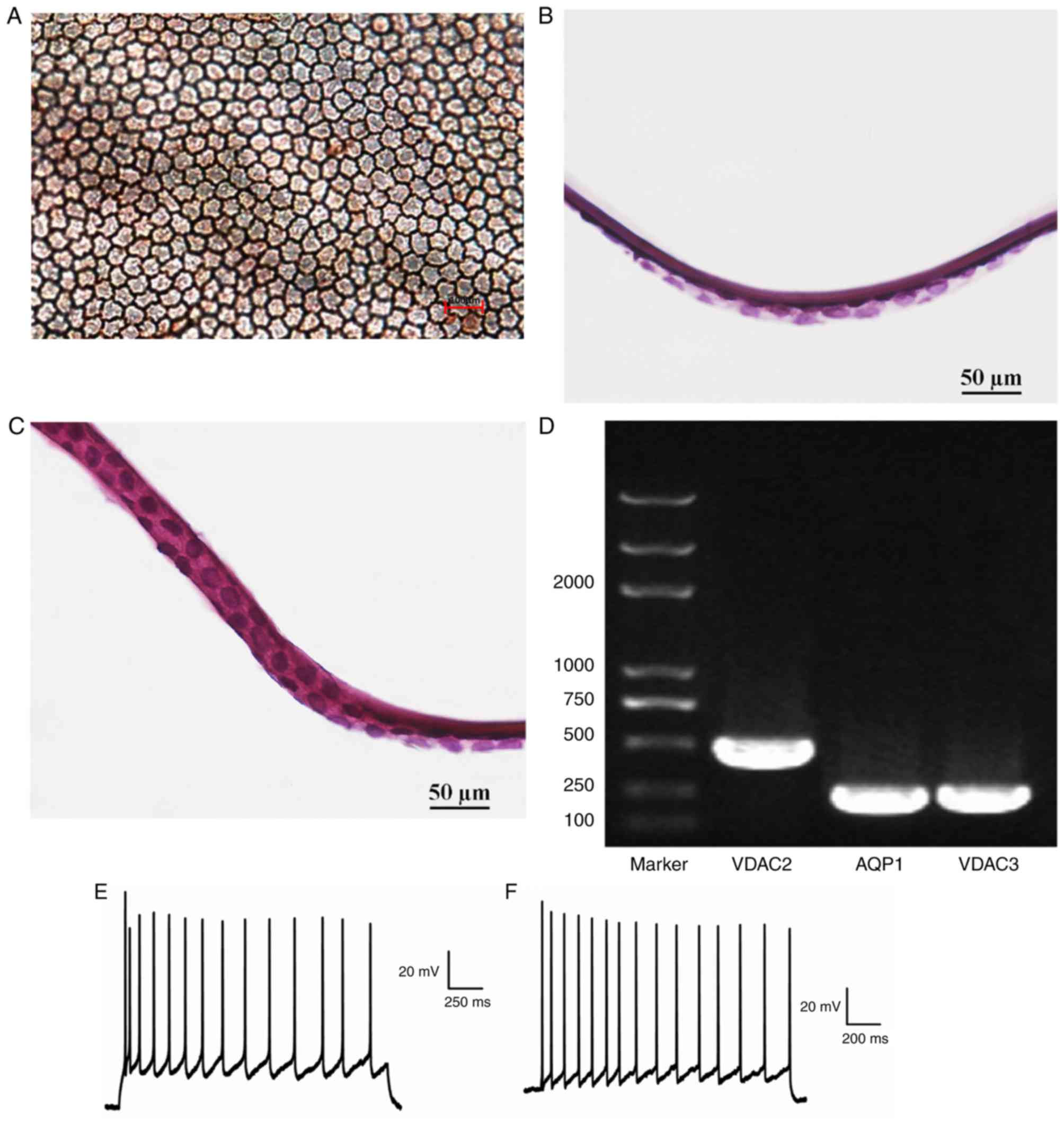
Alizarin red-trypan blue demonstrated that implanted
RCECs adhered to the endothelium side of the porcine DM as a
monolayer, which were tightly connected with a cell density of
2,000–2,500/mm2 and cells exhibited hexagonal and
cobblestone-like morphology (Fig.
