Introduction
Psoriasis vulgaris is a chronic immune-mediated
inflammatory disease with polygenic and multifactorial background
and implies negative impact on the quality of life of psoriatic
patients (1–3). The disease affects approximately 2–4%
of the population (4,5), but several studies indicate that there
are differences between Asia and other regions, with a lower
incidence of psoriasis in Asians (0.3–1.2% in China) (6). It is very important to take into
consideration the genetic factor because it affects not only the
overall risk, but also the clinical type, age of onset, severity of
the disease and the risk for psoriatic arthritis (4,7). Many
genetic studies demonstrate the major role of the immune system in
the pathogenesis of psoriasis. Genome-wide association studies
(GWAS) have identified approximately 50 genetic loci associated
with psoriasis risk (8–12). Numerous studies have demonstrated
that there is a strong link between certain polymorphisms of tumor
necrosis factor-α (TNF-α) or interleukin-23 (IL-23) and the
severity of the disease or the response to anti-TNF-α treatment.
Another group of interleukins with proven implications in the
pathogenesis of psoriasis is the IL-17 family which consists of six
members (IL-17A to IL17F) and these interleukins are attached to
five receptors (IL-17RA to IL-17RE) (4,13,14). The
most important member of the family is IL-17A, followed by IL-17F,
and both of them have pro-inflammatory qualities. These
interleukins are produced by a varied number of cells, but, by far,
the most important are Th17 cells.
Th17 pathway and IL-17 family
Th17 pathway
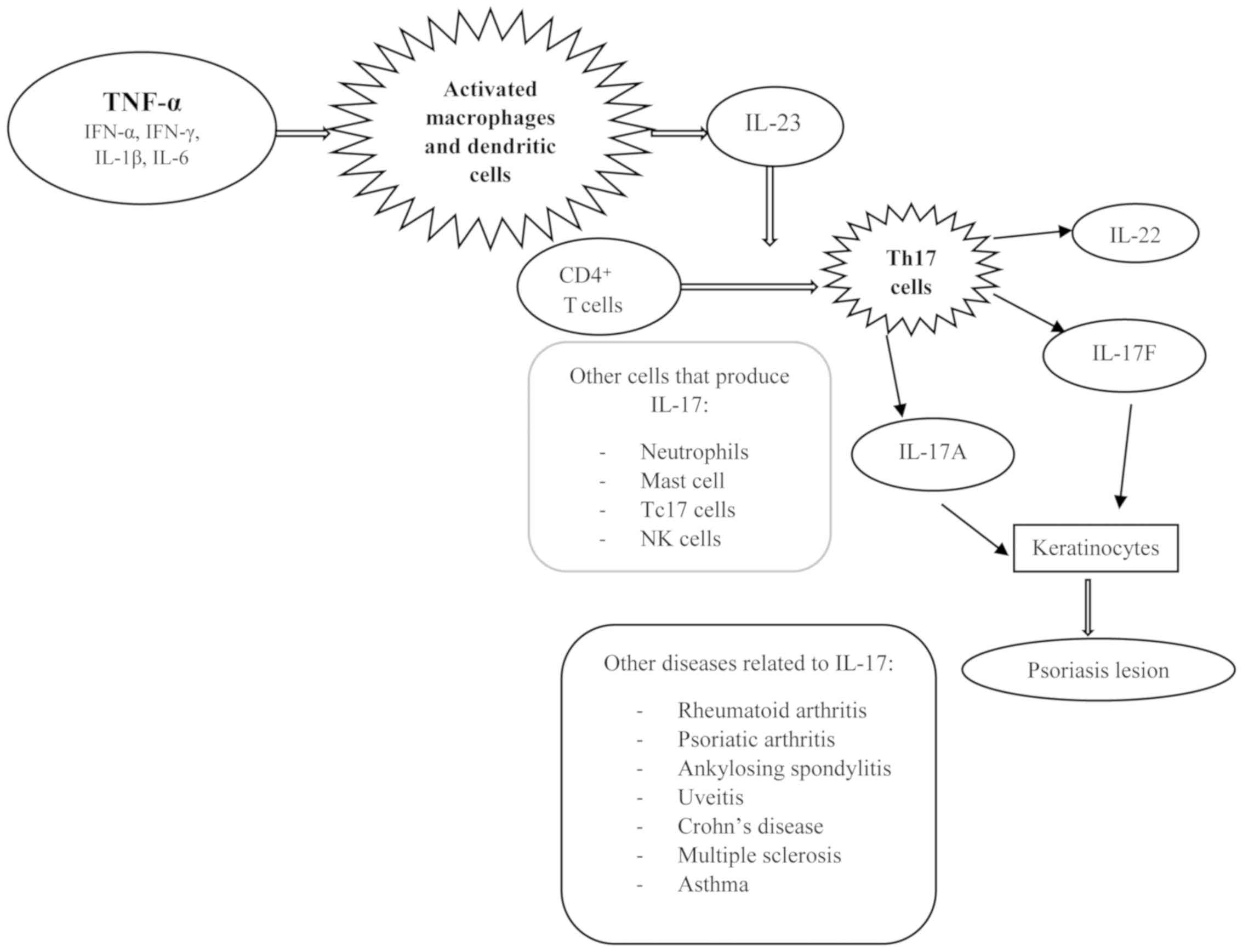
After activation, macrophages and dendritic cells
produce IL-23 which play a mandatory role in differentiation of
naïve CD4+ T cells into Th17 cells. This novel subset of
Th17 cells produce IL-17A, IL-17F and IL-22, the main players in
the formation of characteristic psoriasis lesions (1,13,15,16).
