Introduction
Cervical cancer is the second most common type of
malignant tumor in females worldwide and the most common in a
number of developing countries, representing a large socioeconomic
burden (1). The International Agency
for Research on Cancer reported that ~300,000 mortalities are
attributed to cervical cancer annually in China (2,3).
Human papillomavirus (HPV)-18 is regarded as an
important strain of HPV that causes precancerous lesions, which
subsequently develop into cervical cancer and is associated with
>90% of all cervical cancer cases (4). The prevalence of HPV-18 infection in
women continues to increase globally; however, in the majority of
cases infection does not progress to the disease stage (4). It is therefore important to determine
factors aside from infection that lead to carcinogenesis and the
progression of cervical cancer (5).
Recent studies have revealed that the
phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt) signal
transduction pathway (6) serves a
key role in a number of cellular activities, including apoptosis,
aging and proliferation (7). The
PI3K/Akt signal transduction pathway responds to various
intracellular and extracellular survival pressures to adaptively
regulate these processes (7). A
previous study demonstrated that ionizing radiation, ultraviolet
rays and cytotoxic drugs may activate the PI3K/Akt signal
transduction pathway (8).
Mammalian target of rapamycin (mTOR) is a type of
serine/threonine protein kinase that is highly evolutionarily
conserved and is observed in a wide variety of organisms (9). The mTOR signaling pathway serves an
important role in the growth and proliferation of normal cells,
however it is also closely associated with the growth,
proliferation, differentiation, apoptosis and metabolism of a
number of types of malignant tumor (10). mTOR expression has been reported to
be upregulated in multiple tumors, including breast cancer, colon
cancer and lymphoma (10).
Puerarin is an isoflavonoid monomer that may be
isolated and extracted from the leguminous plant Pueraria
lobate (11). Puerarin has
strong pharmacological activity (12) and a wide range of pharmacological
effects, including anti-arrhythmia, anti-myocardial infarction,
anti-angiectasis, antitumor, microcirculation improvement, blood
fat reduction, increased cerebral blood flow, protection against
oxidation, regulation of bone metabolism and reduction of
intraocular pressure (11,12). Huang et al (13) demonstrated that Puerarin induces cell
apoptosis in human chondrosarcoma cells via inhibiting the PI3K/Akt
signaling pathway. The aim of the present study was to explore the
effects of Puerarin on apoptosis in HPV-positive cervical cancer
cells and the molecular mechanisms responsible.
Materials and methods
Cell culture
A HeLa HPV-18-positive cervical cancer cell line was
obtained from the American Type Culture Collection (cat. no.
CRM-CCL-2; Manassas, VA, USA) and cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 2 mM/L-glutamine, 100 U/ml penicillin and 100 mg/ml
streptomycin (both Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
at 37°C in a humidified atmosphere containing 5%
CO2.
MTT assay and lactate dehydrogenase
(LDH) activity
HeLa cells were seeded at a density of
5×103 cells/well in 96-well plates and incubated
overnight at 37°C. The cells were treated with 0, 0.25, 0.50, 1.00
or 2.00 mM Puerarin (Sigma-Aldrich; Merck KGaA) for 24, 48 and 72 h
at 37°C as previously described (14). A total of 20 µl MTT solution
(Sigma-Aldrich; Merck KGaA) was added to each well and the cells
were incubated for a further 4 h. A total of 150 µl dimethyl
sulfoxide was added to each well to dissolve the purple formazan
and the absorption was measured using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) at 492 nm.
HeLa cells were seeded in 6-well plates at a density
of 5×106 cells/well and were treated with 0.50, 1.00 or
2.00 mM Puerarin for 48 h at 37°C. The LDH activity level was
subsequently measured using an LDH activity kit (C0016; Beyotime
Institute of Biotechnology, Haimen, China) and the absorption was
measured with a microplate reader at 450 nm.
Annexin V/fluorescein
isothiocyanate/propidium iodide (PI) analysis
HeLa cells were seeded in 6-well plates overnight
(5×106 cells/well) at 37°C. The cells were treated with
0.50, 1.00 or 2.00 mM Puerarin for 48 h at 37°C, fixed with 4%
paraformaldehyde for 15 min at room temperature, stained with
Annexin V/phycoerythrin and PI (Sigma-Aldrich; Merck KGaA) at room
temperature for 15 min and analyzed using flow cytometry (FACScan;
BD Biosciences, San Jose, CA, USA). Data were analyzed using FlowJo
7.6.1 (FlowJo LLC, Ashland, OR, USA).
Migration assay
HeLa cells (1×105) were seeded in the
upper chamber of 24-well plates with Transwell inserts (pore size,
8 µm; Millipore; Merck KGaA) containing DMEM. The lower chamber
contained DMEM supplemented with 10% FBS. Following 48 h, cells
from the lower surface of the inserts were fixed with 4%
paraformaldehyde for 10 min at room temperature and stained with 1%
crystal violet for 30 min at room temperature. Migrating cells were
visualized using a light microscope (magnification, ×100).
Measuring caspase-3/9 activity and a
DAPI assay
Total protein was extracted from cells using a
radioimmunoprecipitation buffer (Sigma-Aldrich; Merck KGaA). A
total of 10 µg protein was incubated with caspase-3 and caspase-9
activity kits (C1115 and C1158; Beyotime Institute of
Biotechnology) for 2 h at 37°C, according to the manufacturer's
instructions. The absorption was measured using a plate reader at
405 nm.
