Introduction
Asthma is a common chronic respiratory disease in
children. Its main pathological features are airway inflammation
and airway remodeling. Eosinophils, neutrophils and other
inflammatory cells participate in asthma-induced airway remodeling
(1–3). As a chronic inflammatory disease,
asthma may progress to irreversible airway remodeling without the
appropriate treatment (4). Research
has identified that asthma pathogenesis involves multiple
mechanisms, including genetic mechanisms, the immune response,
chronic airway inflammation, airway hyper responsiveness, airway
neuromodulation disorders and neural signaling pathway (5–7). The
immune response is the leading pathogenic factor.
High mobility group box 1 (HMGB1) is a highly
conserved nuclear protein that is released by mononuclear cells,
macrophages and other immune cells following stimulation by
lipopolysaccharide, tumor necrosis factor-α (TNF-α) or interleukin
(IL)-1. HMGB1 is also passively released from damaged and necrotic
tissue cells to further promote the secretion of a number of
inflammatory factors. As a vital endogenous pro-inflammatory factor
and inflammatory mediator, HMGB1 participates in the pathological
processes of sepsis, pneumonia, arthritis and other diseases
(8,9). Extracellular HMGB1 promotes cytokine
release via the mitogen-activated protein kinase (MAPK), ERK1/2 and
NF-κB pathways (10). HMGB1 also
activates Τoll-like receptor (TLR) 2 and TLR4, which in turn leads
to a downstream inflammatory response via targeting myeloid
differentiation primary response gene 88 (MyD88) and NF-κB
(11). It has been demonstrated that
TLR4 is an essential receptor in HMGB1-induced inflammation
(12). HMGB1 exerts its
pro-inflammatory role via binding to TLR4 leading to release of
inflammatory cytokines such as IL-β, IL-10, IL-12 and TNFα
(13,14). The present study hypothesized that
the HMGB1/TLR4/NF-κB pathway may be of great significance in the
pathogenesis of asthma.
In recent years, resveratrol (RES) has attracted
research focus due to its low toxicity and wide range of action.
Beneficial effects of RES have been demonstrated in pulmonary
fibrosis, chronic obstructive pulmonary disease, pulmonary
hypertension and other lung diseases (15–17). RES
can effectively inhibit eosinophil activation and degranulation
(18), and alleviate airway
inflammation and airway hyper-responsiveness in an acute asthma
model (19). In addition, RES
regulates the differentiation of type 1 T helper (Th1)/Th2 cells
through Tbet/GATA binding protein 3 pathway, suggesting that RES
may have a role in protecting against bronchial asthma (20). However, the specific mechanisms of
RES in regards to asthma remains not fully understood. The present
study first constructed an asthma rat model. Following intervention
with different doses of RES, the alterations in rat airway
remodeling and the HMGB1/TLR4/NF-κB pathway were observed. These
findings provided a theoretical basis for improving clinical asthma
outcomes in children.
Materials and methods
Animal procedures
A total of 40 male Sprague Dawley rats (age, 4
weeks; weight, 80±15 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. and housed at 24±3°C and
humidity of 40–60% on a 12-h light/dark cycle (lights on at 06:00
am). The rats were provided food and water ad libitum. Rats
were acclimated for 1 week and subsequently randomly assigned into
the sham, asthma, 10 µmol/l RES or 50 µmol/l RES groups (n=10).
Asthma rat model construction
A total of 0.2 ml of mixed antigen [2 mg ovalbumin
(OVA) + 40 mg aluminum hydroxide] solution (Sigma-Aldrich; Merck
KGaA) was intraperitoneally injected into rats of the asthma group,
10 µmol/l RES (Sigma-Aldrich; Merck KGaA) group and 50 µmol/l RES
group on day 1, 8 and 15, respectively. Rats in the sham group
received intraperitoneal injection of 0.2 ml of saline. On day 22,
rats in the asthma group, 10 µmol/l RES group and 50 µmol/l RES
group received atomization inhalation of 1% OVA to induce the
asthma model. For the RES group rats, 30 min before inhalation of
OVA, 0.2 ml of either 10 µmol/l or 50 µmol/l RES was injected
intraperitoneally into rats. Rats in the sham group and asthma
group were injected with 0.2 ml of saline. The experiment lasted
until day 35.
Bronchoalveolar lavage fluid (BALF)
sample collection
Rats were anesthetized with 1% sodium pentobarbital
(40 mg/kg) intraperitoneally prior to collection of BALF. In brief,
1 ml of saline was injected into rats then repeat suction was
performed three times. BALF was recycled and centrifuged at 400 × g
for 15 min at 4°C. A sample was considered as suitable for
experimentation when the recycled amount was >0.8 ml. Cell
counting was performed within 1 h. Cell sedimentation was
resuspended with PBS solution, then 10 µl was used for measurement
of total cell number. Then 0.1 ml was used for cell pellets smear.
The cell pellets smear was fixed and stained with Wright's
staining. Cells were differential counted in three visual field and
the values were averaged as previously described in the literature
(19).
Hematoxylin and eosin (H&E)
staining
The right lung tissue of rats was fixed with 10%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) at room temperature
for 48 h. Then the tissue was dehydrated in an ascending series of
ethanol, embedded in paraffin and sectioned (5 µm). Following
deparaffinization in xylene and rehydration in a descending series
of alcohol, lung tissues were stained with H&E. Inflammatory
cell infiltration and airway epithelial injury were observed under
a light microscope.
Masson staining
The aforementioned paraffin embedded slices (5 µm)
were stained with Weigert solution (Sigma-Aldrich; Merck KGaA) for
5–10 min. After being fully washed, sections were treated with
Ponceau fuchsin acid solution for 5–10 min, immersed in 2% acetic
acid aqueous solution for 1 min, then differentiated in 1%
phosphomolybdic acid aqueous solution for 3–5 min. Without washing
with water, the sections were treated with aniline blue for 5 min
then immersed in 0.2% acetic acid aqueous solution for 1 min.
Slices were permeabilized with xylene and mounted with neutral
resin.
