Introduction
Neonatal respiratory distress syndrome (NRDS) is a
very serious disease during the neonatal period, which can even
lead to neonatal death in severe cases (1). According to epidemiological studies,
the incidence of respiratory distress syndrome (RDS) is between 0.7
and 1.6% (2), and the incidence can
increase to 3–4 times when complicated with respiratory diseases
(3). In recent years, the rapid
development of overall medical technology and wide application of
pulmonary surfactant (PS) and mechanical ventilation in clinical
practice have greatly improved the survival rate of children with
extremely low birth weight in China (4,5).
However, continuous oxygen supply and mechanical ventilation are
risk factors for NRDS to further develop into bronchopulmonary
dysplasia (BDP) (6). At present, PS,
as an alternative therapy, has achieved certain curative effect in
clinical practice. Still, there are some children whose condition
has not been well controlled and develops into BDP. Severe
impairment of pulmonary function reduces the life quality and
survival rate of children (7). In
recent years, related studies have found that changes in serum
glycoproteins and related cytokines are of great significance to
early prediction and evaluation of NRDS prognosis of premature
infants (8).
Transforming growth factor β1 (TGF-β1), a group of
multifunctional cytokines that regulate cell growth and
differentiation, is mainly from macrophages, epithelial cells,
endothelial cells and fibroblasts (9). Related research has found that TGF-β1
plays an important role in the occurrence and development of
various diseases, which can lead to acute and chronic lung injury
through a series of signal transduction in vivo (10). Interleukin-6 (IL-6), as a
glycoprotein, is mainly produced by T cells, mononuclear
macrophages and endothelial cells. It can activate neutrophils,
thus promoting the liver to secret acute proteins and causing acute
inflammatory reactions. Moreover, it is a key inflammatory factor
in acute respiratory distress syndrome (ARDS) (11). The physiological characteristics of
IL-6 are to induce T cells to activate, proliferate and
differentiate, to induce B cells to differentiate and produce
antibodies, thus participating in the body's immune response as a
trigger of inflammatory reaction (12). Therefore, the expression of TGF-β1
and IL-6 plays an important role in ARDS. However, there are few
studies on the expression of these two factors in NRDS and their
correlation.
In this study, the correlation between TGF-β1 and
IL-6 in NRDS was examined by means of retrospective study in order
to provide reference for clinical diagnosis and treatment.
Patients and methods
General data
A total of 75 NRDS children born in the Xiangyang
Central Hospital (Xiangyang, China) from July 2015 to August 2017
were included in the study. The NRDS diagnostic criteria refer to
the relevant criteria in Practical Neonatology (13). Of these, 45 NRDS children who were
given PS within 12 h after birth were treated as PS group,
including 25 males and 20 females, with a gestational age of
30.4±1.1 weeks and a birth weight of 1.4±0.4 g. A total of 30 NRDS
children who were not treated with PS due to family difficulties or
other factors were taken as non-PS group, including 19 males and 11
females, with a gestational age of 30.8±0.9 weeks and a birth
weight of 1.4±0.5 g. In addition, 32 premature infants without NRDS
were selected as the control group, including 18 males and 14
females, with a gestational age of 30.9±1.3 weeks and a birth
weight of 1.6±0.7 g. There were no significant differences in sex,
average birth time, gestational age, birth weight and birth mode
between the three groups (P>0.05), which were comparable.
Inclusion criteria: i) combining with clinical
symptoms (shortness of breath, dyspnea, cyanosis and other
symptoms), children were diagnosed with NRDS by chest X-ray and
blood biochemical examination; ii) patients with hypoxemia
confirmed by blood gas analysis (14); iii) patients with normal body mass
index (BMI); and iv) patients with or without carbon dioxide
retention.
Exclusion criteria: i) patients with aspiration
pneumonia; ii) serious congenital heart disease; iii) respiratory
malformation; iv) severe asphyxia and respiratory failure; v)
pneumonia caused by meconium and other factors; vi) intracranial
hemorrhage; and vii) family members who refused to sign informed
consent.
This study was approved by the Ethics Committee of
the hospital and the experimental contents of the subjects were
described in detail. The parents of the child subjects agreed and
signed an informed consent.
Treatment methods
During the hospital stay, the patient's condition
was observed at all times, and effective breathing maintenance,
infection prevention measures, vitamin supplementation for
resistance enhancement were conducted. The children were kept warm
and breastfeeding was recommended. Proper acidosis correction and
blood volume expansion were carried out to maintain the balance of
water and electrolytes in the body, and dopamine and dobutamine
were used in time to stabilize blood pressure. i) Non-PS group:
conventional symptomatic and supportive treatment was given to the
children, and infant incubator, far-infrared radiation bed and
rescue equipment were allocated. Ventilator-assisted breathing,
warmth preservation, sputum suction, oxygen inhalation and
nutritional support was provided, and water, electrolyte and
acidolysis disorder was corrected to maintain internal environment
stability. Clinical indicators such as heart rate, respiratory
condition and oxygen saturation in children were continuously
monitored, regular biochemical, blood routine and chest X-ray
examinations were performed, and effective antibiotic treatment was
given to infected children. ii) Healthy control group: children
were fed with breast milk or 20% glucose water, and other measures
were the same as the observation group. iii) PS group: on the basis
of the observation group, children were treated with PS (Curosurf)
(imported drug registration no. H20080428; Chiesi Farmaceutici
S.p.A.) at a dose of 100 mg/kg. Before treatment, the children were
placed on a rescue table and respiratory secretions were cleaned
quickly with a sputum aspirator. The drug was placed in an
incubator for heating before administration, and after the
temperature reached 37°C, medicine was instilled intratracheally by
a sterile syringe in each position of the child. In order to reduce
the loss of drugs, children can not be treated with sputum
excretion care for 6 h after medication. According to the clinical
manifestations, chest X-ray and blood gas analysis results, the
auxiliary ventilation model and parameters were selected. Blood gas
and sternum were reviewed regularly and disease changes were
observed to adjust auxiliary ventilation model and parameters. When
the condition improved, the ventilator parameters were gradually
reduced, and the ventilator removed in time.
