Introduction
As a metabolic disorder of sugar, fat and protein
with polydipsia, polyuria, more food and weight loss as main
clinical features, diabetes is divided into type I and type II
diabetes (1). With the improvement
of living standards, the increase in the aging population and the
changes in life structure, the incidence of diabetes continues to
increase and diabetes is expected to become the seventh leading
cause of death in the world by 2030, seriously endangering human
health (2). Most patients with type
II diabetes are caused by obesity, however, type I diabetes is not
related to it, because of insulin resistance, absolutely
insufficient insulin and genetic factors, most patients with type I
diabetes are non-obese diabetic (NOD) patients (3).
Insulin is secreted by islet β cells, which are the
main targets for the clinical treatment of diabetes (4). Exendin-4, a polypeptide that promotes
islet cell regeneration and proliferation and stimulates insulin
secretion from islet β cells, has a strong hypoglycemic effect and
a long duration and half-life. Many studies have shown that
Exendin-4 has good prospects for the treatment of type II diabetes
(5,6). As a small, non-coding, endogenous RNA
that is widely expressed in eukaryotic cell organisms, microRNAs
regulate gene expression by causing mRNA degradation or
translational suppression following the formation of RNA-induced
silencing complex with proteins. There is increasing evidence that
microRNAs are important for the production, secretion and function
of insulin, as well as the proliferation, development and apoptosis
of islet cells (7,8). A recent study reported that Exendin-4
inhibits miR-192 secretion from injured tubular epithelial cells
and improves high glucose-induced tubular epithelial cell fibrosis,
thereby inhibiting the downregulation of glucagon-like peptide-1
(GLP-1) and protecting renal cells (9). In addition, Exendin-4 regulates glucose
metabolism, which may improve the function and quality of islet β
cells and protect the cells by downregulating miR-7, miR-9 and
miR-375 (10). Those studies
indicate that microRNAs are effective for the treatment of diabetes
with Exendin-4. miR-19b is also a molecule closely related to
diabetes. It has been reported that GLP-1 regulates cholesterol
homeostasis by inhibiting the downregulation of miR-19b-induced
ATP-binding cassette transporter A1 (ABCA1) that plays an important
role in regulating cholesterol efflux to apolipoprotein A-I and is
the major maintenance mechanism of cellular cholesterol homeostasis
(11). Exendin-4 has high affinity
and homology with GLP-1 (12).
Therefore, it is speculated that miR-19b also affects the role of
Exendin-4.
The aim of the study was to determine the effect of
miR-19b on the protective effect of Exendin-4 on islet cells in
non-obese diabetic (NOD) mice using ELISA and TUNEL. The results
showed that, an increase in the dose of Exendin-4 can further
improve its therapeutic effect on NOD.
Materials and methods
Subjects
Twenty-four NOD/LT mice were purchased from the
Institute of Medical Laboratory Animals, Chinese Academy of Medical
Sciences, and fed with ordinary nutritional feed (Beijing Zhecheng
Technology Co., Ltd., Beijing, China) with acidified water with a
pH between 2.5 and 3.0 after autoclaving as drinking water. The
average age of the mice was 22.6±2.4 days, and the average body
weight was 24.1±1.5 g, with a temperature of 18–22°C and a relative
humidity of 40–70%. The mice were kept separately in the terrarium,
and the pads were changed every morning and evening. The
environmental noise was <85 decibels (dB), ammonia concentration
did not exceed 20 ppm, and the ventilation was 8–12 times per hour;
the nests were changed 1–2 times and cleaned and disinfected each
week; the noise did not exceed 60 dB, ammonia concentration was
less than 14 ppm, and the ventilation per hour was not less than 15
times, with a 12-h day/night cycle under a fluorescent lamp. The
mice were divided into the control group, the low-dose group, the
medium-dose group and the high-dose group (n=6 each group),
according to the random number table.
The study was approved by the Ethics Committee of
Zhangzhou Affiliated Hospital of Fujian Medical University
(Zhangzhou, China).
Intervention methods
All mice were adaptively fed with ordinary
nutritional feed for one week and then interfered with miR-19b
expression (the interference group) except for the control group.