HeLa cells (5×106 cells/well) were seeded
in 6-well plates and incubated overnight at 37°C. The cells were
then treated with 0.50, 1.00 or 2.00 mM Puerarin for 48 h at 37°C,
washed with PBS, fixed with 4% paraformaldehyde for 15 min at room
temperature and stained with DAPI (5 mg/ml) for 30 min in darkness
at room temperature. Cells were observed using a fluorescence
microscope (magnification, ×100).
Western blotting
Total protein was extracted from the cells as
described above. The proteins (50 µg) were separated using 10%
SDS-PAGE gels and transferred to nitrocellulose membranes. The
membranes were blocked with 5% non-fat milk in TBST for 1 h at 37°C
and incubated with primary antibodies against Bax (sc-6236;
1:10,000), PI3K (sc-293172; 1:2,000), phosphorylated (p)-Akt
(sc-7985-R; 1:1,000), Akt (sc-135829; 1:1,000), p-mTOR (sc-293133;
1:1,000), mTOR (sc-1549; 1:1,000), and GADPH (sc-47724; 1:1,000;
all Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at
4°C. The membranes were subsequently incubated with goat
anti-rabbit IgG-horseradish peroxidase secondary antibodies
(sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h
and developed using an enhanced chemiluminescent detection system
(Beyotime Institute of Biotechnology). The protein bands were
scanned using a Fujifilm LAS-3000 Imaging system (Fujifilm
Corporation, Tokyo, Japan) and analyzed with Image Lab 3.0 (Bio-Rad
Laboratories, Inc.).
Cell transfection
The PI3K plasmid was purchased from Sangon Biotech
Co., Ltd. (Shanghai, China). HeLa cells were transfected with 100
nM PI3K plasmid using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. At 4 h following transfection, medium was
replaced and supplemented with 0.50, 1.00 or 2.00 mM Puerarin and
cells were cultured for 44 h at 37°C.
Statistical analysis
Data are expressed as the mean ± standard deviation
and are representative of three replicates. SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA) was used in data analyses. Comparisons were made
using one-way analysis of variance, with a Bonferroni post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Puerarin reduces cell proliferation in
HeLa cells
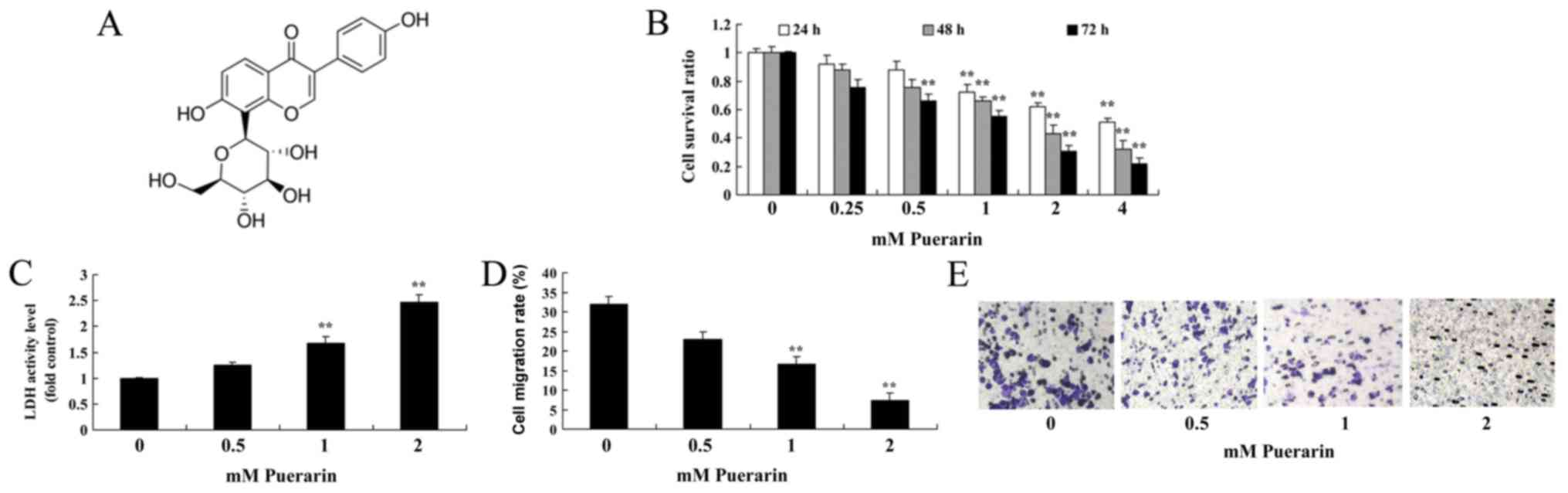
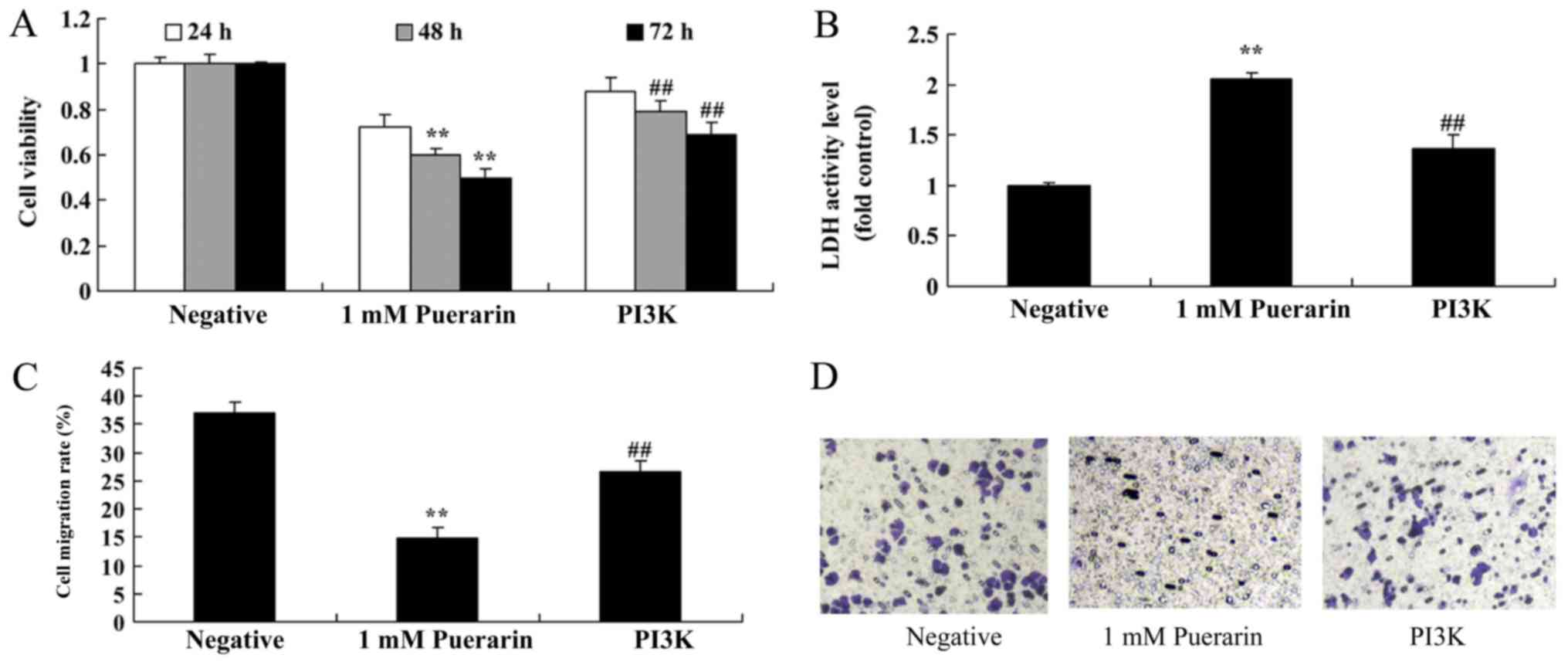
