Introduction
Glioblastoma (GBM) is the most commonly diagnosed
type of malignant primary brain tumor (1). GBM is a high-grade tumor (World Health
Organization grade IV), and prognosis of patients with GBM is
relatively poor compared with other types of brain cancer (2). The median overall survival time is
<1 year following diagnosis and the median progression-free
survival is ~6 months (3,4). Conventional therapeutic approaches for
patients with GBM include chemotherapy, radiotherapy and surgical
resection (5). Unfortunately,
current treatments are unsatisfactory due to resistance development
and cancer recurrence (6). Decades
of study on the molecular mechanism of GBM have greatly enhanced
our knowledge of GBM pathogenesis and provided new insights for
development of targeted therapy (7).
For example, it has been demonstrated that GBM cells with strong
migration potential exhibit pro-apoptotic stimuli resistance
(8) with several publications
confirming that inhibitors that target migration-related molecules
are effective methods to overcome resistance (9,10).
Therefore, further investigation into the molecular mechanism of
GBM is still urgently required to meet clinical needs.
MicroRNAs (miRNAs) are a class of small non-coding
RNAs first discovered in Caenorhabditis elegans (11). In many eukaryotic cells, miRNAs
function as negative regulators of gene expression via directly
binding to sites on the 3′untranslated region (UTR) of mRNAs
(12). Dysregulation of miRNAs is
implicated in the initiation and development of various types of
cancer including GBM (13). Several
miRNAs have been identified as oncogenes or tumor suppressors in
GBM (14,15). In addition, expression of certain
miRNAs is a promising predictor of patient GBM outcome and therapy
response (16,17). miR-485-3p is mapped to the 14q32.31
chromosome region where mutations are frequently observed in
cancers, which suggests that miR-485-3p might demonstrate tumor
suppressor potential (18). A recent
study determined that levels of miR-485-3p in serum could predict
prognosis of patients with GBM (19). The focus of the present study was
identifying the role and molecular mechanism of miR-485-3p in GBM
cells.
Ring finger protein 135 (RNF135) is a RING finger
domain-containing E3 ubiquitin ligase which has a critical role in
many cellular biological processes via regulating protein
degradation (20). For example,
during viral infection, RNF135 ubiquitinates retinoic
acid-inducible gene I to promote interferon-β induction (21). Aberrant RNF135 expression results in
altered expression of genes leading to several diseases (22,23). In
GBM, RNF135 functions as an oncogene through activating the
mitogen-activated protein kinase (MAPK)/ERK pathway and controlling
expression of key cell cycle regulators, such as cyclin dependent
kinase 4, cyclin dependent kinase inhibitor 1A, cyclin dependent
kinase inhibitor 1B (24). However,
how RNF135 is regulated in GBM cells remains unknown; therefore,
the present study aimed to determine the underlying mechanism.
Materials and methods
Collection of patient tissue
A total of 20 GBM and 20 normal tissues were
collected at the Cancer Hospital of China Medical University
between October 2014 to January 2017. The patients with GBM (15
males; 5 females; age, 25–48 years old) had undergone surgery, and
patients with severe traumatic brain injury (13 males; 7 females;
age, 23–56 years old) underwent internal decompression surgery.
None of the patients sampled had undergone chemotherapy or
radiotherapy prior to having surgery. Fresh tissue samples were
histopathologically examined then immediately stored at −80°C prior
to RNA extraction. All patients provided written consent and the
study was conducted under the supervision of the Ethics Committee
of Cancer Hospital of China Medical University.
Bioinformatic analysis
Data for miR-485-3p expression levels in 5 normal
tissues and 82 GBM tissues were downloaded from GSE25631 in the
Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/). The comparison of
miR-485-3p expression between normal and GBM tissues was conducted
using GraphPad Prism v7.05 (GraphPad Software, Inc.).
U251-MG cell culture
The GBM cell line U251-MG was purchased from the
American Type Culture Collection. The cells were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS; Hyclone;
GE Healthcare Life Sciences) and 1% penicillin-streptomycin
solution in a 37°C humidified incubator with 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
miRNeasy Mini kit (Qiagen, Inc.) was used to extract
total RNA from patient tissues and U251-MG cells, following the
manufacturer's instructions. The concentration and quality of
extracted RNA was measured using NanoDrop 2000 (Thermo Fisher
Scientific, Inc.). TransScript First-Strand cDNA Synthesis SuperMix
(Transgen Biotech Co. Ltd.) was used to reverse transcribe RNA into
cDNA, according to the manufacturer's protocol. qPCR was performed
using SYBR Premix Ex Taq (Takara Bio, Inc.) on a CFX96 Touch™
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). The
thermocycling conditions were: Predenaturing step at 95°C for 30
sec, followed by denaturing step at 95°C for 5 sec then annealing
and elongation at 60°C for 30 sec, repeated for 35 cycles. Gene
expression was quantified using the 2−ΔΔCq method
(25). The stem-loop method was used
to detect miRNA expression. U6 and GAPDH were used as internal
controls for miRNA and mRNA, respectively. Sequences of primers are
listed in Table I.
 | Table I.List of the primer sequences. |
Table I.
List of the primer sequences.
| Primer | Sequence
(5′-3′) |
|---|
| Stem-loop
primer |
CTCAACTGGTGTCGTGGAGTCGG |
|
|
CAATTCAGTTGAGAGAGAGG |
| miR-485-3p F |
GCCGAGGUCAUACACGGCUCU |
| miR-485-3p R |
CTCAACTGGTGTCGTGGA |
| RNF135 F |
CTGCGGAAGAACACGCTACT |
| RNF135 R |
GCTCAGTTCGTTGTCTGGTCC |
| U6 F |
CTCGCTTCGGCAGCACA |
| U6 R |
AACGCTTCACGAATTTGCGT |
| GAPDH F |
CCACTCCTCCACCTTTGAC |
| GAPDH R |
ACCCTGTTGCTGTAGCCA |
Overexpression of miR-485-3p
miR-485-3p mimic and miR-negative control (NC) mimic
were synthesized and purchased from Guangzhou RiboBio Co., Ltd. For
the overexpression of miR-485-3p, 10 µl miR-485-3p mimic (50
pmol/µl) was incubated with Lipofectamine 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in serum-free medium for 15 min and then
added to each well in 96-well plates or 6-well plates.
