Introduction
Liver fibrosis is a common pathological consequence
of continued damage to the liver tissue due to infection [primarily
hepatitis B virus (HBV) and hepatitis C virus (HCV)],
toxic/drug-induced injury, or metabolic or autoimmune factors, and
the associated chronic activation of the wound healing reaction
(1). With ongoing liver damage,
fibrosis may progress to cirrhosis, which is characterized by a
distortion of the liver vasculature and architecture and is the
major determinant of morbidity and mortality in patients with liver
disease, predisposing them to liver failure and primary liver
cancer (2,3). At present, the limited available
curative treatment options primarily include antiviral therapy for
chronic HBV and HCV infection, weight loss and exercise for
non-alcoholic steatohepatitis or liver transplantation (4). However, certain patients with liver
fibrosis are either not sensitive to these causal drug treatments
or are diagnosed at late end-stages, when satisfactory therapeutic
strategies are not available, ultimately resulting in mortality
(5). In addition, liver
transplantation is considered to be the only therapy to
significantly improve lifespan, but the inadequate availability of
organs, increasing numbers of patients requiring transplants, and
issues of compatibility and comorbidity factors mean that not all
patients are eligible for transplantation (6). Therefore, the development of novel
effective and safe therapeutic regimens for liver fibrosis are
urgently required.
MicroRNAs (miRNAs/miR), a group of endogenous, small
(18–23 nucleotides in length), non-coding RNAs, have been
identified in a variety of eukaryotic organisms and
post-transcriptionally regulate gene expression by interacting with
the 3′-untranslated region (3′-UTR) of target gene mRNAs to repress
translation or increase mRNA cleavage (7). A growing body of evidence has revealed
that miRNAs may regulate a large number of biological processes,
including cell proliferation, differentiation, and apoptosis
(8) and that aberrant miRNA
expression was closely associated with the development of multiple
diseases, including liver fibrosis (9). For example, miR-130a-3p inhibited
transforming growth factor-β (TGF-β)/Mothers against
decapentaplegic (Smad) signalling by directly targeting TGF-β
receptors 1 and 2, which may contribute to the pathogenesis of
hepatic fibrosis and provide a potential novel drug target for the
treatment of non-alcoholic steatohepatitis (10). In addition, restoration of miR-9
expression inhibited the activation of hepatic stellate cells
(HSCs), the primary extracellular matrix (ECM)-producing cells in
the fibrotic liver, by targeting multidrug resistance-associated
protein 1; therefore, miR-9 may serve a suppressive role in liver
fibrosis (11); members of the
miR-34 family (miR-34a, miR-34b and miR-34c) were identified to be
the most upregulated compared with other present miRs and may be
involved in lipid/fatty acid metabolism by targeting acyl-CoA
synthetase long-chain family member 1 in the progression of hepatic
fibrosis. These studies indicated that dysregulated miRNAs exert an
important role in the fibrotic process, and miRNA gene therapies
have also been proposed as a promising therapeutic approach for the
treatment of liver fibrosis (12).
Previously, miR-152 was suggested to be a regulator
in certain fibrotic diseases (13,14). For
example, miR-152 levels were significantly downregulated in a rat
model of peritoneal fibrosis, indicating that miR-152 may be
associated with the pathogenesis of this disease (15). Furthermore, it was also identified
that miR-152 contributed to DNA methyltransferase 1 downregulation
and epigenetically regulated Patched1, resulting in the inhibition
of epithelial-mesenchymal transition (EMT) in liver fibrosis
(13). Hedgehog (Hh) signalling is
critically important in hepatic fibrogenesis, and GLI family zinc
finger 3 (Gli3) may function as an Hh signalling-independent
transcriptional activator (16,17).
Nevertheless, the interaction and underlying mechanisms between
miR-152 and Gli3 in the progression of liver fibrosis remain
unclear. Therefore, the present study examined the expression of
miR-152 in clinical samples, and in in vivo animal and in
vitro cell models, verified the interaction between miR-152 and
Gli3 and additionally explored the role of miR-152 in the process
of liver fibrosis.
Materials and methods
Study population and serum sample
preparation
Clinical samples were collected from two independent
cohorts recruited from the First People's Hospital of Kunming City
(Kunming, China) between January 2015 and June 2016. Cohort 1
comprised 25 patients with liver fibrosis, whereas cohort 2
comprised 25 healthy people. All patients were diagnosed on the
basis of history, clinical and pathological examination, by at
least two experienced clinicians. Following collection of the liver
samples via resection, tissues were partially embedded with
paraffin and preserved in liquid nitrogen. Diagnoses of the samples
were confirmed by pathological examination. The presence of liver
fibrosis in a sample was the first inclusion criterion. In
addition, patients with liver cancer, autoimmune hepatitis,
drug-induced injury or alcohol abuse were excluded. Informed
written consent was obtained from all participants prior to
enrolment in the study, and the study was approved by the Ethical
Committee of the First People's Hospital of Kunming City.
Fasting venous blood samples were collected by
trained laboratory technicians. Peripheral blood samples (5 ml)
were incubated at 4°C for 12 h, and then the sera in the upper
layers were aspirated and centrifuged at 400 × g for 10 min at 4°C.
Sera were aliquoted and stored at −80°C until examination.
Animal grouping and model
preparation
Male Sprague-Dawley (SD) rats (n=30; 150–200 g) were
purchased from Shanghai Laboratory Animal Centre (Shanghai, China)
and housed with 5 animals per cage under specific pathogen-free
conditions. All animal experiments were approved by the Animal Care
and Use Committee of the First People's Hospital of Kunming City,
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (18). Animals were housed in a
temperature-controlled environment (20–22°C) at 75±2% relatively
humidity with a 12 h light/dark cycle and free access to food and
purified water. Rats were acclimated for 1 week prior to the
experimentation. Then, a liver fibrosis model was generated, and
rats were randomly separated into the following six treatment
groups (n=5 animals per group): i) Model-0 week; ii) Model-2 week;
iii) Model-4 week; iv) Model-8 week; v) Model + Negative control
(NC; injected with NC plasmid); vi) Model + miR-152 (injected with
miR-152 mimic).
All groups, excluding the Model-0 week group,
received a subcutaneous injection of 10 ml/kg carbon tetrachloride
(CCl4) dissolved in olive oil (25%, v/v). The Model-2
week, Model-4 week and Model-8 week groups received CCl4
twice a week for 2, 4 and 8 weeks, respectively as described
previously (18,19). However, the Model + negative control
(NC) and Model + miR-152 groups were injected intraperitoneally
with an NC plasmid and miR-152 mimic, respectively, during the
period of CCl4 treatment twice a week for 8 weeks.