The lentivirus was used to mediate the targeted miR-19b gene, shRNA
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), in order to
silence mouse miR-19b gene expression, with RT-qPCR detecting the
interference results and the blood glucose level (BGL) (blood
glucose meter) (OneTouch UltraVue). The mice were tail
intravenously injected with different doses of Exendin-4 at 2
µg/kg•day (the low-dose group), 4 µg/kg•day (the medium-dose group)
and 8 µg/kg•day (the high-dose group) for intervention. Exendin-4
reagent was purchased from Kangtai Biology (Beijing, China), and
mice in the control group were given Exendin-4 at a dose of 4
µg/kg•day. After 8 weeks, the mice were intraperitoneally injected
with sodium pentobarbital (100 mg/kg; Shanghai Rongbai Biological
Technology Co., Ltd., Shanghai, China) for anesthesia, and then the
peripheral blood was collected from the tail vein before the
intervention, and after 4 and 8 weeks of intervention. The mice
were sacrificed via cervical dislocation to isolate the pancreatic
tissue under aseptic conditions.
Detection of T cell subsets
The isolated pancreatic tissue was washed twice with
Hank's solution, cut with ophthalmic scissors and repeatedly beaten
to free islet cells that were filtered with 300-mesh net and
collected, and then prepared into a cell suspension of not less
than 1×106/ml. Flow cytometry was used to detect T cell
subsets in the pancreatic tissue and the peripheral blood, which
was performed by instrument engineers. FITC-anti mouse CD4 (catalog
no. AB133616; diluted, 1:100) and Per CP-Cy5.5 anti-mouse CD8
(catalog no. AB22378; diluted, 1:100) (both from Abcam, Cambridge,
MA, USA); and flow cytometry (Beijing Kexue Yingye Science and
Technology Development, Beijing, China) were used to detect T cell
subset.
Detection of T cell-related
cytokines
IL-2 and IL-10 levels in the peripheral blood of
mice were detected by ELISA in strict accordance with the kit
instructions. The IL-2 and IL-10 ELISA detection kits were
purchased from Shanghai Biotechnology (Shanghai, China).
Detection of islet cell apoptosis
The pancreatic tissue was cut into paraffin sections
that were stained with TUNEL, and the specific method referred to
protocol. Image analysis software (Image-proPlus 5.0) was used to
calculate the number of TUNEL-positive cells and the total number
of islet cells in five 40×10× visual fields in order to calculate
the apoptotic rate of islet cells. The TUNEL detection kit was
purchased from Xiamen Huijia Biotechnology Co., Ltd. (Xiamen,
China; item no. 4815-30-K).
RT-qPCR
Mouse peripheral blood and TRIzol lysate (Shanghai
Ying Gong Reagent Co., Ltd., Shanghai, China) were added at a ratio
of 3:1 for extracting total RNA, the purity and concentration of
which were detected after RNA was purified by miRNeasy Mini Kit.
The quality was determined using a 2100 bioanalyzer (Agilent
Technology Co., Ltd., New York, NY, USA) with A260/A280 values
between 1.8 and 2.1 considered to meet the experimental
requirements. Reverse transcription reaction was carried out after
the RNA extraction, and PCR amplification was performed after the
first-strand cDNA was synthesized by RT2 Easy First
Strand Kit. The PCR amplification system was as follows: 1 µl of
cDNA template, 12.5 µl of 2X RT2 SYBR-Green qPCR Master
Mix, each 0.4 µl of upstream and downstream primers, and double
distilled water added to 25 µl; pre-denaturation at 95°C for 10
min, denaturation at 95°C for 15 sec, annealing at 60°C for 1 min
and extension at 72°C 30 sec, for a total of 40 cycles. Melting
curve analysis was performed after the experiment during which
GAPDH was used as a reaction internal reference. Experiments were
repeated 3 times and the results were analyzed by 2−ΔCq
(13). The ABI 7500 real-time
fluorescence quantitative PCR was purchased from Shanghai Ke Hua
Experimental Systems Co., Ltd. (Shanghai, China). The primer
sequences were designed and produced by Wcgene Biotechnology Co.,
Ltd. (Shanghai, China) (Table
I).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Upstream primer | Downstream
primer |
|---|
| miR-19b |
5′-TTGCAGATTTGCAGTTCAGCGT-3′ |
5′-TCCCACAATCAGTTTTGCATGG-3′ |
| GAPDH |
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
Statistical methods
SPSS 21.0 statistical software (IBM SPSS, Armonk,
NY, USA) was used. Measurement data were expressed as %, and
χ2 test was used for the comparison of ratio. Count data
were expressed as mean ± standard deviation (mean ± SD). The t-test
was used for comparison between the two groups, analysis of
variance (ANOVA) for comparison between groups, LSD test for post
hoc test, ANOVA with repeated measures for comparison at different
time-points in the group. P<0.05 indicates the difference is
statistically significant.