Detection of TGF-β1 and IL-6 in
serum
After the children were diagnosed with NRDS, 2 ml of
fasting venous blood was collected at 0, 1, 3 and 7 days after
birth, and then placed in anticoagulation tubes and sent to the
clinical laboratory. In the control group, 2 ml of fasting venous
blood was taken from the children in the morning on the day of
physical examination. After coagulation for 60 min (20–25°C),
centrifugation was carried out at 3,000 × g at 4°C for 10 min.
Supernatant was collected and placed at −80°C for testing. Repeated
freezing and thawing were avoided. Serum TGF-β1 and IL-6 levels
were determined by enzyme linked immunosorbent assay (ELISA).
TGF-β1 and IL-6 kits were provided by Jiangsu Baolai Biotechnology
Co., Ltd. (Jiangsu, China), with the cargo numbers of MM-0090H1 and
MM-0049H2, respectively. The instrument was BS-1101 ELISA analyzer
from Beijing Limao Technology Co., Ltd. (Beijing, China). All
operations were strictly carried out in accordance with the
instructions of the kits.
Assessment of severity of NRDS
children (15)
The severity of NRDS children was assessed according
to the chest X-ray examination results. The higher the grade, the
more serious the disease was. Grade I: all the children show fine
miliary and ground-glass shadows with clear cardiac shadows and
decreased pulmonary brightness. Grade II: in addition to miliary
shadows, the air bronchogram sign was visible and extended to the
outer zone of the lung field. Grade III: in addition to the above
images, the diaphragmatic and cardiac boundaries were blurred, the
lungs were ground glassy, and the air bronchogram sign was obvious.
Grade IV: the whole lung field was seen as an extensive white
shadow called ‘white lung’, air bronchogram sign was more and more
obvious, thorax was well expanded and diaphragmatic position was
normal. Grade I and II indicated mild illness, while Grade III and
IV indicated severe one.
Outcome measures
The expression levels of TGF-β1 and IL-6 in
children's serum at various time points were observed, the
expression of these two factors in PS, non-PS and control groups
were compared, and the correlation between the severity of illness
and their expression and the correlation between TGF-β1 and IL-6
were analyzed.
Statistical analysis
SPSS 17.0 statistical software (Tianjin KSoft
Science and Technology Co., Ltd., Tianjin, China) was used to
statistically analyze the experimental data. The enumeration data
were expressed as n (%), the inter-group comparison was performed
by Chi-square test. The measurement data were expressed in mean ±
standard deviation, and the single-factor variance analysis was
used for the multi-group average comparison, Pearson's correlation
coefficient was used for the bivariate normal distribution data,
and Spearman correlation coefficient was used for the ranked data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of clinical indexes
The clinical indexes of newborns in each group were
collected. It was concluded that there were no significant
differences between the three groups in clinical baseline data such
as sex, gestational age, birth weight, average birth time and birth
mode (P>0.05), but there were significant differences between
the three groups in multiple pregnancy, premature rupture of
membranes, intrauterine distress or asphyxia, gestational diabetes,
amniotic fluid aspiration, umbilical cord abnormality, placental
abnormality, intrauterine infection or prenatal glucocorticoid use
(P<0.05; Table I).
 | Table I.Comparison of clinical indexes between
PS, non-PS and control groups [n (%)]/(mean ± standard
deviation). |
Table I.
Comparison of clinical indexes between
PS, non-PS and control groups [n (%)]/(mean ± standard
deviation).
| Variables | PS group (n=45) | Non-PS group
(n=30) | Control group
(n=32) | χ2/F | P-value |
|---|
| Sex |
|
|
|
0.501 | 0.779 |
| Male | 25
(55.6) | 19
(63.3) | 18
(56.3) |
|
|
|
Female | 20
(44.4) | 11
(36.7) | 14
(43.7) |
|
|
| Gestational age | 30.4±1.1 | 30.8±0.9 | 30.9±1.3 |
2.204 | 0.1155 |
| Birth weight |
1.4±0.4 |
1.4±0.5 |
1.6±0.7 |
1.583 | 0.2104 |
| Average birth
time |
3.7±0.9 |
3.9±1.1 |
4.1±1.6 |
1.045 | 0.3553 |
| Birth mode |
|
|
|
3.337 | 0.185 |
| Natural
delivery | 11
(24.4) | 14
(46.7) | 10
(31.2) |
|
|
| Cesarean
section | 34
(75.6) | 16
(53.3) | 22
(68.8) |
|
|
| Multiple
pregnancy | 16
(35.6) | 6 (20) | 2
(6.3) |
9.372 | <0.05 |
| Premature rupture
of membranes | 21
(46.7) | 10
(33.3) | 1
(3.1) | 17.15 | <0.05 |
| Intrauterine
distress or asphyxia | 39
(86.7) | 17
(56.7) | 0 (0) | 56.52 | <0.05 |
| Gestational
diabetes | 18 (40) | 7
(23.3) | 0 (0) | 16.71 | <0.05 |
| Amniotic fluid
aspiration | 21
(46.7) | 9 (30) | 0 (0) | 20.27 | <0.05 |
| Umbilical cord
abnormality | 12
(26.7) | 5
(16.7) | 0 (0) |
9.97 | 0.007 |
| Placental
abnormality | 9
(20) | 3 (10) | 0 (0) |
7.57 | 0.023 |
| Intrauterine
infection | 17
(37.8) | 6 (20) | 0 (0) | 15.87 | <0.05 |
| Prenatal
glucocorticoid use | 23
(51.1) | 16
(53.3) | 1
(3.1) | 26.22 | <0.05 |
Comparison of TGF-β1 expression in the
three groups
As shown in Table
II, TGF-β1 expression was low in the healthy control group at
all time points in vivo, and there was no significant
difference in its expression at any time point in the group
(P>0.05). The expression level of TGF-β1 in the serum of
children in PS group was significantly higher than that in the
control group on days 1 and 3 after birth (P<0.05), and
decreased on day 7, but there was no significant difference
compared with that in the control group (P>0.05). The expression
of TGF-β1 in children in non-PS group increased with the increasing
number of days, the expression was significantly higher than that
in the control group on days 1, 3 and 7 after birth, and the
difference was statistically significant (P<0.05). The
expression was significantly higher than that in PS group on days 3
and 7 after birth (P<0.05; Fig.
