Introduction
Acute lung injury (ALI) can be caused by several
factors and imposes a burden on public health, with in-hospital
mortality >40% (1,2). The overproduction of inflammatory
mediators causes endothelial and epithelial injury and induces
vascular leakage, edema and vasodilatation, which serve a
fundamental role in the pathogenesis of ALI (3,4). Sepsis,
which is a leading cause of morbidity and mortality, is the
systemic inflammatory response to microbial infection (5). The lungs are particularly vulnerable to
the septic inflammatory response, and sepsis is the underlying
cause of ALI in most patients (6).
Therefore, the identification of therapeutic and preventive
approaches that are innovative, safe and effective is crucial for
the treatment of sepsis-induced organ injury.
Innate immune cell activation depends primarily on
Toll-like receptors (TLRs), a family of pattern recognition
receptors (PRRs) that can recognize specific pathogen-associated
molecular patterns (PAMPs), which include nucleic acids, proteins
and lipids (7,8). TLRs are key regulators of both the
innate and adaptive immunity and one of the most well studied PRRs
(9). To date, >10 members of the
TLR family have been identified in mammals, with each receptor
recognizing a specific set of PAMPs (10). TLR2 recognizes peptidoglycan and
lipoteichoic acid, while TLR4 recognizes lipopolysaccharide
(11). TLR3 recognizes
double-stranded RNA within endosomes, while TLR9 recognizes
unmethylated bacterial CpG-DNA (12–14).
TLR3 and 9 are intracellular receptors, while TLR2 and 4 are cell
surface receptors (15). However,
activation of different TLRs induces similar pro-inflammatory
responses with the produces cytokines, which include TNF-α and IL-6
(16). TLRs and their associated
signalling pathways constitute an interlaced network, which makes
it complicated and difficult to identify novel therapeutic targets
(17). Furthermore, inflammation is
often caused by multiple PAMPs (18). Therefore, multiple TLRs may be
activated in a disease, which adds complexity to potential
treatment strategies. It is therefore necessary to identify which
TLRs are involved in the process of an inflammatory disease.
Sepsis is one of the leading risk factors for ALI,
and there are many animal models of sepsis, which include the
administration of live bacteria and bacterial products (19–21).
Cecal ligation and puncture (CLP) has become the most widely used
model for sepsis, in which the cecum is ligated and punctured three
to five times with a needle and PAMPs from bacterial products can
be released into the blood (22).
CLP causes acute lung injury similar to ALI; however, the actual
bacterial inoculum in CLP has not been identified (23). Blood cultures from these models are
usually positive for specific Gram-positive and Gram-negative
bacteria, the components of which can increase the production of
multiple inflammatory cytokines by different TLRs, which include
IL-6 and TNF-α (24).
Given the results of previous studies, the aim of
the current study was to investigate the role of different TLRs
during sepsis and sepsis-induced ALI. A CLP-induced ALI model in
C57BL/6 WT, TLR2−/−, TLR3−/−,
TLR4−/− and TLR9−/− C57BL/6 mice was
established. The current study demonstrated that multiple TLRs may
contribute to the pathogenesis of sepsis-induced ALI, and TLRs
serve a specific role in this process. Therefore, novel therapeutic
targets, which inhibit the activation of multiple TLRs
simultaneously, require investigation.
Materials and methods
Animals
A total of 60 wild-type (WT) and 140 TLR knockout
(TLR−/−), including TLR2−/−,
TLR3−/−, TLR4−/− and TLR9−/−,
C57BL/6 male mice (8–10 weeks; weight, 18–20 g) were obtained from
the Chinese Academy of Inspection and Quarantine (Beijing, China).
All mice were housed in plastic cages at 20–25°C, and a relative
humidity of 40–70% under a 12 h light/dark cycle. All mice were
housed in the Experimental Animal Centre of the Medical Research
Center at the Provincial Hospital Affiliated to Shandong University
(Jinan, China) and kept under specific-pathogen-free conditions
with free access to food and water. Following subsequent
experimental procedures, all mice were euthanized by
CO2. All experimental procedures were performed in
accordance with the National and Institutional Guidelines for
Animal Care and Use and approved by the Institutional Animal Ethics
Committee of Shandong University (Jinan, China).