Results
Results of interference with
miR-19b
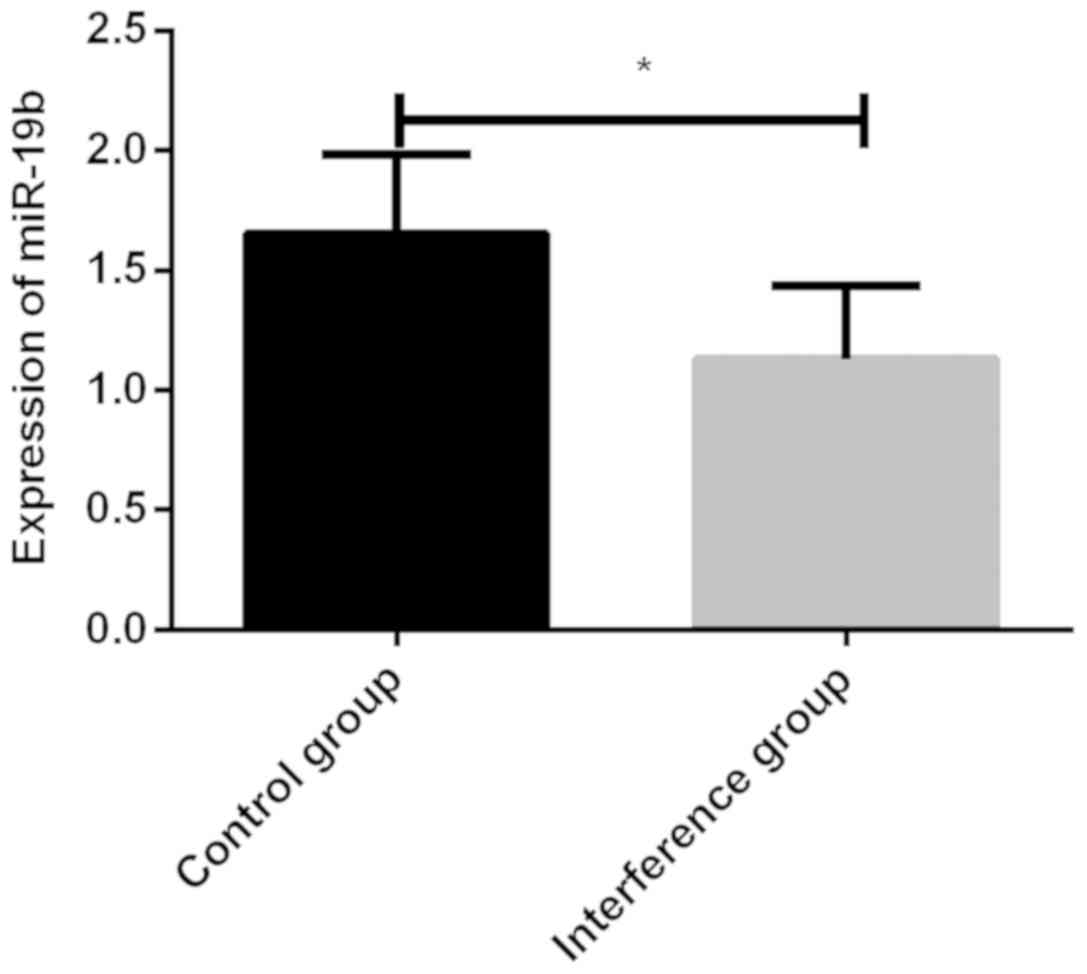
The miR-19b expression level in the mouse peripheral
blood was 1.653±0.329 in the control group, significantly higher
than 1.128±0.306 in the interference group, which indicated a
successful interference (Fig.