It was investigated whether Puerarin (Fig. 1A) exerted any effects on the
proliferation of HeLa cells. Puerarin inhibited the cell
proliferation of HeLa cells in a dose- and time-dependent manner.
Treatment with 0.5 mM Puerarin significantly inhibited the
proliferation of HeLa cells compared with the control group at 72
h, however treatment with 1–4 mM Puerarin significantly inhibited
the cell proliferation at all time points (Fig. 1B). In addition, treatment with 1 or 2
mM Puerarin significantly increased the LDH activity (Fig. 1C) and significantly reduced the
migration rate of HeLa cells compared with the control group
(Fig. 1D and E).
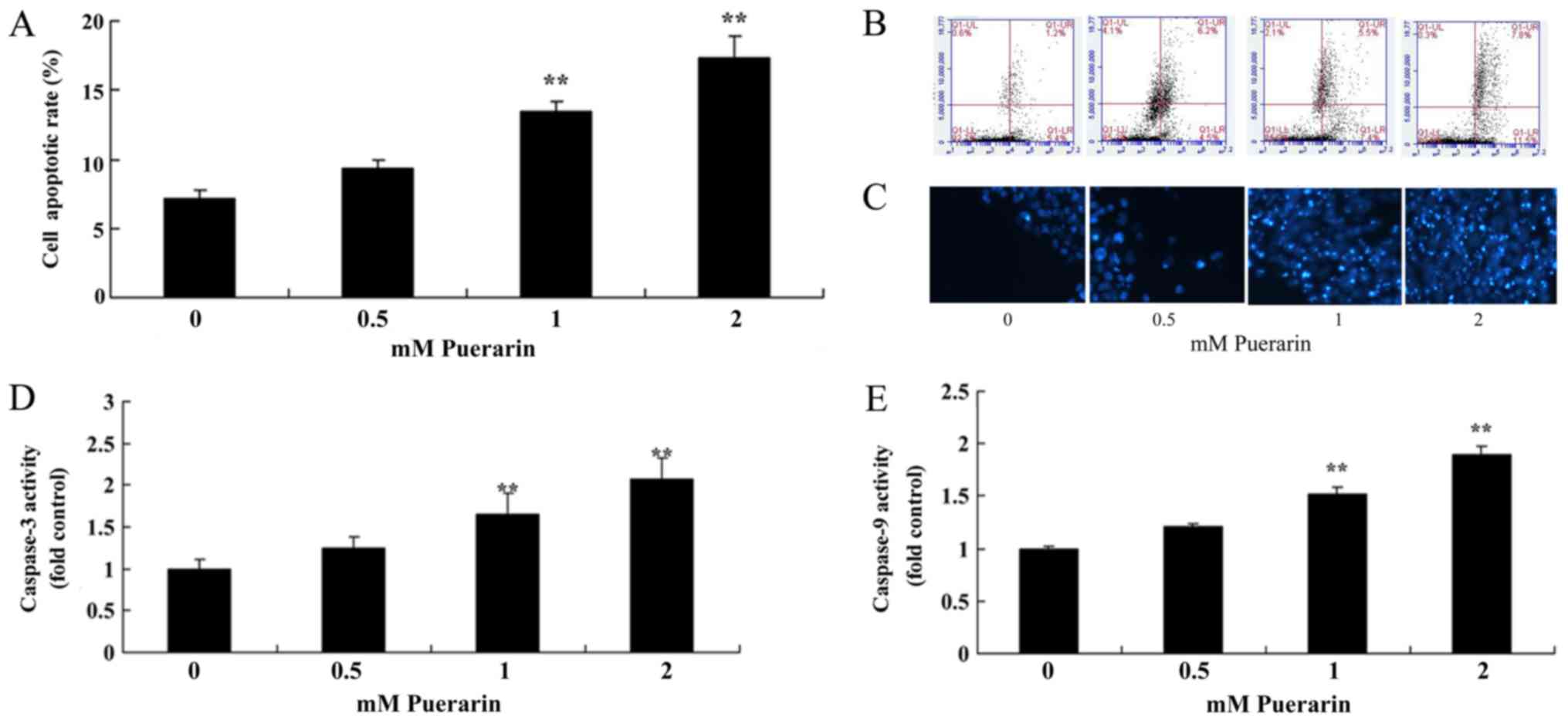
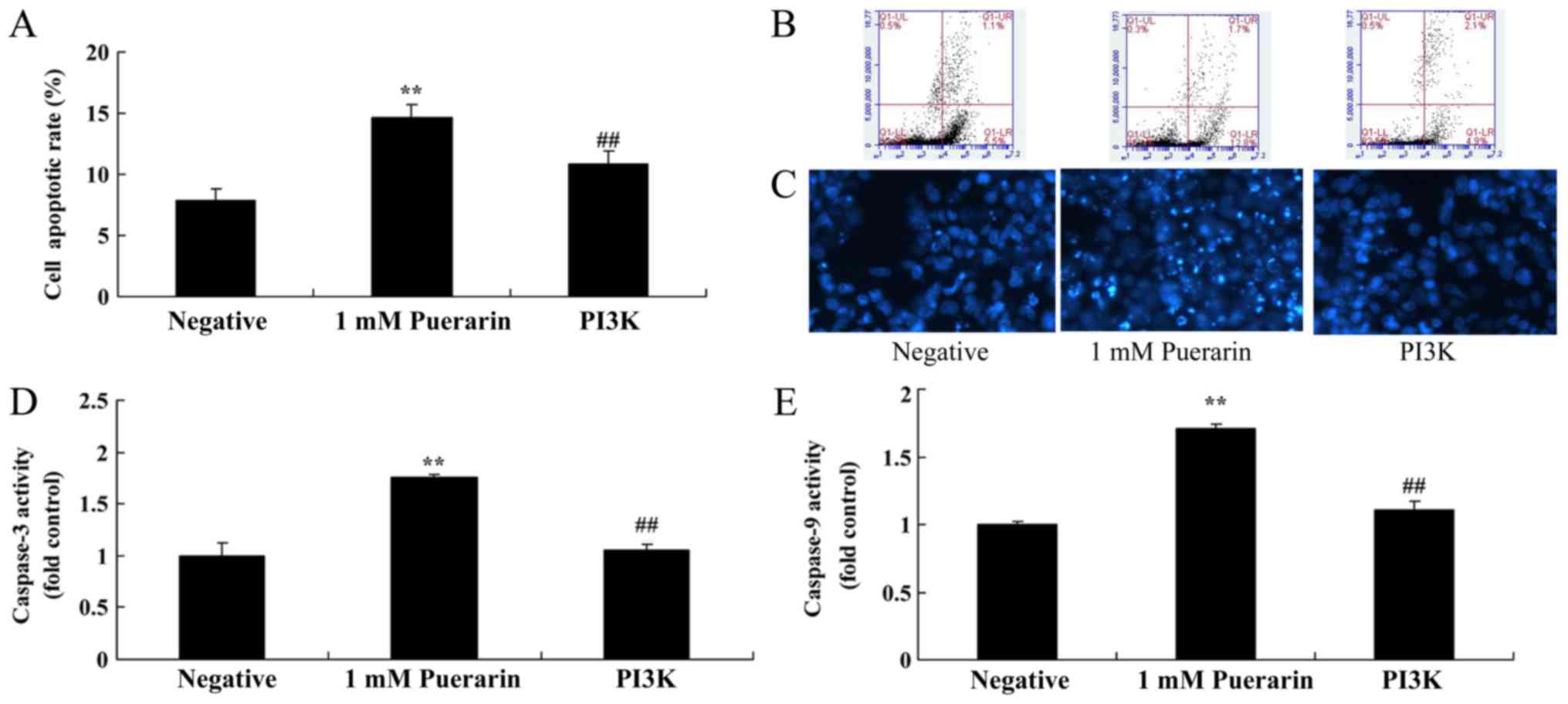
Puerarin induces apoptosis in HeLa
cells
Flow cytometry analysis was performed to determine
whether Puerarin affects the apoptosis of HeLa cells. The apoptosis
rate in cells treated with Puerarin (1–2 mM) was significantly
increased compared with the control group (Fig. 2A). In addition, Puerarin (1–2 mM)
significantly increased Ccaspase-3 and −9 activity in HeLa cells
compared with the control group (Fig. 2D
and E).