Measurement of the thicknesses of
airway wall and smooth muscle
The intact small bronchioles were identified using a
light microscope. Three transverse sections were randomly selected
in each rat. Basement membrane perimeter (Pbm), total bronchial
wall area (Wat1), bronchial luminal area
(Wat2), external smooth muscle area (Wam1)
and internal smooth muscle area (Wam2) of each rat were
accessed using Image Pro Plus v.6.0 software (Media Cybernetics,
Inc.). The thickness of airway wall (Wan) and smooth muscle (Wat)
were then calculated using the following formulas:
(1)
Wan=(Wat1-Wat2)/Pbm
(2)
Wat=(Wam1-Wam2)/Pbm
Determination of inflammatory factors
in lung homogenate
Harvested rat lung tissue (0.5 g) was ground and
centrifuged at 4,500 × g for 10 min at 4°C for preparation of lung
homogenate. Levels of IL-1, IL-10, IL-12 and TNF-α in rat lung
homogenate were detected using the respective ELISA kits (Rat IL-1
ELISA kit, cat. no. RAB0272; Rat IL-10 ELISA kit, cat. no. RAB0246;
Rat TNF-α ELISA kit cat. no. RAB0480; all from Sigma-Aldrich; Merck
KGaA; and Rat IL-12 ELISA kit, cat. no. KRC0121; Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions.
Reverse transcription-quantitative PCR (RT-qPCR).
The lung tissue (0.5 g) was cut and homogenized; TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract
total RNA. Total RNA underwent reverse transcription according to
the instructions of PrimeScript RT reagent Kit (Takara Bio, Inc.).
qPCR was performed using SYBR®-Green Master Mix (Takara
Bio, Inc.) according to the manufacturer's protocol; amplification
was performed under the recommended parameters: Initial
denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 5
sec, 60°C for 15 sec and 72°C for 15 sec, and a final extension at
94°C for 15 sec. The expression level of the target gene was
calculated using the 2−ΔΔCq method (21). Primers are listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | PCR size (bp) | Primer sequence
(5′→3′) |
|---|
| HMGB1 | 356 |
F:CGGATGCTTCTGTCAACT |
|
|
|
R:TCAGCTTGGCAGCTTTCT |
| TLR4 | 310 |
F:GGTGAGAAATGAGCTGGTA |
|
|
|
R:TCTGCTAAGAAGGCGATA |
| MyD88 | 113 |
F:CGTCGCATGGTGGTGGTTGTTT |
|
|
|
R:GGGATCAGTCGCTTCTGTTGGA |
| NF-κB | 425 |
F:GCGCATCCAGACCAACAATAAC |
|
|
|
R:GCCGAAGCTGCATGGACACT |
| GAPDH | 526 |
F:CCACTTGAAGGGTGGAGC |
|
|
|
R:TGAAGTCGCAGGAGACAA |
Determination of inflammatory factors
in serum of children
A total of 34 pediatric patients with asthma (age,
4–14 years; 19 male, 15 female), admitted to The Affiliated
Changzhou No. 2 People's Hospital of Nanjing Medical University
(Changzhou, China) from January 2018 to May 2018 were selected as
the asthma group. The diagnosis of childhood asthma conforms to the
diagnostic criteria in the Guidelines for the Diagnosis and
Prevention of Childhood Bronchial Asthma, formulated by the
Respiratory Group of the Chinese Medical Association in 2008.
Exclusion criteria: i) Patients with other tracheal, bronchial or
pulmonary diseases; ii) patients with severe multiple organ,
nervous or psychiatric diseases. Subjects were all in the acute
asthmatic stage regardless of the severity of the attack. For the
control group, 24 healthy children (age, 4–12 years; 13 male, 11
female) were selected during the same period. From each subject, 5
ml of venous blood sample was collected for detecting serum levels
of IL-1, IL-10, IL-12 and TNF-α by ELISA. Written informed consent
was obtained from the legal guardians for all patients and healthy
controls.
Statistical analysis
Data were analyzed by SPSS v.17.0 statistical
software (SPSS Inc.). Quantitative data were presented as mean ±
standard deviation. Student's t-test was used for comparing
differences between two groups. One-way analysis of variance was
performed for assessing differences amongst multiple groups,
followed by Fisher's least significant difference analysis or
Dunnett's test. P<0.05 was considered to indicate statistical
significance.
Results
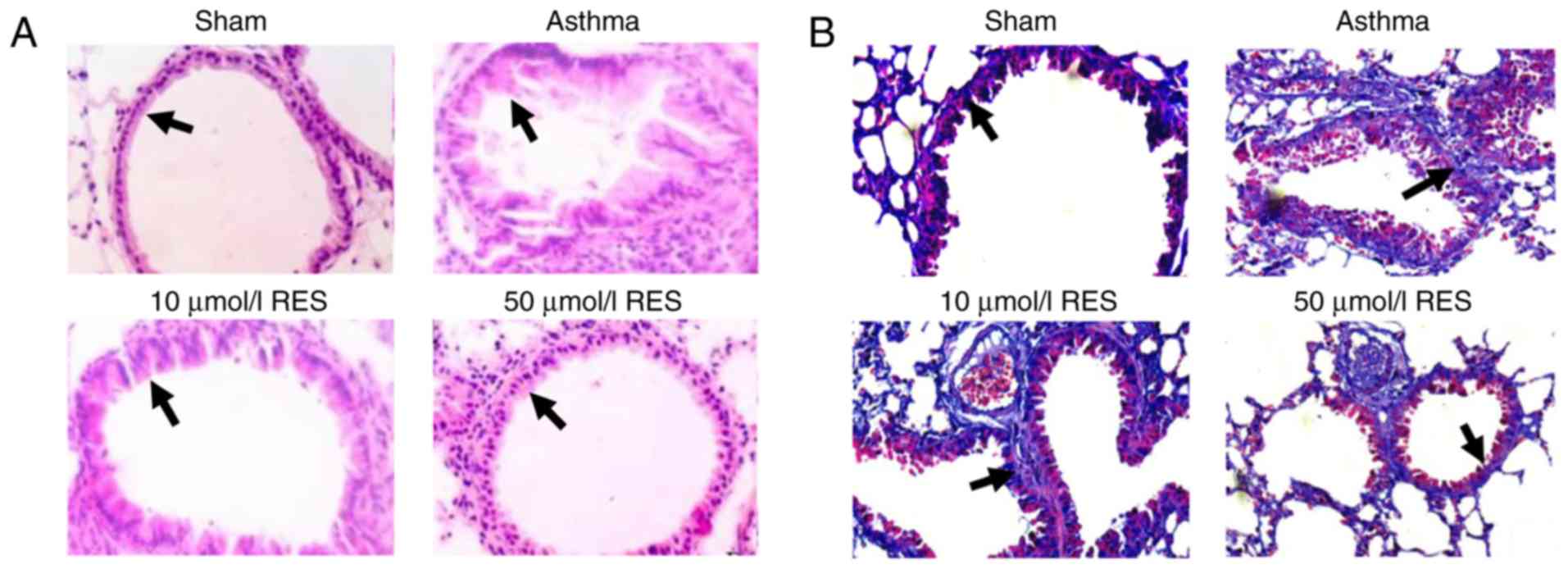
High dose RES treatment attenuates
pathological lesions in a rat asthma model
Pronounced pathological lesions were observed in the
lung tissues of the asthma group, manifesting as edema and
abscission of airway epithelium, airway constriction, multiple
inflammatory cell infiltration and tracheal smooth muscle
thickening (Fig. 1A). In addition,
marked capillary congestion, alveolar fusion enlargement and
alveolar septum widening were observed in asthma rats (Fig. 1A). Pathological changes were similar
in the 10 µmol/l RES group. By contrast, pathological changes in
lung tissues were less pronounced in the 50 µmol/l RES group
compared with the asthma group (Fig.