1).
Results of BGL detection
Before Exendin-4 intervention, there was no
statistically significant difference in BGL between the four groups
(P>0.05), whereas after 4 and 8 weeks of intervention, there was
a significant difference (all P<0.001). Post hoc test showed
that after 4 and 8 weeks of intervention, mice in the medium and
high-dose groups had lower BGL than those in the control group (all
P<0.05), whereas the high-dose group had lower BGL than the
medium-dose group (P<0.05). After 4 weeks of intervention, there
was no difference in BGL between the control and low-dose groups
(P>0.05), whereas after 8 weeks of intervention, the low-dose
group had lower BGL than the control group (P<0.05). The
comparison at different time-points in the group showed that the
mouse blood glucose in the four groups continued to decrease (all
P<0.05) (Table II).
 | Table II.Analysis of mouse BGL detection
(µg/l). |
Table II.
Analysis of mouse BGL detection
(µg/l).
| Time period | Control group | Low-dose group | Medium-dose
group | High-dose group | F-value | P-value |
|---|
| Before
intervention | 23.83±3.52 | 22.01±3.56 | 21.94±3.54 | 21.89±3.53 | 0.426 | 0.736 |
| After 4 weeks of
intervention |
19.12±2.14a |
17.33±1.89a |
14.06±1.42a,c,d |
11.81±1.34a,c,d,e | 21.477 | <0.001 |
| After 8 weeks of
intervention |
16.24±1.84a,b |
14.24±1.64a–c |
12.26±1.37a–d |
10.39±1.21a–e | 16.207 | <0.001 |
Results of T lymphocyte subset
detection
After 8 weeks of Exendin-4 intervention, there were
statistically significant differences in CD4+ and
CD8+ cell levels in the pancreatic tissue and the
peripheral blood of mice between the four groups (P<0.05). Post
hoc test showed that the CD4+ cell level in the control
group was lower than that in the medium and high-dose groups
(P<0.05), but not different from that in the low-dose group
(P>0.05), and the medium-dose group had lower CD4+
cell level than the high-dose group (P<0.05); the
CD8+ cell level in the control group was higher than
that in the medium and high-dose groups (P<0.05), but lower than
that in the low-dose group (P>0.05), and the medium-dose group
had higher CD8+ cell level than the high-dose group
(P<0.05) (Tables III and
IV).
 | Table III.Results of T lymphocyte subset
detection in the mouse pancreatic tissue (%). |
Table III.
Results of T lymphocyte subset
detection in the mouse pancreatic tissue (%).
| Cell | Control group | Low-dose group | Medium-dose
group | High-dose group | F-value | P-value |
|---|
|
CD4+ | 11.42±1.02 | 10.23±1.03 |
13.45±1.02a,b |
15.33±1.05a–c | 28.622 | <0.001 |
|
CD8+ | 13.83±1.04 | 14.62±1.01 |
11.45±1.04a,b |
9.33±1.05a–c | 31.774 | <0.001 |
 | Table IV.Results of T lymphocyte subset
detection in the mouse peripheral blood (%). |
Table IV.
Results of T lymphocyte subset
detection in the mouse peripheral blood (%).
| Cell | Control group | Low-dose group | Medium-dose
group | High-dose
group | F-value | P-value |
|---|
|
CD4+ | 25.33±1.45 | 24.47±1.38 |
26.75±1.46a,b |
29.17±1.53a–c | 16.352 | <0.001 |
|
CD8+ | 31.19±1.77 | 32.43±1.69 |
28.62±1.68a,b |
25.74±1.57a–c | 18.696 | <0.001 |
Results of T cell-related cytokine
detection
After 8 weeks of Exendin-4 intervention, there were
significant differences in IL-2 and IL-10 levels between the four
groups of mice (all P<0.001). Post hoc test showed that after 8
weeks of intervention, compared with the control group, the medium
and high-dose groups had lower IL-2 level (both P<0.05), but
higher IL-10 level (P<0.05), whereas compared with the
medium-dose group, the high-dose group had lower IL-2 level
(P<0.05), but higher IL-10 level (P<0.05). Compared with the
low-dose group, the medium-dose group and the high-dose group had
lower IL-2 level (P<0.05), but higher IL-10 level (P<0.05).