Experimentation commenced 48 h following transfection. The
miR-485-3p mimic sequence was 5′-GUCAUACACGGCUCUCCUCUCU-3′ and the
miR-NC mimic sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′.
Western blot analysis
Cell lysates were prepared using
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology). The concentration of protein lysate was determined
with Pierce Bicinchoninic Acid Protein Assay kit (Thermo Fisher
Scientific, Inc.). A total of 20 µg protein were loaded per lane
and separated via SDS-PAGE on an 8% gel. The separated proteins
were then transferred to polyvinylidene difluoride membranes and
subsequently blocked with 5% non-fat milk at room temperature for 1
h. Then membranes were incubated with primary antibodies
anti-RNF135 (cat. no. ab28636; 1:1,000; Abcam), anti-GAPDH (cat.
no. G8795; 1:8,000; Sigma-Aldrich; Merck KGaA), ERK1/2 (cat. no.
4695; 1:1,000; Cell Signaling Technology, Inc.) and phosphorylated
(p-) ERK1/2 (cat. no. 9101; 1:1,000; Cell Signaling Technology,
Inc.) at 4°C overnight. Following primary incubation, membranes
were incubated with horseradish peroxidase-labeled secondary mouse
antibody (cat. no. AP308P; 1:10,000; Sigma-Aldrich; Merck KGaA) and
rabbit antibody (cat. no. SAB3700852; 1:10,000; Sigma-Aldrich;
Merck KGaA) at room temperature for 1 h. Subsequently, the bands
were visualized using enhanced chemiluminescence western blotting
detection reagents (GE Healthcare Life Sciences). Images were
captured and analyzed using ImageQuant TL 7.0 (GE Healthcare Life
Sciences). GAPDH served as a loading control for protein
quantification.
Dual luciferase reporter assay
To investigate whether miR-485-3p directly regulated
RNF135, bioinformatic analysis (TargetScan V7.2; www.targetscan.org) was performed to compare the
miR-485-3p sequence with the 3UTR of RNF135 mRNA. Dual luciferase
assay was performed using the Dual-Luciferase® Reporter
Assay System (Promega Corporation). U251-MG cDNA was prepared by
isolating RNA from U251-MG cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) followed by reverse-transcription
into first-strand cDNA using RevertAid RT Reverse Transcription kit
(Thermo Fisher Scientific, Inc.) according to manufacturer's
protocol. The 3UTR containing putative binding sites of miR-485-3p
on RNF135 mRNA was amplified from the U251-MG cDNA and ligated into
pGL3-basic vector (Addgene, Inc.) using XbaI enzyme (New England
Biolabs, Inc.). The primer sequences of the RNF135 mRNA were:
forward, 5′-CTCTAGACCTATCGCTGGAGCTGTGAG-3′ and reverse,
5′-CTCTAGAAGGAATTCGACACCAGCCTG-3′. 3UTR mutant (Mut) 1 and Mut 2
were constructed by introducing 2 site mutations into putative
binding site 1 and putative binding site 2 respectively with
Q5® Site-Directed Mutagenesis kit (New England BioLabs,
Inc.). In brief, U251-MG cells were cultured in 24-well plates and
co-transfected with miR-485-3p mimic or miR-NC mimic with
pGL3-RNF135 3′UTR-wild type (WT) or pGL3-RNF135 3′UTR-Mut 1 or
pGL3-RNF135 3′UTR-Mut 2 and pRL-TK vector (Promega Cooperation)
using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.). Following 48 h, cells were collected and the dual-luciferase
activity was examined using Dual Luciferase Reporter Assay system
(Promega Cooperation) following the manufacturer's protocol with
Renilla luciferase as the internal control.
Construction of plasmid and
overexpression of RNF135
The full length RNF135 cDNA from U251-MG cells was
cloned into pcDNA3.1 plasmid (Addgene, Inc.) to construct the
pcDNA3.1-RNF135 plasmid. For overexpression of RNF135 with or
without overexpression of miR-485-3p, 2 µg pcDNA3.1-RNF135 and/or
10 µl miR-485-3p mimics (50 pmol/µl) were transfected into U251-MG
cells using Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol.
Cell proliferation assay
Cell proliferation was measured using Cell Counting
kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) according to
manufacturer's protocol. In brief, 2,000 U251-MG cells were seeded
into 96-well plates then incubated under standard conditions. The
next day, cells were transfected with pcDNA3.1-RNF135 with or
without miR-485-3p mimic. At 0, 24, 48 and 72 h post transfection,
10 µl CCK-8 solution was added to each well and the cells were
incubated for another 2 h at 37°C. The absorbance at 450 nm was
detected using a microplate reader (Bio-Rad Laboratories, Inc.) to
determine the number of cells.