1A).
High dose RES treatment decreases
airway collagen deposition area
Masson staining is commonly used to stain collagen
fibers, mucus and cartilage blue. Muscle fibers, cellulose and red
blood cells are stained red, whilst cell nuclei are stained
blue-black. Airway collagen deposition was more marked in the
asthma group compared with the sham group (Fig. 1B). No significant difference in
airway collagen deposition area was observed between the asthma
group and 10 µmol/l RES group (Fig.
1B). By contrast, the airway collagen deposition area was
markedly reduced in the 50 µmol/l RES group compared with the
asthma group (Fig. 1B).
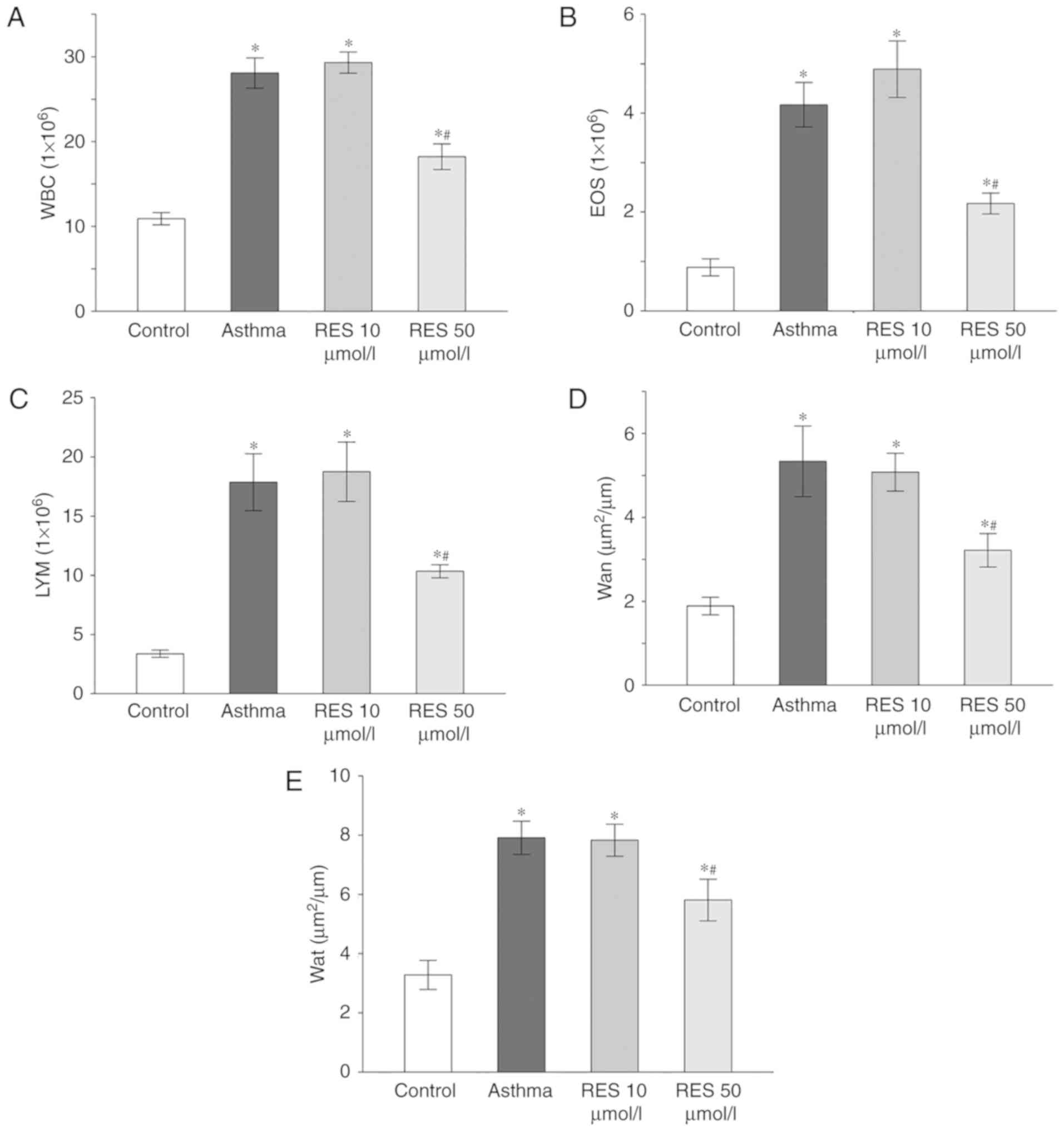
High dose RES treatment decreases
inflammatory cell levels in the BALF of asthma model rats
Total amounts of inflammatory cells, eosinophils and
lymphocytes were elevated in the asthma group and the 10 µmol/l RES
group compared with the sham group (P<0.05; Fig. 2A-C). By contrast, the amounts of
inflammatory cells, eosinophils and lymphocytes were significantly
decreased in the 50 µmol/l RES group (P<0.05; Fig. 2A-C).