CLP-induced ALI model
C57BL/6 mice were divided into six groups: WT sham,
WT CLP, TLR2−/− CLP, TLR3−/− CLP,
TLR4−/− CLP and TLR9−/− CLP. CLP was used to
establish the ALI mouse model used in the current study. For the
CLP procedure, mice were fasted, with only free access to water for
12 h prior to surgery. Subsequently, mice were anesthetized with 5%
isoflurane (Shanghai Yuyan instruments, Co., Ltd., Shanghai, China)
and maintained at 1.5% isoflurane in 70% N2O and 30%
O2 using a small animal anesthetic machine (Midmark
Corporation, Kettering, OH, USA). Following anaesthesia, skin was
disinfected and a median incision was made in the abdomen, allowing
the cecum to be exposed and a 4-0 braided silk suture was passed
through the midpoint between the colon root and cecum terminal. A
21-gauge 1-inch needle was inserted into the cecum ligation and a
small drop of the intestinal content was squeezed to induce
infection. Finally, the, cecum was repositioned and the incision
was closed. For the sham group, the abdomen was opened, cecum
exposed and repositioned, and the incision was closed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Animals were sacrificed and lungs were isolated at
20 h post-surgery. Total RNA was extracted from lung samples using
TRIzol® reagent, according to the manufacturer's
protocol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Total RNA was reverse transcribed into cDNA using the
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). mRNA expression was evaluated by
qPCR using the Maxima SYBR Green/ROX qPCR Master Mix 2X (Thermo
Fisher Scientific, Inc.) and the Mastercycler® Realplex
system (Eppendorf, Hamburg, Germany). Primer pair sequences are
summarised in Table I. qPCR quality
and genomic DNA contamination was examined using intron-spanning
primers, reverse transcriptase-negative samples from cDNA synthesis
and melting curve analysis obtained from each reaction. The
amplification conditions were as follows: 95°C (10 sec), followed
by 40 cycles at 55°C (20 sec), 72°C (25 sec). mRNA levels were
quantified using the 2−∆∆Cq method (25) and normalized to the internal
reference gene β-actin. The results are expressed relative to the
values of the sham (CLP-induced sepsis) group.
 | Table I.Primer pair sequences for
quantitative polymerase chain reaction. |
Table I.
Primer pair sequences for
quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| TLR2 | F:
CGATGACTACCGCTGTGACTC |
|
| R:
CCTTCCTGGGCTTCCTCTT |
| TLR3 | F:
GGGACTGTTGACCTGTT |
|
| R:
GTTGGCTGTATCTCGTAA |
| TLR4 | F:
TGCCTTCACTACAGGGACTTT |
|
| R:
TGGGACACCACGACAATAAC |
| TLR9 | F:
CGTGACAATTACCTGGCCTTC |
|
| R:
CAGGGCCTTCAGCTGGTTTC |
| β-actin | F:
ATGAAGATCCTGACCGAGCG |
|
| R:
TACTTGCGCTGAGGAGGAGC |
Western blot analysis
Total protein was extracted from lung tissue using a
T-PER Tissue Protein Extraction Reagent kit according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc). Total
protein was quantified using a bicinchoninic acid (BCA) assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Samples were
then separated on 12% SDS-Polyacrylamide gel (20 µg per lane) and
transferred on to PVDF membranes in an ice bath. After blocking the
non-specific site with blocking solution (5% non-fat dry milk) at
room temperature for 2 h, the membrane was incubated overnight at
4°C with the following specific primary antibodies: Primary
antibodies against TLR2 (1:1,000; cat. no. ab213676; Abcam,
Cambridge, MA, USA), TLR3 (1:500; cat. no. ab62566; Abcam), TLR4
(1:500; cat. no. ab13556; Abcam) and TLR9 (1:500; cat. no. ab37154;
Abcam). Samples were then incubated with horseradish
peroxidase-labelled IgG secondary antibody (cat. no. A0208;
1:2,000; Beyotime Institute of Biotechnology) for 1 h at room
temperature. Chemiluminescent liquid (cat. no. WBKLS0100; EMD
Millipore) was prepared in a ratio of 1:1. Protein expression was
quantified and scanned using the ChemiDoc XRS+ system (Bio-Rad, CA,
USA) with GAPDH (1:500; cat. no. ab8245; Abcam) as the loading
control. Finally, The band density was quantified using Image J
software (v1.8.0; National Institutes of Health, Bethesda, MD,
USA).
ELISA
Mouse plasma and lung tissue samples were analysed
for TNF-α (cat. no. 88-7324-22) and IL-6 (cat. no. 88-7064-88)
expression using ELISA kits obtained from eBioscience (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocol.
Collection of bronchoalveolar lavage
fluid (BALF) samples
BALF samples were collected from mice following
bronchoalveolar lavage as previously described (26). Briefly, mice were anesthetised and
lungs were exposed. The tracheas were cannulated and the lungs were
lavaged twice with 0.8 ml normal saline. The BALF samples were
collected and centrifuged at 800 × g for 10 min at 4°C, and the
supernatant was removed and stored at −80°C until further use. The
cell pellet was resuspended and the total number of cells was
determined using a hemocytometer. The total number of leukocytes in
the BALF sample was counted and classified into five categories
including, neutrophils, basophils, eosinophils, macrophages and
lymphocytes on a Hemavet 950 instrument (Drew Scientific Inc.,
Miami Lakes, FL, USA). Total protein in the BALF samples was
quantified using a BCA assay kit (Beyotime Institute of
Biotechnology).