At the end of the treatments, all animals were
anaesthetized with ketamine hydrochloride (50 mg/kg) and sodium
pentobarbital (30 mg/kg, iv). At the end of the study period,
animals were sacrificed via an overdose of pentobarbital. Blood
samples were immediately collected into tubes and then centrifuged
at 400 × g for 10 min at 4°C for serum preparation. Specimens were
removed from the liver and washed immediately with ice-cold PBS to
remove blood. Then, one-half of each specimen was fixed in a 10%
formalin solution at 4°C overnight for histopathological analysis,
and one-half was stored at −80°C for reverse transcription
quantitative polymerase chain reaction (RT-qPCR) and western blot
(WB) analysis.
Cell culture and treatments
Human normal hepatocytes, including AML12 and L02
cell lines, and the HSC LX2 cell line were purchased from American
Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in
Petri dishes (Corning, Inc., Corning, NY, China) containing
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 100 U/ml
penicillin, 100 µg/ml streptomycin, 0.25 g/l glutamine and 10%
foetal bovine serum (FBS; Hyclone GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in the presence of 95% air and 5%
CO2. Culture medium was changed every 2 or 3 days.
THP-1 cells purchased from the ATCC were cultivated
in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% FBS, 1% penicillin/streptomycin and
maintained in a humidified incubator at 37°C with 5%
CO2. THP-1 cells were differentiated into macrophages
over 48 h in RPMI-1640 medium containing 5–25 ng/ml phorbol
12-myristate 13-acetate, as described previously (20). Then, LX2 cells with the phenotype of
activated HSCs were pre-cocultured with the treated THP-1 cells and
challenged with LPS (1 µg/ml) as described by Prestigiacomo et
al (21). After 0, 6, 12, 24 and
48 h, the cells were collected for subsequent analysis.
293T cells obtained from the ATCC were grown in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) containing 10%
FBS, L-glutamine and 1% penicillin/streptomycin at 37°C in a
humidified incubator with 5% CO2 for the dual luciferase
assay.
Determination of hepatic
hydroxyproline content
Hydroxyproline is an amino acid that stabilizes
collagen deposited in the liver and is exclusively associated with
collagenous connective tissue; therefore, it is a good surrogate
for the quantification of collagen deposition (22). Briefly, liver samples were weighed
and hydrolysed in 2.5 ml 6N HCl at 110°C for 18 h in Teflon-coated
tubes. The hydrolysate was centrifuged at 300 × g at room
temperature for 10 min, and then the pH of the resulting
supernatant was adjusted to pH 7.4. Finally, the optical density
was measured at an absorbance wavelength of 558 nm by a microplate
reader (Tecan GENios 2032218; Tecan Group Ltd., Männedorf,
Switzerland). Total hydroxyproline content was calculated against a
standard curve prepared with a trans4hydroxyLproline
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) preparation and
expressed per mg of wet tissue weight.
Liver histology and morphometric
collagen determination
The liver tissues were fixed in 4% paraformaldehyde
at 4°C overnight and embedded in paraffin, sectioned at 4 µm and
stained with haematoxylin-eosin (H&E) at room temperature for
10 min and Masson's trichrome at room temperature for 30 min
according to the manufacture's protocol (C0105; Beyotime Institiute
of Biotechnology, Haimen, China). The extent of fibrosis was
evaluated on slides by a member of the Department of Pathology from
the First People's Hospital of Kunming City blinded to the
experimental conditions. Fibrosis was determined histologically by
observing the intensity of fibrosis in 5 digital images by a light
microscopy (magnification, ×200) captured from slides from each
tissue sample stained with Masson's trichrome for visual
assessment.
Immunohistochemistry
To detect the immunohistochemical localization of
α-smooth muscle actin (α-SMA), sections from 4% formalin-fixed at
room temperature for 10 min, paraffin-embedded specimens were
deparaffinized and rehydrated in decreasing concentrations (90, 50,
10 and 0%) of ethyl alcohol at room temperature. All tissue
sections were treated with fresh 3% hydrogen peroxide
(H2O2) for 20 min at room temperature to
eliminate endogenous peroxidase activity and then washed with PBS.
The sections were then sequentially incubated with 2% bovine serum
albumin (BSA) for 30 min, then rabbit anti-rat α-SMA monoclonal
antibody (1:300 dilution; ab5694; Abcam, Cambridge, MA, USA) for 2
h, and then with the appropriate horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:200 dilution; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 40 min, followed by
incubation with 3,3′-diaminobenzidine (DAB) as a substrate for 10
min. All the incubation steps were performed at room temperature,
and three washes with PBS were applied between each step. In the
negative control experiments, the primary antibodies were replaced
with PBS. Finally, the sections were counterstained with
haematoxylin at room temperature for 6 min, covered with a
glycerine gel and observed under light microscopy (magnification,
×200) by a pathologist blinded to the experimental conditions. The
readout was recorded visually and was not quantitatively
analyzed.
RNA extraction and RT-qPCR
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was utilized to extract total RNA from clinical
samples, rat tissues and cell lines. RNA concentration and purity
were determined using an Agilent 2100 Bioanalyzer and RNA 6000
Nano/Pico LabChip (Agilent Technologies GmbH, Waldbronn, Germany).
The expression levels of miR-152, α-SMA, albumin and Gli3 were
quantified by RT-qPCR. For miRNA analysis, a total of 100 ng RNA
was reverse transcribed using an miScript RT-II kit (Qiagen GmbH,
Hilden Germany) in a reaction volume of 20 µl and subjected to 60
min incubation at room temperature, followed by 5 min incubation at
95°C. miR-152 expression was assessed with an miScript miRNA PCR
Array (Qiagen GbmH) in accordance with the manufacturer's protocol
in 96-well plates using an ABI PRISM® 7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 30 min at 95°C, followed by 40 cycles of
denaturation for 15 sec at 94°C, annealing for 30 sec at 55°C,
elongation for 30 sec at 70°C and final extension at 70°C for 5
min.
For mRNA analysis, 2 µg total RNA was reverse
transcribed to cDNA using an Moloney-Murine Leukaemia Virus reverse
transcriptase kit (Promega Corporation, Madison, WI, USA), and then
PCR was performed using a specific primer set to examine the
expression levels of mRNAs with a SYBR Green qPCR SuperMix kit
(Invitrogen; Thermo Fisher Scientific, Inc.) on an ABI
PRISM® 7500 Sequence Detection System. The PCR
thermocycler conditions were 95°C for 5 min, then 40 cycles of
denaturation at 94°C for 2 min and annealing and extension at 62°C
for 30 sec, followed by extension at 72°C for 30 sec. The
gene-specific primer pairs used for RT-qPCR in the present study,
listed in Table I, were designed
according to the published GenBank database (https://www.uniprot.org/database/DB-0028) using Primer
Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA).
The 2−ΔΔCq method was performed using U6 and β-actin as
the internal reference for miRNA and mRNA, respectively, and the
following formula: ΔΔCq=ΔCq experimental group-ΔCq control group,
where ΔCq=Cq detected gene-Cq internal reference (22). All experiments were performed in
triplicate.
 | Table I.Sequences of primers used for the
RT-quantitative polymerase chain reaction assays. |
Table I.