After 8 weeks of intervention, there was no difference in IL-2
level between the control and low-dose groups (P>0.05), whereas
after 8 weeks of intervention, the low-dose group had higher IL-10
level than the control group (P<0.05) (Table V).
 | Table V.Results of T cell-related cytokine
detection in the mouse peripheral blood. |
Table V.
Results of T cell-related cytokine
detection in the mouse peripheral blood.
| Cytokine | Control group | Low-dose group | Medium-dose
group | High-dose
group | F-value | P-value |
|---|
| IL-2 (pg/ml) |
57.25±8.36a | 53.24±7.24 |
44.33±6.56c,d |
35.42±5.25c–e | 11.777 | <0.001 |
| IL-10 (pg/ml) |
204.47±20.93b | 223.56±21.47 |
258.38±22.31c,d |
294.21±23.25c–e | 17.946 | <0.001 |
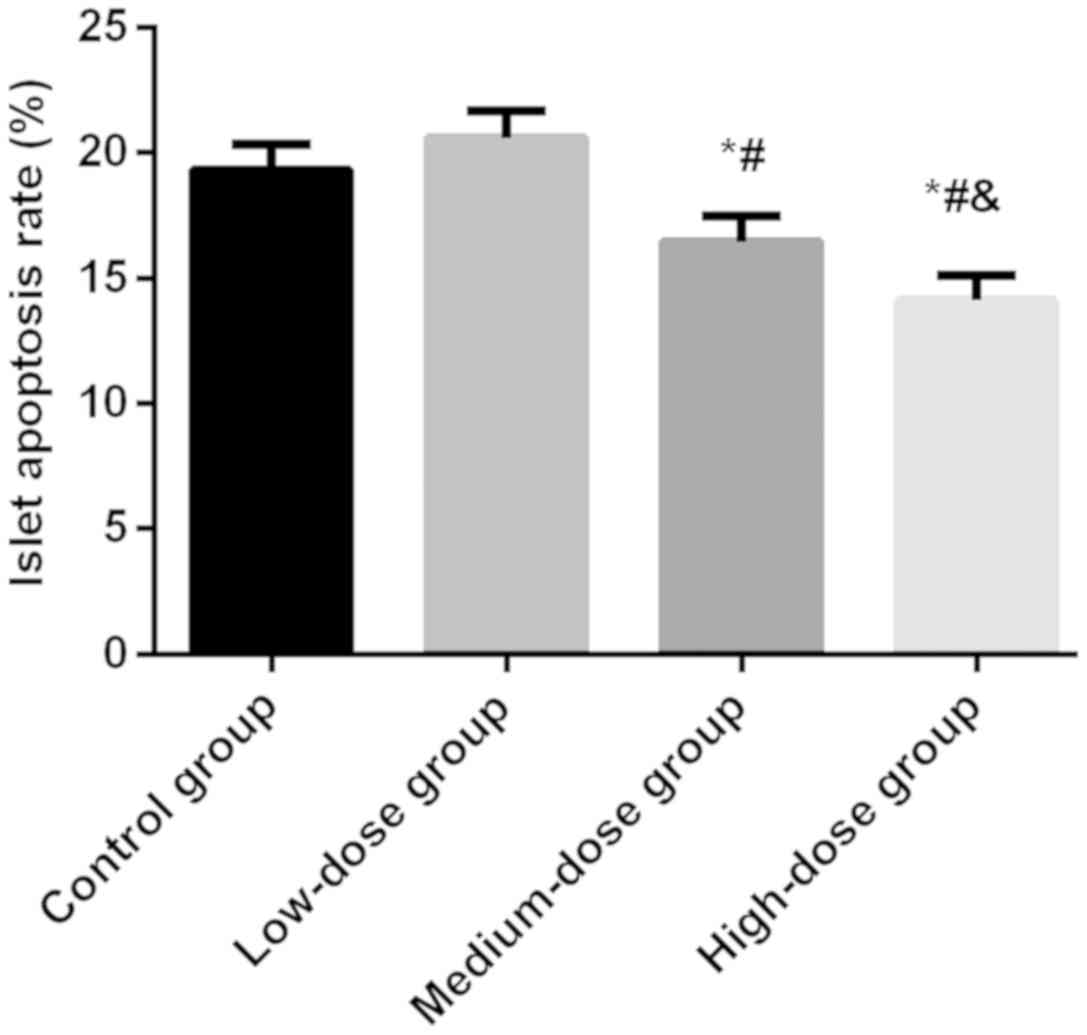
Results of islet cell apoptosis
detection
After 8 weeks of Exendin-4 intervention, the
apoptosis rate of islet cells was 19.33±1.04% in the control group,
20.59±1.06% in the low-dose group, 16.45±1.07% in the medium-dose
group and 14.12±1.02% in the high-dose group, with a significant
difference between the four groups (P<0.05). The apoptosis rate
in the control group was significantly higher than that in the
medium and high-dose groups (P<0.05), but not different from
that in the low-dose group (P>0.05). In addition, the
medium-dose group had a higher apoptosis rate than the high-dose
group (P<0.05) (Fig. 2).
Analysis of adverse reactions
No significant adverse reactions were found in the
mice during the study.
Discussion
Diabetes, a global public health problem with a high
incidence, is difficult to cure, and the number of diabetic
patients worldwide is estimated to exceed 440 million by 2030
(14,15). At different stages of NOD, pancreatic
tissue morphology changes generally. The current traditional
treatment cannot fundamentally prevent the reduction of islet cell
number and improve functional impairment in patients, but normal
islet structure and tissue form is the basis for ensuring the
normal function of the body's islet physiological function
(16).
Due to the islet cells being destroyed by autoimmune