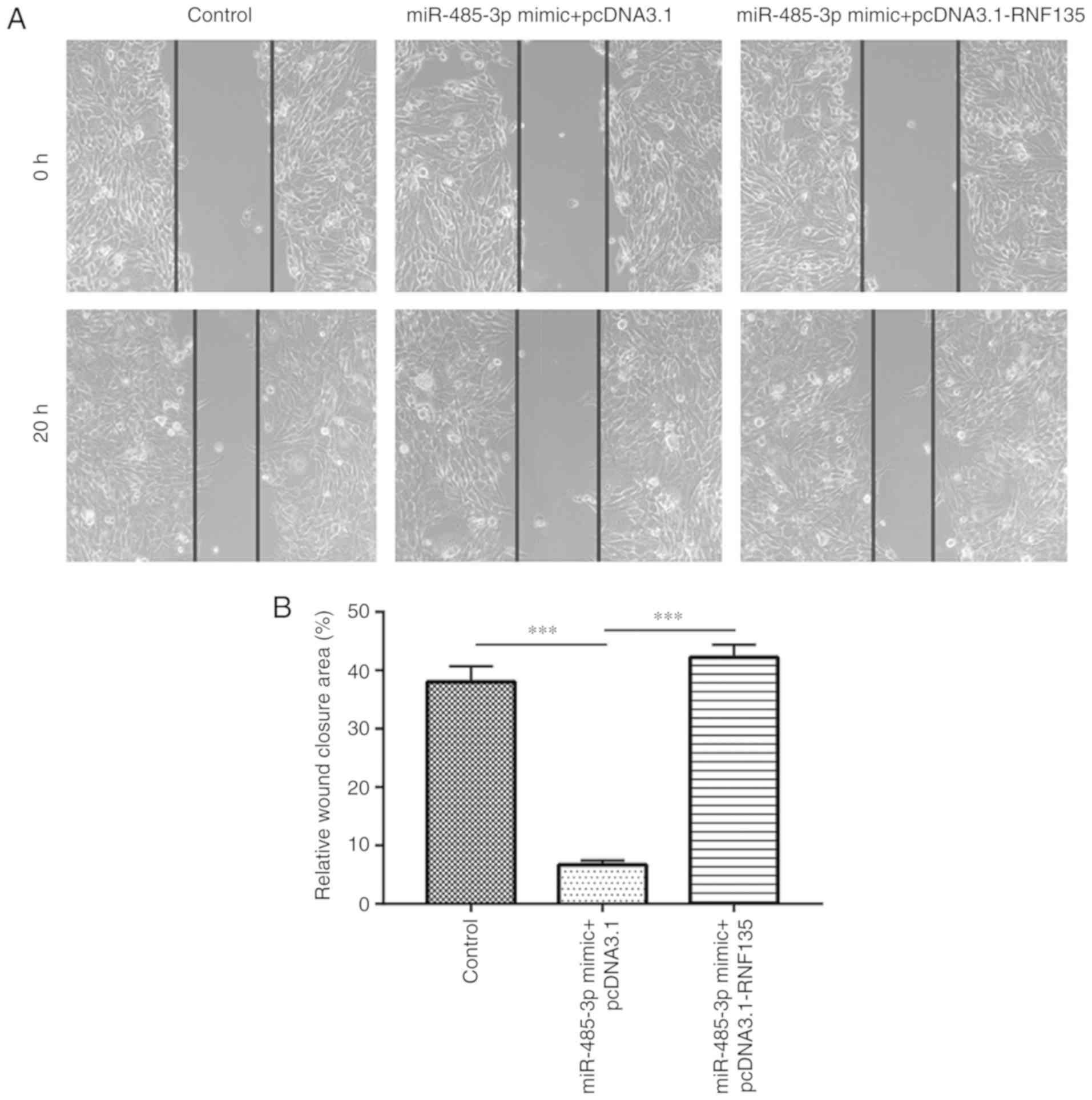
Cell migration assay
Cell migration ability was detected using a
wound-healing assay. U251-MG cells (1×106) were seeded
into 6-well plates and cultured under standard conditions until 90%
confluence. The cells were then transfected with miR-485-3p mimic
with or without pcDNA3.1-RNF135. The following day, a wound area
was made by scratching the center of each well with a 10 µl pipette
tip. The wells were washed with PBS then culture medium containing
1% FBS was added. At 0 and 20 h, images of the scratch area were
captured. Subsequently, the percentage of the relative wound
closure area was analyzed using Image Pro Plus 6 (Media
Cybernetics, Inc.).
Statistical analysis
All statistical analyses were carried out using
GraphPad Prism 7 software (GraphPad Software, Inc.). Data were
presented as the mean ± standard deviation. Student's t-test was
used to compare 2 groups and multiple groups were compared using
one-way analysis of variance followed by the Newman-Keuls post hoc
test. P<0.05 was considered to indicate statistical
significance.
Results
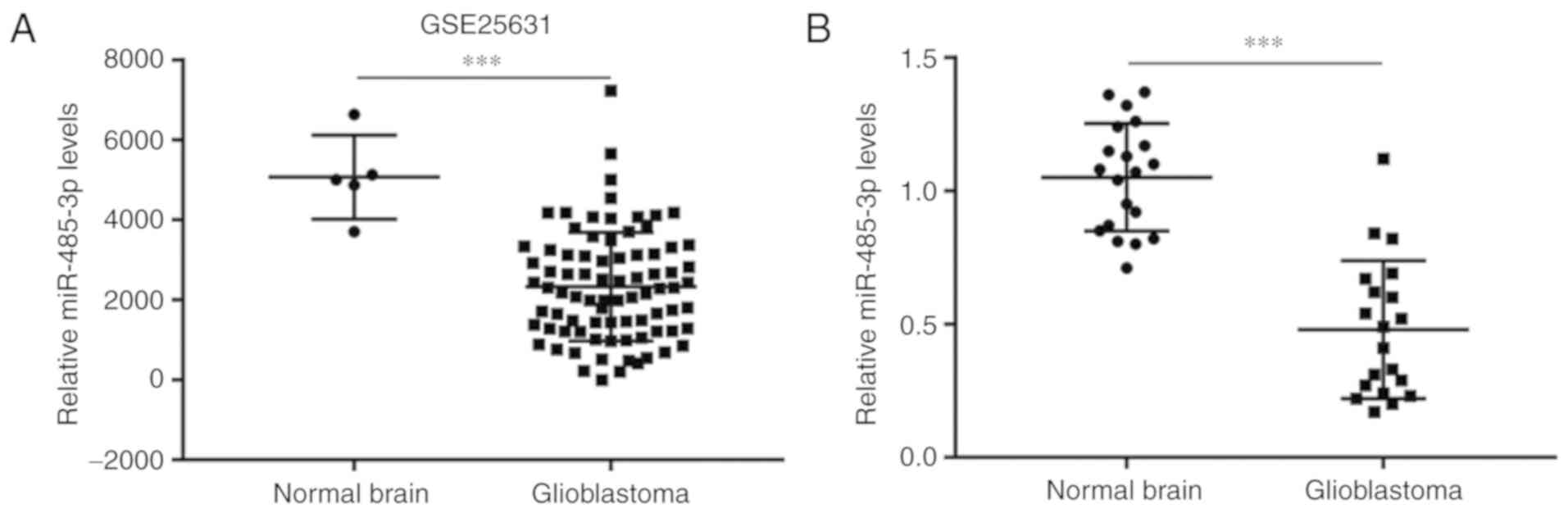
miR-485-3p is downregulated in GBM
compared with normal brain tissues
Decreased expression of miR-485-3p has been
identified in several cancer types (26,27)
including GBM (19). To evaluate the
expression of miR-485-3p in GBM tissues, miR-485-3p expression data
was downloaded from GSE25631 which contained miRNA microarray data
for 5 normal brain tissues and 82 GBM tissues. Compared with the
normal brain group, miR-485-3p levels were significantly decreased
in GBM tissues (Fig. 1A). To further
validate this finding, RT-qPCR was performed to compare miR-485-3p
expression in 20 GBM tissues and 20 normal brain tissues collected
at our institution. Significant downregulation of miR-485-3p was
detected in GBM tissues compared with normal brain tissues
(Fig. 1B), which suggested that
miR-485-3p may have a tumor suppressor role in GBM.