1).
 | Table II.Comparison of TGF-β1 expression in
the three groups at different time points (mean ± standard
deviation). |
Table II.
Comparison of TGF-β1 expression in
the three groups at different time points (mean ± standard
deviation).
| Group | Day 0 | Day 1 | Day 3 | Day 7 |
|---|
| PS group
(n=45) | 38.67±6.82 |
41.43±5.31a,c |
42.12±6.47a,c | 38.01±6.28 |
| Non-PS group
(n=30) | 38.75±6.36 |
42.87±6.13a,c |
47.01±5.59a–c |
48.98±3.03a–c |
| Control group
(n=32) | 35.78±5.35 | 35.81±5.21 | 35.71±5.18 | 35.67±5.29 |
| F | 2.41 | 14.90 | 29.95 | 61.06 |
| P-value |
0.0953 | <0.05 | <0.05 | <0.05 |
Comparison of IL-6 expression in the
three groups
Table III shows
that IL-6 was slightly expressed in the healthy control group at
various time points in vivo, and there was no significant
difference in its expression at various time points in the group
(P>0.05). The expression level of serum IL-6 in PS group peaked
on day 3 after birth, decreased on day 7, and was significantly
higher than that in the control group on days 1 and 3 (P<0.05).
The expression level of IL-6 in the non-PS group increased
continuously after birth, and was significantly higher than that in
the control group on days 1, 3 and 7 (P<0.05), and higher than
that in the PS group on days 3 and 7 (P<0.05, Fig. 2).
 | Table III.Comparison of IL-6 expression in the
three groups at different time points (mean ± standard
deviation). |
Table III.
Comparison of IL-6 expression in the
three groups at different time points (mean ± standard
deviation).
| Group | Day 1 | Day 2 | Day 3 | Day 7 |
|---|
| PS group
(n=45) | 13.67±4.78 |
14.79±4.98a |
16.69±5.01a,c | 15.03±4.68 |
| Non-PS group
(n=30) | 13.87±4.63 |
14.93±3.46a |
18.75±3.11a–c |
20.94±5.01a–c |
| Control group
(n=32) | 12.58±3.65 | 12.57±3.70 | 12.55±3.73 | 12.59±3.63 |
| F | 0.80 |
3.25 | 18.01 | 28.38 |
| P-value |
0.4516 | <0.05 | <0.05 | <0.05 |
Comparison and correlation analysis of
TGF-β1 and IL-6 expression levels in NRDS children with different
severity
A total of 75 NRDS children were graded according to
the disease severity examined by chest X-ray, and the results
showed that there were 32 children in Grade I, 24 in Grade II, 11
in Grade III and 8 in Grade IV. Table
IV, shows that there were significant differences in the
expression levels of TGF-β1 and IL-6 in the groups (P<0.05).