8A). H&E staining following implantation revealed that in
the experimental group, RCECs adhered to the surface of porcine DM
uniformly (Fig. 8B), similar to that
observed in the control group of fresh RCEC-DM complexes (Fig. 8C). The expression levels of
voltage-dependent anion-selective channel-2 (VDAC2),
voltage-dependent anion-selective channel-3 (VDAC3) and AQP1 in
implanted RCECs were determined by RT-qPCR. The positive expression
of VDAC2, VDAC3 and AQP1 in implanted RCECs correspond with typical
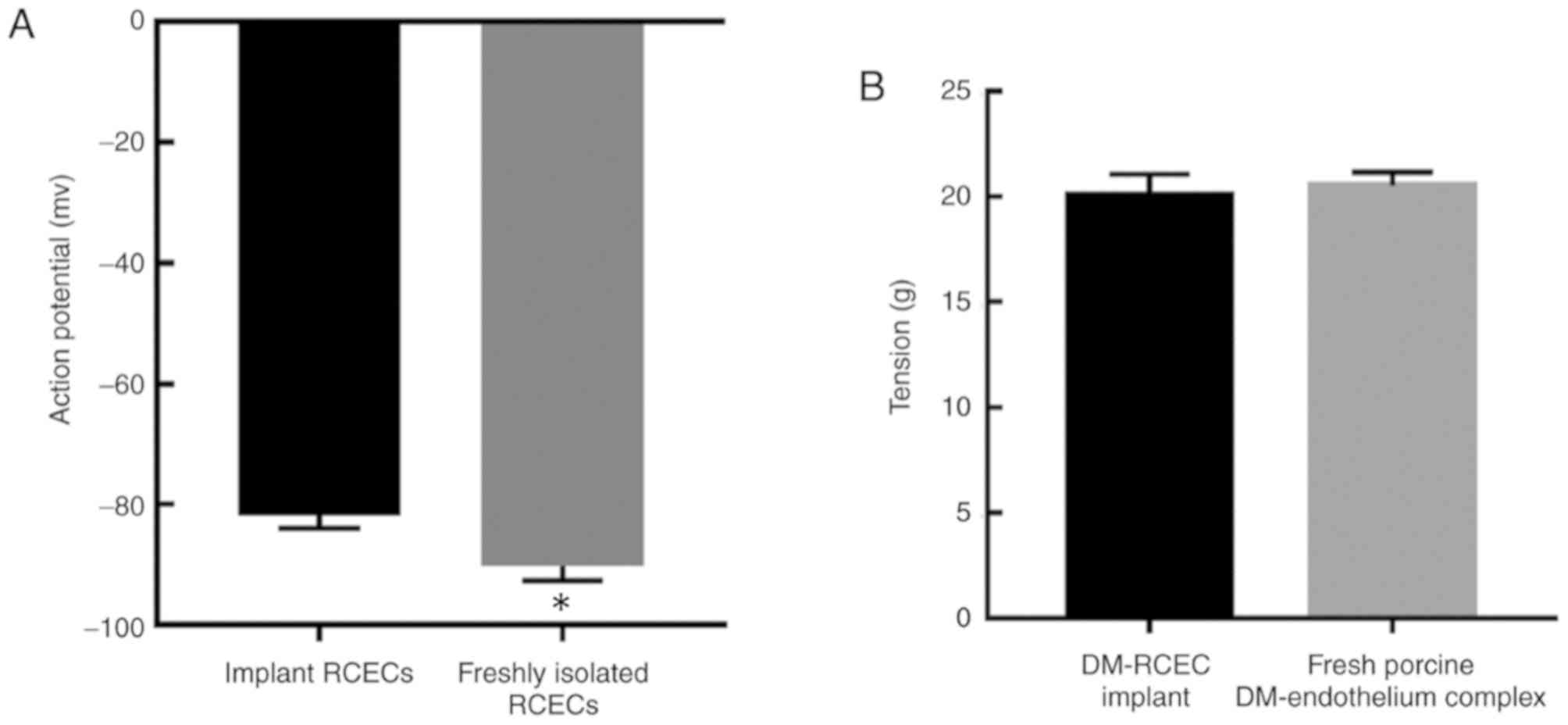
RCEC gene expression characteristics (Fig. 8D). The action potential amplitudes of
the implant RCECs (experimental group) and the freshly isolated
RCECs (control group) were over −80 mV (Fig. 8E and F), and the experimental group
had a significantly higher amplitude of the action potential
compared with the control group (P<0.05; Fig. 9A and Table III). The tension in the DM-RCEC
implant (experimental group) and the fresh porcine DM-endothelium
complex (control) was measured, however, there was no significant
difference between the experimental and control groups observed
(P>0.05; Fig. 9B and Table IV).