cells and then absolutely insufficient insulin secretion, patients
with type I diabetes need exogenous insulin supportive treatment
for life (17). GLP-1 is one of the
most central mediators of glycemic control after gastric bypass in
diabetic patients (18). Exendin-4
is a GLP-1 analogue and not inactivated by dipeptidyl peptidase-4,
which has an incretin effect, controls blood glucose and protects
islet β cells (19,20), but its specific mechanism of action
remains unclear. Previous findings have shown that improving
pancreatic inflammation and fibrosis and changing microRNA
expression level in the pancreatic tissue and blood, Exendin-4
intervention in NOD mice upregulates miR-1 level, downregulates
miR-19a, miR-19b and miR-22 levels and inhibits the differentiation
and maturation of islet β cells, as well as promotes the recovery
of insulin secretion function (21).
This is speculated to be one of the mechanisms of action of
Exendin-4. In recent years, studies have verified some microRNA.
Additionally, miR-204 enhances the effect of GLP-1 receptor,
promotes insulin secretion and maintains glucostasis (22). The effect of miR-19b on the role of
Exendin-4 was analyzed in this study.
Insulin-dependent NOD mice were studied, and then a
lentiviral vector was constructed to interfere with miR-19b
expression in some of the mice. RT-qPCR results showed a successful
intervention. First, the effect of interference with miR-19b
expression on the role of Exendin-4 was analyzed. The analysis of
the mice in the control and medium-dose groups showed that by
increasing the hypoglycemic effect of Exendin-4 and effectively
improving CD4+ and CD8+ cell levels in the
mouse pancreatic tissue and blood, the decrease in miR-19b
expression inhibited the release of IL-2, promoted that of IL-10
and reduced islet cell apoptosis. Our results show that reducing
miR-19b expression improves the therapeutic effect of Exendin-4.
Then, the therapeutic effect of Exendin-4 at different
concentrations was analyzed when miR-19b expression was inhibited.