There are other cells that are capable of producing IL-17: mast
cells, NK cells, Tc17 cells, and neutrophils (17,18).
Besides cutaneous psoriasis, IL-17 is also involved in other
immune-mediated diseases: rheumatoid arthritis, psoriatic
arthritis, ankylosing spondylitis, uveitis, Crohn's disease,
multiple sclerosis, and asthma (19–24)
(Fig. 1).
Interleukin-17A
IL-17A is a reference member of its family and a
dimeric glycoprotein which in circulation can be found as a
heterodimer of IL-17A with IL-17F or as a homodimer of two IL-17A
chains (19,25–27). The
homodimer of IL-17A has increased potency in induction of
keratinocyte gene expression, compared to IL-17A/F heterodimer or
IL-17F homodimer (28). IL-17A
stimulate fibroblasts, epithelial and endothelial cells to produce
inflammatory mediators (4,29,30).
Likewise, by creating a connection between innate and adaptive
immune system, mobilizes, recruits and activates neutrophils
(31). In the presence of
extracellular bacteria and fungi IL-17A increases the expression of
chemokines on keratinocytes and leads to an immune response in the
skin (32). The close relationship
between IL-17A and TNF-α is highlighted by the increased response
of keratinocytes to inflammatory cytokines (14,33).
Though, it should be considered that in psoriasis the
pro-inflammatory effects on keratinocytes and neutrophils is due to
both IL-17A and IL-17F (4,34).
Interleukin-17F
IL-17F is another member of the IL-17 family, which
is also secreted by Th17 lymphocytes (16). The structure of this pro-inflammatory
interleukin is almost identical to that of IL-17A, sharing 50%
homology (14). It induces the
release of neutrophil-mobilizing and pro-inflammatory cytokines
(32). IL-17A and IL-17F bind to the
same receptor, which consists of two subunits: IL-17RA and IL-17RC
(28). They also manifest their
pro-inflammatory effects on other types of cells, such as
fibroblasts, endothelial cells, chondrocytes, osteoblasts,
monocytes, and synovial cells (16,19,35,36).
Interleukin-17B, interleukin-17E and
interleukin-17C
IL-17B has a pro-inflammatory role, but its
mechanism of action is not fully elucidated. This interleukin
interferes with embryonic development, tumor progression and
autoimmune diseases (37). IL-17D is
linked to viral and tumor surveillance (37). IL-17E, also called IL-25, is related
to Th2 cells and has the task to produce Th2 cytokines and to
recruit eosinophils, thus it has a role in defending against
parasitic infections (37–39). Also it exerts an inhibitory role on
Th17 cells (26).
It is assumed that IL-17C, a newer member of the
IL-17 family, is also involved in the pathogenesis of psoriasis.
IL-17C exert its role in epithelial cells and mucosa, and modulates
the innate immune system (14). It
has 23% of the IL-17A structure and binds to its receptor: IL-17RC
(40). Skin biopsies from psoriatic
lesions revealed a significantly increased expression of IL-17C, up
to 125 times higher than that of IL-17A (41).