 | Table III.Action potential amplitudes of the
implant RCECs (experimental group) and the freshly isolated RCECs
(control group). |
Table III.
Action potential amplitudes of the
implant RCECs (experimental group) and the freshly isolated RCECs
(control group).
| Group | Action potential
(mV) | n |
|---|
| Experimental
group | −81.90±2.025 | 10 |
| Control group | −90.30±2.406 | 10 |
| P-value | <0.05 |
|
 | Table IV.Tension of the DM-RCEC implant
(experimental group) and the fresh porcine DM-endothelium complex
(control group). |
Table IV.
Tension of the DM-RCEC implant
(experimental group) and the fresh porcine DM-endothelium complex
(control group).
| Group | Tension (g) | n |
|---|
| Experimental
group | 20.0248±1.048 | 10 |
| Control group | 20.5013±0.657 | 10 |
| P-value | 0.848 |
|
Discussion
CECs form a barrier between the aqueous humour and
the corneal stroma to maintain corneal clarity and thickness
(8). Loss of these cells due to
disease, injury or aging could result in requiring endothelial
keratoplasty. However, a global shortage of donor corneas has
limited the availability of endothelial keratoplasty (1). This problem can be addressed by
fabricating tissue-engineered CEC implants. The CEC implants
primarily include actively proliferating seed cells with a
biocompatible carrier (19). The
earliest method of transplanting in vitro-cultured CECs
involved injecting a cell suspension into the anterior chamber of
the eye. Unfortunately, the CECs adhered to the DM and to crystals,
irises and angles, causing complications such as anterior chamber
fibrin exudation and glaucoma (20).
Over the years, scientists have searched for suitable carriers for
seeding and transplanting CECs. The optimal carrier should meet the
following requirements: i) The carrier must be permeable to ensure
the effective exchange of substances between cells and the
environment; ii) the carrier must be penetrable to refracting
media; iii) the carrier must have sufficient tension to tolerate
the transplant operation; and iv) the antigenicity of the carrier
should be minimized so that the transplant is not rejected
(3).
A previous study demonstrated that the peripheral
cornea contains corneal endothelial precursor cells with strong
renewing capacity that are suitable for transplant experiments
(21). However, in
vitro-cultured HCECs have reduced proliferative capacity and
are subject to morphological changes (22). HCECs are affected by various factors,
including endothelin and fibroblast growth factors (23–25).
Y-27632, a selective ROCK inhibitor, has been shown to promote CEC
adhesion and proliferation and reduce apoptosis (26,27).
This phenomenon may occur as Y-27632 can affect molecular
compositions that regulate the cell cycle (28), although this hypothesis requires
further verification. In the current study, Y-27632 was added to
the cell culture medium, and the results demonstrated that Y-27632
promoted RCEC proliferation, reduced apoptosis and facilitated the
cells to maintain their original morphology.