Sequences of primers used for the
RT-quantitative polymerase chain reaction assays.
| miRNA/genes | Primer
sequences |
|---|
| miR-152 | RT:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCAAGTTC-3′ |
|
| Forward:
5′-ACACTCCAGCTGGGTCAGTGCATGACAGAACT-3′ |
|
| Reverse:
5′-CTCAACTGGTGTCGTGGA-3′ |
| U6 | RT:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAAAAATATGG-3′ |
|
| Forward:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| Reverse:
5′-AACGCTTCACGAATTTGCGT-3′ |
| α-SMA (Human) | Forward:
5′-GTTCCAGCCATCCTTCATCGG-3′ |
|
| Reverse:
5′-CCTTCTGCATTCGGTCGGCAA-3′ |
| Albumin
(Human) | Forward:
5′-CAGAATGCGCTATTAGTTCG-3′ |
|
| Reverse:
5′-CTGGCGTTTTCTCATGCAA-3′ |
| Gli3 (Human) | Forward:
5′-TTTTCCCCTTTAATCTTGCCAT-3′ |
|
| Reverse:
5′-CCAGTGGCAAATCAACCTCC-3′ |
| β-actin
(Human) | Forward:
5′-CTCCATCCTGGCCTCGCTGT-3′ |
|
| Reverse:
5′-GCTGTCACCTTCACCGTTCC-3′ |
| α-SMA (Rat) | Forward:
5′-GCGTGACTCACAACGTGCCTA-3′ |
|
| Reverse:
5′-CCCATCAGGCAGTTCGTAGCTCT-3 |
| Albumin (Rat) | Forward:
5′-GATCTGCCCTCAATAGCTG-3′ |
|
| Reverse:
5′-TGGCTTCATATTTCTTAGCAA-3′ |
| Gli3 (Rat) | Forward:
5′-CTCGACCATTTCCACGGCAAC-3′ |
|
| Reverse:
5′-TCAGCACAGTGAAGTCTACACC-3′ |
| β-actin (Rat) | Forward:
5′-TCAGGTCATCACTATCGGCAAT-3′ |
|
| Reverse:
5′-AAAGAAAGGGTGTAAAACGCA-3′ |
WB analysis
A total of 3 independent samples from each group
were harvested, and proteins were extracted using
radioimmunoprecipitation assay lysis buffer (50 mM Tris-Cl, pH 8.0,
150 mM NaCl, 5 mM EDTA, 0.1% SDS, 1% NP-40) supplemented with
protease inhibitor cocktail on ice for 30 min. Cell lysates were
centrifuged at 1,200 × g for 10 min at 4°C. The supernatants were
retained, and protein concentrations were determined by a BCA
protein assay (Promega Corporation). Protein extracts (30 µg) were
resuspended and subjected to 10–12% SDS-PAGE and then blotted onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) at 200 mA for 1.5 h by wet electrophoretic transfer. Following
blocking with 5% non-fat milk in TBS with 0.1% Tween-20 (TBST) for
1 h at room temperature, membranes were immunoblotted with primary
antibodies against GAPDH (1:1,000; mAbM20028; Abmart, Berkeley
Heights, NJ, USA), α-SMA (1:1,000; ab5694; Abcam), albumin
(1:1,500; ab106582; Abcam) and Gli3 (1:2,000; ab69838; Abcam)
overnight at 4°C, and then additionally incubated with secondary
horseradish peroxidase-conjugated antibodies (1:12,000; M21002;
Abmart) at room temperature for 2 h. Finally, protein bands were
detected by developing the blots with an enhanced chemiluminescence
WB detection kit (Beyotime Institute of Biotechnology, Haimen,
China). The band intensity was analysed by Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Luciferase reporter assay
The sequences of the 3′-UTR of wild-type (WT) and
mutant Gli3 mRNA containing the putative miR-152 binding sites were
synthesized by Sangon Biotech Co., Ltd., (Shanghai, China) and
cloned downstream of the luciferase gene in a psiCHECK-2 reporter
vector (Promega Corporation) to generate the vectors
psiCHECK-2-Gli3-3′-UTR-WT and psiCHECK-2-Gli3-3′-UTR-Mutant. The
aforementioned 293T cells were seeded into 96-well plates (1,000
cells/well) 24 h prior to transfection and then co-transfected with
50 ng WT or mutant luciferase vector containing Gli3 3′-UTR and 20
µM miR-152 mimics (5′-AGGUUCUGUGAUACACUCCGACU-3′), miR-152
inhibitor (5-AGUCGGAGUGUAUCACAGAACCU-3′) or their respective
controls (5′UUCUCCGAACGUGUCACGUTT-3′; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China). Cell transfection was using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
cells were harvested 48 h after co-transfection and then the
luciferase activity was measured with a Dual-Luciferase reporter
assay system (Promega Corporation) following the protocol of the
manufacturer. The fluorescence intensity was normalized to
Renilla intensity.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Data are
presented as the mean ± standard deviation from triplicate
experiments. A student's t-test was used for the comparison of two
groups, and one-way analysis of variance followed by a Bonferroni
post-hoc test was used for the comparison of >two groups.
P<0.05 was considered to indicate a statistically significant
difference. Mean values were obtained using SPSS v. 19.0 software
(IBM Corp., Armonk, NY, USA).