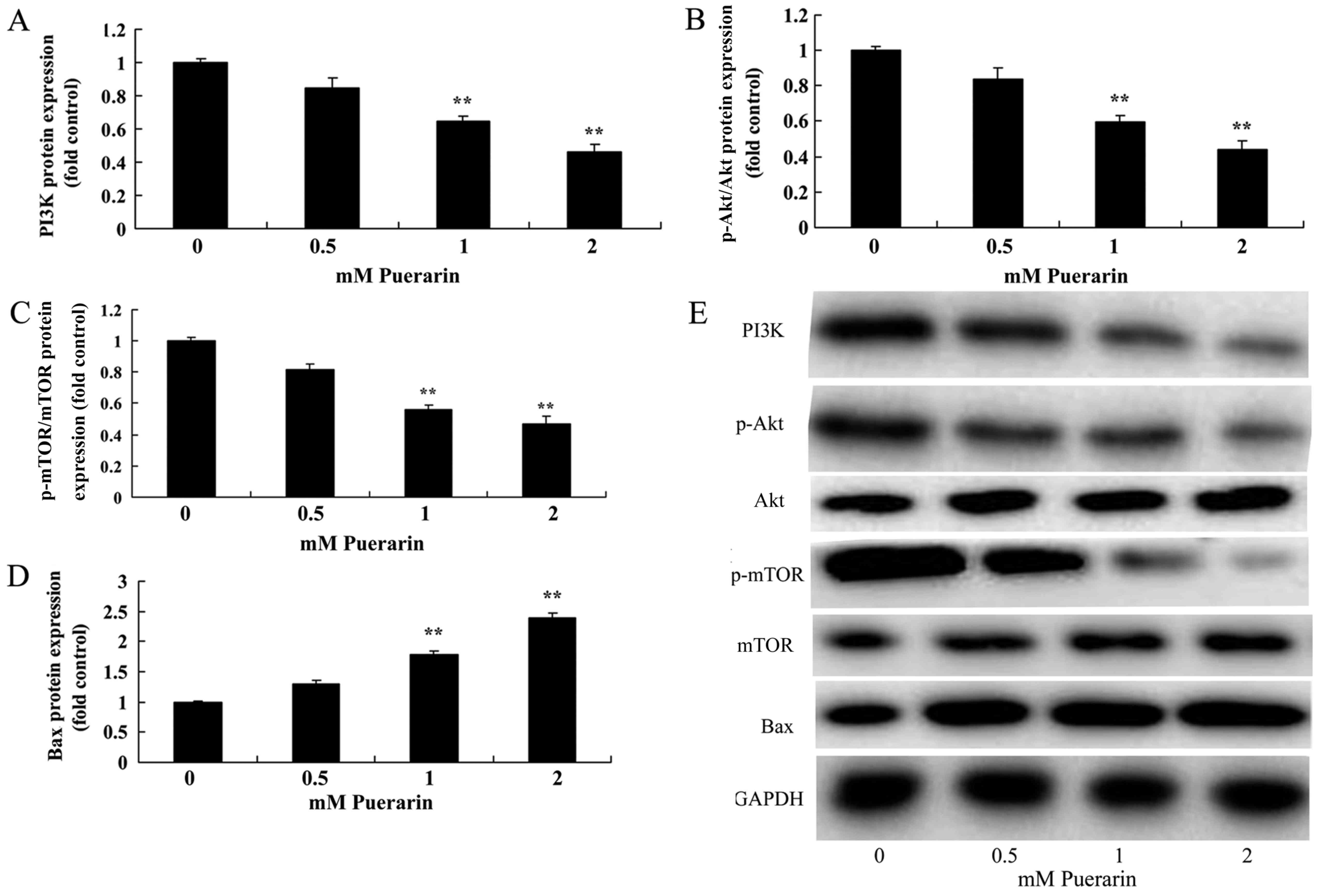
Puerarin suppresses PI3K, p-Akt and
p-mTOR protein expression in HeLa cells
The results of western blotting demonstrated that
treatment with Puerarin (1–2 mM) significantly reduced PI3K
(Fig. 3A), p-Akt (Fig. 3B) and p-mTOR (Fig. 3C) protein expression and
significantly increased Bax (Fig.
3D) protein expression in HPV-18 positive HeLa cells compared
with the control group (Fig.
3E).