Primary cultured RCECs are easily contaminated by
other cells, including corneal epithelial cells and stroma cells.
Na+-K+-ATPase is highly expressed on CECs and
is primarily distributed on both sides of the cell membrane
(29). AQP1 is the only member of
the AQP channel family present on CECs, and CEC transmembrane
functional activity is reflected by the expression level of AQP1
(30). DM is primarily composed of
collagen α2 (IV) and collagen α1 (VIII), which are also positively
expressed on CECs (8). Keratin-12 is
a known marker for corneal epithelial cells, however, it is not
expressed on CECs (31). The spindle
morphology of corneal stroma cells can be used to identify and
isolate them from CECs (29). In the
current study, morphological observations and expression of
endothelial-associated markers demonstrated that the cultured cells
were uncontaminated RCECs.
Previous studies have examined different carriers
for RCEC implant fabrication; however, these approaches are
associated with certain limitations, which include unsatisfactory
biocompatibility and unsuitable differences in structure and
biochemical composition (32).
McCulley et al (33) reported
the use of a gelatine film as a carrier to transplant in
vitro-cultured CECs, however, the adhesive elements
demonstrated toxicity, and a liquid gap formed between the implant
bed and the gelatine film that induced fibroblast ingrowth. Liang
et al (34) used a hydrogel
as a carrier, however, this material caused corneal allograft
rejection and changed the postoperative corneal curvature. In 2004,
Ishino et al (35) used an
amniotic membrane to seed CECs, resulting in high-density CECs with
normal morphology and tight junctions. However, the amniotic
membrane is a thick and opaque membrane, which did not conform to
the cornea's physiological characteristics. In the present study,
the use of a porcine DM as a carrier demonstrated several
advantages. Porcine DM is widely available and easy to obtain. In
addition, porcine DM has a similar thickness and shape to human DM
as well as being transparent and permeable (36). DM is composed of the same collagens
that are secreted by CECs (37). DM
has no cellular components and is therefore characterised by a low
antigenicity (36).
To use a heterogeneous DM as a carrier, the
indigenous CECs must first be removed. During this process, the
structure and functional proteins of the extracellular matrix
should be carefully preserved (38).
Gospodarowicz and Greenburg (39)
revealed that chemical processes can be used to remove indigenous
CECs, however, the chemicals also destroyed the fibronectin on the
DM surface (40). A mechanical
erasing process was previously identified as an appropriate method
to prepare a carrier prior to the seeding of cultured CECs
(41). In the current study, the
carrier was preserved in glycerol after liquid nitrogen storage.
This method was used to reduce antigenicity. The H&E staining
revealed that the carrier had no immunogenicity and potentially
only a small chance of rejection.
To confirm whether an implant can be used for
endothelial transplantation, cultured CECs must have an adequate
density and possess normal electrophysiological activity. In
addition, an implant must have sufficient tension to withstand the
transplant operation. In the current study, alizarin red-trypan
blue staining demonstrated that implanted RCECs maintained the
structure and morphology associated with RCECs. VDAC2 and VDAC3 are
members of the mitochondrial porin family, which are located in the
mitochondrial outer membrane (42).
CECs contain a large number of mitochondria and express high levels
of VDAC2 and VDAC3 (43). In the
current study, the expression levels of AQP1, VDAC2 and VDAC3 of
the implanted RCECs were consistent with that of in vivo
RCECs.
Neher and Akamann (44) established the patch clamp technique
to detect cellular membrane potential. In the current study, the
membrane potential of the in vitro-cultured RCECs was
recorded using the whole-cell mode patch clamp technique. Action
potentials were observed in the implanted RCECs, however the
amplitude was significantly lower than that of freshly isolated
RCECs. Furthermore, the tension of implanted RCECs was examined
using a homemade tension detector. The results demonstrated that
the porcine DM-RCEC complex possessed sufficient tension to
tolerate a transplant operation.