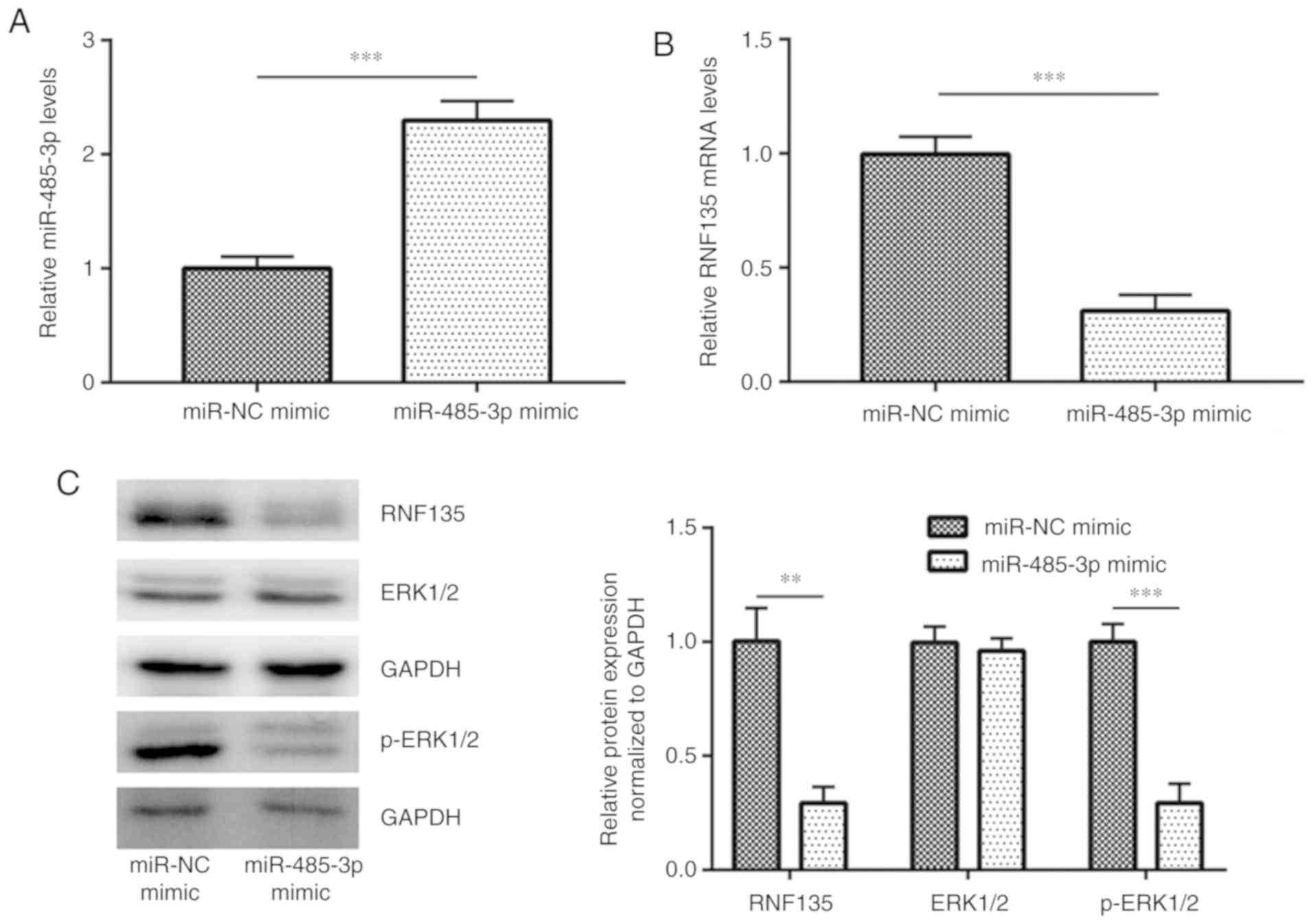
Overexpression of miR-485-3p decreases
RNF135 expression and inactivates the MAPK/ERK pathway in GBM
cells
RNF135 is a recently identified GBM oncogene
(24). Transfection of miR-485-3p
mimic increased miR-485-3p expression in U251-MG cells (Fig. 2A), accompanied with a decreased
expression of RNF135 mRNA (Fig. 2B).
Western blot analysis demonstrated that overexpression of
miR-485-3p also decreased RNF135 protein expression (Fig. 2C). Overactivation of MAPK/ERK
signaling promotes GBM progression, which is regulated by RNF135
(21). In addition to downregulation
of RNF135, overexpression of miR-485-3p also decreased p-ERK1/2
levels, indicating an inactivation of MAPK/ERK signaling (Fig. 2C). These results suggested that
miR-485-3p negatively regulated RNF135 to inhibit GBM cells.