High dose RES treatment decreases
airway wall and smooth muscle thickness in asthma model rats
Airway wall and smooth muscle thickness were
increased in the asthma group and 10 µmol/l RES group compared to
the sham group (P<0.05; Fig. 2D and
E). Treatment with 50 µmol/l RES decreased airway wall and
smooth muscle thickness (P<0.05; Fig.
2D and E).
High dose RES treatment decreases
inflammatory factor levels in lung tissues of asthma model
rats
ELISA results illustrated that IL-1, IL-10 and TNF-α
levels in lung tissues of the asthma and 10 µmol/l RES groups were
elevated, whereas IL-12 level was reduced (P<0.05; Fig. 3). In the 50 µmol/l RES group, IL-1,
IL-10 and TNF-α levels were downregulated but IL-12 was upregulated
in rat lung tissues following establishment of the asthma model
(P<0.05; Fig. 3).
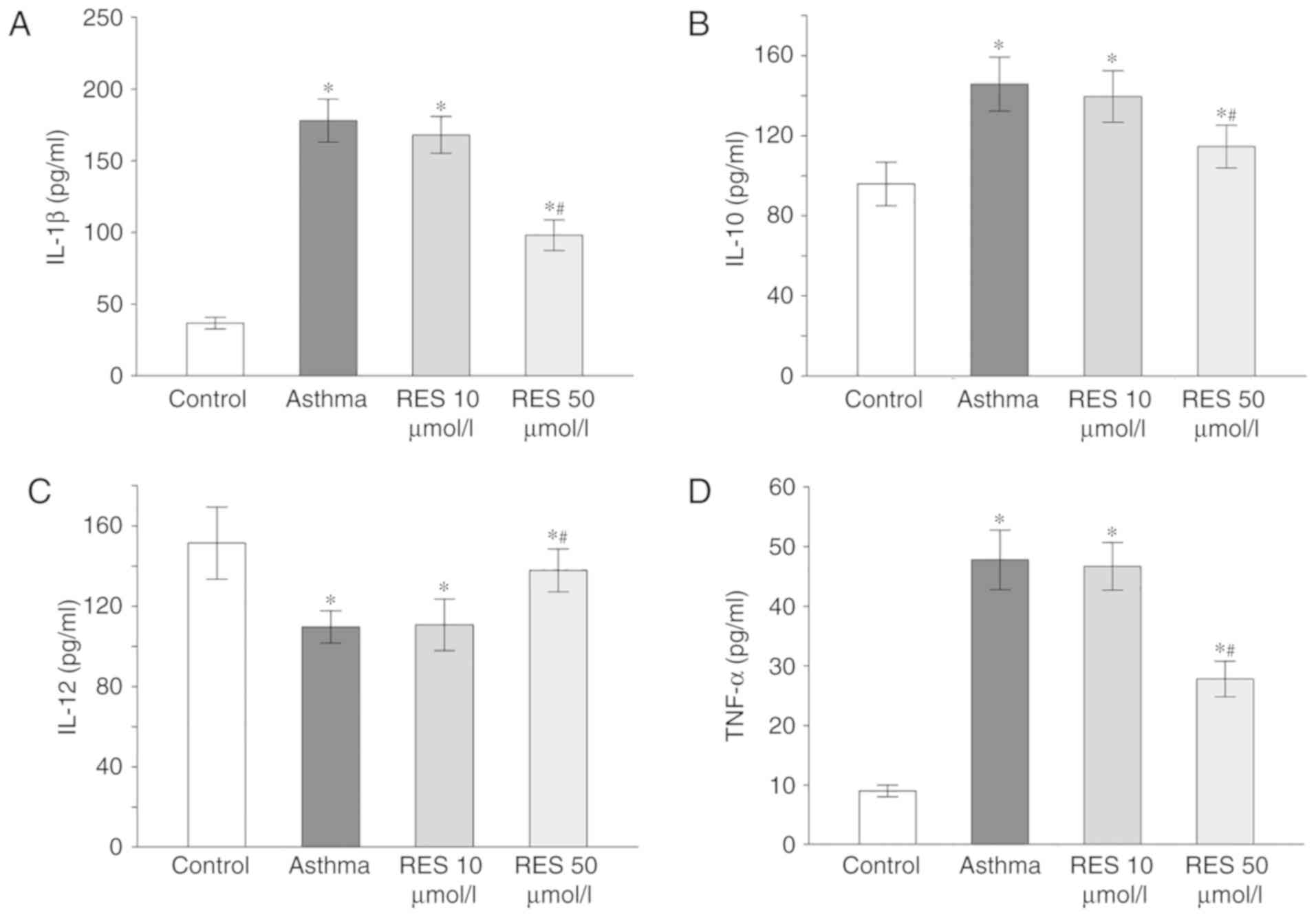 | Figure 3.IL-1, IL-10, IL-12 and TNF-α levels
in rat lung tissues for sham, asthma, 10 µmol/l RES and 50 µmol/l
RES groups. (A) IL-1β, (B) IL-10, (C) IL-12, and (D) TNF-α levels
in rat lung tissues from the different experimental groups.
*P<0.05 vs. control; #P<0.05 vs. asthma group. IL,
interleukin; TNF-α, tumor necrosis factor-α; RES, resveratrol. |
High dose RES treatment reduces HMGB1,
TLR4, MyD88 and NF-κB expression in asthma model rats
HMGB1, TLR4, MyD88 and NF-κB mRNA expression levels
were remarkably elevated in rats of the asthma and 10 µmol/l RES
groups compared with the sham group (P<0.05; Fig. 4). By contrast, 50 µmol/l RES
treatment significantly decreased HMGB1, TLR4, MyD88 and NF-κB mRNA
expression in rat lung tissue following establishment of the asthma
model (P<0.05; Fig. 4).