Results
Expression patterns of miR-152 in
patients with liver fibrosis and cell lines
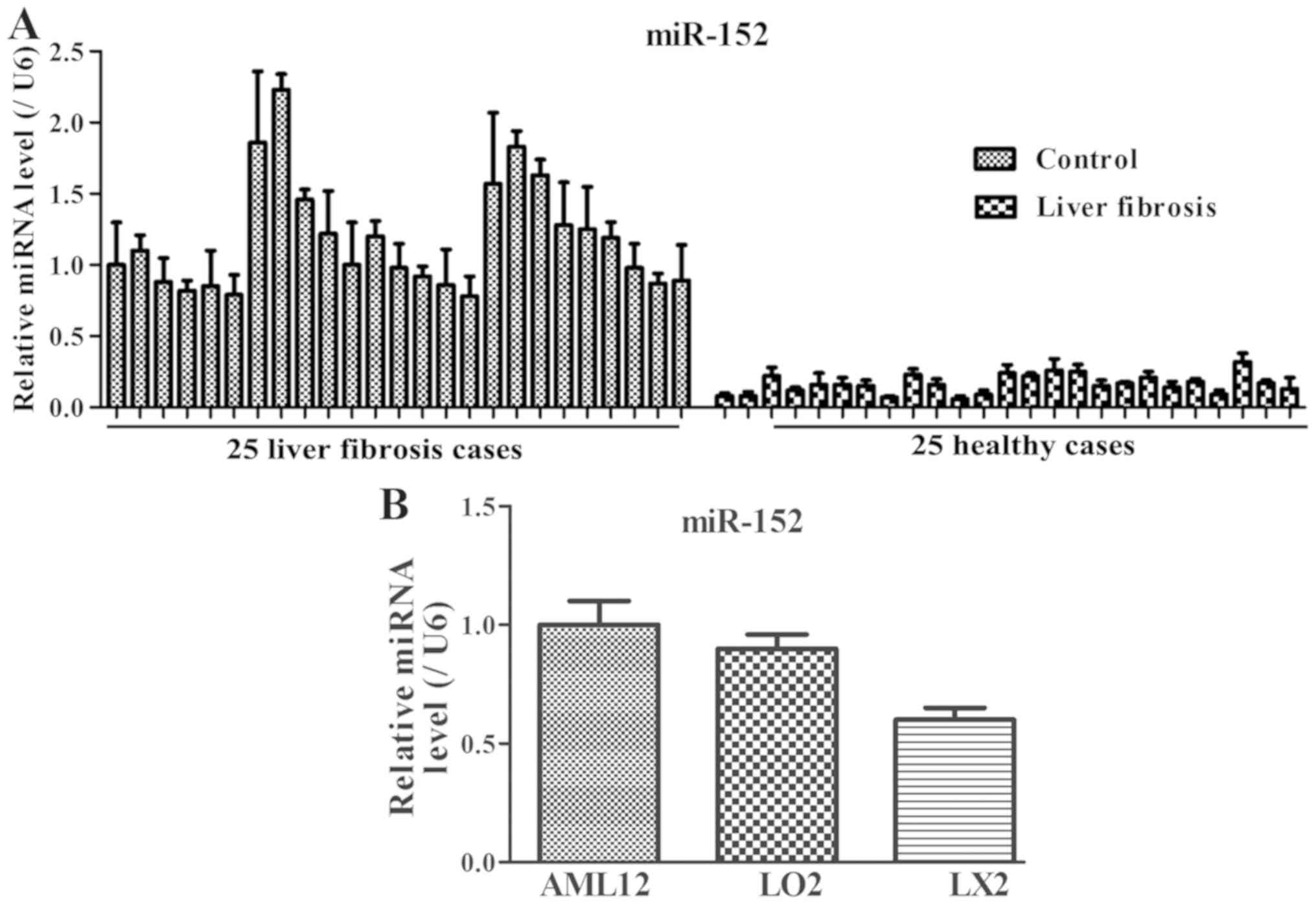
To investigate the miR-152 expression levels in
serum samples of patients with liver fibrosis and cellular samples,
RT-qPCR was performed in vivo and in vitro. Among the
25 patients with liver fibrosis, the miR-152 expression level was
decreased compared with that in the healthy controls (Fig. 1A). In addition, compared with that in
the normal immortalized human liver AML12 and L02 cells, miR-152
expression was markedly decreased in LX2 cells (Fig. 1B). These results suggested that
decreased miR-152 expression may be closely associated with the
progression of liver fibrosis.
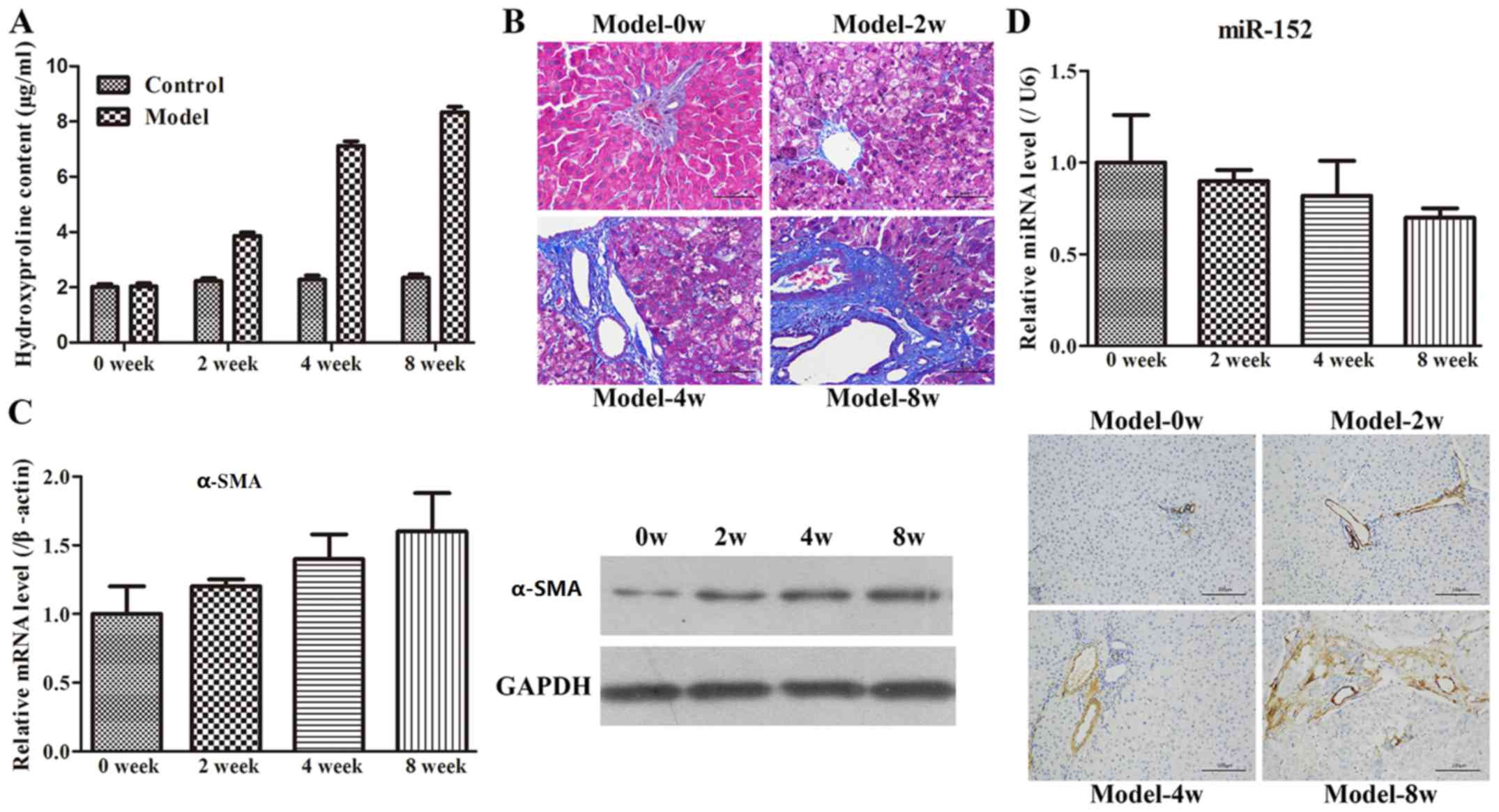
Evaluation of the animal model
Administration of CCl4 is frequently used to
construct liver fibrosis models (18), and hydroxyproline content is commonly
measured to assess liver fibrosis (23). The hydroxyproline contents of the
Model-0 week, Model-2 week, Model-4 week and Model-8 week groups
were 2.04±0.49, 3.86±0.52, 7.12±0.63 and 8.34±0.72 µg/mg,
respectively (Fig. 2A). The
hydroxyproline level in the CCl4-treated groups was
increased compared with that in the control group. In addition, the
increase in hydroxyproline following CCl4 treatment was
observed to occur in a time-dependent manner. Subsequently, H&E
or Masson's trichrome staining was performed to detect collagen
fibres. The results indicated that the rats in the Model-0 week
group exhibited normal, clear and complete liver tissue structures
with large and round nuclei and abundant cytoplasm, and with
limited collagen deposition at the venous walls and bile duct walls
in the portal area (Fig. 2B).
