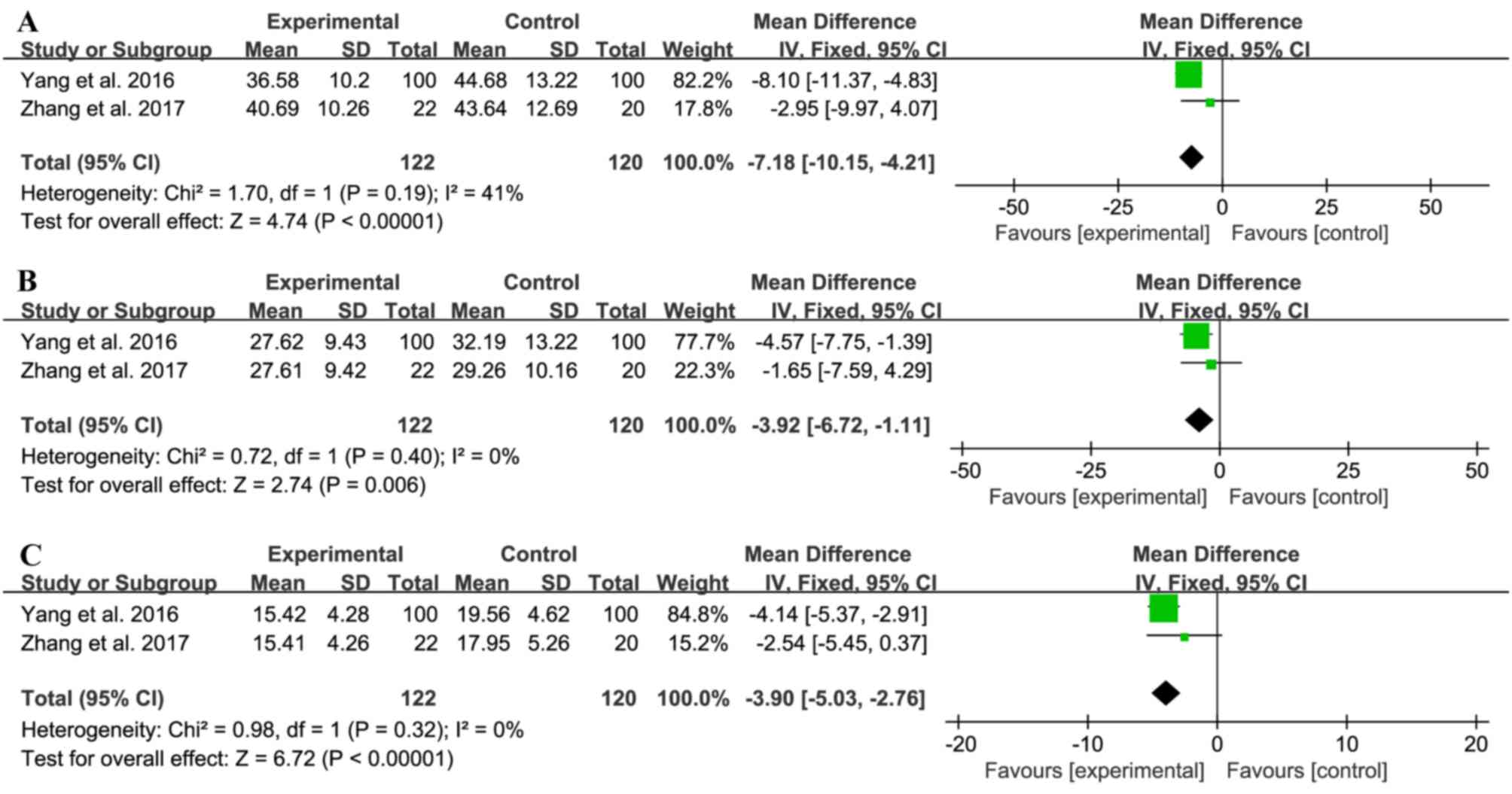
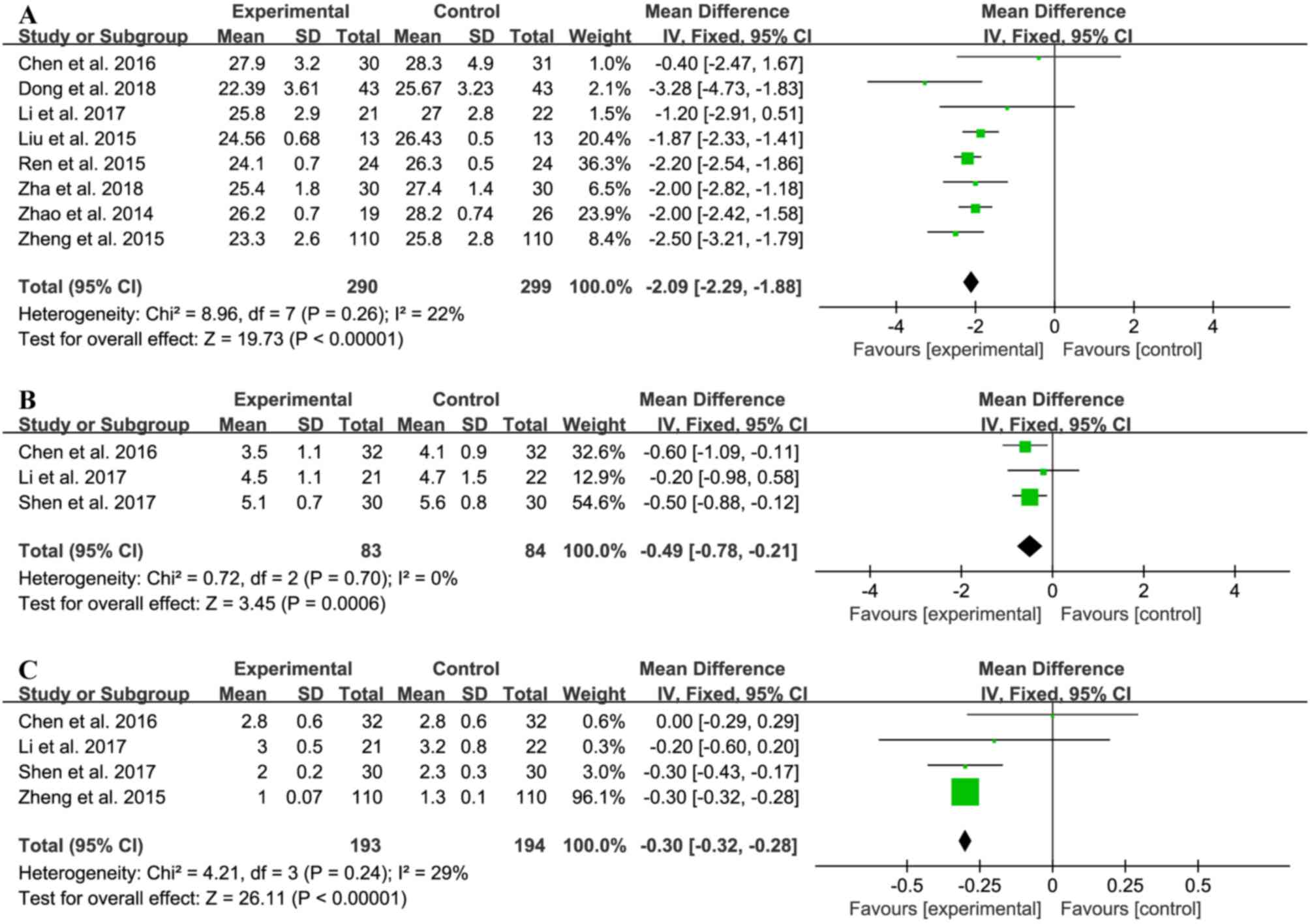
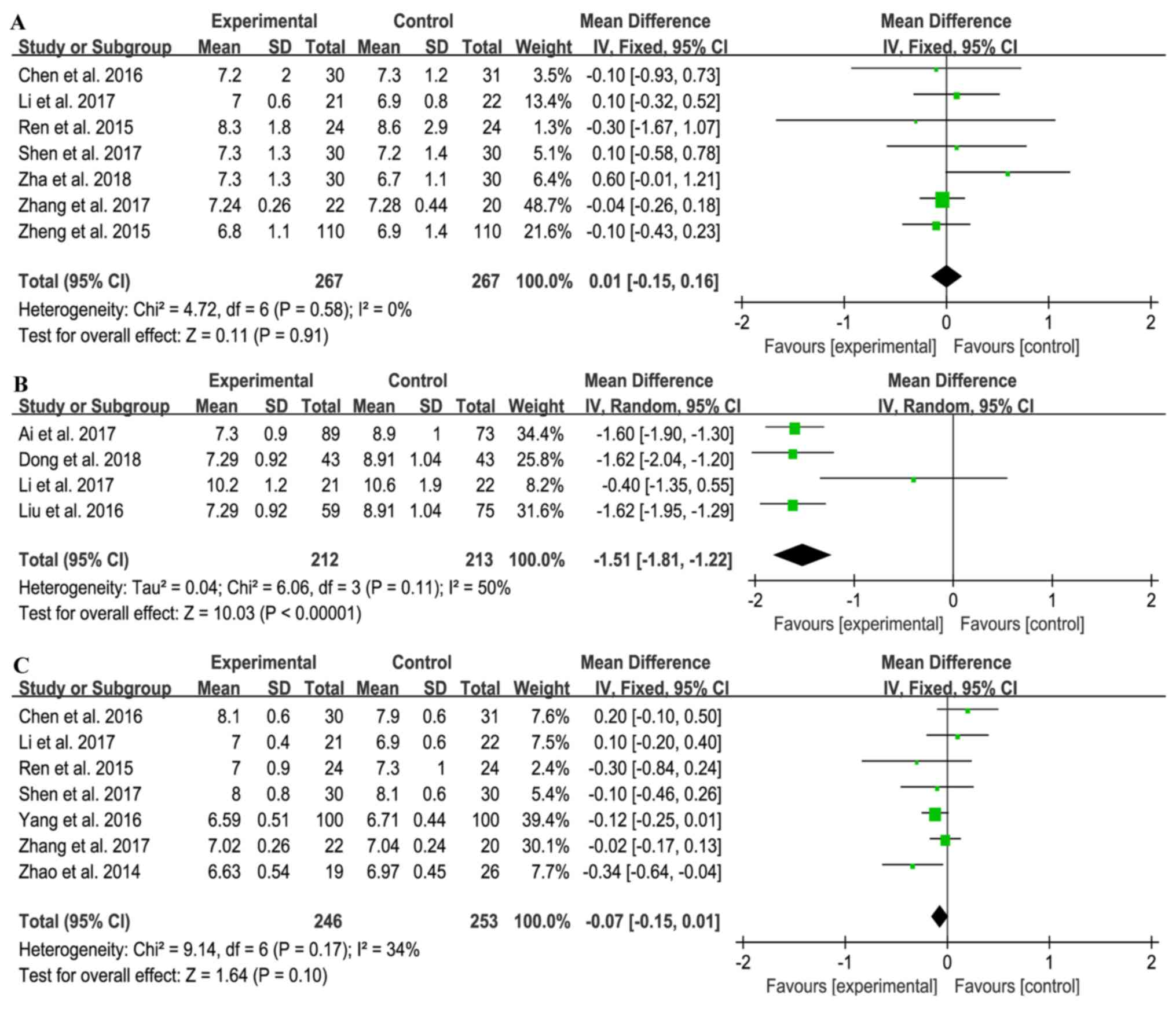
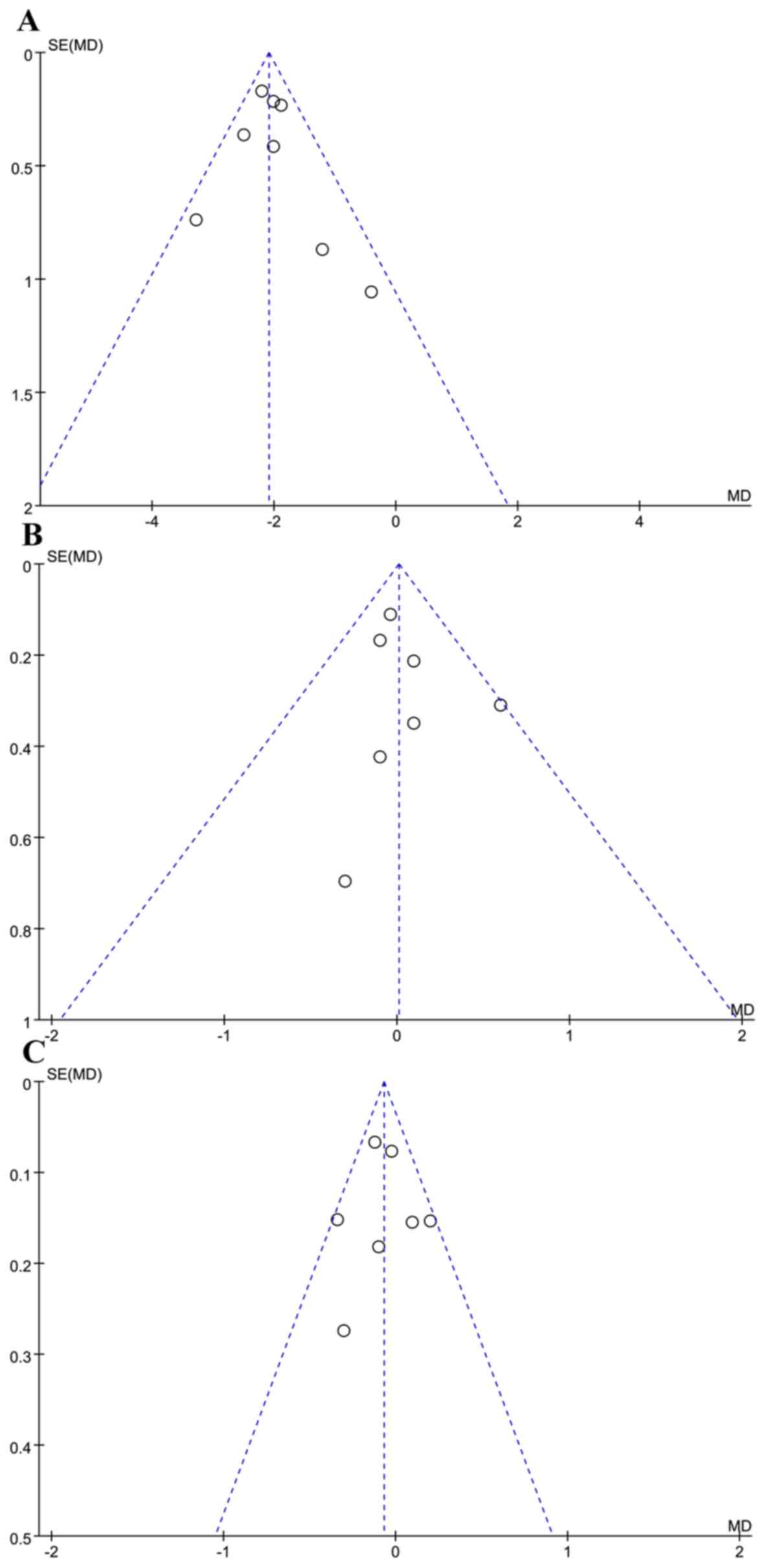
|
1
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF Diabetes atlas: Global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tong L and Adler S: Glycemic control of
type 2 diabetes mellitus across stages of renal impairment:
Information for primary care providers. Postgrad Med. 130:381–393.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
U.S. Food Drug Administration, .
Drugs@FDA: FDA Approved Drug Products. VICTOZA (Liraglutide).