To the best of our knowledge, this is the first
report describing the fabrication of a porcine DM-RCEC complex, as
well as the use of a novel tension detection method. The long-term
effects associated with the use of porcine DM-RCEC complex
transplants in vivo remains unknown. Therefore, further
animal experiments are required to observe the long-term effects.
In addition, future work is required to improved cell culture
techniques and cell sourcing, which may increase seed cell
viability.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Shanghai Scientific and Technical Innovation Plan 2016,
experimental animal research project (grant no. 16140900900) and
the Municipal Human Resources Development Program for Outstanding
Leaders in Medical Disciplines in Shanghai (grant no.
2017BR060).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL collected and analyzed the data, and prepared the
manuscript. ST, GN and JZ performed the animal experiments and
obtained tissue samples. XH and YZ performed laboratory experiments
and collected the data. YB analyzed the results and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Tongji University School of Medicine (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interest
The authors declared that they have no competing
interests.
References
|
1
|
Melles GR, Ong TS, Ververs B and van der
Wees J: Descemet membrane endothelial keratoplasty (DMEK). Cornea.
25:987–990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gospodarowicz D, Greenburg G and Alvarado
J: Transplantation of cultured bovine corneal endothelial cells to
species with nonregenerative endothelium. The cat as an
experimental model. Arch Ophthalmol. 97:2163–2169. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niu G, Choi JS, Wang Z, Skardal A,
Giegengack M and Soker S: Heparin-modified gelatin scaffolds for
human corneal endothelial cell transplantation. Biomaterials.
35:4005–4014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan T, Zhao J, Ma X, Xu X, Zhao W and Xu
B: Establishment of a continuous untransfected human corneal
endothelial cell line and its biocompatibility to denuded amniotic
membrane. Mol Vis. 17:469–480. 2011.PubMed/NCBI
|
|
5
|
Watanabe R, Hayashi R, Kimura Y, Tanaka Y,
Kageyama T, Hara S, Tabata Y and Nishida K: A novel gelatin
hydrogel carrier sheet for corneal endothelial transplantation.
Tissue Eng Part A. 17:2213–2219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honda N, Mimura T, Usui T and Amano S:
Descemet stripping automated endothelial keratoplasty using
cultured corneal endothelial cells in a rabbit model. Arch
Ophthalmol. 127:1321–1326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnston MC, Noden DM, Hazelton RD,
Coulombre JL and Coulombre AJ: Origins of avian ocular and
periocular tissues. Exp Eye Res. 29:27–43. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edelhauser HF: The balance between corneal
transparency and edema: The proctor lecture. Invest Ophthalmol Vis
Sci. 47:1754–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joyce NC, Meklir B, Joyce SJ and Zieske
JD: Cell cycle protein expression and proliferative status in human
corneal cells. Invest Ophthalmol Vis Sci. 37:645–655.
1996.PubMed/NCBI
|
|
10
|
Mimura T, Yamagami S and Amano S: Corneal
endothelial regeneration and tissue engineering. Prog Retin Eye
Res. 35:1–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peh GS, Beuerman RW, Colman A, Tan DT and
Mehta1 JS: Human corneal endothelial cell expansion for corneal
endothelium transplantation: An overview. Transplantation.
91:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa H, Yoshioka K, Miyahara E,
Fukushima Y, Tamura M and Itoh K: Intrathecal Administration of
Y-27632, a specific Rho-associated kinase inhibitor, for rat
neoplastic meningitis. Mol Cancer Res. 3:425–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng ZH, Zhang XH, Zhao JQ and Ma JZ:
Involvement of Rho-associated coiled-coil kinase signaling
inhibition in TGF-β1/Smad2, 3 signal transduction in vitro. Int J
Ophthalmol. 10:1805–1811. 2017.PubMed/NCBI
|
|
14
|
Okumura N, Inoue R, Okazaki Y, Nakano S,
Nakagawa H, Kinoshita S and Koizumi N: Effect of the Rho kinase
inhibitor Y-27632 on corneal endothelial wound healing. Invest
Ophthalmol Vis Sci. 56:6067–6074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Cai H, Sun L, Zhan P, Chen M,
Zhang F, Ran Y and Wan J: LGR5, a novel functional glioma stem cell
marker, promotes EMT by activating the Wnt/β-catenin pathway and
predicts poor survival of glioma patients. J Exp Clin Cancer Res.