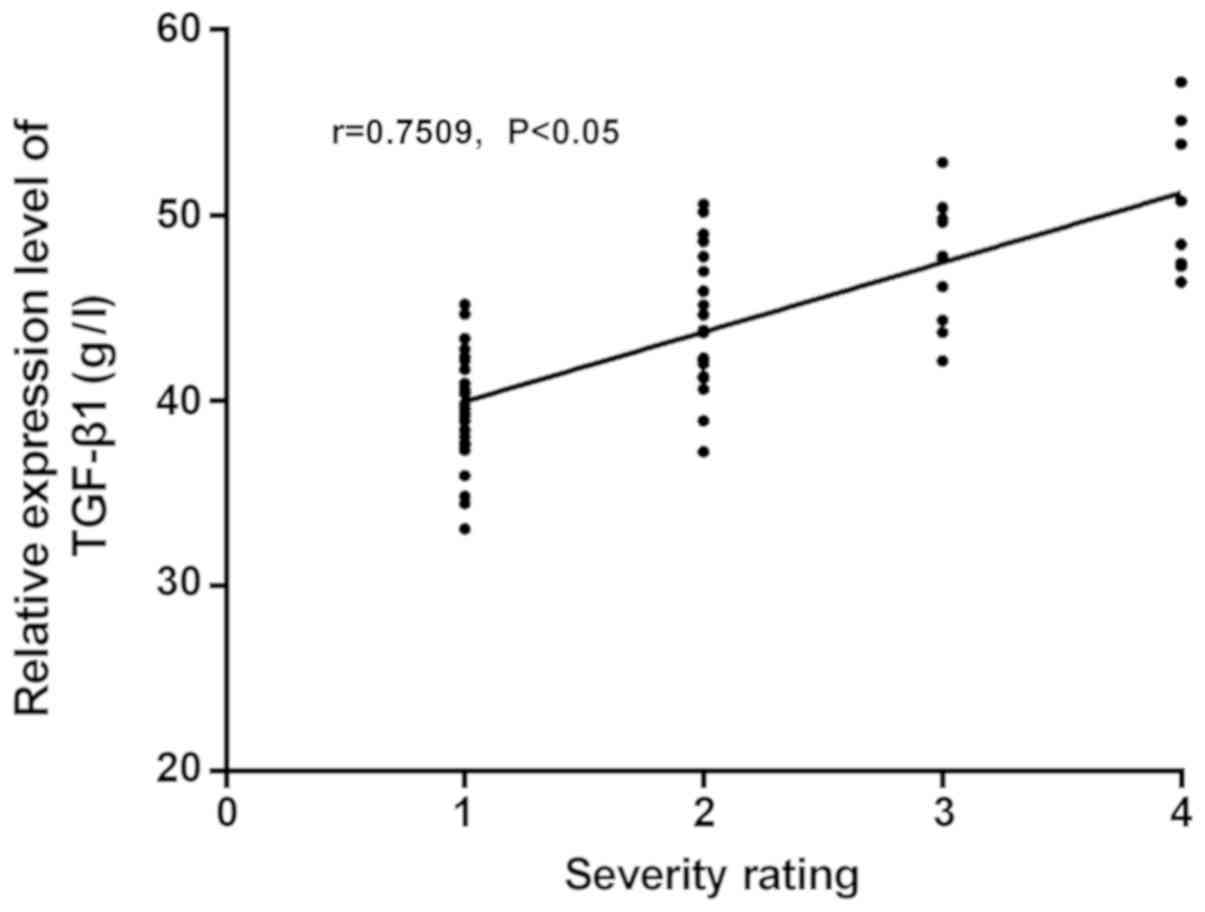
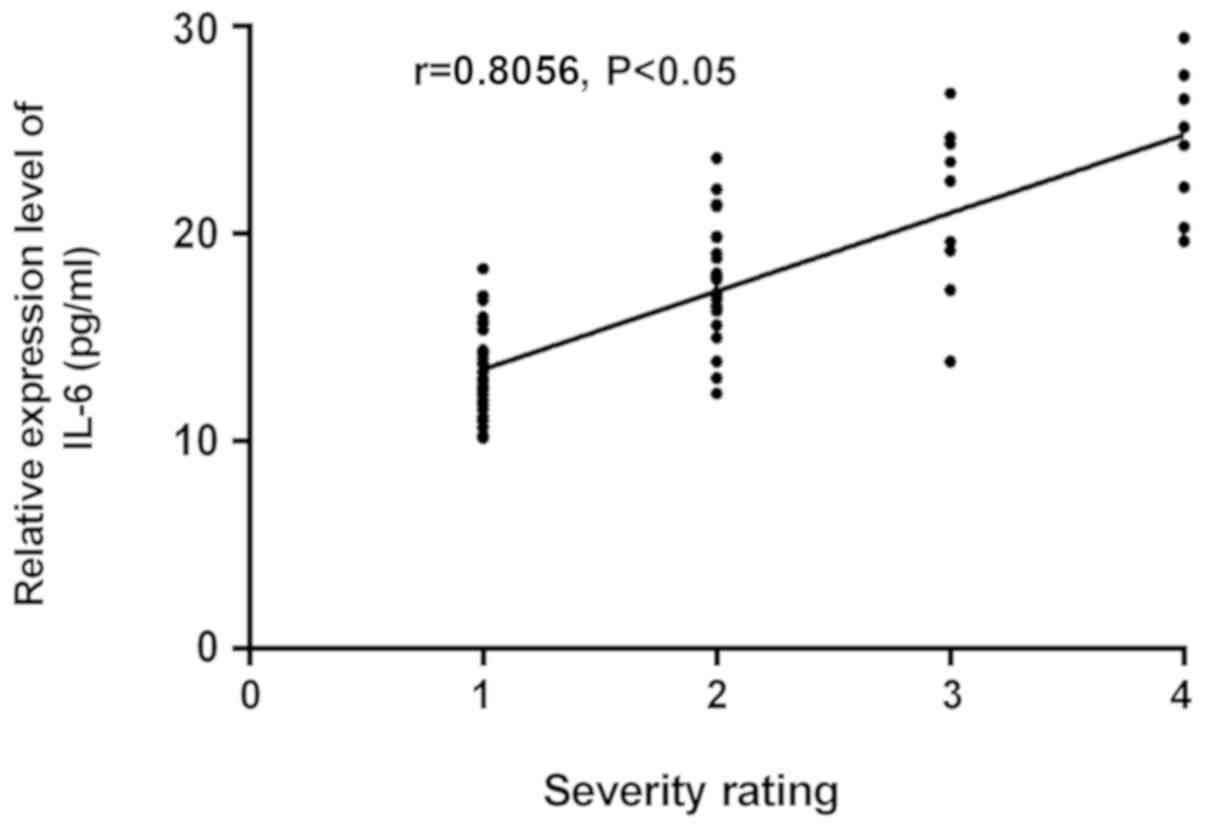
Correlation analysis between the severity of the disease and the
expression levels of TGF-β1 and IL-6 showed that, the expression
level of TGF-β1 increased with the increase of disease severity, so
there was a positive correlation between them (r=0.7509,
P<0.05). As the expression level of IL-6 increased with the
increase of disease severity, there was also a positive correlation
between them (r=0.8056, P<0.05) (Fig.