http://www.accessdata.fda.gov/July 11–2016
|
|
4
|
Mann JFE, Ørsted DD, Brown-Frandsen K,
Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B and Buse JB;
LEADER Steering Committee and Investigators, : Liraglutide and
renal outcomes in type 2 diabetes. N Engl J Med. 377:839–848. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davies MJ, Bain SC, Atkin SL, Rossing P,
Scott D, Shamkhalova MS, Bosch-Traberg H, Syrén A and Umpierrez GE:
Efficacy and safety of liraglutide versus placebo as add-on to
glucose-lowering therapy in patients with type 2 diabetes and
moderate renal impairment (LIRA-RENAL): A randomized clinical
trial. Diabetes Care. 39:222–230. 2016.PubMed/NCBI
|
|
6
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions version 5.1.2. The cochrane
collaboration, 2011. http://handbook.cochrane.org/April 8–2018
|
|
8
|
Zhang JH, Liu XR, Sheng CX and Liu YE:
Analysis on difference in gastrointestinal hormone levels of
patients with the history of diabetes and concurrent nephropathy
and study on the role of liraglutide. Eur Rev Med Pharmacol Sci.
21:3523–3529. 2017.PubMed/NCBI
|
|
9
|
Zha M, Zhang S, Ruan Y, Shi M, Zhou L and
Huang LJ: Clinical effects of combination therapy of Huangkui
capsules and liraglutide on patients with early diabetic
nephropathy. Chin Trad Patent Med. 40:1493–1495. 2018.(In
Chinese).
|
|
10
|
Dong L and Zhao JH: Effects of liraglutide
on renal function in patients with microalbuminuria of diabetic
nephropathy. Lin Chang Hui Cui. 33:420–423. 2018.
|
|
11
|
Li Q, Cao WJ, Zhou GY and Yang LH: Effects
of liraglutide on early diabetic nephropathy and its relationship
with the changes of VEGF and VEGF-A. Xiangnan Xue Yuan Xue Bao (Yi
Xue Ban). 19:6–11. 2017.
|
|
12
|
Zheng Y and Yu SD: Clinical effects of
combination therapy of insulin glargine and liraglutide on type 2
diabetic patients with nephropathy. Xiandai Shi Yong Yi Xue.