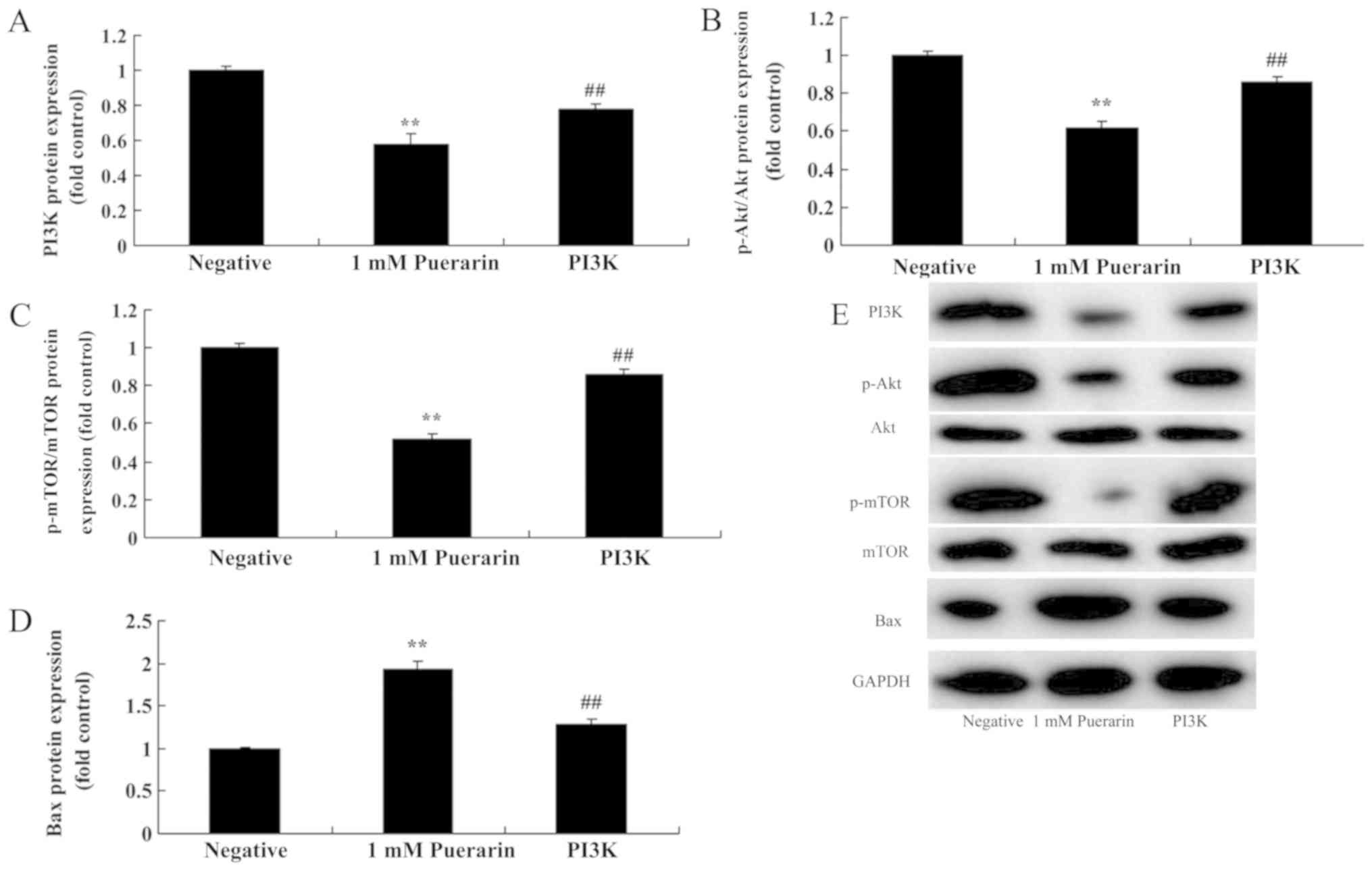
An increase in PI3K reduces the
anticancer effect of Puerarin in HeLa cells
The role of PI3K/p-Akt/p-mTOR signaling in the
anticancer effects of Puerarin was further investigated.
Transfection with the PI3K plasmid significantly increased the
protein expression of PI3K (Fig.
4A), p-Akt (Fig. 4B) and p-mTOR
(Fig. 4C), while Bax (Fig. 4D) expression was significantly
reduced compared with the Puerarin treated group (Fig. 4E). It was also revealed that
transfection with PI3K plasmids significantly ameliorated the
effects of Puerarin on cell viability (Fig. 5A) and also significantly decreased
the LDH activity compared with the Puerarin group (Fig. 5B). PI3K plasmid transfection
significantly decreased the migration rate of HeLa cells compared
with the Puerarin group (Fig. 5C and
D). It was also demonstrated that PI3K transfection
significantly decreased the anticancer effect of Puerarin on the
rate of apoptosis and caspase-3/9 activities in HeLa cells compared
with the Puerarin group (Fig.
6).
Discussion
Cervical cancer associated with HPV-infection is a
potentially preventable malignancy, as a vaccination for HPV is
currently available and undergoing further development. In
addition, if identified at an early stage cervical cancer is
readily curable (15). In the
present study, it was revealed that Puerarin inhibited cell
proliferation, increased the LDH activity, reduced the migration
rate and increased apoptosis rate in HeLa cells in a dose- and
time-dependent manner. A MTT assay, an LDH activity assay and
Annexin V-FITC/PI analysis were utilized to analyze cell growth and
apoptosis. Hu et al (16)
reported that Puerarin inhibits non-small cell lung cancer cell
growth via apoptosis induction. However, in future studies it is
recommended that the number of experimental methods be expanded to
include Ki-67 and Cyclin D analysis. As the current study was only
performed with HeLa cells further investigations are required to
validate the reported findings.