The results showed that the therapeutic effect of Exendin-4 was
improved with the rise in its concentration, suggesting that
Exendin-4 exerts a therapeutic effect on NOD through multiple
targets. The mechanism of action of Exendin-4 remains to be
explored more extensively. Previous studies have reported the
improvement of Exendin-4 on glycemic control, inflammatory response
and immune function in diabetic patients (23,24), and
its protective effect on islet cells has also been confirmed in an
animal experiment (25). However,
there are few studies directly related to the effect of miR-19b on
the treatment of diabetes with Exendin-4. It has been reported that
GLP-1 regulates cholesterol homeostasis by inhibiting the
downregulation of miR-19b-induced ABCA1 (11). Cholesterol homeostasis is the key to
normal cell function, the improper distribution or metabolism of
which has serious consequences for cells and the body, and the
increase in cholesterol is also one of the important causes of
diabetes (26). Exendin-4, which
shares 53% homology with human GLP-1, binds to GLP-1 receptor and
produces a physiological effect similar to human GLP-1 (20). This also verifies the correctness of
our results to some extent.
However, there are shortcomings in this study.
Although NOD mice are a common model for studying diabetes, there
are differences between mice and humans, so the results of this
study still require more validation of clinical trial data. The
study time was short, and research on the safety of adenovirus
vectors and different doses of Exendin-4 in long-term treatment is
still required. Although no significant adverse reactions were
found in the mice during the study, the exploration concerning the
safety still cannot be ignored.
In conclusion, the decrease in miR-19b expression
can improve the therapeutic effect of Exendin-4 on NOD, control
blood glucose effectively and improve inflammatory response and
immune function, as well as reduce islet cell injury. The increase
in the dose of Exendin-4 can further improve its therapeutic effect
on NOD.
Acknowledgements
Not applicable.
Funding
This study was supported by Fujian Natural Science
Foundation (grant no. 2016J01650).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH wrote the manuscript. JH, YK and CL performed
RT-qPCR and ELISA. JW and HZ were responsible for flow cytometry.
XY contributed to statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhangzhou Affiliated Hospital of Fujian Medical University
(Zhangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association: Standards
of medical care in diabetes - 2014. Diabetes Care. 37 (Suppl
1):S14–S80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pai LW, Hung CT, Li SF, Chen LL, Chung Y
and Liu HL: Musculoskeletal pain in people with and without type 2
diabetes in Taiwan: A population-based, retrospective cohort study.
BMC Musculoskelet Disord. 16:3642015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 32 (Suppl
1):S62–S67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, York NW, Nichols CG and Remedi MS:
Pancreatic β cell dedifferentiation in diabetes and
redifferentiation following insulin therapy. Cell Metab.
19:872–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kendall DM, Riddle MC, Rosenstock J,
Zhuang D, Kim DD, Fineman MS and Baron AD: Effects of exenatide
(exendin-4) on glycemic control over 30 weeks in patients with type
2 diabetes treated with metformin and a sulfonylurea. Diabetes
Care. 28:1083–1091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tate M, Robinson E, Green BD, McDermott BJ
and Grieve DJ: Exendin-4 attenuates adverse cardiac remodelling in
streptozocin-induced diabetes via specific actions on infiltrating
macrophages. Basic Res Cardiol. 111:12016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ortega FJ, Mercader JM, Moreno-Navarrete
JM, Rovira O, Guerra E, Esteve E, Xifra G, Martínez C, Ricart W,
Rieusset J, et al: Profiling of circulating microRNAs reveals
common microRNAs linked to type 2 diabetes that change with insulin
sensitization. Diabetes Care. 37:1375–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lima TI, Araujo HN, Menezes ES, Sponton
CH, Araújo MB, Bomfim LH, Queiroz AL, Passos MA, E Sousa TA,
Hirabara SM, et al: Role of microRNAs on the regulation of
mitochondrial biogenesis and insulin signaling in skeletal muscle.
J Cell Physiol. 232:958–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia Y, Zheng Z, Guan M, Zhang Q, Li Y,
Wang L and Xue Y: Exendin-4 ameliorates high glucose-induced
fibrosis by inhibiting the secretion of miR-192 from injured renal
tubular epithelial cells. Exp Mol Med. 50:562018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo C, Sun YQ, Li Q and Zhang JC: MiR-7,
miR-9 and miR-375 contribute to effect of Exendin-4 on pancreatic
β-cells in high-fat-diet-fed mice. Clin Invest Med. 41:E16–E24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao Y, Li Q, Wang W, Zhang J, Gao P and Xu
Y: Glucagon-like peptide-1 modulates cholesterol homeostasis by
suppressing the miR-19b-induced downregulation of ABCA1. Cell
Physiol Biochem. 50:679–693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Chu J, Wang YH, Wang H, Zhuang YP
and Zhang SL: Purification and bioactivity of exendin-4, a peptide
analogue of GLP-1, expressed in Pichia pastoris. Biotechnol Lett.