Many cutaneous biopsy studies show that the
psoriasis lesions contain cells that secrete IL-17, especially Th17
lymphocytes, in a higher number compared to normal skin (34,37,42–45).
Thus, IL-17A is highly expressed in the affected skin compared to
unaffected tegument (32). Patients
with psoriasis have increased plasma levels of IL-17, as well as
higher levels of circulating IL-17 producing cells, compared to
healthy people (36,42,46–48).
Therapy against IL-17
Martin H. Fischer, a specialist in psychology, said
that the amount of doctor's medical knowledge is not important for
the patient; for him the most important thing is if the doctor can
cure his disease (49). Nowadays,
different therapeutic strategies are available: topical agents,
ultraviolet light treatment or systemic therapies. Of the patients
with psoriasis 70–80% suffer from mild disease (28) and in this case the topical treatment
and phototherapy are sufficient. For about 20–30% of the patients,
which present moderate-to-severe psoriasis, systemic medication
(phototherapy, methotrexate, cyclosporine, acitretin or biologic
agents) is mandatory (13,28). Psoriatic arthritis affects between
6–42% of patients and if early treatment is not established, local
inflammation will produce joint destruction and disability
(50). During the treatment, some
patients can experience side effects, relapses or insufficient
response (13). For example,
methotrexate is an effective and well-tolerated drug but it also
presents disadvantages such as: important side effects (severe
headache, pancytopenia, mental/mood changes, seizures, allergic
reaction, hepatitis and lung fibrosis) or large variability in
response to treatment (51,52). Another example is acitretin, a drug
commonly used to treat psoriasis. This therapy may seem harmless
but it should be always considered that the response occurs slowly
and is not recommended for women and men at reproductive age
because of its major side effect of teratogenicity (53). Thus, it is very important to
investigate and to take into consideration new possible therapeutic
targets.
The discovery of Th17 pathway had a major impact on
treatment and prognosis of psoriasis (6). Anti-psoriatic agents against IL-17 or
its receptors are a novel group of biologic agents; these include
ixekizumab, secukinumab and brodalumab (54), agents that have been approved for
treating moderate-to-severe psoriasis. They present quite a rapid
response and so far are proved to be successful therapies (4), which also supports the important role
of Th17 axis in the pathogenesis of psoriasis (6). Unlike anti-TNF-α agents, anti-IL-17
therapy have a specific target and do not produce global
immunosuppression (6). After
administration of IL-17 blockers more than half of the 1200 genes
identified in the affected skin were normalized, demonstrating its
superior effect compared to anti-TNF-α agents (13); also, as predicted, these agents
decrease the levels of Th17 cells and IL-17 in affected skin
(37).
Ixekizumab
Ixekizumab is an IgG4 humanized monoclonal antibody
that has high affinity for both IL-17A and IL-17A/F (55). Numerous studies paid attention to
this innovative therapeutic agent including three randomized,
double blind, placebo-controlled phase 3 trials (UNCOVER-1,
UNCOVER-2, and UNCOVER-3) (56).
These studies have shown that ixekizumab is an effective treatment
for moderate-to-severe psoriasis compared to placebo and etanercept
(anti-TNF agent) (57–59). Kemény et al have recently
reported that ixekizumab has a high level of efficacy when given
for up to 156 weeks. It can be said that ixekizumb is a treatment
that offers a high safety profile and a high level of response when
given to patients with moderate-to-severe plaque psoriasis
(56).
Brodalumab
Brodalumab is a fully humanized monoclonal IgG2
antibody, anti-interleukin-17RA. By binding to the IL-17 receptor
subunit IL-17RA, this therapeutic agent neutralizes the activity of
IL-17A, IL-17C, IL-17F, IL-17A/F, and IL-17E (13,37,54).
Response rates after 12 weeks of treatment were similar to that of
ixekizumab and secukinumab, but higher than those seen with
classical treatment or other generations of biologic agents
(37).
Secukinumab
Secukinumab is a fully human IgG1k monoclonal
antibody that has high affinity for IL-17A and blocks the activity
of the cytokine (54). Compared to
placebo, secukinumab is a safe treatment, regarding toxicity no
difference compared to placebo and no important adverse events
compared to placebo (13).
It is worth mentioning that anti-TNF-α drugs exert
inhibitory effects on IL-17 signaling pathway (32). IL-17A acts in synergism with TNF-α
and the efficacy of etanercept is due to suppressive effect on Th17
cells (28).