37:225–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Serafini S, Santos MM, Aoun Tannuri AC,
Zerbini MCN, de Mendonça Coelho MC, de Oliveira Gonçalves J and
Tannuri U: Is hematoxylin-eosin staining in rectal mucosal and
submucosal biopsies still useful for the diagnosis of Hirschsprung
disease? Diagn Pathol. 12:842017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leyrer-Jackson JM, Olive MF and Gipson CD:
Whole-cell patch-clamp electrophysiology to study ionotropic
glutamatergic receptors and their roles in addiction. Methods Mol
Biol. 1941:107–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esipov RS, Beĭrakhova KA, Chupova LA,
Likhvantseva VK, Stepanova EV and Miroshnikov AI: Recombinant
fragment of pigment epithelium-derived factor (44–77) prevents
pathological corneal neovascularization. Bioorg Khim. 38:78–85.
2012.(In Russian). PubMed/NCBI
|
|
20
|
Singh N, Higgins E, Amin S, Jani P,
Richter E, Patel A, Kaur R, Wang J, Ambati J, Dong Z and Ambati BK:
Unique homologous siRNA blocks hypoxia-induced VEGF upregulation in
human corneal cells and inhibits and regresses murine corneal
neovascularization. Cornea. 26:65–72. 2007.PubMed/NCBI
|
|
21
|
Mimura T, Yamagami S, Yokoo S, Araie M and
Amano S: Comparison of rabbit corneal endothelial cell precursors
in the central and peripheral cornea. Invest Ophthalmol Vis Sci.
46:3645–3648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okumura N, Kay EP, Nakahara M, Hamuro J,
Kinoshita S and Koizumi N: Inhibition of TGF-β signaling enables
human corneal endothelial cell expansion in vitro for use in
regenerative medicine. PLoS One. 8:e580002013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rieck P, Oliver L, Engelmann K, Fuhrmann
G, Hartmann C and Courtois Y: The role of exogenous/endogenous
basic fibroblast growth factor (FGF2) and transforming growth
factor beta (TGF beta-1) on human corneal endothelial cells
proliferation in vitro. Exp Cell Res. 220:36–46. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soldano S, Paolino S, Pizzorni C,
Trombetta AC, Montagna P, Brizzolara R, Corallo C, Giordano N,
Sulli A and Cutolo M: Dual endothelin receptor antagonists contrast
the effects induced by endothelin-1 on cultured human microvascular
endothelial cells. Clin Exp Rheumatol. 35:484–493. 2017.PubMed/NCBI
|
|
25
|
Hsieh P and Baum J: Effects of
fibroblastic and endothelial extracellular matrices on corneal
endothelial cells. Invest Ophthalmol Vis Sci. 26:457–463.
1985.PubMed/NCBI
|
|
26
|
Okumura N, Koizumi N, Ueno M, Sakamoto Y,
Takahashi H, Hirata K, Torii R, Hamuro J and Kinoshita S:
Enhancement of corneal endothelium wound healing by Rho-associated
kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 95:1006–1009.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bi YL, Zhou Q, Du F, Wu MF, Xu GT and Sui
GQ: Regulation of functional corneal endothelial cells isolated
from sphere colonies by Rho-associated protein kinase inhibitor.
Exp Ther Med. 5:433–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joyce NC and Harris DL: Decreasing
expression of the G1-phase inhibitors, p21Cip1 and p16INK4a,
promotes division of corneal endothelial cells from older donors.