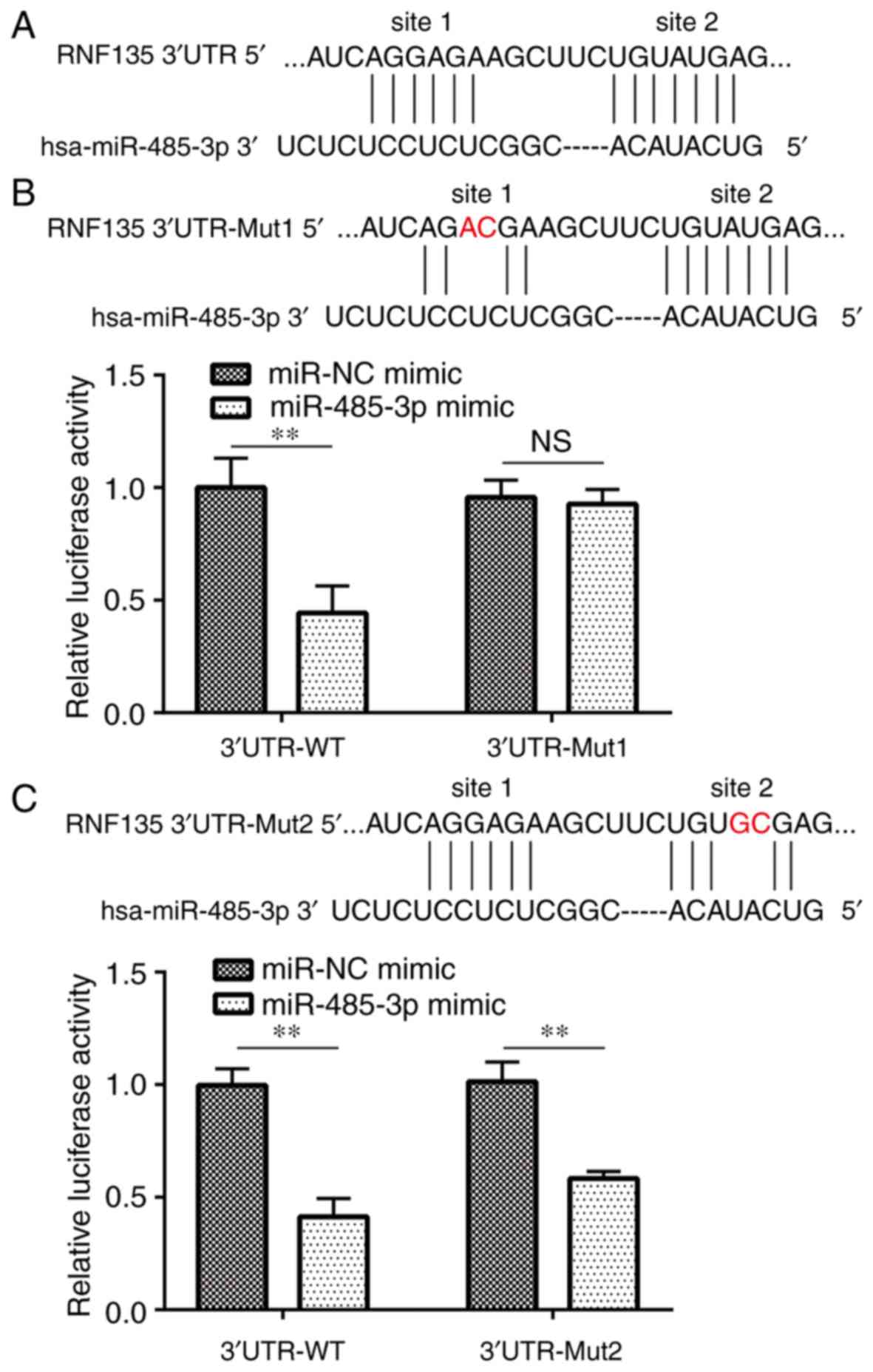
RNF135 is a target gene of
miR-485-3p
There were two putative binding sites between
miR-485-3p and 3UTR of RNF135 (Fig.
3A). Dual luciferase reporter assays demonstrated that
overexpression of miR-485-3p significantly decreased luciferase
activity of RNF135 3UTR-WT in U251-MG cells (Fig. 3B and C). Additionally, miR-485-3p
mimic induced decreased luciferase activity of RNF135 3UTR-Mut2 but
not RNF135 3UTR-Mut1, which suggested that site 1 was a direct
binding site for miR-485-3p on the 3UTR of RNF135 in GBM cells
(Fig. 3B and C).