All three anti-IL-17 molecules, through their
targeted action, reduce the level of IL-17 both systemically and in
plaque psoriasis, demonstrating that IL-17 is a key cytokine. These
agents may also influence the expression of some genes in the
affected skin, but their exact role is still debated.
Polymorphisms of IL-17
Among the first steps that have been taken in order
to understand the link between genetics and the pathogenesis of
psoriasis, the identification of PSOR family was included. It
consists of ten chromosomal loci (noted from 1 to 10), which are
related to the onset of the disease (28). The first locus for this inflammatory
disease, PSOR1, was mapped to chromosome 6p21.3 (60). Another important genetic risk factor
for psoriasis is HLA-C*06:02 (60).
More recently, GWAS have detected a multitude of susceptibility
genes for psoriasis (28).
A question arises: what is the best strategy that
clinicians can choose for non-responders to classical treatment or
to other biologic treatments? Thus, the necessity of individualized
treatment plan, for each patient, is imperative. A possibility is
to identify markers that can predict the response to treatment or
the course of the disease. Gene analysis studies suggest the direct
link between genetics and response to treatment (61). Many polymorphisms of IL-17 family
have been correlated with the risk of inflammatory, infectious,
autoimmune or neoplastic pathologies (4,34). The
genes of IL-17A and IL-17F are both located on 6p12 (32,62).
Kim et al, evaluated the presence of 11
polymorphisms in Korean population: IL-17A (rs2275913, rs3819025,
rs3804513, and rs3748067), IL-17F (rs763780 and rs2397084), IL-17RA
(rs6518660, rs2241046, rs2241049, rs879574, and rs882643) in 208
patients diagnosed with psoriasis and in 266 healthy control. They
demonstrated that the IL-17F polymorphism rs763780 T/C is strongly
associated with psoriasis (1). The
study found no association between the five evaluated polymorphism
of IL-17RA or with the other evaluated polymorphisms and psoriasis
(1).
Contrary, a study conducted by Shibata et al,
which included 153 Japanese with psoriasis and 103 healthy
controls, found no association between the IL-17F rs763780
polymorphism and psoriasis (16).
In 2015, Prieto-Pérez et al, studied for the
first time the influence of IL-17F rs763780 on the response to
biologic treatment with anti-TNF-α drugs or ustekinumab (a
monoclonal antibody against IL-12 and IL-23) (32,63), in
a Caucasian population. The study included 194 patients with
psoriasis and 197 healthy people. Subjects who were carriers of the
C allele were reported as non-responders to treatment with
adalimumab or ustekinumab, but they were responders to treatment
with infliximab (32).
In 2015, Batalla et al reported a study that
was conducted on 580 Spanish patients diagnosed with psoriasis and
567 healthy controls. The subjects were genotyped for IL-17RA
(rs4819554, rs879577), IL-17A (rs7747909), IL-17F (rs763780,
rs2397084), and IL-17E (rs79877597) genes. The IL-17RA rs4819554 G
carrier patients were much more common in psoriasis group. This
polymorphism was also related to the Cw6 status: 48% of the Cw6
positive subjects presented it compared to 40% of the Cw6 negative
subjects (4). The patients who were
carriers for IL-17F rs2397084 TT genotype were also Cw-6 positive
(4). The presence of Cw-6 is closely
related to the early onset of the disease, but in this study
IL-17RA rs4819554 and the age of onset were independent factors. On
the contrary, IL-17F rs2397084 was linked to an early age of onset.
The IL-17RA rs879557, IL-17A rs7747909 and IL-17F rs763780
polymorphisms were not included in the group of susceptibility loci
because they did not show association with the disease (4). IL-17RA rs4819554, IL-17RA rs879557, and
IL-17F rs2397084 were not linked to the severity of the disease,
psoriatic arthritis or other characteristics of the patients
(4). Among the IL-17E rs79877597
allele C carriers a more severe form of psoriasis and a higher risk
for psoriatic arthritis were observed (4).
One year later, in another study, Batalla et
al genotyped 238 Caucasian patients who underwent anti-TNF-α
treatment with adalimumab, etanercept or infliximab. The
investigated polymorphisms were IL-17RA rs4819554 and rs879577. The
rs4819554 polymorphism was directly linked to the response to
anti-TNF-α agents at week 12 (34).