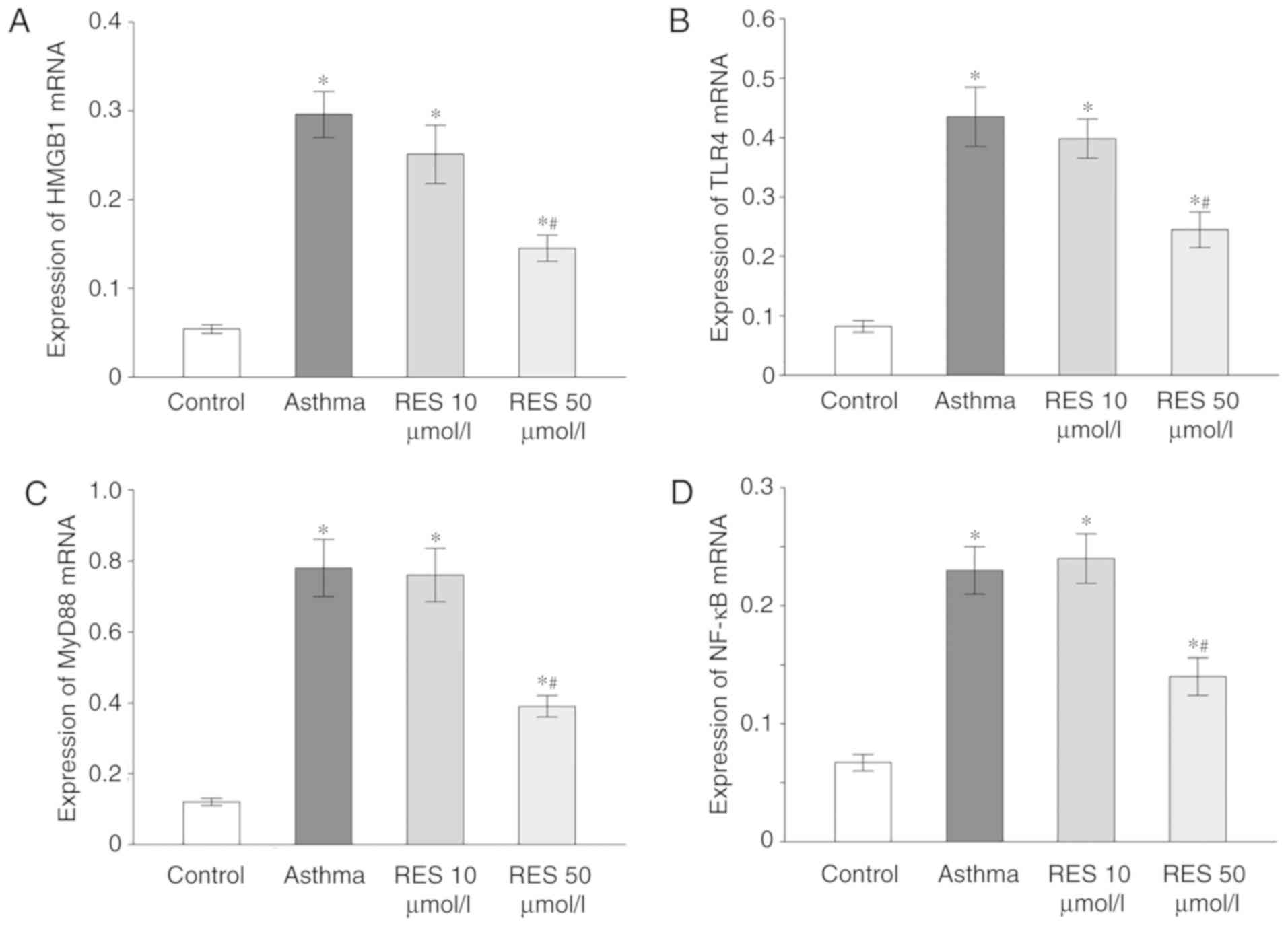 | Figure 4.HMGB1, TLR4, MyD88 and NF-κB mRNA
expression in rat lung tissues for sham, asthma, 10 µmol/l RES and
50 µmol/l RES groups. (A) HMGB1, (B) TLR4, (C) MyD88 and (D) NF-κB
mRNA expression levels in rat lung tissues from the different
experimental groups. *P<0.05 vs. control; #P<0.05
vs. asthma group. HMGB1, high mobility group box 1; TLR4, Τoll-like
receptor; MyD88, myeloid differentiation primary response gene 88;
RES, resveratrol. |
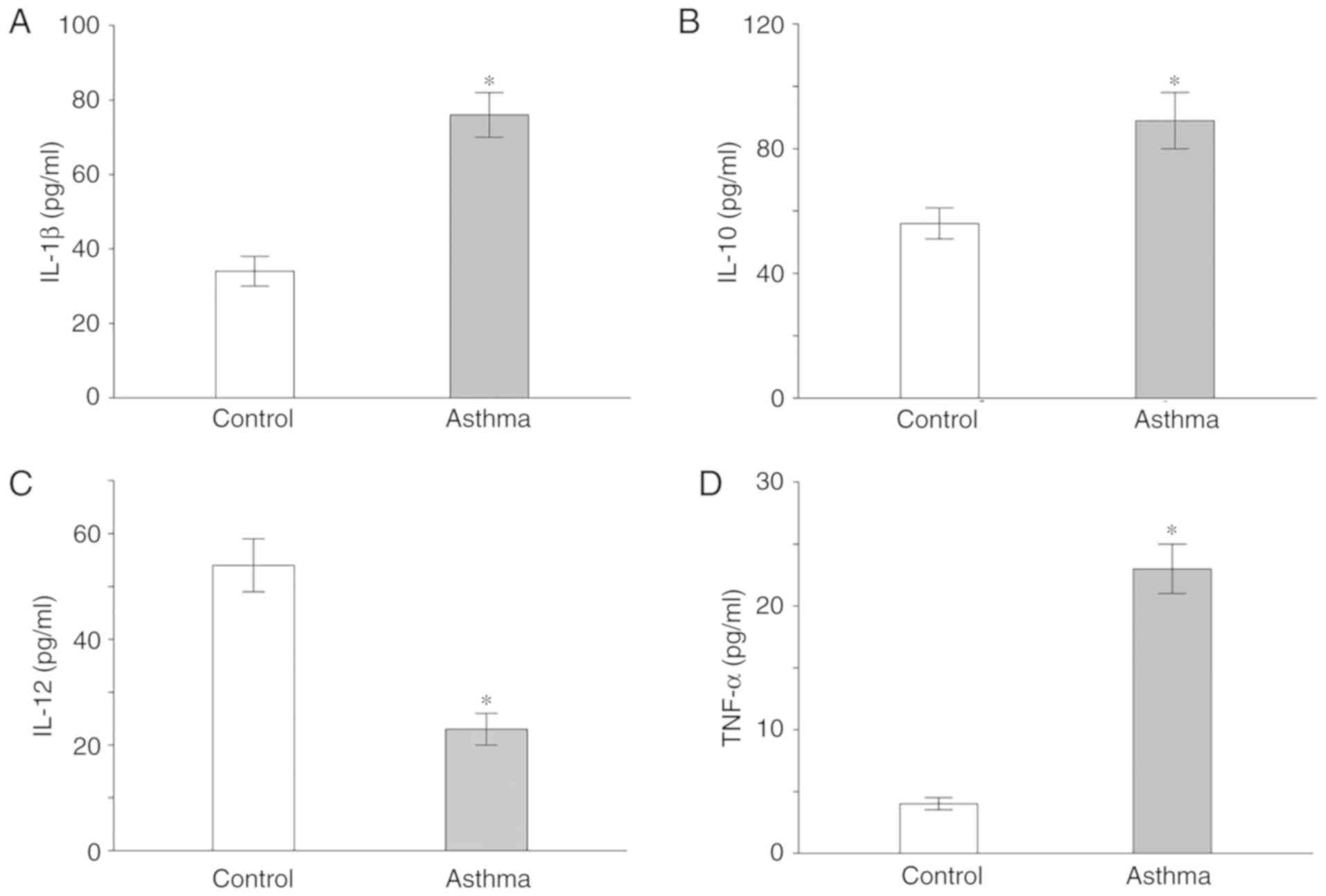
Inflammatory factor levels increase in
serum samples of asthmatic children
Serum levels of IL-1, IL-10, IL-12 and TNF-α in
asthma children and healthy children were analyzed. Asthmatic
children had increased IL-1, IL-10 and TNF-α serum levels, as well
as decreased IL-12 levels compared with healthy children
(P<0.05; Fig. 5).
Discussion
Bronchial asthma in children is a common disease
that can severely influence daily life (1). Asthma can progress to adulthood if
proper and timely treatment is not received. Pathologically, asthma
is an airway chronic inflammatory disease involving a variety of
cells and cellular components. Airway inflammation and remodeling
are important pathological features of chronic asthma. IL-1β has an
important role in the development of allergic asthma by inducing
the formation of Th17 cells (22).
IL-10 can regulate immune function and improve airway inflammatory
response (23). IL-12 is a Th1
cytokine, which increases airway hyper responsiveness due to
exogenous injury and decreases eosinophil aggregation around the
airway (24). The present study
determined that serum levels of IL-1, IL-10 and TNF-α in lung
tissues of asthma model rats and serum samples of asthmatic
children were significantly elevated. By contrast, IL-12 level was
decreased. These results indicated that the inflammatory response
is involved in the occurrence and progression of asthma.
HMGB1 is widely present in the cell nucleus and
cytoplasm. Nuclear membrane permeability is increased by
inflammation, which in turn promotes the translocation of HMGB1 to
the cell cytoplasm where it exerts pro-inflammatory effects
(14,25,26).
HMGB1 stimulates mononuclear cell and macrophage-secreted
inflammatory mediators, such as IL-1β, IL-10, IL-12 and TNF-α
(27). In addition, HMGB1 promotes
major histocompatibility complex II, CD80, CD83, CD86 and other
costimulatory molecules in dendritic cells (DCs), inducing DC