2). Therefore, the higher the grade, the higher the expression
levels of TGF-β1 and IL-6, and the higher the risk of illness.
 | Table IV.Comparison of TGF-β1 and IL-6
expressions in NRDS children with different severity (mean ±
standard deviation). |
Table IV.
Comparison of TGF-β1 and IL-6
expressions in NRDS children with different severity (mean ±
standard deviation).
| Grade | n | TGF-β1 (g/l) | IL-6 (pg/ml) |
|---|
| I | 32 | 39.11±3.12 | 14.01±2.83 |
| II | 24 | 43.95±3.34 | 17.23±3.05 |
| III | 11 | 46.83±3.62 | 19.97±3.62 |
| IV | 8 | 50.11±4.23 | 23.32±4.47 |
| F |
| 31.13 | 22.70 |
| P-value |
| <0.05 | <0.05 |
Correlation between TGF-β1 and
IL-6
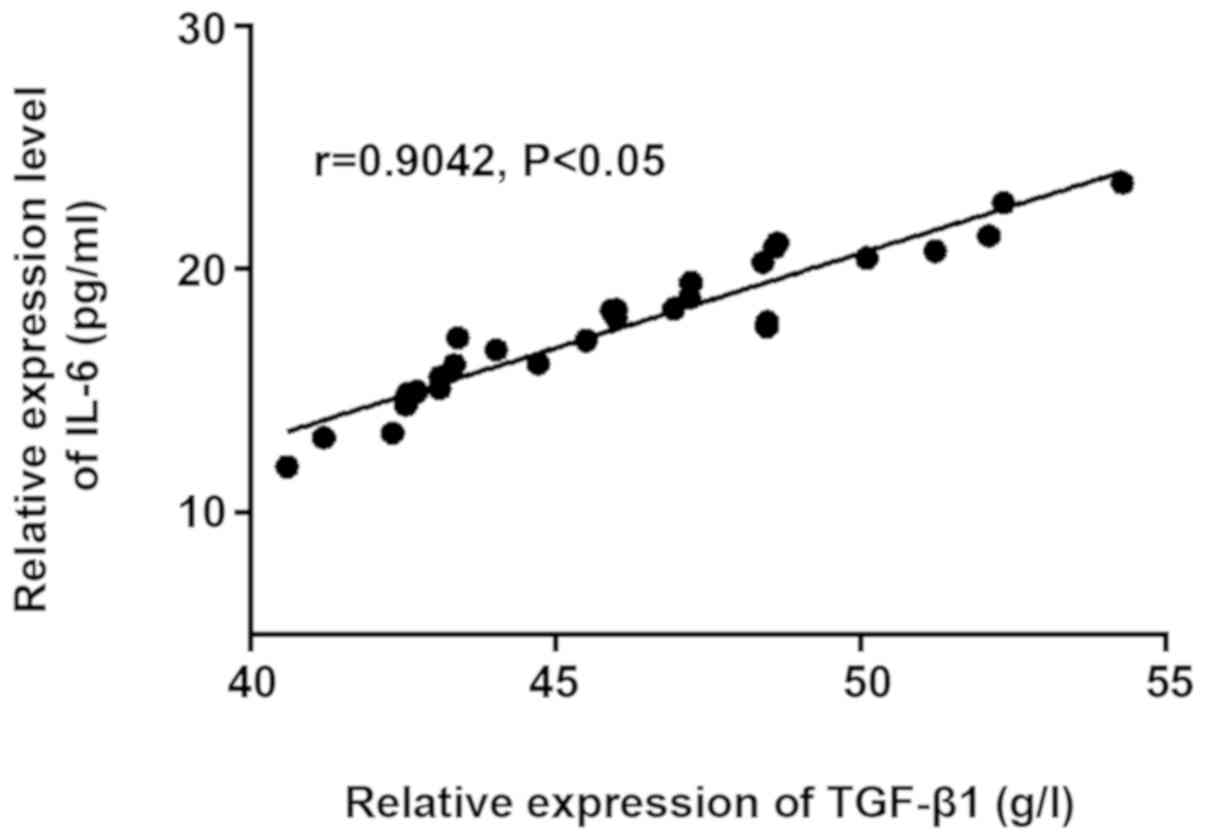
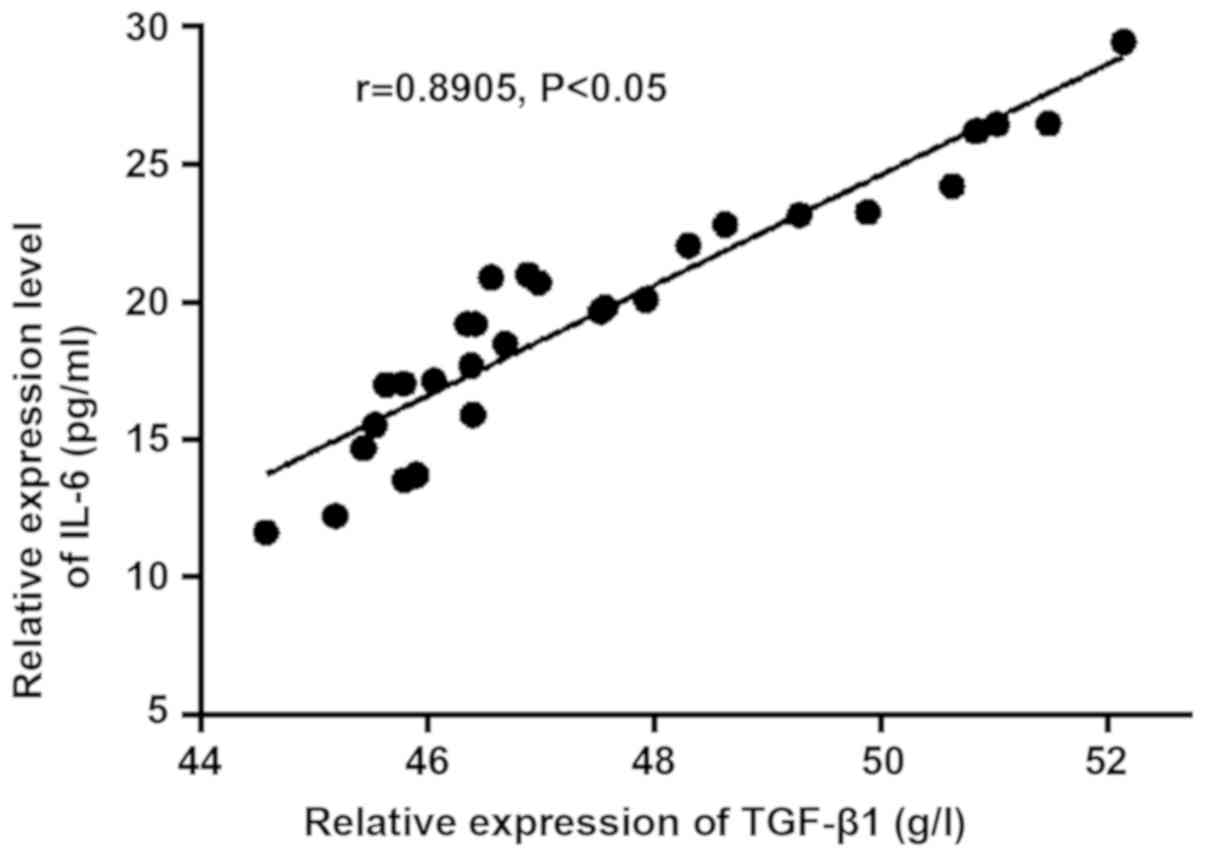
The correlation analysis chart was made according to
the expression levels of TGF-β1 and IL-6 in PS and non-PS groups
(Figs. 3 and 4). TGF-β1 and IL-6 were positively
correlated in PS group (r=0.9042, P<0.05), and they were also
positively correlated in non-PS group (r=0.8905, P<0.05). The
expression level of IL-6 increased gradually with the increase of
the expression level of TGF-β1 in vivo.
Discussion
NRDS is mainly characterized by respiratory failure
and progressive dyspnea shortly after birth (16). The occurrence of RDS is mainly
related to the long-term injury of respiratory epithelial cells and
insufficient secretion of surfactant in alveolar epithelium.
Genetics is also an important factor leading to RDS. Especially in
high risk groups such as premature infants, its incidence is higher
than that in the general population (55–75%) (17,18).
Therefore, early prediction and evaluation of NRDS in clinical
treatment is of great significance in minimizing the occurrence of
BPD. Infection, mechanical ventilation and high oxygen
concentration may interfere with the normal programmed development
of immature lung (19), and then
produce a series of inflammatory reactions, resulting in acute and
chronic lung injury in newborn children.
As a cytokine, TGF-β1 plays a vital role in the
multifunctional regulation of cell growth and differentiation
(20). Moreover, it is also very