Haematoxylin and eosin (H&E)
staining
Lung tissue samples from CLP-induced ALI mice at 20
h were fixed in 10% formalin for ≥24 h at 22°C and embedded in
paraffin. Paraffin-embedded tissue samples were cut into 5-µm-thick
sections. Tissue sections were subsequently deparaffinized and
stained with haematoxylin erythrosine saffron and morphological
changes were observed under the light microscope (magnification,
×200). Lung injury was scored by two independent pathologists
according to the following four items: Alveolar congestion,
hemorrhage, infiltration or aggregation of neutrophils in airspaces
or vessel walls, and thickness of alveolar wall or hyaline membrane
formation. Each item was graded according to a four-point scale: 0
= minimal damage/appears normal; 1 = mild damage; 2 = moderate
damage; 3 = severe damage; and 4 = maximal damage. The total lung
injury score was calculated by adding up the individual scores for
each category, ranging from 0 to 16 (most severe).
Mortality
For survival studies, each mouse was monitored daily
for 7 days following CLP or sham surgery. Observations for survival
were performed every 24 h for 7 days.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. All statistical analyses were performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The
independent-sample t-test was used to analyse the statistical
difference between two groups. One-way analysis of variance
followed by Fisher's least significant difference post hoc test was
used to analyse differences among multiple groups. Overall survival
was analysed using the Kaplan-Meier method and the log-rank method
was used to compare 1-week survival distribution between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TLR2, 4 and 9 mRNA and protein
expression levels increased in lung tissue following CLP-induced
ALI
CLP is the most widely used model of sepsis, whereby
multiple PAMPs from bacterial products are released into the blood
(27). CLP-induced ALI mode can be
used to identify upregulated TLRs, which are regulated by PAMPs. To
detect the expression of TLR2, 3, 4 and 9 in CLP-induced ALI, total
RNA and protein were isolated from the lung tissue of WT and
CLP-induced ALI mice. The mRNA expression levels of TLR2, 4 and 9