maturation to produce pro-inflammatory cytokines (28). Eosinophils are recruited by HMGB1 to
the inflammatory sites further aggravating the inflammatory
response (29,30). Recent studies have identified that
HMGB2 deteriorates airway inflammation by upregulating TNF-α, VEGF,
MMP-9 and TSLP expression. HMGB1 knockdown remarkably decreases
relative indicators of airway inflammation, mucus secretion,
collagen deposition, and airway smooth muscle thickness (31). TLR4 was the first TLR identified in
mammals; it comprises an extracellular domain, a transmembrane
domain and an intracellular domain (32,33).
Relative studies have demonstrated that TLR4 is involved in the
regulation of airway inflammation through regulating DC maturation
and activation, antigen presentation and T cell immune responses.
TLR4 is overexpressed in airway smooth muscle cells by elastase
stimulation, leading to thickening of the airway wall via
activation of NF-κB (34) therefore
it is evident that TLR4 participates in airway remodeling. NF-κB is
a nuclear transcription factor with various biological activities.
NF-κB pathway regulates genes involved in asthma-related airway
inflammation (35). Inhibition of
NF-κB activity alleviates the airway inflammatory response,
thereafter improving airway remodeling. TLR4 activates NF-κB
through MyD88-dependent and MyD88-independent ways, eventually
stimulating the production of pro-inflammatory factors. HMBG1
induces the immune and inflammatory response after binding to its
receptor TLR4 (36,37). It is hypothesized that the
HMGB1/TLR4/NF-κB pathway may serve an important role in the
development and progression of asthma.
RES is a plant polyphenolic substance present in
grape skins, berries and nuts (38).
Numerous studies have demonstrated that RES exerts anti-apoptosis,
anti-inflammatory and anti-oxidative effects in vitro
(39–43). RES also alleviates airway
inflammation and airway hyper responsiveness in vivo,
partially through inhibiting eosinophil activation and
degranulation (18,19). The most pronounced effect of RES is
inflammation alleviation, mainly via NF-κB pathway regulation
(44,45). RES is capable of inhibiting TLR4
expression in cardiomyocytes with hypoxia-reoxygenation injury
(46). However, the pathogenesis of
asthma and the mechanism of RES on asthma are complex. In the
present study, airway lesions were obvious in asthma model rats,
manifesting as abundant inflammatory cell infiltration.