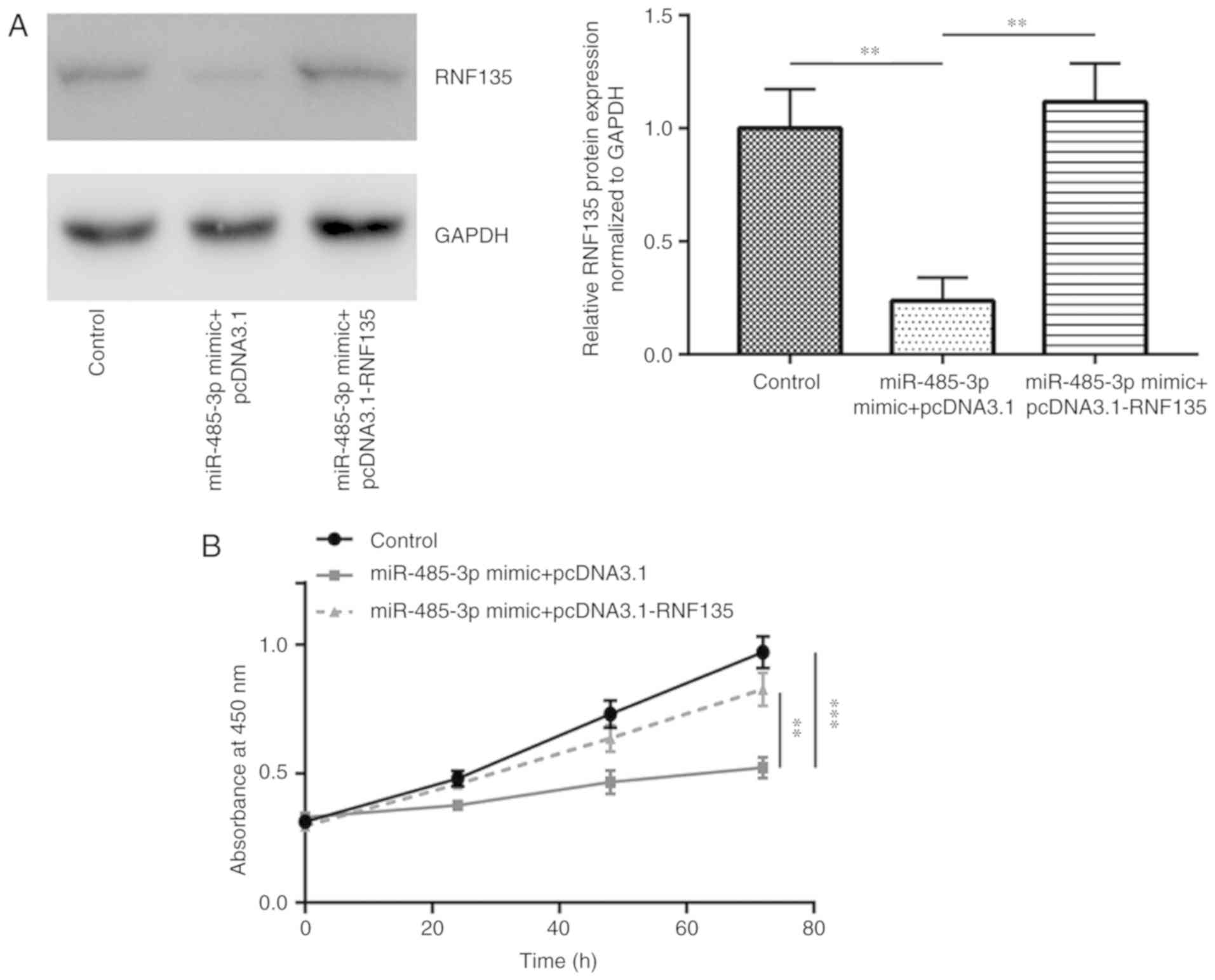
Overexpression of miR-485-3p inhibits
GBM cell proliferation and migration via targeting RNF135
Recombinant RNF135 was constructed and
co-transfected with miR-485-3p mimic to study the functions of
miR-485-3p/RNF135 in GBM cells. Transfection with miR-485-3p mimic
decreased RNF135 protein expression whereas co-transfection of
recombinant RNF135 and miR-485-3p mimic rescued RNF135 expression
in U251-MG cells (Fig. 4A). In the
cell proliferation assay, overexpression of miR-485-3p decreased
the cell viability compared with control, whilst overexpression of
RNF135 partially recovered the cell viability (Fig. 4B). These results suggested that
miR-485-3p inhibited GBM cell proliferation via repression of
RNF135. In the cell migration assay, overexpression of miR-485-3p
inhibited cell migration. Transfection with recombinant RNF135
attenuated this inhibitory effect (Fig.
5A and B). These results demonstrated that miR-485-3p inhibited
GBM cell proliferation and migration via repression of RNF135.
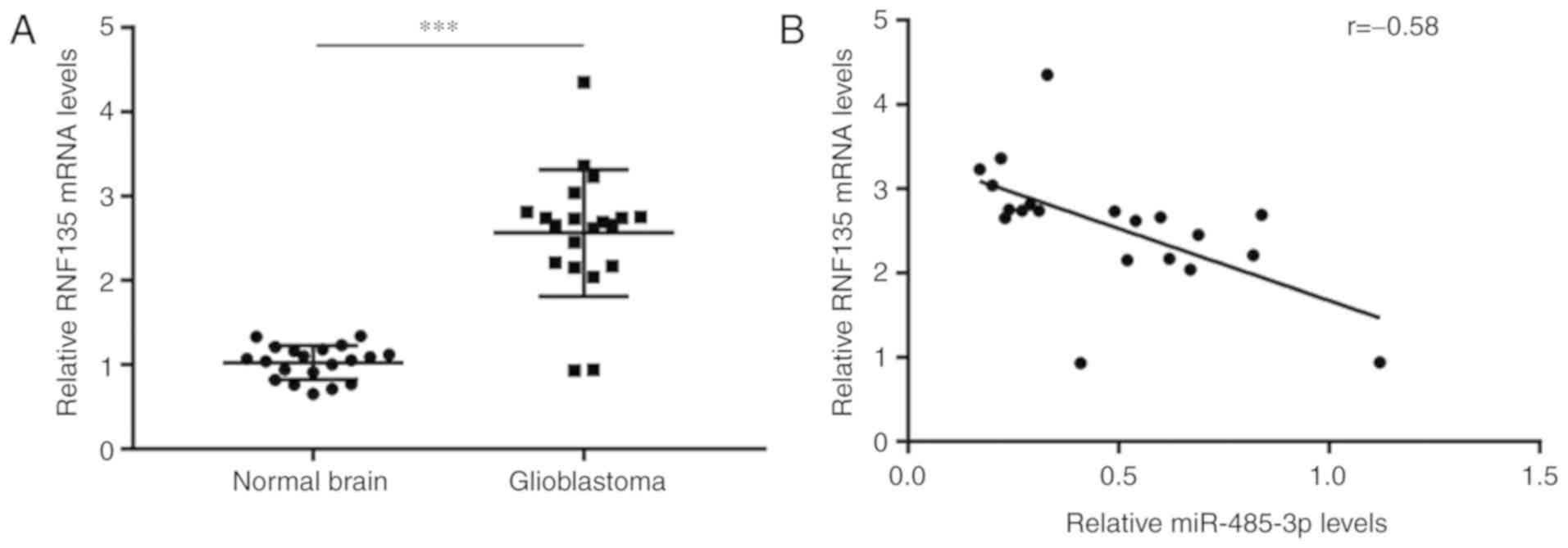
Expression of miR-485-3p is negatively
correlated with RNF135 in GBM tissues
Next, the potential association between miR-485-3p
and RNF135 in GBM tissues was investigated. RT-qPCR was performed
on the 20 GBM and 20 normal tissues collected in the present study,
to detect RNF135 mRNA levels. Significant upregulation of RNF135
mRNA levels were observed in GBM tissues compared with normal
tissues (Fig. 6A). Crucially,
Pearson correlation analysis demonstrated that miR-485-3p
expression was negatively correlated with RNF135 mRNA levels in GBM
tissues (r=−0.58; P<0.01; Fig.