were significantly increased in the CLP-operated group compared
with the sham-operated group (Fig.
1A). Similarly, increased in the CLP-operated group the protein
expression levels of TLR2, 4 and 9 were increased compared with the
sham-operated group (Fig. 1B).
However, there were no significant changes in the mRNA and protein
expression level of TLR3 in the CLP-operated group compared with
the sham-operated group (Fig. 1A and
B). These results suggest that PAMPs in CLP-induced ALI may
induce TLR2, 4 and 9 mRNA and protein expression.
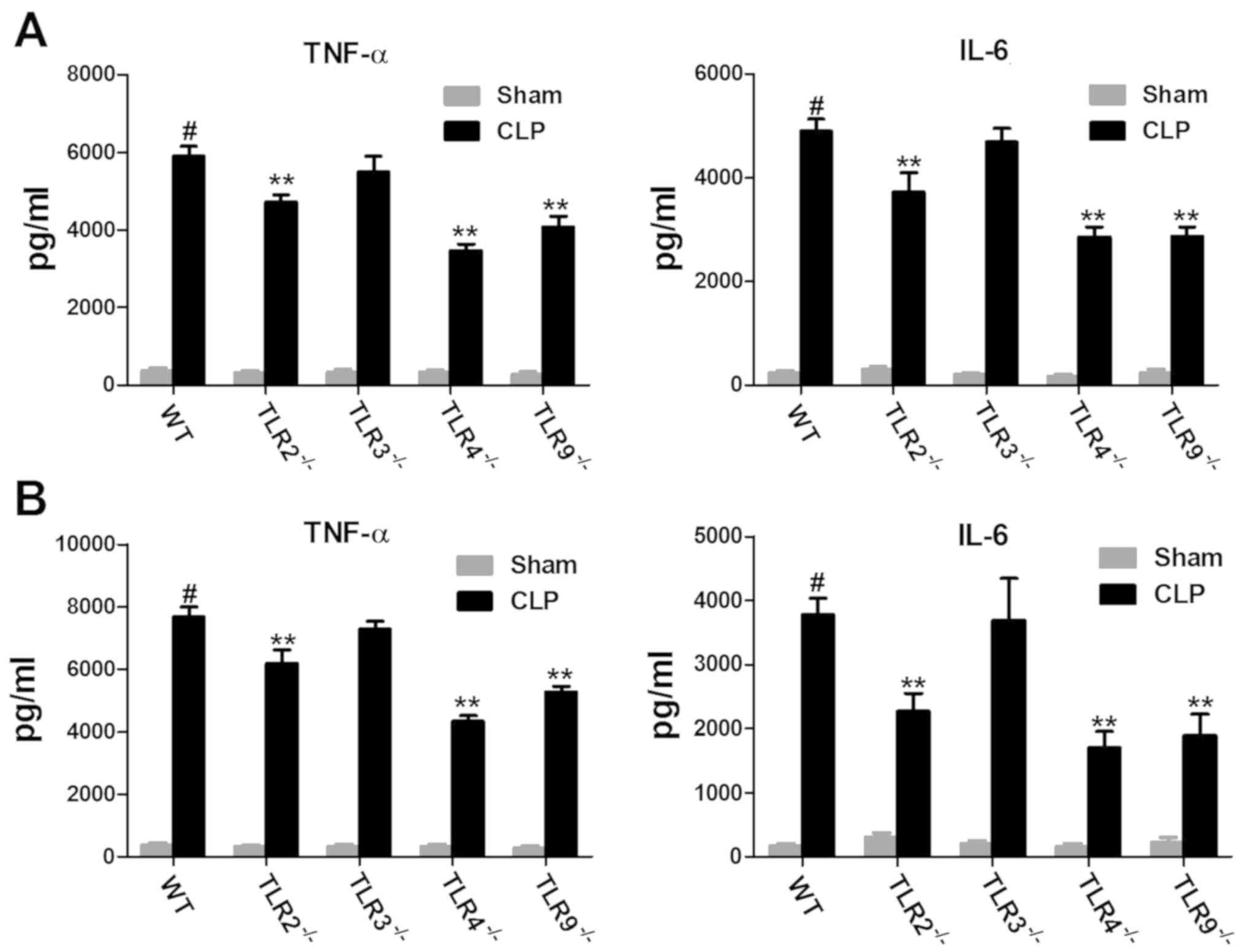
TLR2, 9 and 4 increased cytokine
production in CLP-induced ALI
To investigate the role of TLRs in the inflammatory
response in CLP-induced ALI, the inflammatory response in
TLR-deficient and WT mice was examined. Mouse plasma and lung
tissue samples were analysed for TNF-α and IL-6 expression. The
expression levels of TNF-α and IL-6 in the plasma of CLP-induced
ALI WT mice were significantly increased compared with the
sham-operated mice (Fig. 2A and B).
In addition, the expression levels of TNF-α and IL-6 in the plasma
of CLP-induced ALI TLR-deficient mice were markedly increased
compared with the sham-operated mice (Fig. 2A and B). However, the expression
levels of TNF-α and IL-6 in the plasma of CLP-induced ALI in TLR2-,
4- and 9-deficient mice were significantly decreased compared with
CLP-induced ALI WT mice. Furthermore, there was no difference in
the expression levels of TNF-α and IL-6 in the plasma of
CLP-induced ALI in TLR3-deficient mice compared with CLP-induced
ALI WT mice. Similarly, the trend in the expression levels of TNF-α
and IL-6 in plasma was also observed in lung tissue samples
(Fig. 2B). These results suggest
that some TLRs including TLR2, 4 and 9 may increase CLP-induced
cytokine production and aggravate ALI, however TLR3 may not.
Knockdown of TLR2, 4 or 9 may
attenuate lung injury
The main features associated with CLP-induced lung
injury are hypoxemia, immune cell infiltration, as well as
interstitial and alveolar edema. In the CLP-induced ALI groups, the
total BALF cell count, including, neutrophils and macrophages as
well as protein concentration were increased compared with the sham