important in the occurrence and development of acute and chronic
lung injury (21). It has been found
that TGF-β1, interleukin 8 (IL-8) and staphylococcal protein A
(SPA) are highly expressed in pulmonary lavage fluid of BDP
children (22). TGF-β1 can not only
promote the process of pulmonary fibrosis, but also induce the high
expression of connective tissue growth factor (CTGF) in lung,
thereby promoting the development of pulmonary fibrosis (23). Studies have shown that TGF-β1 is
activated at the early stage of ARDS, the occurrence of pulmonary
fibrosis suggests strong expression of TGF-β1 (24,25), and
the degree of pulmonary fibrosis is closely related to the
mortality of ARDS (26). As an
inflammatory cytokine with multiple effects, IL-6 is a member of
the immunomodulatory cytokine family and has anti-inflammatory and
pro-inflammatory effects (27). The
binding of IL-6 to its receptor (IL-6R) activates the target cell
and further recruits the dimer formed by human glycoprotein 130
(gp130), thereby initiating the downstream signaling pathway
(28).
Comparison of clinical baseline data in this study
showed that there were no significant differences between the three
groups in general data such as sex, gestational age, birth weight,
average birth time or birth mode. While there were significant
differences in multiple pregnancy, premature rupture of membranes,
intrauterine distress or asphyxia, gestational diabetes, amniotic
fluid aspiration, umbilical cord abnormality, placental
abnormality, intrauterine infection and prenatal glucocorticoid use
(P<0.05), indicating that these factors may be the cause of NRDS
(29,30). In this study, the expression levels
of TGF-β1 and IL-6 in the three groups were compared and analyzed,
and it was found that expression of TGF-β1 and IL-6 was low in the
healthy control group at all time points, indicating that they were
secreted in normal lung tissue. The expression level of serum
TGF-β1 in PS group was significantly higher than that in control
group on days 1 and 3 after birth, and decreased on day 7, which
indicated that PS treatment could alleviate the inflammatory
reaction in vivo (31).