In 2016, Białecka et al, determined the
association between the IL-17A rs2275913, IL-17F rs763780,
rs11465553, rs2397084 polymorphisms and psoriasis susceptibility,
but also the influence of these polymorphism on topical treatment
or combined therapy (topical and NB-UVB), in Polish subjects. These
polymorphisms could not predict the response to treatment. Subjects
carrying the IL-17F rs2397084 variant C allele, required a greater
number of NB-UVB sessions in order to observe clinical improvement,
than those who were carriers of TT genotype (48).
Catanoso et al, found a poor association
between IL-17A rs7747909 and IL-17RA rs9606615, rs2241046,
rs2241049 and peripheral psoriatic arthritis in a group of 118
patients (64). Three years later,
Batalla et al did not find any association of IL-17A
rs774909 with psoriatic arthritis (4).
Conclusions
There is a significant difference in the presence or
absence of susceptibility loci in different population. The genetic
background and the environmental factors have a major impact on
disease susceptibilities in different populations. This leads to
increased medical and socioeconomic costs and influences the
patient's quality of life (65).
IL-17F polymorphism rs763780 is strongly associated
with psoriasis in Korean population (1), with a good response to treatment with
infliximab and with no response to treatment with adalimumab or
ustekinumab in Caucasian population (32). On the other hand, no association
between this polymorphism and the disease was found in Japanese and
Spanish population (4,16).
In Spanish population, the IL-17RA rs4819554 was
more commonly identified in the group of patients with psoriasis
and was also linked to Cw6 status (4). In the same population, IL-17E
rs79877597 was associated with severe forms of psoriasis and an
increased risk for psoriatic arthritis (4). In Caucasian population, the rs4819554
polymorphism was linked to the response to anti-TNF-α agents
(34).
The published data show the presence of a link
between certain alleles and demographic, clinical and therapeutic
features, but due to the limited number of published studies, the
data are insufficient. In order to fully elucidate the role of
polymorphisms in psoriasis, several comparative prospective studies
are needed. To observe the general influence of polymorphisms these
studies should be conducted in different populations on a larger
number of patients.
In conclusion, the existence of many IL-17 related
genes that have been linked to psoriasis (13), in association with anti-IL-17 agents
that have proven their efficacy and safety, confirm the central
role of IL-17 in psoriasis.
Acknowledgements
The authors wish to acknowledge support from the
‘Iuliu Hațieganu’ University of Medicine and Pharmacy (doctoral
research project no. 7690/91/15.04.2016; Cluj-Napoca, Romania).
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ADP was responsible for the conception and design of
the study, the analysis and interpretation of the data, as well as
the drafting and writing of the manuscript, and revising it for
important intellectual content. CP and IIR contributed to the
conception and design of the study, the analysis and interpretation
of the data, as well as the drafting of the manuscript. AC, CV, MS
and RIO were responsible for the conception of the study, and were
involved in drafting of the manuscript and revising it for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GWAS
|
genome-wide association studies
|
|
HLA
|
human leukocyte antigen
|
|
IL
|
interleukin
|
|
NB-UVB
|
narrowband UVB phototherapy
|
|
NK
|
natural killer
|
|
Th17
|
T helper 17
|
|
TNF
|
tumor necrosis factor
|
References
|
1
|
Kim SY, Hur MS, Choi BG, Kim MJ, Lee YW,
Choe YB and Ahn KJ: A preliminary study of new single polymorphisms
in the T helper type 17 pathway for psoriasis in the Korean
population. Clin Exp Immunol. 187:251–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|
|
3
|
Boda D, Negrei C, Nicolescu F and Balalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
4
|
Batalla A, Coto E, González-Lara L,
González-Fernández D, Gómez J, Aranguren TF, Queiro R,
Santos-Juanes J, López-Larrea C and Coto-Segura P: Association
between single nucleotide polymorphisms IL17RA rs4819554 and IL17E
rs79877597 and psoriasis in a Spanish cohort. J Dermatol Sci.
80:111–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Căruntu C: Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl 3):1191–1196.