group (Fig. 3A-D). However, compared
with the CLP-induced ALI WT mice, the total BALF cell count, as
well as BALF neutrophil and macrophage counts were significantly
decreased in the CLP-induced ALI TLR2-, 4- and 9-deficient mice
(Fig. 3A-C). In addition, the BALF
protein concentration was significantly decreased in the
CLP-induced ALI TLR2-, 4- and 9-deficient mice compared with the
CLP-induced ALI WT mice (Fig.
3D).
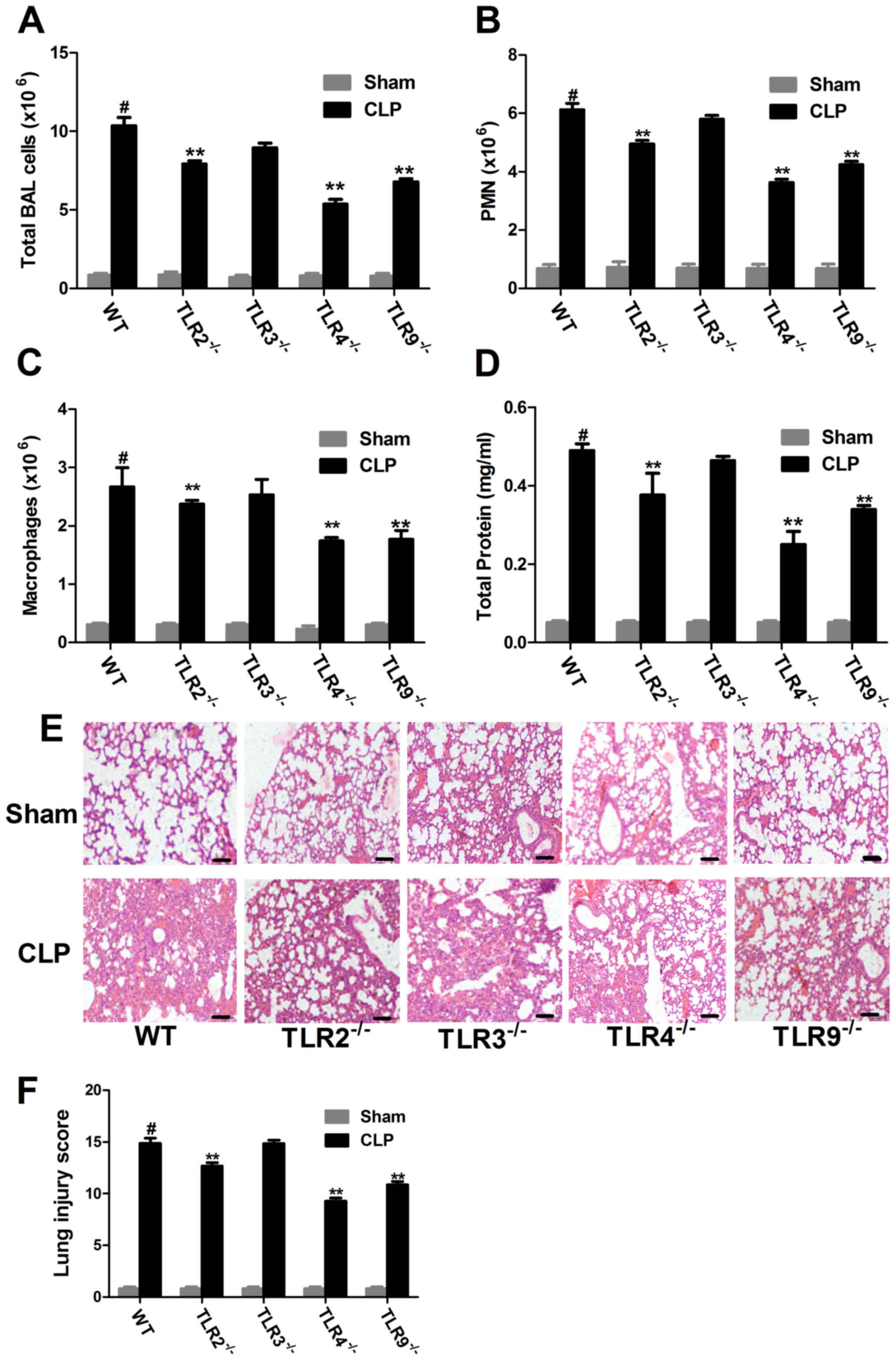 | Figure 3.Immune cell infiltration and lung
injury in CLP-induced ALI in WT and TLR−/− mice. BALF
samples were collected from WT and TLR−/− mice 20-h
post-surgery and the (A) total, (B) PMN and (C) macrophage count
was examined in each group. (D) BALF protein concentration was
examined. (E) Morphological changes were observed following H&E
staining in lung tissue sections (magnification, ×200). Scale bar,
200 µm. Data from at least three independent experiments. (F)
CLP-induced lung injury scores were examined. Data are presented as
the mean ± standard error of the mean (n=5). #P<0.05
vs. the WT sham group; **P<0.01 vs. WT CLP group. CLP, cecal
ligation and puncture; ALI, acute lung injury; WT, wild-type; TLR,
Toll-like receptor; PMN, polymorphonuclear cells; BALF,
bronchoalveolar lavage fluid; H&E, hematoxylin and eosin. |
H&E stained lung sections demonstrated that
there were a large number of infiltrating inflammatory cells in the
CLP-induced ALI WT group compared with the sham group (Fig. 3E). In addition, the lung injury score
was significantly increased in the CLP-induced ALI WT group
compared with the sham group (Fig.
3F). However, the number of infiltrating inflammatory cells
decreased in the CLP-induced ALI TLR2-, 4- and 9-deficient mice,
with a significantly decreased lung injury score compared with the
CLP-induced ALI WT group (Fig. 3E and
F). By contrast, the pathological injury in the CLP-induced ALI
TLR3-deficient mice was not improved, and there was no statistical
difference in the lung injury score compared with the CLP-induced
ALI group (Fig. 3E and F). Taken
together, these results suggest that knockdown of TLR2 and 9, and
especially TLR4, may attenuate lung injury in CLP-induced ALI.