However, the expression level of serum TGF-β1 in non-PS group
increased continuously with the increase of the number of days,
indicating that the inflammatory reaction in the children's body
was always present and showed a high expression, which would easily
lead to the occurrence of BDP. In this study, the expression level
of IL-6 in the three groups were compared and analyzed, the results
showed that the expression level of serum IL-6 in the PS group
peaked on day 3 after birth, decreased at day 7, and there was no
significant difference when compared with the control group,
suggesting that the child was recovering gradually. The expression
level of IL-6 in the PS group was significantly higher than that in
the control group on days 1 and 3 (P<0.05). The expression level
of IL-6 in the non-PS group increased continuously after birth and
was significantly higher than that in the control group on days 1,
3 and 7 (P<0.05), and higher than that in the PS group on days 3
and 7 (P<0.05). Because many families can not afford the high
cost of PS treatment, it is crucial to find a more suitable
alternative for the general population to control the inflammatory
reaction of the body. Yu et al (32) found that serum tumor necrosis factor
α (TNF-α) and IL-6 play an important role in the pathogenesis of
ARDS in children and are closely related to the occurrence,
development and severity of ARDS. In this study, by comparing the
expression of TGF-β1 and IL-6 in NRDS children with different
severity, it was found that both TGF-β1 and IL-6 were positively
correlated with the severity of the disease. In other words, the
more severe the disease, the higher their expression. There are few
references on the correlation between TGF-β1 and IL-6 with the
severity of NRDS, so they are of great research value. Correlation
analysis of the expression of TGF-β1 and IL-6 in PS and non-PS
groups showed that, TGF-β1 and IL-6 were correlated in the two
groups. IL-6 expression level increased with the increase of TGF-β1
in vivo, with a positive correlation between them. Findings
have shown that TGF-β1 and signal transduction protein (smad3) are
closely related to fibrosis (33),
and IL-6 can activate the signal transduction pathway of
TGF-β1/smad3 (34). However, there
is no reference concerning the correlation between these two
factors in NRDS children. Therefore, this study was conducted to
provide reference value for clinical research.
Understanding the trends and correlations of TGF-β1
and IL-6 in NRDS can help early clinical prevention and treatment
and improve the prognosis of children. There were some limitations
in this study such as few samples and short observation time.
Therefore, in order to understand the condition of the children in
detail, it is necessary to perform regularly follow-up and
pulmonary function test after the discharge.
In conclusion, TGF-β1 and IL-6 are associated with
NRDS, which can provide some references for clinical practice.
TGF-β1 and IL-6 have important value in identifying NRDS. It is
suggested that decreasing TGF-β1 or IL-6 level by treatment has
certain significance for the development of the children's
condition.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FC interpreted the data and drafted the manuscript.
FH conceived and designed the study. FC and FH collected and
analyzed the data. FZ was responsible for the detection of TGF-β1
and IL-6 in serum. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xiangyang Central Hosptial (Xiangyang, China). Patients who
participated in this study had complete clinical data. Signed
informed consents were obtained from the parents of the child
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lupton-Smith A, Argent A, Rimensberger P,
Frerichs I and Morrow B: Prone positioning improves ventilation
homogeneity in children with acute respiratory distress syndrome.
Pediatr Crit Care Med. 18:e229–e234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang CH, Du LZ, Ma XL, Shi LP, Tong XM,
Liu H, Ding GF, Yi B, Pan XN, Zhong DN, et al: Analysis of
in-hospital neonatal death in the tertiary neonatal intensive care
unit in China: a multicenter retrospective study. Chin Med J
(Engl). 129:2652–2658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stephenson J, Heslehurst N, Hall J,
Schoenaker DAJM, Hutchinson J, Cade JE, Poston L, Barrett G,
Crozier SR, Barker M, et al: Before the beginning: nutrition and
lifestyle in the preconception period and its importance for future
health. Lancet. 391:1830–1841. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma L, Liu C, Wang Y, Li S, Zhai S, Gu X,
Liu F, Yan A, Guo W, Li Y, et al Hebei Neonatal Network Study
Group, : Mortality of neonatal respiratory failure related to
socioeconomic factors in Hebei province of China. Neonatology.
100:14–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steurer MA, Adams M, Bacchetti P, Schulzke
SM, Roth-Kleiner M and Berger TM; Swiss Neonatal Network, : Swiss
medical centres vary significantly when it comes to outcomes of
neonates with a very low gestational age. Acta Paediatr.
104:872–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lassi ZS, Middleton PF, Crowther C and
Bhutta ZA: Interventions to improve neonatal health and later
survival: an overview of systematic reviews. EBioMedicine.
2:985–1000. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salam RA, Das JK, Lassi ZS and Bhutta ZA:
Adolescent health and well-being: Background and methodology for
review of potential interventions. J Adolesc Health 59 (4S).
S4–S10. 2016. View Article : Google Scholar
|
|
8
|
Armenia S, Thangamathesvaran L, Caine AD,
King N, Kunac A and Merchant AM: The role of high-fidelity
team-based simulation in acute care settings: a systematic review.
Surg J NY. 4:e136–e151. 2018. View Article : Google Scholar
|
|
9
|
Gaede KI, Amicosante M, Schürmann M,
Fireman E, Saltini C and Müller-Quernheim J: Function associated
transforming growth factor-beta gene polymorphism in chronic
beryllium disease. J Mol Med (Berl). 83:397–405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kinnula VL: Focus on antioxidant enzymes
and antioxidant strategies in smoking related airway diseases.
Thorax. 60:693–700. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iannuzzi MC and Rybicki BA: Genetics of
sarcoidosis: Candidate genes and genome scans. Proc Am Thorac Soc.