However, the rats in the other three groups demonstrated increased
levels of hyperplasia of fibrous connective tissue, fatty
degeneration, steatosis, cell necrosis, infiltration of
inflammatory cells and a larger number of collagen fibres, which
were primarily deposited in the portal area and interlobular septa
in comparison with the Model-0 group. In addition, longer modelling
time intervals exhibited more marked changes compared with the
shorter modelling time intervals. Finally, to examine the rat model
of liver fibrosis in more detail, the expression of α-SMA at the
mRNA and protein levels was examined by RT-qPCR, WB and
immunohistochemistry methods. As demonstrated in Fig. 2C, the α-SMA expression was
significantly increased with increases in the modelling time
intervals. Furthermore, the immunohistochemistry result also
revealed that limited α-SMA-positive tissues were detected at the
vascular walls of the liver tissues in the Model-0 week group,
whereas the expression of α-SMA was not only identified in the
vascular walls but also widely spread throughout the portal area,
fibrous septum and the adjacent hepatic sinusoids in the other
three groups. Therefore, these results indicated that the rat model
of liver fibrosis was successfully established.
miR-152 changes in the rat model of
fibrosis
Based on the miR-152 results in the clinical
samples, the expression level of miR-152 in the rat model of
fibrosis was examined using RT-qPCR. It was identified that miR-152
expression gradually decreased with increasing time intervals
(Fig. 2D). This result implied that
the dynamic change in miR-152 expression may be involved in the
development of liver fibrosis.
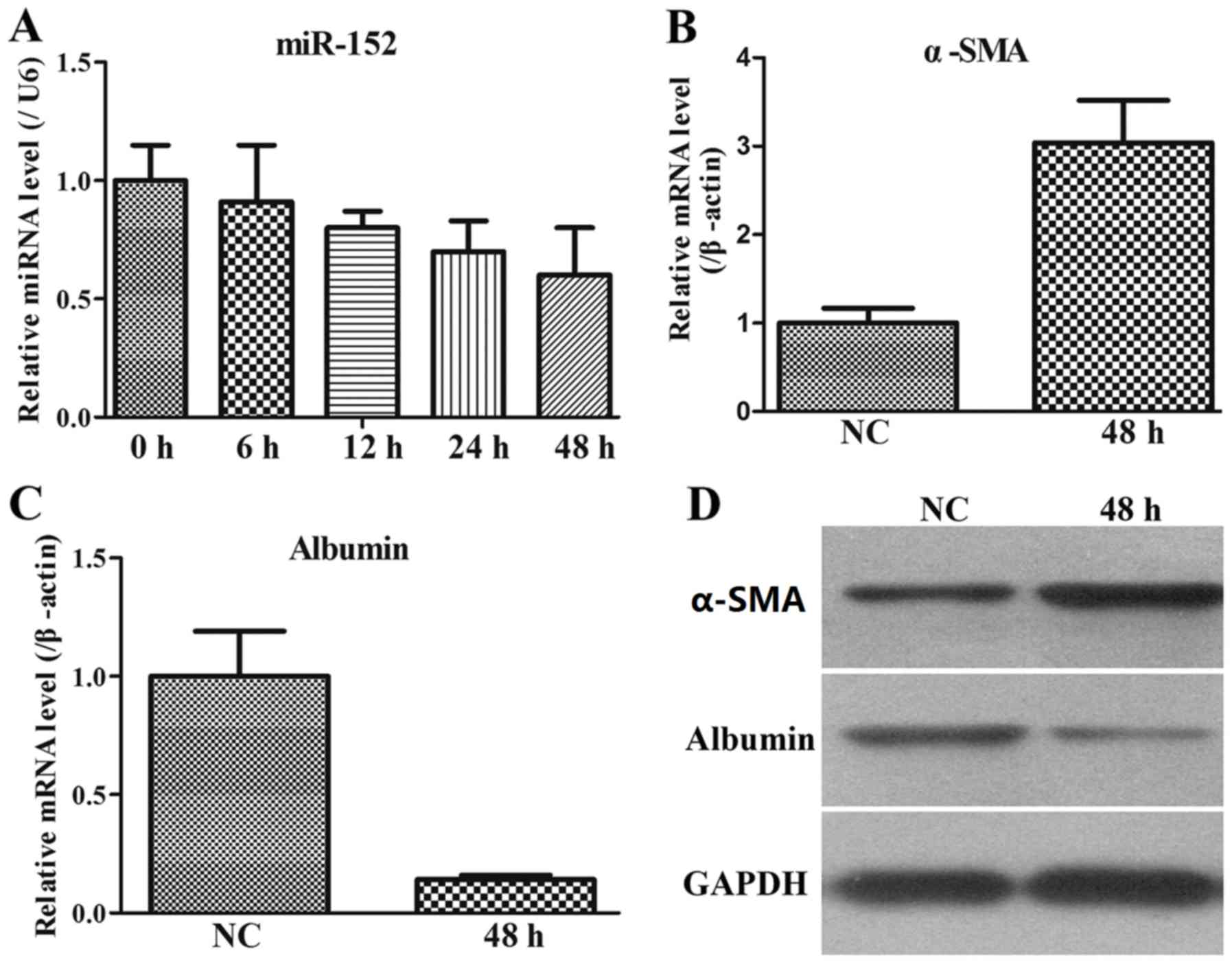
miR-152 and fibrosis-associated gene
expression in stimulated LX2 cells
The LX-2 human HSC line has been widely
characterized and maintains key features of hepatic stellate
cytokine signalling, retinoid metabolism and fibrogenesis, making
it a suitable model of human hepatic fibrosis. Therefore, the
miR-152 expression was additionally assessed by RT-qPCR in
stimulated LX2 cells. The results indicated that in the co-culture
system of LX2 and THP-1 cells, miR-152 expression was gradually
decreased with increasing time intervals (Fig. 3A).
As α-SMA is the most well-established marker for
activated LX2 cells (24), the
levels of α-SMA in stimulated LX2 cells at 48 h were monitored. It
was demonstrated that α-SMA expression at the mRNA and protein
levels was upregulated in stimulated LX2 cells compared with that
in the NC group (Fig. 3B and C). In
addition, albumin level is also a biochemical marker during liver
fibrosis, and therefore, its expression pattern was measured. The
results indicated that albumin expression at the mRNA and protein
levels was downregulated in stimulated LX2 cells compared with that
in the NC group (Fig. 3C and D).