6B).
Discussion
GBM is a lethal cancer type (28) and aberrant expression of miRNAs is
experimentally identified as a major step towards development of
the disease (29). miR-485-3p,
mapped to chromosome 14q32.31 region, is a well-characterized tumor
suppressor in several cancer types (30). Recently, miRNA microarray data of 14
GBM tissues identified miR-485-3p as one of the significantly
downregulated miRNAs in tissues from short-survival patients
compared with long-survival patients (31). Low expression of serum miR-485-3p
predicts poor overall survival in patients with GBM (19). The present study determined that
miR-485-3p was downregulated in GBM. RT-qPCR and western blot
analysis demonstrated that RNF-135 was negatively regulated by
miR-485-3p. Dual luciferase assay confirmed RNF135 as a target gene
of miR-485-3p. Functional assays indicated that miR-485-3p
inhibited cell proliferation and migration of GBM cells via
repression of RNF135. The present findings suggested that
miR-485-3p was a tumor suppressor in GBM.
In order to investigate the role of miR-485-3p in
GBM, analysis of miRNA expression in normal brain tissues and GBM
tissues from a previously published microarray dataset was
performed. Results determined that miR-485-3p expression was
significantly lower in GBM tissues compared with normal tissues. To
validate this result, the present study collected and analyzed 20
pairs of normal brain and GBM tissues. Therefore, in addition to
the reported decreased miR-485-3p expression in serum and tissues
of short-survival patients (19,31), the
present study identified miR-485-3p as a downregulated miRNA in GBM
tissues compared with normal brain tissues. In addition, functional
analysis of miR-485-3p demonstrated that miR-485-3p overexpression
inhibited cell proliferation and migration of GBM cells. GBM is
highly aggressive with cells displaying strong proliferative
capacity (28) therefore the current
findings suggested that miR-485-3p may be involved in the
development of GBM.
E3 ubiquitin ligases regulate turnover of many
target genes via protease degradation (32). In GBM, dysregulation of E3 ligases
results in accumulation of oncogenes or downregulation of tumor
suppressors, leading to GBM progression (33,34).
RNF135 is a member of E3 ubiquitin ligases and regulates protein
stability (24). High expression of
RNF135 is associated with poor prognosis for GBM patients (24). RNF135 activates the MAPK/ERK
signaling pathway and facilitates progression of the GBM cell cycle
to promote proliferation (24). The
present study identified that overexpression of miR-485-3p
decreased RNF135 expression in GBM cells. Bioinformatic analysis
and the dual luciferase reporter assay predicted and confirmed
RNF135 as a target gene of miR-485-3p. In GBM tissues, a
significant negative correlation between RNF135 mRNA and miR-485-3p
expression was identified. RNF135 function has been previously
reported to be regulated by mutation (20,35). The
present findings demonstrated that RNF135 was also regulated by
miRNA. Crucially, cell proliferation and migration inhibition
induced by miR-485-3p mimic was reversed by overexpression of
RNF135, which indicated that miR-485-3p exerted its tumor
suppressive function through RNF135.
In conclusion, the present study provided evidence
to suggest that miR-485-3p may have a role as a tumor suppressor in
GBM via regulation of RNF135.
Acknowledgements
Not applicable.
Funding
The study was funded by the Clinical Capability
Construction Project for Liaoning Provincial Hospitals (grant no.
LNCCC-B04-2015).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
Clinical sample collection was performed by JS and
HL. The study was designed by HP and RS. Data acquisition and
analysis were performed by HP, YZ and YC. The manuscript was
prepared, edited and reviewed by HP and JS.
Ethics approval and consent to
participate
All patients provided written informed consent and
the Ethic Committee of Cancer Hospital of China Medical University
approved the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wick W and Platten M: Understanding and
treating glioblastoma. Neurol Clin. 36:485–499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buckner JC: Factors influencing survival
in high-grade gliomas. Semin Oncol. 30:10–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das S and Marsden PA: Angiogenesis in
glioblastoma. N Engl J Med. 369:1561–1563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fine HA: The basis for current treatment
recommendations for malignant gliomas. J Neurooncol. 20:111–120.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wick W, Osswald M, Wick A and Winkler F:
Treatment of glioblastoma in adults. Ther Adv Neurol Disord.
11:17562864187904522018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen HS, Shabani S, Awad AJ, Kaushal M
and Doan N: Molecular markers of therapy-resistant glioblastoma and
potential strategy to combat resistance. Int J Mol Sci. 19(pii):
E17652018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osswald M, Jung E, Sahm F, Solecki G,
Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M,
et al: Brain tumour cells interconnect to a functional and
resistant network. Nature. 528:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lefranc F, Le Rhun E, Kiss R and Weller M:
Glioblastoma quo vadis: Will migration and invasiveness reemerge as
therapeutic targets? Cancer Treat Rev. 68:145–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onken J, Torka R, Korsing S, Radke J,
Krementeskaia I, Nieminen M, Bai X, Ullrich A, Heppner F and
Vajkoczy P: Inhibiting receptor tyrosine kinase AXL with small
molecule inhibitor BMS-777607 reduces glioblastoma growth,
migration and invasion in vitro and in vivo. Oncotarget.