2014.PubMed/NCBI
|
|
6
|
Bilal J, Berlinberg A, Bhattacharjee S,
Trost J, Riaz IB and Kurtzman DJB: A systematic review and
meta-analysis of the efficacy and safety of the interleukin
(IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab,
ixekizumab, brodalumab, guselkumab and tildrakizumab for the
treatment of moderate to severe plaque psoriasis. J Dermatolog
Treat. 29:569–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coto E, Santos-Juanes J, Coto-Segura P and
Alvarez V: New psoriasis susceptibility genes: Momentum for
skin-barrier disruption. J Invest Dermatol. 131:1003–1005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsoi LC, Spain SL, Knight J, Ellinghaus E,
Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, et al
Collaborative Association Study of Psoriasis (CASP); Genetic
Analysis of Psoriasis Consortium; Psoriasis Association Genetics
Extension; Wellcome Trust Case Control Consortium 2, :
Identification of 15 new psoriasis susceptibility loci highlights
the role of innate immunity. Nat Genet. 44:1341–1348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin X, Low HQ, Wang L, Li Y, Ellinghaus E,
Han J, Estivill X, Sun L, Zuo X, Shen C, et al: Genome-wide
meta-analysis identifies multiple novel associations and ethnic
heterogeneity of psoriasis susceptibility. Nat Commun. 6:69162015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harden JL, Krueger JG and Bowcock AM: The
immunogenetics of psoriasis: A comprehensive review. J Autoimmun.
64:66–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strange A, Capon F, Spencer CC, Knight J,
Weale ME, Allen MH, Barton A, Band G, Bellenguez C, Bergboer JG, et
al Genetic Analysis of Psoriasis Consortium and the Wellcome Trust
Case Control Consortium 2, : A genome-wide association study
identifies new psoriasis susceptibility loci and an interaction
between HLA-C and ERAP1. Nat Genet. 42:985–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anbunathan H and Bowcock AM: The molecular
revolution in cutaneous biology: The era of genome-wide association
studies and statistical, big data, and computational topics. J
Invest Dermatol. 137:e113–e118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lønnberg AS, Zachariae C and Skov L:
Targeting of interleukin-17 in the treatment of psoriasis. Clin
Cosmet Investig Dermatol. 7:251–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malakouti M, Brown GE, Wang E, Koo J and
Levin EC: The role of IL-17 in psoriasis. J Dermatolog Treat.
26:41–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibata S, Saeki H, Tsunemi Y, Kato T,
Nakamura K, Kakinuma T, Kagami S, Fujita H, Tada Y, Sugaya M, et
al: IL-17F single nucleotide polymorphism is not associated with
psoriasis vulgaris or atopic dermatitis in the Japanese population.
J Dermatol Sci. 53:163–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Girolomoni G, Mrowietz U and Paul C:
Psoriasis: Rationale for targeting interleukin-17. Br J Dermatol.
167:717–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Anderson DE, Baecher-Allan C,
Hastings WD, Bettelli E, Oukka M, Kuchroo VK and Hafler DA: IL-21
and TGF-beta are required for differentiation of human T(H)17
cells. Nature. 454:350–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaffen SL, Kramer JM, Yu JJ and Shen F:
The IL-17 cytokine family. Vitam Horm. 74:255–282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tesmer LA, Lundy SK, Sarkar S and Fox DA:
Th17 cells in human disease. Immunol Rev. 223:87–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patel DD, Lee DM, Kolbinger F and Antoni
C: Effect of IL-17A blockade with secukinumab in autoimmune
diseases. Ann Rheum Dis. 72 (Suppl 2):ii116–ii123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leipe J, Grunke M, Dechant C, Reindl C,
Kerzendorf U, Schulze-Koops H and Skapenko A: Role of Th17 cells in
human autoimmune arthritis. Arthritis Rheum. 62:2876–2885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gaffen SL: Recent advances in the IL-17
cytokine family. Curr Opin Immunol. 23:613–619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gaffen SL: Structure and signalling in the
IL-17 receptor family. Nat Rev Immunol. 9:556–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wright JF, Bennett F, Li B, Brooks J,
Luxenberg DP, Whitters MJ, Tomkinson KN, Fitz LJ, Wolfman NM,
Collins M, et al: The human IL-17F/IL-17A heterodimeric cytokine
signals through the IL-17RA/IL-17RC receptor complex. J Immunol.
181:2799–2805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiricozzi A: Pathogenic role of IL-17 in
psoriasis and psoriatic arthritis. Actas Dermosifiliogr. 105 (Suppl
1):9–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Starnes T, Robertson MJ, Sledge G, Kelich
S, Nakshatri H, Broxmeyer HE and Hromas R: Cutting edge: IL-17F, a
novel cytokine selectively expressed in activated T cells and
monocytes, regulates angiogenesis and endothelial cell cytokine
production. J Immunol. 167:4137–4140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arisawa T, Tahara T, Shibata T, Nagasaka
M, Nakamura M, Kamiya Y, Fujita H, Nakamura M, Yoshioka D, Arima Y,
et al: The influence of polymorphisms of interleukin-17A and
interleukin-17F genes on the susceptibility to ulcerative colitis.