Therefore, these data from the co-culture system of activated LX2
cells and THP-1 cells indicated that miR-152 served an important
role in the process of liver fibrosis.
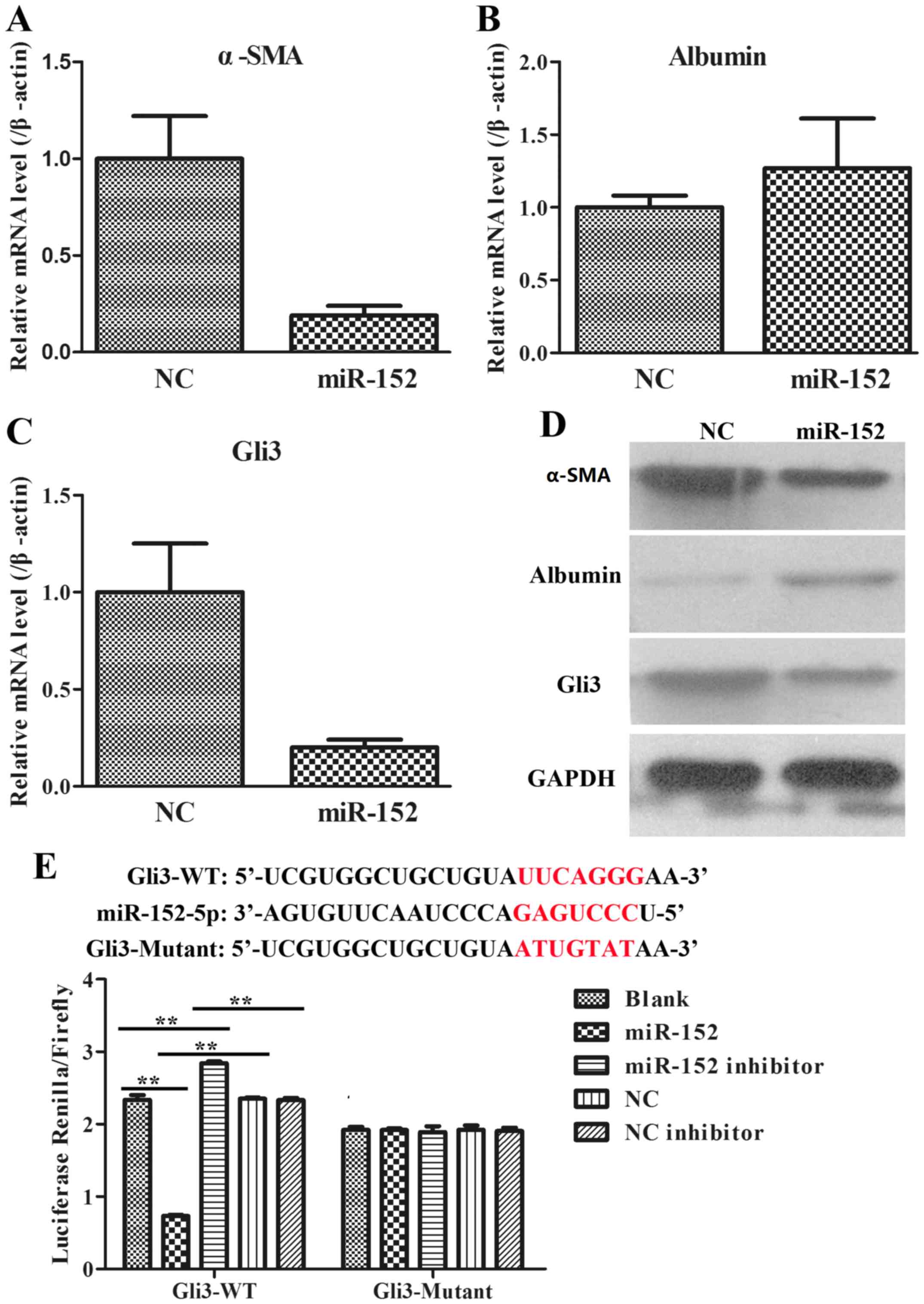
Effects of miR-152 on activated LX2
cells
To additionally explore the role of miR-152 in the
formation of liver fibrosis, fibrosis-associated genes, including
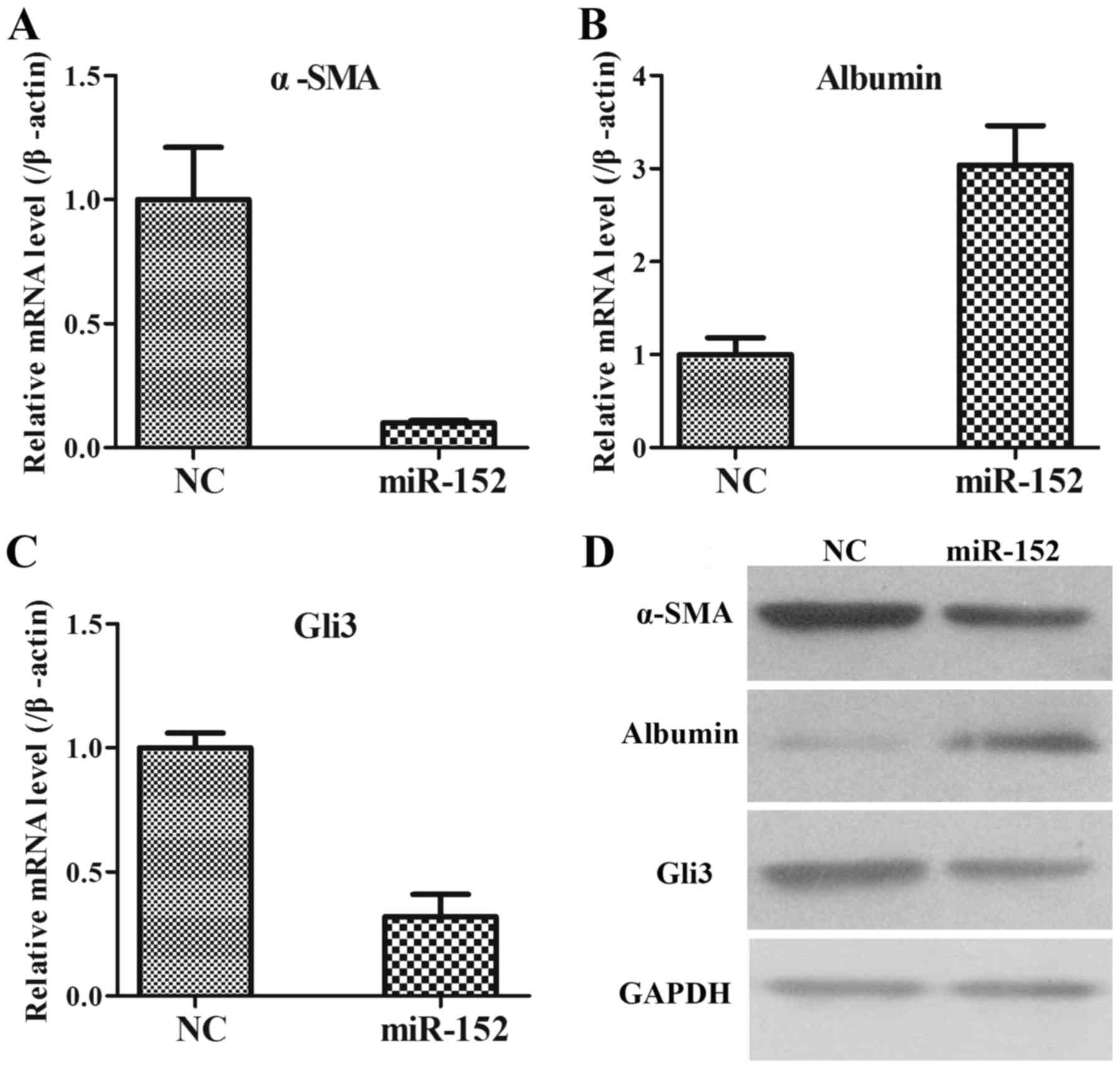
α-SMA, albumin and Gli3, were analysed using RT-qPCR and WB in LX2
cells transfected with an NC plasmid and miR-152 mimic. It was
observed that when compared with those in NC group, the mRNA
expression of α-SMA was significantly decreased (Fig. 4A); the mRNA expression of Albumin was
increased (Fig. 4B); and the mRNA
expression of Gli3 was significantly reduced in miR-152 mimic
transfected cells (Fig. 4C). The
protein expression levels of these genes were consistent with the
mRNA expression levels (Fig. 4D).