Pathological lesions in rat airways were remarkably alleviated by
treatment with 50 µmol/l RES. However, 10 µmol/l RES treatment did
not present therapeutic effect on asthma-induced inflammation.
HMGB1, TLR4, MyD88 and NF-κB mRNA levels in 50 µmol/l RES group
were significantly lower compared with the asthma group. Treatment
with 10 µmol/l RES did not affect HMGB1, TLR4, MyD88 and NF-κB mRNA
levels following establishment of an asthma model. The results
confirmed that HMGB1 and TLR4 were involved in asthma-induced
airway inflammation. However, the present study used only RT-qPCR
to detect HMGB1, TLR4, MyD88 and NF-κB levels; additional
experimental methods should be investigated in future work. In
addition, detection of the remodeling-associated proteins and
verification of which cell type is targeted by RES require to be
further elucidated. Finally, the relationship of the
HMGB1/TLR4/MyD88/NF-κB axis and RES will be further studied in
future work.
In conclusion, the present study demonstrated that
large dose RES alleviated asthma-induced airway inflammation and
airway remodeling by inhibiting the release of inflammatory
cytokines via the HMGB1/TLR4/NF-κB pathway. The present results
provided evidence for RES as a potential novel treatment for
asthma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
HJ and WZ designed the study and performed the
experiments. HJ, JD and KX established the animal models. HJ and KX
collected the data. HJ and KX analyzed the data. HJ and WZ prepared
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Experimental protocols involving the use of animals
were approved by the Animal Ethics Committee of Nanjing Medical
University Animal Center. Protocols involving the use of human
samples were approved by the Ethics Committee of the Affiliated
Changzhou No. 2 People's Hospital of Nanjing Medical University
(Changzhou, China). Written informed consent was obtained from the
legal guardians of all patients and healthy controls.
Patient consent for publication
Not applicable.
Competing interests
Not applicable.
References
|
1
|
Guilbert TW, Mauger DT and Lemanske RF Jr:
Childhood asthma-predictive phenotype. J Allergy Clin Immunol
Pract. 2:664–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinfield J, Gaskin K, Bentley J and Rouse
J: Recognition and management of asthma in children and young
people. Nurs Stand. 30:50–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Us'Ka VR: Role of psychological factors in
the etiology of asthma in children. Lik Sprava. 148–150. 2015.(In
Ukrainian). PubMed/NCBI
|
|
4
|
Croisant S: Epidemiology of asthma:
Prevalence and burden of disease. Adv Exp Med Biol. 795:17–29.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raedler D and Schaub B: Immune mechanisms
and development of childhood asthma. Lancet Respir Med. 2:647–656.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenberg SL, Miller GE, Brehm JM and
Celedon JC: Stress and asthma: Novel insights on genetic,
epigenetic and immunologic mechanisms. J Allergy Clin Immunol.
134:1009–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan DA: Allergic rhinitis and asthma:
Epidemiology and common pathophysiology. Allergy Asthma Proc.
35:357–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Q, Liu X, Yao Z, Mao S, Wei Q and
Chang Y: Penehyclidine hydrochloride inhibits the release of
high-mobility group box 1 in lipopolysaccharide-activated RAW264.7
cells and cecal ligation and puncture-induced septic mice. J Surg
Res. 186:310–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Musumeci D, Roviello GN and Montesarchio
D: An overview on HMGB1 inhibitors as potential therapeutic agents
in HMGB1-related pathologies. Pharmacol Ther. 141:347–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dumitriu IE, Baruah P, Valentinis B, Voll
RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA and
Rovere-Querini P: Release of high mobility group box 1 by dendritic
cells controls T cell activation via the receptor for advanced
glycation end products. J Immunol. 174:7506–7515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JS, Svetkauskaite D, He Q, Kim JY,
Strassheim D, Ishizaka A and Abraham E: Involvement of Τoll-like
receptors 2 and 4 in cellular activation by high mobility group box
1 protein. J Biol Chem. 279:7370–7377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang H, Hreggvidsdottir HS, Palmblad K,
Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y,
et al: A critical cysteine is required for HMGB1 binding to
Toll-like receptor 4 and activation of macrophage cytokine release.
Proc Natl Acad Sci USA. 107:11942–11947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andersson U, Wang H, Palmblad K, Aveberger
AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M,
Yang H and Tracey KJ: High mobility group 1 protein (HMG-1)
stimulates proinflammatory cytokine synthesis in human monocytes. J
Exp Med. 192:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JS, Arcaroli J, Yum HK, Yang H, Wang
H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ and Abraham
E: Activation of gene expression in human neutrophils by high
mobility group box 1 protein. Am J Physiol Cell Physiol.
284:C870–C879. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, He F, Chen L, Li Q, Jin S, Zheng
H, Lin J, Zhang H, Ma S, Mei J and Yu J: Resveratrol inhibits
pulmonary fibrosis by regulating miR-21 through MAPK/AP-1 pathways.
Biomed Pharmacother. 105:37–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XL, Li T, Li JH, Miao SY and Xiao XZ:
The effects of resveratrol on inflammation and oxidative stress in
a rat model of chronic obstructive pulmonary disease. Molecules.
22:E15292017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang DL, Zhang HG, Xu YL, Gao YH, Yang XJ,
Hao XQ and Li XH: Resveratrol inhibits right ventricular
hypertrophy induced by monocrotaline in rats. Clin Exp Pharmacol
Physiol. 37:150–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh YC, Kang OH, Choi JG, Chae HS, Lee YS,
Brice OO, Jung HJ, Hong SH, Lee YM and Kwon DY: Anti-inflammatory
effect of resveratrol by inhibition of IL-8 production in
LPS-induced THP-1 cells. Am J Chin Med. 37:1203–1214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan Y and Lim LH: Trans-Resveratrol, an
extract of red wine, inhibits human eosinophil activation and
degranulation. Br J Pharmacol. 155:995–1004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colitti M and Stefanon B: Different
anti-adipogenic effects of bio-compounds on primary visceral
pre-adipocytes and adipocytes. EXCLI J. 15:362–377. 2016.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ebenezer AJ, Prasad K, Rajan S and Thangam
EB: Silencing of H4R inhibits the production of IL-1β through
SAPK/JNK signaling in human mast cells. J Recept Signal Transduct
Res. 38:204–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Feng Y, Li L, Ye X, Wang J, Wang
Q, Li P, Li N, Zheng X, Gao X, et al: Immunization with an
adenovirus-vectored TB vaccine containing Ag85A-Mtb32 effectively
alleviates allergic asthma. J Mol Med (Berl). 96:249–263. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CL, Hsiao G, Wang CC and Lee YL:
Imperatorin exerts antiallergic effects in Th2-mediated allergic
asthma via induction of IL-10-producing regulatory T cells by
modulating the function of dendritic cells. Pharmacol Res.