It has been demonstrated that the risk of developing
cervical cancer is positively correlated with the number of sexual
partners an individual has, as this increases their exposure to the
HPV virus. Smoking, economic status, race and geographical location
are all additional risk factors for the development of cervical
cancer (17). Gan and Yin
(14) demonstrated that Puerarin
induced apoptosis in mantle cell lymphoma; based on this, the
authors of the present study hypothesized that Puerarin may
increase the apoptosis rate of HPV-18 positive cervical cancer
cells, which was confirmed in the present study.
The function of Bcl-2 regulates programmed cell
death (18). Members of the Bcl-2
family are inhibitors of apoptosis (19). Initiator and executor caspases are
key molecules associated with regulation of the apoptosis signaling
cascade (19). Apoptosis may occur
through either the intrinsic or extrinsic signaling pathway
(18). The extrinsic pathway is
activated by signals from other cells, while the intrinsic pathway
may be activated by a number of internal cell signals associated
with cell stress, caused by radioactive rays, cytotoxic drugs, the
elimination of growth factors and proteins released by
mitochondrial membranes, including cytochrome C (20). The intrinsic pathway is often
referred to as the mitochondrial apoptosis-signaling pathway.
Cytochrome C combines with apoptosis protease activating factor 1
and inactivated caspase-9 to form a protein complex called the
apoptosome (21). The formation of
apoptosomes activates Caspase-9, which in turn activates of a
series of caspase proteins (caspase-3, caspase-6 and caspase-7) to
trigger changes in cell morphology and biochemistry associated with
apoptosis (22,23). The results of the present study
indicate that Puerarin effectively induced Bax protein expression
and promoted apoptosis in HPV-18 positive cervical cancer cells.
Liu et al (24) revealed that
Puerarin also suppressed lipopolysaccharide-induced breast cancer
cell migration, migration and adhesion.
Activation of the PI3K/mTOR signaling pathway may
promote cell cycle progression, reduce apoptosis and promote the
migration of cancer cells (23),
which are factors associated with the occurrence of multiple tumors
(10). Activated PI3K activates
downstream Akt (23), which infers
an increased tolerance against apoptosis in cancer cells, as well
as inducing cell growth and abnormal metabolism (24). The excessive activation of Akt
activates downstream mTOR, which may cause the rapid proliferation
of cancer cells, increase oncoprotein secretion, accelerate cell
cycle progression and shorten the G1 time interval, which increases
the occurrence and development of tumors (24). In vivo, mTOR realizes its
physiological effects by phosphorylating multiple substrate
proteins (24). The translation
products include translation elements, such as ribosomal proteins
and elongation factors (24).
Previous research indicates that mTOR phosphorylates the 412th
threonine residue of P70S6 kinase, which increases its activity
100-fold and promotes the biosynthesis of proteins (25). The results of the present study
indicate that Puerarin significantly suppressed PI3K, p-Akt and
p-mTOR protein expression in HeLa HPV-18 positive cervical cancer
cells, thereby reducing their proliferative and migration
abilities; however, PI3K upregulation reduced the anticancer effect
of Puerarin on HeLa cells. Huang et al (13) revealed that Puerarin induces cell
apoptosis in human chondrosarcoma cells via inhibition of the
PI3K/Akt signaling pathway, which supports the results of the
present study.
In conclusion, the present study demonstrated that
Puerarin effectively inhibits cell proliferation, increases
apoptosis and promotes caspase-3/9 and Bax protein expression in
HeLa cells, in part by inhibiting the PI3K/Akt/mTOR signaling
pathway. However, further studies are required to provide
additional evidence for the anticancer effects and underlying
mechanisms of Puerarin in HPV-18 positive cervical cancer cells.
Puerarin has potential as a novel drug for the treatment of
cervical cancer in future clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation (grant no. 81302538).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL designed the experiments. LJ, YH and GY performed
the experiments and analyzed the data. PL wrote the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ruifeng G, Yunhe F, Zhengkai W, Ershun Z,
Yimeng L, Minjun Y, Xiaojing S, Zhengtao Y and Naisheng Z:
Chlorogenic acid attenuates lipopolysaccharide-induced mice
mastitis by suppressing TLR4-mediated NF-kappaB signaling pathway.
Eur J Pharmacol. 729:54–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang H, Li J, Chen A, Li Y, Xia M, Guo P,
Yao S and Chen S: Fucosterol exhibits selective antitumor
anticancer activity against HeLa human cervical cell line by
inducing mitochondrial mediated apoptosis, cell cycle migration
inhibition and downregulation of m-TOR/PI3K/Akt signalling pathway.
Oncol Lett. 15:3458–3463. 2018.PubMed/NCBI
|
|
4
|
Kemeny N, Brown K, Covey A, Kim T,
Bhargava A, Brody L, Guilfoyle B, Haag NP, Karrasch M,
Glasschroeder B, et al: Phase I, open-label, dose-escalating study
of a genetically engineered herpes simplex virus, NV1020, in
subjects with metastatic colorectal carcinoma to the liver. Hum
Gene Ther. 17:1214–1224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CH, Shi L, Zhan GL, Rao SZ and Zhang H:
A twenty-four-week, open-label study on ziprasidone's efficacy and
influence on glucolipid metabolism in patients with schizophrenia
and metabolic disorder. Eur Rev Med Pharmacol Sci. 17:2136–2140.