J Clin Immunol. 28:44–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawaguchi M, Adachi M, Oda N, Kokubu F and
Huang SK: IL-17 cytokine family. J Allergy Clin Immunol.
114:1265–1274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prieto-Pérez R, Solano-López G, Cabaleiro
T, Román M, Ochoa D, Talegón M, Baniandrés O, López Estebaranz JL,
de la Cueva P, Daudén E, et al: The polymorphism rs763780 in the
IL-17F gene is associated with response to biological drugs in
patients with psoriasis. Pharmacogenomics. 16:1723–1731. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiricozzi A, Guttman-Yassky E,
Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I, Chimenti S and
Krueger JG: Integrative responses to IL-17 and TNF-α in human
keratinocytes account for key inflammatory pathogenic circuits in
psoriasis. J Invest Dermatol. 131:677–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Batalla A, Coto E, Gómez J, Eirís N,
González-Fernández D, Gómez-De Castro C, Daudén E, Llamas-Velasco
M, Prieto-Perez R, Abad-Santos F, et al: IL17RA gene variants and
anti-TNF response among psoriasis patients. Pharmacogenomics J.
18:76–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martin DA, Towne JE, Kricorian G, Klekotka
P, Gudjonsson JE, Krueger JG and Russell CB: The emerging role of
IL-17 in the pathogenesis of psoriasis: Preclinical and clinical
findings. J Invest Dermatol. 133:17–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harper EG, Guo C, Rizzo H, Lillis JV,
Kurtz SE, Skorcheva I, Purdy D, Fitch E, Iordanov M and Blauvelt A:
Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro
and in vivo: Implications for psoriasis pathogenesis. J Invest
Dermatol. 129:2175–2183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roostaeyan O, Kivelevitch D and Menter A:
A review article on brodalumab in the treatment of
moderate-to-severe plaque psoriasis. Immunotherapy. 9:963–978.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 Cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pukelsheim K, Stoeger T, Kutschke D,
Ganguly K and Wjst M: Cytokine profiles in asthma families depend
on age and phenotype. PLoS One. 5:e142992010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ramirez-Carrozzi V, Sambandam A, Luis E,
Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, et al:
IL-17C regulates the innate immune function of epithelial cells in
an autocrine manner. Nat Immunol. 12:1159–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fujishima S, Watanabe H, Kawaguchi M,
Suzuki T, Matsukura S, Homma T, Howell BG, Hizawa N, Mitsuya T,
Huang SK, et al: Involvement of IL-17F via the induction of IL-6 in
psoriasis. Arch Dermatol Res. 302:499–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wilson NJ, Boniface K, Chan JR, McKenzie
BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel
F, et al: Development, cytokine profile and function of human
interleukin 17-producing helper T cells. Nat Immunol. 8:950–957.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chan JR, Blumenschein W, Murphy E, Diveu
C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S,
Kimball AB, et al: IL-23 stimulates epidermal hyperplasia via TNF
and IL-20R2-dependent mechanisms with implications for psoriasis
pathogenesis. J Exp Med. 203:2577–2587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prieto-Pérez R, Cabaleiro T, Daudén E,
Ochoa D, Roman M and Abad-Santos F: Genetics of psoriasis and
pharmacogenetics of biological drugs. Autoimmune Dis.
2013:6130862013.PubMed/NCBI
|
|
45
|
Asarch A, Barak O, Loo DS and Gottlieb AB:
Th17 cells: A new therapeutic target in inflammatory dermatoses. J
Dermatolog Treat. 19:318–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kagami S, Rizzo HL, Lee JJ, Koguchi Y and
Blauvelt A: Circulating Th17, Th22, and Th1 cells are increased in
psoriasis. J Invest Dermatol. 130:1373–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Johansen C, Usher PA, Kjellerup RB,
Lundsgaard D, Iversen L and Kragballe K: Characterization of the
interleukin-17 isoforms and receptors in lesional psoriatic skin.