4:108–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rincon M and Irvin CG: Role of IL-6 in
asthma and other inflammatory pulmonary diseases. Int J Biol Sci.
8:1281–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esteban-Gorgojo I, Antolín-Amérigo D,
Domínguez-Ortega J and Quirce S: Non-eosinophilic asthma: current
perspectives. J Asthma Allergy. 11:267–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Cao HY, Wang HW and Kong XY: The
role of lung ultrasound in diagnosis of respiratory distress
syndrome in newborn infants. Iran J Pediatr. 24:147–154.
2014.PubMed/NCBI
|
|
15
|
Boskabadi H, Mamoori G, Khatami SF and
Faramarzi R: Serum level of vitamin D in preterm infants and its
association with premature-related respiratory complications: a
case-control study. Electron Physician. 10:6208–6214. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Podraza W, Michalczuk B, Jezierska K,
Domek H, Kordek A, Łoniewska B, Modrzejewska M and Kot J:
Correlation of retinopathy of prematurity with bronchopulmonary
dysplasia. Open Med (Wars). 13:67–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mullany D, Shekar K, Ziegenfuss M, Joyce
C, Pilcher D, Dobson A and Fraser JF: The effects of the
introduction of an adult ECMO program on statewide referral
patterns, casemix and outcomes in patients with acute respiratory
distress syndrome or pneumonia. Intensive Care Med. 43:1065–1066.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jabaudon M, Godet T, Futier E, Bazin JÉ,
Sapin V, Roszyk L, Pereira B and Constantin JM; AZUREA group, :
Rationale, study design and analysis plan of the lung imaging
morphology for ventilator settings in acute respiratory distress
syndrome study (LIVE study): Study protocol for a randomised
controlled trial. Anaesth Crit Care Pain Med. 36:301–306. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Britt RD Jr, Velten M, Tipple TE, Nelin LD
and Rogers LK: Cyclooxygenase-2 in newborn hyperoxic lung injury.
Free Radic Biol Med. 61:502–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Chen Y, Fan L, Ye J, Fan J, Xu X,
You D, Liu S, Chen X and Luo P: Pharmacological mechanism of
roflumilast in the treatment of asthma-COPD overlap. Drug Des Devel
Ther. 12:2371–2379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bin S, Li HD, Xu YB, Qi SH, Li TZ, Liu XS,
Tang JM and Xie JL: BMP-7 attenuates TGF-β1-induced fibroblast-like
differentiation of rat dermal papilla cells. Wound Repair Regen.
21:275–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo J, Lin Q, Shao Y, Rong L and Zhang D:
BMP-7 suppresses excessive scar formation by activating the
BMP-7/Smad1/5/8 signaling pathway. Mol Med Rep. 16:1957–1963. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang HY, Liang XW, Chen ZY, Tan XH, Yang
HH, Liao JY, Cai K and Yu JS: Ultrasound in neonatal lung disease.
Quant Imaging Med Surg. 8:535–546. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al American Heart Association Statistics Committee and Stroke
Statistics Subcommittee, : Heart disease and stroke statistics-2017
update: a report from the American Heart Association. Circulation.
135:e146–e603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dhainaut JF, Charpentier J and Chiche JD:
Transforming growth factor-beta: a mediator of cell regulation in
acute respiratory distress syndrome. Crit Care Med. 31
(Suppl):S258–S264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng XM, Chung AC and Lan HY: Role of the
TGF-β/ BMP-7/Smad pathways in renal diseases. Clin Sci (Lond).
124:243–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chioma OS and Drake WP: Role of microbial
agents in pulmonary fibrosis. Yale J Biol Med. 90:219–227.
2017.PubMed/NCBI
|
|
28
|
Lokau J, Agthe M, Flynn CM and Garbers C:
Proteolytic control of interleukin-11 and interleukin-6 biology.
Biochim Biophys Acta Mol Cell Res 1864 (11 Pt B). 2105–2117. 2017.
View Article : Google Scholar
|
|
29
|
Vignoles P, Gire C, Mancini J, Bretelle F,
Boubli L, Janky E and Carcopino X: Gestational diabetes: a strong
independent risk factor for severe neonatal respiratory failure
after 34 weeks. Arch Gynecol Obstet. 284:1099–1104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anadkat JS, Kuzniewicz MW, Chaudhari BP,
Cole FS and Hamvas A: Increased risk for respiratory distress among
white, male, late preterm and term infants. J Perinatol.
32:780–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lamontagne F, Brower R and Meade M:
Corticosteroid therapy in acute respiratory distress syndrome.
CMAJ. 185:216–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu L, Zeng XL, Cheng ML, Yang GZ, Wang B,
Xiao ZW, Luo X, Zhang BF, Xiao DW, Zhang S, et al: Quantitative
assessment of the effect of pre-gestational diabetes and risk of
adverse maternal, perinatal and neonatal outcomes. Oncotarget.
8:61048–61056. 2017.PubMed/NCBI
|
|
33
|
MacLean J and Pasumarthi KB: Signaling
mechanisms regulating fibroblast activation, phenoconversion and
fibrosis in the heart. Indian J Biochem Biophys. 51:476–482.
2014.PubMed/NCBI
|
|
34
|
Ogunyemi D, Jovanovski A, Liu J, Friedman
P, Sugiyama N, Creps J and Madan I: The contribution of untreated
and treated anxiety and depression to prenatal, intrapartum, and
neonatal outcomes. AJP Rep. 8:e146–e157. 2018. View Article : Google Scholar : PubMed/NCBI
|