2013.PubMed/NCBI
|
|
6
|
Bettencourt C, Santos C, Montiel R, Kay T,
Vasconcelos J, Maciel P and Lima M: The (CAG)n tract of
Machado-Joseph Disease gene (ATXN3): A comparison between DNA and
mRNA in patients and controls. Eur J Hum Genet. 18:621–623. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang KH, Chen WL, Wu YR, Lin TH, Wu YC,
Chao CY, Lin JY, Lee LC, Chen YC, Lee-Chen GJ and Chen CM: Aqueous
extract of Gardenia jasminoides targeting oxidative stress to
reduce polyQ aggregation in cell models of spinocerebellar ataxia
3. Neuropharmacology. 81:166–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukohyama J, Shimono Y, Minami H, Kakeji Y
and Suzuki A: Roles of microRNAs and RNA-binding proteins in the
regulation of colorectal cancer stem cells. Cancers (Basel).
9:E1432017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Tian M, Zhen Y, Yue Y, Sherman J,
Zheng H, Li S, Tanzi RE, Marcantonio ER and Xie Z: The effects of
isoflurane and desflurane on cognitive function in humans. Anesth
Analg. 114:410–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Yao C, Shi J, Yang F, Qi S, Wang L,
Zhang H, Li J, Wang C, Wang C, et al: Comparative study of the
efficacy and safety between blonanserin and risperidone for the
treatment of schizophrenia in Chinese patients: A double-blind,
parallel-group multicenter randomized trial. J Psychiatr Res.
69:102–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haacke A, Broadley SA, Boteva R, Tzvetkov
N, Hartl FU and Breuer P: Proteolytic cleavage of
polyglutamine-expanded ataxin-3 is critical for aggregation and
sequestration of non-expanded ataxin-3. Hum Mol Genet. 15:555–568.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L and Madura K: Evidence for distinct
functions for human DNA repair factors hHR23A and hHR23B. FEBS
Lett. 580:3401–3408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang L, Cao J, Cao L, Gao L, Yang Y and
Xu L: Puerarin induces cell apoptosis in human chondrosarcoma cell
line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol
Lett. 14:5585–5590. 2017.PubMed/NCBI
|
|
14
|
Gan M and Yin X: Puerarin induced in
mantle cell lymphoma apoptosis and its possible mechanisms
involving multi-signaling pathway. Cell Biochem Biophys.
71:367–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun HQ, Li SX, Chen FB, Zhang Y, Li P, Jin
M, Sun Y, Wang F, Mi WF, Shi L, et al: Diurnal neurobiological
alterations after exposure to clozapine in first-episode
schizophrenia patients. Psychoneuroendocrinology. 64:108–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Li X, Lin L, Liang S and Yan J:
Puerarin inhibits non-small cell lung cancer cell growth via the
induction of apoptosis. Oncol Rep. 39:1731–1738. 2018.PubMed/NCBI
|
|
17
|
Cascinu S, Rosati G, Nasti G, Lonardi S,
Zaniboni A, Marchetti P, Leone F, Bilancia D, Iaffaioli RV, Zagonel
V, et al: Treatment sequence with either irinotecan/cetuximab
followed by FOLFOX-4 or the reverse strategy in metastatic
colorectal cancer patients progressing after first-line
FOLFIRI/bevacizumab: An Italian Group for the Study of
Gastrointestinal Cancer phase III, randomised trial comparing two
sequences of therapy in colorectal metastatic patients. Eur J
Cancer. 83:106–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Zhang B, Shan S and Zhao X:
Neuroprotective effects of vitexin against isoflurane-induced
neurotoxicity by targeting the TRPV1 and NR2B signaling pathways.
Mol Med Rep. 14:5607–5613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang M, Sun L, Liu X, Sun W and You X:
Association of common variants in H2AFZ gene with schizophrenia and
cognitive function in patients with schizophrenia. J Hum Genet.
60:619–624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li G, Xue Q, Luo Y, Hu X and Yu B: S6
inhibition contributes to isoflurane neurotoxicity in the
developing brain. Toxicol Lett. 233:102–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chai D, Jiang H and Li Q: Isoflurane
neurotoxicity involves activation of hypoxia inducible factor-1α
via intracellular calcium in neonatal rodents. Brain Res.
1653:39–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scurr M, Pembroke T, Bloom A, Roberts D,
Thomson A, Smart K, Bridgeman H, Adams R, Brewster A, Jones R, et
al: Effect of modified vaccinia ankara-5T4 and low-dose
cyclophosphamide on antitumor immunity in metastatic colorectal
cancer: A randomized clinical trial. JAMA Oncol. 3:e1725792017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Man DW, Law KM and Chung RC: Cognitive
training for Hong Kong Chinese with schizophrenia in vocational
rehabilitation. Hong Kong Med J. 18 (Suppl 6):S18–S22. 2012.
|
|
24
|
Liu F, Guo X, Wu R, Ou J, Zheng Y, Zhang
B, Xie L, Zhang L, Yang L, Yang S, et al: Minocycline
supplementation for treatment of negative symptoms in early-phase
schizophrenia: A double blind, randomized, controlled trial.
Schizophr Res. 153:169–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lane HY, Liu YC, Huang CL, Chang YC, Liau
CH, Perng CH and Tsai GE: Sarcosine (N-methylglycine) treatment for
acute schizophrenia: A randomized, double-blind study. Biol
Psychiatry. 63:9–12. 2008. View Article : Google Scholar : PubMed/NCBI
|