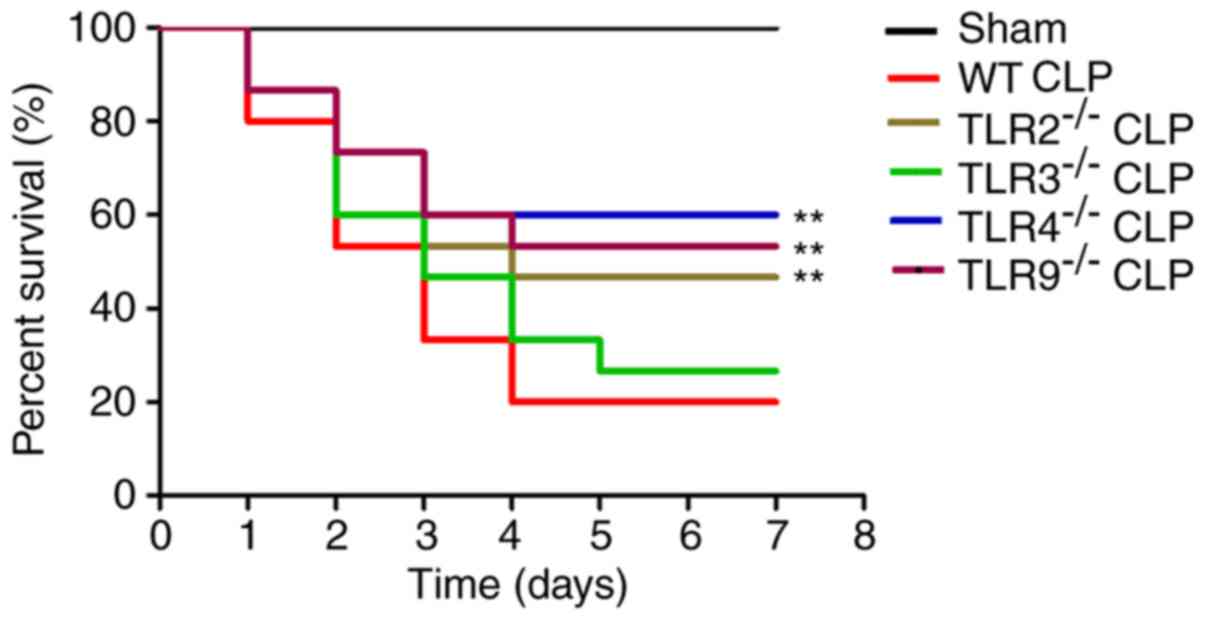
TLR2, 4 and 9 expression increased the
mortality of CLP-induced ALI mice
To investigate the effect of TLRs on the mortality
in CLP-induced ALI, overall survival was examined in TLR-deficient
and WT mice. Overall survival was significantly decreased in the
CLP-induced ALI WT group compared with the sham-operated group;
however, the overall survival was significantly increased in the
CLP-induced ALI TLR2-, 4- and 9-deficient mice compared with the
CLP-induced ALI WT mice (Fig. 4).
There was no statistically significant difference between the
CLP-induced ALI TLR3-deficient mice and the CLP-induced ALI WT
mice. These results suggest that TLR2, 4 and 9 increased mortality
in the CLP-induced ALI mice.
Discussion
Although activation of TLRs is essential to the
innate immune system and serve a role in the host defensive
mechanism against invading microorganisms, overactivation of TLRs
is involved in the pathogenesis of several inflammatory diseases
(28). Acute respiratory distress
syndrome (ARDS) is the most severe form of ALI, which is caused by
a severe systemic inflammatory response due to several risk factors
including sepsis, major surgery and trauma (29). Severe infection and major trauma
induces the systemic release of inflammatory mediators and
subsequent indirect lung injury (30).
The innate immune response provides protection
against invading pathogens and relies on PRRs to recognize
conserved microbial motifs or PAMPs (31). TLRs were one of the first major
families of PRRs discovered in mammals, and are expressed on immune
cells, including DCs, macrophages and B cells, as well as specific
non-immune cells, which include endothelial and epithelial cells
(32). In addition, TLRs 1–10 are
mainly expressed in lung tissue (33) and individual TLRs can be
differentially regulated in specific lung cell populations in
response to microbial stimulation (34). The activation of specific TLRs can
lead to the expression of several proinflammatory cytokines,
including TNF-α, IL-6, IL-12 and IFNs (35).
Although a previous study has investigated the
relationship between TLRs and sepsis or ALI, the current study is
the first to examine multiple TLRs simultaneously and compare their
underlying role in sepsis-induced ALI (36). The current study established a
CLP-induced ALI mouse model to examine the effect of four TLRs,
which included TLR2, 3, 4 and 9, on sepsis-induced ALI. The CLP
sepsis model is currently regarded as the gold standard for
sepsis-related studies because of its high stability, good
repeatability and wide applicability (37). The current study used the CLP-induced
ALI mouse model to examine cellular changes in TLR2, 3, 4 and 9
mRNA and protein expression in the process of pulmonary acute
inflammatory response. The results of the current study
demonstrated that the mRNA and protein expression levels of TLR2, 4
and 9 were significantly increased in the CLP-operated group
compared with the sham-operated group, and activation of these TLRs
also increased cytokine production and mortality in CLP-induced ALI
mice. However, the mRNA and expression protein levels of TLR3 were
not significantly affected. Taken together, these results suggest
that TLRs 2, 4 and 9 may be involved in the pathogenesis of ALI. In
addition, knockdown of TLR2 and 9, and especially TLR4, may
attenuate lung injury. In the current study, TLR2−/−,
3−/−, 4−/− and 9−/− mice were used
to demonstrate that knockdown of TLR2, 4 and 9 significantly
increased the expression levels of TNF-α and IL-6, while knockdown
of TLR3 significantly decreased the expression levels of TNF-α and
IL-6. In addition, the number of infiltrating inflammatory cells
decreased in TLR2−/−, 4−/− and
9−/− CLP-induced ALI mice, with a significantly
decreased lung injury score compared with the CLP-induced ALI WT
mice. Furthermore, the overall survival was significantly increased
in TLR2−/−, 4−/− and 9−/−
CLP-induced ALI mice compared with the WT CLP-induced ALI mice.