27:251–253. 2015.
|
|
13
|
Yang R, Wang YF and Zhang W: Liraglutide
combined with low dosage telmisartan decreases the serum levels of
TNF-α, IL-6 and TGF-β1 in patients with early diabetic nephropathy.
Med J West China. 28:191–194. 2016.(In Chinese).
|
|
14
|
Shen YP, Qiao Q and Lu GY: Effect of
liraglutide on PI3K-Akt-mTOR pathway in patients with diabetic
nephropathy. Huazhong Ke Ji Da Xue Xue Bao (Yi Xue Ban).
46:466–470. 2017.
|
|
15
|
Chen ZP: Efficacy and safety analysis of
Liraglutide in treatment of patients with type 2 diabetes mellitus
and mild to moderate renal disease. Zhongguo Dang Dai Yi Yao.
23:131–133. 2016.
|
|
16
|
Ren W, Guo JJ, Zuo GX, Li YB, Gao LL, Li X
and Liu J: Effects of liraglutide on early diabetic nephropathy
with obese. Chin Remedies & Clinics. 15:1284–1286. 2015.(In
Chinese).
|
|
17
|
Zhao CY, Guo HT, Dai HS, Tian JR and Zhao
YQ: Clinical effects of liraglutide on early diabetic nephropathy.
Shandong Yi Yao. 54:47–49. 2014.(In Chinese).
|
|
18
|
Aiyitan A and Qing Q: Clinical effect of
liraglutide in treating early diabetic nephropathies and the
influence of renal function and adipocytokines. Chin J Clin
Rational Drug Use. 10:10–14. 2017.(In Chinese).
|
|
19
|
Liu R: Protective effect of hypoglycemic
therapy by liraglutide on renal function in early diabetic
nephropathy. J Hainan Med Univ. 22:43–46. 2016.
|
|
20
|
Liu CY: Liraglutide treatment efficacy of
early type 2 diabetic nephropathy and its mechanism analysis.
Dalian Yi Ke Da Xue. 9–11. 2015.
|
|
21
|
Fineberg D, Jandeleit-Dahm KA and Cooper
ME: Diabetic nephropathy: Diagnosis and treatment. Nat Rev
Endocrinol. 9:713–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jorsal T, Rhee NA, Pedersen J, Wahlgren
CD, Mortensen B, Jepsen SL, Jelsing J, Dalbøge LS, Vilmann P,
Hassan H, et al: Enteroendocrine K and L cells in healthy and type
2 diabetic individuals. Diabetologia. 61:284–294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sonne DP, Rehfeld JF, Holst JJ, Vilsbøll T
and Knop FK: Postprandial gallbladder emptying in patients with
type 2 diabetes: Potential implications for bile-induced secretion
of glucagon-like peptide 1. Eur J Endocrinol. 171:407–419. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersen A, Lund A, Knop FK and Vilsbøll
T: Glucagon-like peptide 1 in health and disease. Nat Rev
Endocrinol. 14:390–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holst JJ: The physiology of glucagon-like
peptide 1. Physiol Rev. 87:1409–1439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kreymann B, Williams G, Ghatei MA and
Bloom SR: Glucagon-like peptide-1 7–36: A physiological incretin in
man. Lancet. 2:1300–1304. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lopez-Giacoman S and Madero M: Biomarkers
in chronic kidney disease, from kidney function to kidney damage.
World J Nephrol. 4:57–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Javanmardi M, Azadi NA, Amini S and Abdi
M: Diagnostic value of cystatin C for diagnosis of early renal
damages in type 2 diabetic mellitus patients: The first experience
in Iran. J Res Med Sci. 20:571–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou SJ, Bai L, Lv L, Chen R, Li CJ, Liu
XY, Yu DM and Yu P: Liraglutide ameliorates renal injury in
streptozotocin-induced diabetic rats by activating endothelial
nitric oxide synthase activity via the downregulation of the
nuclear factor-κB pathway. Mol Med Rep. 10:2587–2594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Russell-Jones D, Vaag A, Schmitz O, Sethi
BK, Lalic N, Antic S, Zdravkovic M, Ravn GM and Simó R; Liraglutide
Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group, :
Liraglutide versus insulin glargine and placebo in combination with
metformin and sulfonylurea therapy in type 2 diabetes mellitus
(LEAD-5 met+SU): A randomised controlled trial. Diabetologia.
52:2046–2055. 2009. View Article : Google Scholar : PubMed/NCBI
|