Therefore, these data demonstrated that miR-152 may inhibit α-SMA
and Gli3 expression and promote albumin expression.
miR-152 is predicted to target the
3′-UTR of Gli3
Due to the opposite expression patterns of miR-152
and Gli3, bioinformatic analysis was used to predict the potential
target interaction between miR-152 and Gli3 (Targetscan Human 7.2,
http://www.targetscan.org/). It was
confirmed by luciferase assays that miR-152 decreased the relative
activity of luciferase by directly binding to the 3′-UTR of Gli3 in
293T cells (Fig. 4E). A combined
plasmid with mutations in the predicted binding site was generated
and co-transfected with different groups of miR-152 in luciferase
assays, and no significant differences in luciferase activity
levels among the different groups were observed. These data
suggested that miR-152 may directly target Gli3.
Role of miR-152 in the rat model of
liver fibrosis
Subsequent to demonstrating the effects of miR-152
on activated LX2 cells, the role of miR-152 in the rat liver
fibrosis model was eventually confirmed. As indicated in Fig. 5, the changing expression patterns of
α-SMA, albumin and Gli3 at the mRNA and protein levels were notably
coincident with LX2 cells. Taken together, these data suggested
that miR-152 may suppress α-SMA and Gli3 expression and facilitate
albumin expression in vivo and in vitro.
Discussion
Liver fibrosis is a scarring response to liver
damage (1). It is a common
pathological process for a number of liver disorders (25). A small number of patients will
progress to cirrhosis and/or hepatocellular carcinoma (26). Presently, a considerable number of
studies have focused on the roles of miRNAs in the pathophysiology
of liver fibrosis in view of their regulatory effects on
fibrogenesis-associated genes (27).
For example, the downregulation of miR-145 may contribute to liver
fibrosis in biliary atresia by targeting gamma-adducin (28); and miR-101 suppressed liver fibrosis
by targeting the TGF-β signalling pathway (29). Zheng et al (14) demonstrated that the long non-coding
RNA Pvt1 oncogene epigenetically downregulated protein patched
homolog 1 (PTC1) expression via competitively binding miR-152,
contributing to the EMT process in liver fibrosis. Yu et al
(13) revealed that salvianolic acid
B-induced miRNA-152 inhibited liver fibrosis by attenuating DNA
(cytosine-5)-methyltransferase 1-mediated PTC1 methylation. In the
present study, it was identified that miR-152 was significantly
decreased in serum samples from clinical patients, liver tissues
from CCl4-treated rats and activated LX2 cells,
suggesting that downregulated miR-152 may serve an important role
in liver fibrosis.
Subsequently, fibrogenesis-associated indexes,
including hydroxyproline content, collagen deposition, and α-SMA
and albumin expression levels, were examined in in vivo and
in vitro models. The hydroxyproline content was increased in
the livers of CCl4-treated rats compared with in the
livers of the control rats, and H&E and Masson staining
revealed deposition of excessive collagen fibres in
CCl4-treated livers over time. In addition, the
expression level of α-SMA in liver tissues was markedly increased.
Therefore, these results indicated that CCl4-treated
rats exhibited the apparent features of liver fibrosis.
Additionally, it was identified that α-SMA and albumin expression
levels were notably upregulated and downregulated, respectively, in
LX2 cells co-cultured with treated THP-1 cells. During fibrosis,
HSCs were hypothesized to serve a crucial role, as they are
responsible for the proliferation and migration of HSCs and
excessive deposition of ECM to effectively amplify the fibrotic
response (26). Furthermore,
activation of HSCs is regulated by multiple signalling pathways,
including the TGF-β/Smad, phosphatidylinositol 3-kinase/RAC-alpha
serine/threonine-protein kinase and the Hh signalling pathways
(30). Among these pathways, Hh
pathway components are usually expressed at relatively low levels
in normal liver tissues; however, they progressively increase
during the process of liver injury, and Gli3 is a vital
transcription factor in the Hh pathway (31). The present study primarily focused on
whether miR-152 was involved in the regulation of Gli3, which may
trigger the Hh pathway, in human liver fibrosis samples, stimulated
LX2 cells, a common type of HSCs, and a rat model for verification.
The results demonstrated that overexpression of miR-152 inhibited
α-SMA and Gli3 expression and facilitated albumin expression. In
addition, it was identified that Gli3 was a directly target gene of
miR-152, as indicated by bioinformatical analysis and a
dual-luciferase reporter assay. Finally, in concordance with the
α-SMA, albumin and Gli3 expression patterns exhibited in activated
LX2 cells following administration of an miR-152 mimic to
CCl4-treated rats, it was additionally identified that
these genes presented a similar trend of expression in the liver
tissues, implying that miR-152 may suppress activation of LX2 cells
and liver fibrosis by regulating Gli3.
In conclusion, the present study demonstrated that
miR-152 was markedly decreased during the progression of liver
fibrosis in vivo and in vitro. It was also confirmed
that the interaction target of miR-152 is Gli3. In addition, the
overexpression of miR-152 in a CCl4-induced liver
fibrosis rat model and activated LX2 cells decreased pro-fibrotic
gene expression and increased anti-fibrotic gene expression. Taken
together, the identification of miR-152 and its target gene
provides useful insights into the mechanisms underlying liver
fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Research Fund
from the Yunnan Provincial Department of Education (grant. no.,
2013C239) and a Postdoctoral Supporting Fund from Kunming Human
Resources and Social Security Bureau.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL designed the current study and drafted the
manuscript. LZ and XZ performed the animal experiments. JC and JL
collected clinical and cellular data. GC analyzed the data.