ALI is a major cause of morbidity and mortality in
intensive care units, despite improvements in supportive care
(6,38). Inhibition of the TLR signalling
pathways is likely to be an effective therapeutic target for
ALI/ARDS. There are several TLR antagonists, most of which can only
inhibit the activation of a single TLR. In addition, current
treatment strategies have a limited effect on improving overall
survival in patients with ALI/ARDS (39–41).
Therefore, novel therapeutic targets, which inhibit the activation
of multiple TLRs simultaneously, need to be investigated. The
current study is the first to examine multiple TLRs simultaneously
and compare their underlying role in sepsis-induced ALI, which may
be used to improve the screening of potential therapeutic
targets.
In conclusion, the current study demonstrated that
the mRNA and protein expression levels of TLR2, 4 and 9 were
significantly increased in lung tissue samples of CLP-induced ALI
mice. Furthermore, multiple TLRs, including TLR2 and 9, and
especially TLR4, significantly increased CLP-induced cytokine
production and aggravated ALI. The current study demonstrated that
knockdown of TLR2, 4 or 9 may attenuate lung injury and overall
survival was significantly increased in TLR2−/−,
4−/− and 9−/− CLP-induced ALI mice compared
with the WT CLP-induced ALI mice. Taken together, these results
suggest that multiple TLRs may contribute to the pathogenesis of
sepsis-induced ALI. Therefore, inhibition of multiple TLRs
including TLR2, 9, and especially TLR4 simultaneously, but not
TLR3, may be a potential therapeutic target for the treatment of
sepsis-induced ALI/ARDS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and XL conceived the project. XC and TW designed
the experiments. XC, TW and LS performed the experiments. XC
analysed the data. XC and XL prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the National and Institutional Guidelines for
Animal Care and Use and approved by the Institutional Animal Ethics
Committee of Shandong University (Shandong, China).
Patent consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seeley EJ, Matthay MA and Wolters PJ:
Inflection points in sepsis biology: From local defense to systemic
organ injury. Am J Physiol Lung Cell Mol Physiol. 303:L355–L363.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villar J, Sulemanji D and Kacmarek RM: The
acute respiratory distress syndrome: Incidence and mortality, has
it changed? Curr Opin Crit Care. 20:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: Pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuk HJ, Lee JW, Park HA, Kwon OK, Seo KH,
Ahn KS, Oh SR and Ryu HW: Protective effects of coumestrol on
lipopolysaccharide-induced acute lung injury via the inhibition of
proinflammatory mediators and NF-κB activation. J Funct Foods.
34:181–188. 2017. View Article : Google Scholar
|
|
5
|
Miyashita T, Ahmed AK, Nakanuma S, Okamoto
K, Sakai S, Kinoshita J, Makino I, Nakamura K, Hayashi H, Oyama K,
et al: A Three-phase Approach for the Early Identification of Acute
Lung Injury Induced by Severe Sepsis. In Vivo. 30:341–349.
2016.PubMed/NCBI
|
|
6
|
Butt Y, Kurdowska A and Allen TC: Acute
Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beutler BA: TLRs and innate immunity.
Blood. 113:1399–1407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cognasse F, Nguyen KA, Damien P, McNicol
A, Pozzetto B, Hamzeh-Cognasse H and Garraud O: The Inflammatory
Role of Platelets via Their TLRs and Siglec Receptors. Front
Immunol. 6:832015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akira S and Hemmi H: Recognition of
pathogen-associated molecular patterns by TLR family. Immunol Lett.
85:85–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mukherjee S, Karmakar S and Babu SP: TLR2
and TLR4 mediated host immune responses in major infectious
diseases: A review. Braz J Infect Dis. 20:193–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chattopadhyay S and Sen GC:
dsRNA-activation of TLR3 and RLR signaling: Gene
induction-dependent and independent effects. J Interferon Cytokine
Res. 34:427–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jelinek I, Leonard JN, Price GE, Brown KN,
Meyer-Manlapat A, Goldsmith PK, Wang Y, Venzon D, Epstein SL and
Segal DM: TLR3-specific double-stranded RNA oligonucleotide
adjuvants induce dendritic cell cross-presentation, CTL responses,
and antiviral protection. J Immunol. 186:2422–2429. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vollmer J and Krieg AM: Immunotherapeutic
applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug
Deliv Rev. 61:195–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Shea JJ and Murray PJ: Cytokine
signaling modules in inflammatory responses. Immunity. 28:477–487.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan RST, Ho B, Leung BP and Ding JL: TLR
cross-talk confers specificity to innate immunity. Int Rev Immunol.
33:443–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rai V and Agrawal DK: The role of damage-
and pathogen-associated molecular patterns in inflammation-mediated
vulnerability of atherosclerotic plaques. Can J Physiol Pharmacol.