30:651–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
NCD Risk Factor Collaboration (NCD-RisC),
. Worldwide trends in diabetes since 1980: A pooled analysis of 751
population-based studies with 4.4 million participants. Lancet.
387:1513–1530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nolan CJ, Ruderman NB, Kahn SE, Pedersen O
and Prentki M: Insulin resistance as a physiological defense
against metabolic stress: Implications for the management of
subsets of type 2 diabetes. Diabetes. 64:673–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung HS, Chung KW, Won Kim J, Kim J,
Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, et al:
Loss of autophagy diminishes pancreatic beta cell mass and function
with resultant hyperglycemia. Cell Metab. 8:318–324. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller KM, Foster NC, Beck RW, Bergenstal
RM, DuBose SN, DiMeglio LA, Maahs DM and Tamborlane WV; T1D
Exchange Clinic Network, : Current state of type 1 diabetes
treatment in the U.S.: Updated data from the T1D Exchange clinic
registry. Diabetes Care. 38:971–978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ceriello A, La Sala L, De Nigris V,
Pujadas G, Rondinelli M and Genovese S: GLP-1 reduces
metalloproteinase-9 induced by both hyperglycemia and hypoglycemia
in type 1 diabetes. The possible role of oxidative stress. Ther
Clin Risk Manag. 11:901–903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Hou S, Liu S, Huan Y, Sun S, Liu Q
and Shen Z: The albumin-exendin-4 recombinant protein E2HSA
improves glycemic control and β-cell function in spontaneous
diabetic KKAy mice. BMC Pharmacol Toxicol. 18:482017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Candeias E, Sebastião I, Cardoso S,
Carvalho C, Santos MS, Oliveira CR, Moreira PI and Duarte AI: Brain
GLP-1/IGF-1 signaling and autophagy mediate exendin-4 protection
against apoptosis in type 2 diabetic rats. Mol Neurobiol.
55:4030–4050. 2018.PubMed/NCBI
|
|
21
|
He JS, Lian CW, Fang YL, Wu JZ, Ye XL and
Zhu SB: Influence and significance of intervening diabetes microRNA
expression profile of NOD mice with exendin-4. Eur Rev Med
Pharmacol Sci. 20:4322–4327. 2016.PubMed/NCBI
|
|
22
|
Jo S, Chen J, Xu G, Grayson TB, Thielen LA
and Shalev A: miR-204 controls glucagon-like peptide 1 receptor
expression and agonist function. Diabetes. 67:256–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li PC, Liu LF, Jou MJ and Wang HK: The
GLP-1 receptor agonists exendin-4 and liraglutide alleviate
oxidative stress and cognitive and micturition deficits induced by
middle cerebral artery occlusion in diabetic mice. BMC Neurosci.
17:372016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seo E, Lim JS, Jun JB, Choi W, Hong IS and
Jun HS: Exendin-4 in combination with adipose-derived stem cells
promotes angiogenesis and improves diabetic wound healing. J Transl
Med. 15:352017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lehtonen J, Schäffer L, Rasch MG,
Hecksher-Sørensen J and Ahnfelt-Rønne J: Beta cell specific probing
with fluorescent exendin-4 is progressively reduced in type 2
diabetic mouse models. Islets. 7:e11374152015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lotta LA, Sharp SJ, Burgess S, Perry JRB,
Stewart ID, Willems SM, Luan J, Ardanaz E, Arriola L, Balkau B, et
al: Association between low-density lipoprotein
cholesterol-lowering genetic variants and risk of type 2 diabetes:
A Meta-analysis. JAMA. 316:1383–1391. 2016. View Article : Google Scholar : PubMed/NCBI
|