9:9876–9889. 2016.
|
|
11
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer
A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J
and Califano A: An extensive microRNA-mediated network of RNA-RNA
interactions regulates established oncogenic pathways in
glioblastoma. Cell. 147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue S, Wang L, Zhang H, Min Y, Lou Y, Sun
H, Jiang Y, Zhang W, Liang A, Guo Y, et al: miR-139-5p suppresses
cancer cell migration and invasion through targeting ZEB1 and ZEB2
in GBM. Tumour Biol. 36:6741–6749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu
P, Wang B, Wang G, Jia Z, Pu P, et al: Downregulation of miR-21
inhibits EGFR pathway and suppresses the growth of human
glioblastoma cells independent of PTEN status. Lab Invest.
90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Guo F, Wang P, Hong S and Zhang C:
miR-221/222 confers radioresistance in glioblastoma cells through
activating Akt independent of PTEN status. Curr Mol Med.
14:185–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong X, Deng J, Zeng C, Jiang Y, Tang S
and Sun X: MicroRNA-141 is a tumor regulator and prognostic
biomarker in human glioblastoma. Oncol Lett. 14:4455–4460. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Avet-Loiseau H, Facon T, Grosbois B,
Magrangeas F, Rapp MJ, Harousseau JL, Minvielle S and Bataille R;
Intergroupe Francophone du Myélome, : Oncogenesis of multiple
myeloma: 14q32 and 13q chromosomal abnormalities are not randomly
distributed, but correlate with natural history, immunological
features, and clinical presentation. Blood. 99:2185–2191. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZQ, Zhang MY, Deng ML, Weng NQ, Wang
HY and Wu SX: Low serum level of miR-485-3p predicts poor survival
in patients with glioblastoma. PLoS One. 12:e01849692017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tastet J, Decalonne L, Marouillat S, Malvy
J, Thépault RA, Toutain A, Paubel A, Tabagh R, Bénédetti H,
Laumonnier F, et al: Mutation screening of the ubiquitin ligase
gene RNF135 in French patients with autism. Psychiatr Genet.
25:263–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oshiumi H, Matsumoto M, Hatakeyama S and
Seya T: Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I
to promote interferon-beta induction during the early phase of
viral infection. J Biol Chem. 284:807–817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin J, Zhao L and Li Z: The E3 ubiquitin
ligase RNF135 regulates the tumorigenesis activity of tongue cancer
SCC25 cells. Cancer Med. 5:3140–3146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tastet J, Decalonne L, Marouillat S, Malvy
J, Thépault RA, Toutain A, Paubel A, Tabagh R, Bénédetti H,
Laumonnier F, et al: Mutation screening of the ubiquitin ligase
gene RNF135 in French patients with autism. Psychiat Genet.
6:263–267. 2015. View Article : Google Scholar
|
|
24
|
Liu Y, Wang F, Liu Y, Yao Y, Lv X, Dong B,
Li J, Ren S, Yao Y and Xu Y: RNF135, RING finger protein, promotes
the proliferation of human glioblastoma cells in vivo and in vitro
via the ERK pathway. Sci Rep. 6:206422016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lou C, Xiao M, Cheng S, Lu X, Jia S, Ren Y
and Li Z: MiR-485-3p and miR-485-5p suppress breast cancer cell
metastasis by inhibiting PGC-1α expression. Cell Death Dis.
7:e21592016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong D, Sheng Y, Ding S, Chen J, Tan X,
Zeng T, Qin D, Zhu L, Huang A and Tang H: LINC00052 regulates the
expression of NTRK3 by miR-128 and miR-485-3p to strengthen HCC
cells invasion and migration. Oncotarget. 7:47593–47608. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Batash R, Asna N, Schaffer P, Francis N
and Schaffer M: Glioblastoma multiforme, diagnosis and treatment;
recent literature review. Curr Med Chem. 24:3002–3009. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahir BK, Ozer H, Engelhard HH and Lakka
SS: MicroRNAs in glioblastoma pathogenesis and therapy: A
comprehensive review. Crit Rev Oncol Hematol. 120:22–33. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Formosa A, Markert EK, Lena AM, Italiano
D, Finazzi-Agro' E, Levine AJ, Bernardini S, Garabadgiu AV, Melino
G and Candi E: MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c,
miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p,
mapped to the 14q32.31 locus, regulate proliferation, apoptosis,
migration and invasion in metastatic prostate cancer cells.
Oncogene. 33:5173–5182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Henriksen M, Johnsen KB, Olesen P,
Pilgaard L and Duroux M: MicroRNA expression signatures and their
correlation with clinicopathological features in glioblastoma
multiforme. Neuromolecular Med. 16:565–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y: Targeting E3 ubiquitin ligases for
cancer therapy. Cancer Biol Ther. 2:623–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu T, Wang H, Jiang M, Yan Y, Li W, Xu H,
Huang Q, Lu Y and Chen J: The E3 ubiquitin ligase CHIP/miR-92b/PTEN
regulatory network contributes to tumorigenesis of glioblastoma. Am
J Cancer Res. 7:289–300. 2017.PubMed/NCBI
|
|
34
|
Qi Z, Cai S, Cai J, Chen L, Yao Y, Chen L
and Mao Y: miR-491 regulates glioma cells proliferation by
targeting TRIM28 in vitro. BMC Neurol. 16:2482016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Douglas J, Cilliers D, Coleman K,
Tatton-Brown K, Barker K, Bernhard B, Burn J, Huson S, Josifova D,
Lacombe D, et al: Mutations in RNF135, a gene within the NF1
microdeletion region, cause phenotypic abnormalities including
overgrowth. Nat Genet. 39:963–965. 2007. View Article : Google Scholar : PubMed/NCBI
|