95:1245–1253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matute-Bello G, Frevert CW and Martin TR:
Animal models of acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 295:L379–L399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walley KR, Lukacs NW, Standiford TJ,
Strieter RM and Kunkel SL: Balance of inflammatory cytokines
related to severity and mortality of murine sepsis. Infect Immun.
64:4733–4738. 1996.PubMed/NCBI
|
|
21
|
Altemeier WA, Hung CF and Matute-Bello G:
Mouse Models of Acute Lung Injury. In: Acute Lung Injury and
Repair. Respiratory Medicine. Schnapp L and Feghali-Bostwick C
(eds). Humana Press; Cham: pp. 5–23. 2017
|
|
22
|
Dejager L, Pinheiro I, Dejonckheere E and
Libert C: Cecal ligation and puncture: The gold standard model for
polymicrobial sepsis? Trends Microbiol. 19:198–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhan J, Liu Y, Zhang Z, Chen C, Chen K and
Wang Y: Effect of penehyclidine hydrochloride on expressions of
MAPK in mice with CLP-induced acute lung injury. Mol Biol Rep.
38:1909–1914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiang M and Fan J: Pattern recognition
receptor-dependent mechanisms of acute lung injury. Mol Med.
16:69–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhandari V, Choo-Wing R, Lee CG, Zhu Z,
Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, et
al: Hyperoxia causes angiopoietin 2-mediated acute lung injury and
necrotic cell death. Nat Med. 12:1286–1293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cartwright M, Rottman M, Shapiro NI,
Seiler B, Lombardo P, Gamini N, Tomolonis J, Watters AL, Waterhouse
A, Leslie D, et al: A Broad-Spectrum Infection Diagnostic that
Detects Pathogen-Associated Molecular Patterns (PAMPs) in Whole
Blood. EBioMedicine. 9:217–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Achek A, Yesudhas D and Choi S: Toll-like
receptors: Promising therapeutic targets for inflammatory diseases.
Arch Pharm Res. 39:1032–1049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim WY and Hong SB: Sepsis and Acute
Respiratory Distress Syndrome: Recent Update. Tuberc Respir Dis
(Seoul). 79:53–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torun AC, Tutuncu S, Ustun B and Akdemir
HU: A Study of the Therapeutic Effects of Resveratrol on Blunt
Chest Trauma-Induced Acute Lung Injury in Rats and the Potential
Role of Endocan as a Biomarker of Inflammation. Inflammation.
40:1803–1810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Q, He G, Wang J, Wang Y, Chen W and
Guo T: Down-regulation of toll-like receptor 4 alleviates
intestinal ischemia reperfusion injury and acute lung injury in
mice. Oncotarget. 8:13678–13689. 2017.PubMed/NCBI
|
|
32
|
Medvedev AE, Sabroe I, Hasday JD and Vogel
SN: Tolerance to microbial TLR ligands: Molecular mechanisms and
relevance to disease. J Endotoxin Res. 12:133–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zarember KA and Godowski PJ: Tissue
expression of human Toll-like receptors and differential regulation
of Toll-like receptor mRNAs in leukocytes in response to microbes,
their products, and cytokines. J Immunol. 168:554–561. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang CS, Lee JS, Rodgers M, Min CK, Lee
JY, Kim HJ, Lee KH, Kim CJ, Oh B, Zandi E, et al: Autophagy protein
Rubicon mediates phagocytic NADPH oxidase activation in response to
microbial infection or TLR stimulation. Cell Host Microbe.
11:264–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chalifour A, Jeannin P, Gauchat JF,
Blaecke A, Malissard M, N'Guyen T, Thieblemont N and Delneste Y:
Direct bacterial protein PAMP recognition by human NK cells
involves TLRs and triggers alpha-defensin production. Blood.
104:1778–1783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu M, Shao D, Liu J, Zhu J, Zhang Z and Xu
J: Effects of ketamine on levels of cytokines, NF-kappaB and TLRs
in rat intestine during CLP-induced sepsis. Int Immunopharmacol.
7:1076–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng D, Li X, Liu C, Zhai Z, Li B, Kuang
M, Li P, Shang S, Song Y, Cen Y, et al: Systematic investigation on
the turning point of over-inflammation to immunosuppression in CLP
mice model and their characteristics. Int Immunopharmacol.
42:49–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou D, Qiu J, Liang Y, Dai W, Yuan D and
Zhou J: Epidemiological analysis of 9,596 patients with acute lung
injury at Chinese Military Hospitals. Exp Ther Med. 13:983–988.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Creagh-Brown BC, Quinlan GJ, Evans TW and
Burke-Gaffney A: The RAGE axis in systemic inflammation, acute lung
injury and myocardial dysfunction: An important therapeutic target?
Intensive Care Med. 36:1644–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Devaney J, Contreras M and Laffey JG:
Clinical review: gene-based therapies for ALI/ARDS: where are we
now? Crit Care. 15:224–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Staples KJ: Editorial: Therapeutics for
acute lung injury: time to call in the DRs? J Leukoc Biol.
101:351–353. 2017. View Article : Google Scholar : PubMed/NCBI
|