Ethics approval and consent to
participate
Informed written consent was obtained from all
participants prior to enrolment in the study, and the study was
approved by the Ethical Committee of the First People's Hospital of
Kunming City. All animal experiments were approved by the Animal
Care and Use Committee of the First People's Hospital of Kunming
City, in accordance with the National Institutes of Health Guide
for the Care and Use of Laboratory Animals (18).
Patient consent for publication
Informed written consent was obtained from all
participants prior to enrolment in the study.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elpek GÖ: Cellular and molecular
mechanisms in the pathogenesis of liver fibrosis: An update. World
J Gastroenterol. 20:7260–7276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seki E and Brenner DA: Recent advancement
of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat
Sci. 22:512–518. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trautwein C, Friedman SL, Schuppan D and
Pinzani M: Hepatic fibrosis: Concept to treatment. J Hepatolo 62 (1
Suppl). S15–S24. 2015.
|
|
5
|
Friedman SL: Mechanisms of disease:
Mechanisms of hepatic fibrosis and therapeutic implications. Nat
Clin Pract Gastroenterol Hepatol. 1:98–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coletta M, Nicolini D, Benedetti
Cacciaguerra A, Mazzocato S, Rossi R and Vivarelli M: Bridging
patients with hepatocellular cancer waiting for liver transplant:
All the patients are the same? Transl Gastroenterol Hepatol.
2:782017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Treiber T, Treiber N, Plessmann U,
Harlander S, Daiß JL, Eichner N, Lehmann G, Schall K, Urlaub H and
Meister G: A Compendium of RNA-binding proteins that regulate
MicroRNA biogenesis. Mol Cell. 66:270–284.e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Fortin K and Mourelatos Z:
MicroRNAs: Biogenesis and molecular functions. Brain Pathol.
18:113–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murakami Y and Kawada N: MicroRNAs in
hepatic pathophysiology. Hepatol Res. 47:60–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Du J, Niu X, Fu N, Wang R, Zhang
Y, Zhao S, Sun D and Nan Y: MiR-130a-3p attenuates activation and
induces apoptosis of hepatic stellate cells in nonalcoholic
fibrosing steatohepatitis by directly targeting TGFBR1 and TGFBR2.
Cell Death Dis. 8:e27922017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun J, Zhang H, Li L, Yu L and Fu L:
MicroRNA-9 limits hepatic fibrosis by suppressing the activation
and proliferation of hepatic stellate cells by directly targeting
MRP1/ABCC1. Oncol Rep. 37:1698–1706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li WQ, Chen C, Xu MD, Guo J, Li YM, Xia
QM, Liu HM, He J, Yu HY and Zhu L: The rno-miR-34 family is
upregulated and targets ACSL1 in dimethylnitrosamine-induced
hepatic fibrosis in rats. FEBS J. 278:1522–1532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu F, Lu Z, Chen B, Wu X, Dong P and Zheng
J: Salvianolic acid B-induced microRNA-152 inhibits liver fibrosis
by attenuating DNMT1-mediated Patched1 methylation. J Cell Mol Med.
19:2617–2632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng J, Yu F, Dong P, Wu L, Zhang Y, Hu Y
and Zheng L: Long non-coding RNA PVT1 activates hepatic stellate
cells through competitively binding microRNA-152. Oncotarget.
7:62886–62897. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin F, Wu X, Zhang H, You X, Zhang Z, Shao
R and Huang C: A microrna screen to identify regulators of
peritoneal fibrosis in a rat model of peritoneal dialysis. BMC
Nephrol. 16:482015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Renault MA, Roncalli J, Tongers J, Misener
S, Thorne T, Jujo K, Ito A, Clarke T, Fung C, Millay M, et al: The
Hedgehog transcription factor Gli3 modulates angiogenesis. Circ
Res. 105:818–826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Syn WK, Jung Y, Omenetti A, Abdelmalek M,
Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, et al:
Hedgehog-mediated epithelial-to-mesenchymal transition and
fibrogenic repair in nonalcoholic fatty liver disease.
Gastroenterology. 137:1478–1488.e8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang X, Wang X, Lv Y, Xu L, Lin J and
Diao Y: Protection effect of kallistatin on carbon
tetrachloride-induced liver fibrosis in rats via antioxidative
stress. PLoS One. 9:e884982014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahreen S, Khan MR and Khan RA: Evaluation
of Rumex hastatus leaves against hepatic fibrosis: A rat model. BMC
Complement Altern Med. 17:4352017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park EK, Jung HS, Yang HI, Yoo MC, Kim C
and Kim KS: Optimized THP-1 differentiation is required for the
detection of responses to weak stimuli. Inflamm Res. 56:45–50.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prestigiacomo V, Weston A, Messner S,
Lampart F and Suter-Dick L: Pro-fibrotic compounds induce stellate
cell activation, ECM-remodelling and Nrf2 activation in a human
3D-multicellular model of liver fibrosis. PLoS One.
12:e01799952017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knight V, Lourensz D, Tchongue J, Correia
J, Tipping P and Sievert W: Cytoplasmic domain of tissue factor
promotes liver fibrosis in mice. World J Gastroenterol.
23:5692–5699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buniatian G, Hamprecht B and Gebhardt R:
Glial fibrillary acidic protein as a marker of perisinusoidal
stellate cells that can distinguish between the normal and
myofibroblast-like phenotypes. Biol Cell. 87:65–73. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ismail MH and Pinzani M: Reversal of
hepatic fibrosis: Pathophysiological basis of antifibrotic
therapies. Hepat Med. 3:69–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang XP, Ai WB, Wan LY, Zhang YQ and Wu
JF: The roles of microRNA families in hepatic fibrosis. Cell
Biosci. 7:342017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye Y, Li Z, Feng Q, Chen Z, Wu Z, Wang J,
Ye X, Zhang D, Liu L, Gao W, et al: Downregulation of microRNA-145
may contribute to liver fibrosis in biliary atresia by targeting
ADD3. PLoS One. 12:e01808962017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu X, Zhang H and Zhang J, Zhao S, Zheng
X, Zhang Z, Zhu J, Chen J, Dong L, Zang Y and Zhang J: MicroRNA-101
suppresses liver fibrosis by targeting the TGFβ signalling pathway.
J Pathol. 234:46–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Q, Qin CY, Zhao ZH, Fan YC and Wang
K: Epigenetic modifications in hepatic stellate cells contribute to
liver fibrosis. Tohoku J Exp Med. 229:35–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang JJ, Tao H and Li J: Hedgehog
signaling pathway as key player in liver fibrosis: New insights and
perspectives. Expert Opin Ther Targets. 18:1011–1021. 2014.
View Article : Google Scholar : PubMed/NCBI
|