Mol Vis. 16:897–906. 2010.PubMed/NCBI
|
|
29
|
Ding V, Chin A, Peh G, Mehta JS and Choo
A: Generation of novel monoclonal antibodies for the enrichment and
characterization of human corneal endothelial cells (hCENC)
necessary for the treatment of corneal endothelial blindness. MAbs.
6:1439–1452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song Q, Yuan S, An Q, Chen Y, Mao FF, Liu
Y, Liu Q and Fan G: Directed differentiation of human embryonic
stem cells to corneal endothelial cell-like cells: A transcriptomic
analysis. Exp Eye Res. 151:107–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu RL, Zhu G, Galvin S, Xu C, Haseba T,
Chaloin-Dufau D, Dhouailly D, Wei ZG, Lavker RM, Kao WY, et al:
Lineage-specific and differentiation-dependent expression of K12
keratin in rabbit corneal/limbal epithelial cells: cDNA cloning and
northern blot analysis. Differentiation. 55:137–144. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahearne M, Wilson SL, Liu KK, Rauz S, El
Haj AJ and Yang Y: Influence of cell and collagen concentration on
the cell-matrix mechanical relationship in a corneal stroma wound
healing model. Exp Eye Res. 91:584–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCulley JP, Maurice DM and Schwartz BD:
Corneal endothelial transplantation. Ophthalmology. 87:194–201.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang Y, Liu W, Han B, Yang C, Ma Q, Song
F and Bi Q: An in situ formed biodegradable hydrogel for
reconstruction of the corneal endothelium. Colloids Surf B
Biointerfaces. 82:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishino Y, Sano Y, Nakamura T, Connon CJ,
Rigby H, Fullwood NJ and Kinoshita S: Amniotic membrane as a
carrier for cultivated human corneal endothelial cells
transplantation. Invest Ophthalmol Vis Sci. 45:800–806. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Diao YM and Hong J: Feasibility and safety
of porcine Descemet's membrane as a carrier for generating
tissue-engineered corneal endothelium. Mol Med Rep. 12:1929–1934.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi HJ, Kim MK, Lee HJ, Ko JH, Jeong SH,
Lee JI, Oh BC, Kang HJ and Wee WR: Efficacy of pig-to-rhesus
lamella corneal xenotransplantation. Invest Ophthalmol Vis Sci.
52:6643–6650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murphy SV and Atala A: Organ
engineering-combining stem cells, biomaterials, and bioreactors to
produce bioengineered organs for transplantation. Bioessays.
35:163–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gospodarowicz D and Greenburg G: The
coating of bovine and rabbit corneas denuded of their endothelium
with bovine corneal endothelial cells. Exp Eye Res. 28:249–265.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen KH, Azar D and Joyce NC:
Transplantation of adult human corneal endothelium ex vivo: A
morphologic study. Cornea. 20:731–737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jumblatt MM, Maurice DM and McCulley JP:
Transplantation of tissue-cultured corneal endothelium. Invest
Ophthalmol Vis Sci. 17:1135–1141. 1978.PubMed/NCBI
|
|
42
|
Thinnes FP: Neuroendocrine differentiation
of LNCaP cells suggests: VDAC in the cell membrane is involved in
the extrinsic apoptotic pathway. Mol Genet Metab. 97:241–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McIntosh Ambrose W, Salahuddin A, So S, Ng
S, Ponce Márquez S, Takezawa T, Schein O and Elisseeff J: Collagen
Vitrigel membranes for the in vitro reconstruction of separate
corneal epithelial, stromal, and endothelial cell layers. J Biomed
Mater Res B Appl Biomater. 90:818–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Neher E and Sakmann B: Single channel
currents recorded from membrane of denervated frog muscle fibers.
Nature. 260:799–802. 1976. View Article : Google Scholar : PubMed/NCBI
|