Br J Dermatol. 160:319–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Białecka M, Ostasz R, Kurzawski M,
Klimowicz A, Fabiańczyk H, Bojko P, Dziedziejko V, Safranow K,
Machoy-Mokrzyńska A and Droździk M: IL17A and IL17F gene
polymorphism association with psoriasis risk and response to
treatment in a Polish population. Dermatology. 232:592–596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Raţiu MP, Purcărea I, Popa F, Purcărea VL,
Purcărea TV, Lupuleasa D and Boda D: Escaping the economic turn
down through performing employees, creative leaders and growth
driver capabilities in the Romanian pharmaceutical industry.
Farmacia. 59:119–130. 2011.
|
|
50
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
51
|
Negrei C, Caruntu C, Ginghina O,
Dragomiroiu GTAB, Toderescu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. Rev Chim.
66:607–610. 2015.
|
|
52
|
Negrei C, Ginghină O, Căruntu C, Burcea
Dragomiroiu GTA, Jinescu G and Boda D: Investigation relevance of
methotrexate polyglutamates in biological systems by high
performance liquid chromatography. Rev Chim. 66:766–768. 2015.
|
|
53
|
Negrei C, Arsene AL, Toderescu CD, Boda B
and Ilie M: Acitretin treatment in psoriazis may influence the cell
membrane fluidity. Farmacia. 60:767–771. 2012.
|
|
54
|
Thomas LW, Lee EB and Wu JJ: Systematic
review of anti-drug antibodies of IL-17 inhibitors for psoriasis. J
Dermatolog Treat. 18:1–7. 2018.
|
|
55
|
Liu L, Lu J, Allan BW, Tang Y, Tetreault
J, Chow CK, Barmettler B, Nelson J, Bina H, Huang L, et al:
Generation and characterization of ixekizumab, a humanized
monoclonal antibody that neutralizes interleukin-17A. J Inflamm
Res. 9:39–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kemény L, Berggren L, Dossenbach M,
Dutronc Y and Paul C: Efficacy and safety of ixekizumab in patients
with plaque psoriasis across different degrees of disease severity:
Results from UNCOVER-2 and UNCOVER-3. J Dermatolog Treat. 4:1–8.
2018.
|
|
57
|
Gordon KB, Blauvelt A, Papp KA, Langley
RG, Luger T, Ohtsuki M, Reich K, Amato D, Ball SG, Braun DK, et al
UNCOVER-1 Study Group, : UNCOVER-2 Study Group; UNCOVER-3 Study
Group: Phase 3 trials of ixekizumab in moderate-to-severe plaque
psoriasis. N Engl J Med. 375:345–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Griffiths CE, Reich K, Lebwohl M, van de
Kerkhof P, Paul C, Menter A, Cameron GS, Erickson J, Zhang L,
Secrest RJ, et al UNCOVER-2 and UNCOVER-3 investigators, :
Comparison of ixekizumab with etanercept or placebo in
moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results
from two phase 3 randomised trials. Lancet. 386:541–551. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Olteanu R, Zota A and Constantin M:
Biosimilars: An update on clinical trials (review of published and
ongoing studies). Acta Dermatovenerol Croat. 25:57–66.
2017.PubMed/NCBI
|
|
60
|
Nair RP, Stuart PE, Nistor I, Hiremagalore
R, Chia NVC, Jenisch S, Weichenthal M, Abecasis GR, Lim HW,
Christophers E, et al: Sequence and haplotype analysis supports
HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet.
78:827–851. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nishikawa R, Nagai H, Bito T, Ikeda T,
Horikawa T, Adachi A, Matsubara T and Nishigori C: Genetic
prediction of the effectiveness of biologics for psoriasis
treatment. J Dermatol. 43:1273–1277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Olteanu R, Constantin MM, Zota A,
Dorobantu DM, Constantin T, Serban ED, Balanescu P, Mihele D and
Gheuca-Solovastru L: Original clinical experience and approach to
treatment study with interleukin 12/23 inhibitor in
moderate-to-severe psoriasis patients. Farmacia. 64:918–921.
2016.
|
|
64
|
Catanoso MG, Boiardi L, Macchioni P,
Garagnani P, Sazzini M, De Fanti S, Farnetti E, Casali B,
Chiarolanza I, Nicoli D, et al: IL-23A, IL-23R, IL-17A and IL-17R
polymorphisms in different psoriatic arthritis clinical
manifestations in the northern Italian population. Rheumatol Int.
33:1165–1176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1–9. 2014. View Article : Google Scholar
|