110:111–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abraham E, Arcaroli J, Carmody A, Wang H
and Tracey KJ: HMG-1 as a mediator of acute lung inflammation. J
Immunol. 165:2950–2954. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JY, Park JS, Strassheim D, Douglas I,
Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama
I, et al: HMGB1 contributes to the development of acute lung injury
after hemorrhage. Am J Physiol Lung Cell Mol Physiol.
288:L958–L965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bauer EM, Shapiro R, Zheng H, Ahmad F,
Ishizawar D, Comhair SA, Erzurum SC, Billiar TR and Bauer PM: High
mobility group box 1 contributes to the pathogenesis of
experimental pulmonary hypertension via activation of Toll-like
receptor 4. Mol Med. 18:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dumitriu IE, Bianchi ME, Bacci M, Manfredi
AA and Rovere-Querini P: The secretion of HMGB1 is required for the
migration of maturing dendritic cells. J Leukoc Biol. 81:84–91.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Messmer D, Yang H, Telusma G, Knoll F, Li
J, Messmer B, Tracey KJ and Chiorazzi N: High mobility group box
protein 1: An endogenous signal for dendritic cell maturation and
Th1 polarization. J Immunol. 173:307–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz
D, Lee JJ, Rubartelli A, Schrezenmeier H and Lotze MT: Eosinophils
oxidize damage-associated molecular pattern molecules derived from
stressed cells. J Immunol. 183:5023–5031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang Y, Hou C, Kong J, Wen H, Zheng X, Wu
L, Huang H and Chen Y: HMGB1 binding to receptor for advanced
glycation end products enhances inflammatory responses of human
bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol
Cell Biochem. 405:63–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Granucci F, Zanoni I, Feau S and
Ricciardi-Castagnoli P: Dendritic cell regulation of immune
responses: A new role for interleukin 2 at the intersection of
innate and adaptive immunity. EMBO J. 22:2546–2551. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
MacLeod H and Wetzler LM: T cell
activation by TLRs: A role for TLRs in the adaptive immune
response. Sci STKE. 2007:pe482007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee KY, Ho SC, Lin HC, Lin SM, Liu CY,
Huang CD, Wang CH, Chung KF and Kuo HP: Neutrophil-derived elastase
induces TGF-beta1 secretion in human airway smooth muscle via
NF-kappaB pathway. Am J Respir Cell Mol Biol. 35:407–414. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mazarati A, Maroso M, Iori V, Vezzani A
and Carli M: High-mobility group box-1 impairs memory in mice
through both Τoll-like receptor 4 and receptor for advanced
Glycation end products. Exp Neurol. 232:143–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu M, Wang H, Ding A, Golenbock DT, Latz
E, Czura CJ, Fenton MJ, Tracey KJ and Yang H: HMGB1 signals through
Τoll-like receptor (TLR) 4 and TLR2. Shock. 26:174–179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yadav M, Jain S, Bhardwaj A, Nagpal R,
Puniya M, Tomar R, Singh V, Parkash O, Prasad GB, Marotta F and
Yadav H: Biological and medicinal properties of grapes and their
bioactive constituents: An update. J Med Food. 12:473–484. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eo SH, Cho H and Kim SJ: Resveratrol
inhibits nitric oxide-induced apoptosis via the NF-kappa B pathway
in rabbit articular chondrocytes. Biomol Ther (Seoul). 21:364–370.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Csaki C, Keshishzadeh N, Fischer K and
Shakibaei M: Regulation of inflammation signalling by resveratrol
in human chondrocytes in vitro. Biochem Pharmacol. 75:677–687.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shakibaei M, John T, Seifarth C and
Mobasheri A: Resveratrol inhibits IL-1 beta-induced stimulation of
caspase-3 and cleavage of PARP in human articular chondrocytes in
vitro. Ann NY Acad Sci. 1095:554–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Elmali N, Esenkaya I, Harma A, Ertem K,
Turkoz Y and Mizrak B: Effect of resveratrol in experimental
osteoarthritis in rabbits. Inflamm Res. 54:158–162. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Im HJ, Li X, Chen D, Yan D, Kim J, Ellman
MB, Stein GS, Cole B, Kc R, Cs-Szabo G and van Wijnen AJ:
Biological effects of the plant-derived polyphenol resveratrol in
human articular cartilage and chondrosarcoma cells. J Cell Physiol.
227:3488–3497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Manna SK, Mukhopadhyay A and Aggarwal BB:
Resveratrol suppresses TNF-induced activation of nuclear
transcription factors NF-kappa B, activator protein-1 and
apoptosis: Potential role of reactive oxygen intermediates and
lipid peroxidation. J Immunol. 164:6509–6519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Estrov Z, Shishodia S, Faderl S, Harris D,
Van Q, Kantarjian HM, Talpaz M and Aggarwal BB: Resveratrol blocks
interleukin-1beta-induced activation of the nuclear transcription
factor NF-kappaB, inhibits proliferation, causes S-phase arrest,
and induces apoptosis of acute myeloid leukemia cells. Blood.
102:987–995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang C, Lin G, Wan W, Li X, Zeng B, Yang
B and Huang C: Resveratrol, a polyphenol phytoalexin, protects
cardiomyocytes against anoxia/reoxygenation injury via the
TLR4/NF-κB signaling pathway. Int J Mol Med. 29:557–563. 2012.
View Article : Google Scholar : PubMed/NCBI
|