Introduction
Vascular calcification increasingly affects the
aging and dysmetabolic population worldwide (1,2).
Vascular calcification, which occurs in the intima and media of the
artery, is considered an independent risk factor of cardiovascular
and cerebrovascular diseases (1).
Although angiotensin-converting enzymes and statins can stabilize
atherosclerosis, they cannot reverse vascular calcification
(3,4). The molecular complexity involved in the
regulation of vascular calcification is still unclear (4,5).
Notably, the arterial remodeling (AR) process serves an important
role in the pathogenesis of vascular calcification (6).
The AR process includes smooth muscle cell (SMC)
migration and proliferation, and extracellular matrix (ECM)
production (7). During the AR
process, the phenotypic transformation of vascular smooth muscle
cells (VSMCs) causes abnormal development of the ECM in blood
vessels (7). Studies have indicated
that elevated extracellular calcium and phosphate levels impact the
survival and phenotype of VSMCs, which results in cellular
adaptations and damage that ultimately promotes calcification
(8). As a result, the normal
contractile SMCs are converted into synthetic SMCs (9), with significantly decreased α-SMA
expression and significantly increased OPN expression (10).
Little is known regarding how the alterations in ECM
composition and VSMCs impact vascular calcification, even though
>50% of the neointimal hyperplasia consists of ECM proteins
(7). Furthermore, the mechanisms of
VSMC activation in the AR process remain unclear. Studies have
indicated that VSMCs can be activated by persistent injury or other
factors, including the renin-angiotensin aldosterone system
(11), transforming growth factor-β
(12), connective tissue growth
factor (13) and matrix
metalloproteinases (14). In
addition, the change in the proportion of ECM components of the
vascular wall is also vital for the transformation of the VSMC
phenotype (9).
ECM is a complex mixture of structural and
functional proteins, glycoproteins, and proteoglycans arranged in a
unique, tissue specific three-dimensional (3D) ultrastructure
(15). The natural 3D spatial
structure provides a convenient platform for cells to regenerate
(16). Similar to the native ECM
(17–19), decellularized scaffolds are
completely depleted of cells and their components by chemical or
physical methods, but preserve the majority of the 3D spatial
structures of tissues and organs (20). The decellularized scaffolds have a
good biological capacitance, low immunogenicity, and are widely
used in the field of tissue engineering (21). Due to the complexity of the ECM
components and the spatial structure of the vascular wall, it is
difficult to elucidate the impact of the ECM on VSMC phenotypic
transformation following artery reconstruction in vitro
(22,23). Therefore, in the current study,
decellularized vascular scaffolds were used to determine whether
ECM could impact the phenotypic transformation of VSMCs following
AR in vascular calcification.
Materials and methods
Rat model of vascular
calcification
The rat model of arterial calcification was
constructed as described previously (17). A total of 22 male 6-week-old Sprague
Dawley rats (Animal Experimental Center of Wenzhou Medical
University, Wenzhou, China) with mean body weight of ~180 g were
used to evaluate the effects of arterial calcification. Animals
were maintained at a controlled temperature (22±2°C) and humidity
(55±5%) with a 12-h light/dark cycle under specific-pathogen-free
conditions with free access to food and water. Following a week
acclimation period, 22 rats were divided into the arterial
calcification group (experimental group; n=16) and normal arterial
group (control group; n=6). The animals in the arterial
calcification group were injected with vitamin K (Chengdu Bite
Pharmaceutical Group Co., Ltd.) 11.5 mg/(100 g body weight/day)
subcutaneously from the first day to the sixth, on the third day,
subcutaneous injection of vitamin D3 (Beijing Solarbio Science
& Technology Co., Ltd.; 30,000 U/(100 g body weight/day) was
continued once per day until the fifth day, and warfarin (Sangon
Biotech Co., Ltd.) 150 mg/kg was administered twice per day orally
until the sixth day; and normal arterial group were injected with
0.9% saline of the same volume with the same method. All rats were
anesthetized with 300 mg/kg 10% chloral hydrate (cat. no. A600288;
Sangon Biotech Co., Ltd., Shanghai, China) and euthanized by
cervical dislocation on the 7th day following the model
establishment. Following sacrifice, the rats were immersed in 75%
alcohol for 5 min at room temperature. The thoracic cavity of the
rats was opened on a sterile clean bench. The thoracic aorta and
abdominal aorta were removed as soon as possible, and the residual
blood clots were washed with PBS at 4°C three times, and PBS was
exchanged every 5 min. A small section of each segment of the
vessels (the normal and the calcifiated vessels) was fixed with 4%
paraformaldehyde for ~30 min at room temperature using for Von
Kossa staining. The remaining samples were dried using a Drying
Oven (model DHG-9023A) for 15 min and stored in at −20°C until
use.
Von Kossa staining
Aortic vessels were examined by Von Kossa staining
as follows: The sections (8-µm-thick) were attached to a glass
slide in a 37°C water bath, and dried at 37°C for 30 min. The
sections were incubated with 1% silver nitrate for 1 h at room
temperature under a UV lamp, then rinsed with distilled water >3
times, washed off the black material on the surface with distilled
water, then incubated with 5% sodium thiosulfate at room
temperature for 2 min. Samples were subsequently counterstained
with 1% neutral red at room temperature for 10 min and images were
captured under a fluorescent microscope (Olympus Corporation).
Preparation of aortic decellularized
biological scaffold
Half of the normal and calcified blood vessels were
continuously perfused (perfusion pressure, 3.6 mmHg) with 0.1%
trypsin solution and 0.5–1% SDS solution at 37°C for 12 h. The
decellularization solution (trysin and SDS) was refreshed every 6
h. The color and shape of the blood vessels at various time points
(0, 30, 60, 120, 180 and 240 min) were observed during perfusion
in vitro. When the vessels became clean and transparent, the
decellularization solution was replaced with 0.9% saline at room
temperature for 1 h before use.
To confirm that decellularized scaffolds were
successfully obtained, the DNA band in the normal arterial and
calcified arterial decellularized scaffolds was detected by agarose
gel electrophoresis. Total DNA was purified from normal arterial
decellularized or non-decellularized scaffolds and calcified
arterial decellularized or non-decellularized scaffolds, using a
Mammalian genomic DNA extraction kit (Beyotime Institute of
Biotechnology). Extracted DNA was applied to 1% agarose gel 100 V
for 1 h at room temperature. The gel with DNA fragments was
visualized and analyzed on the ChemiDoxTM XRS+ system (Bio-Rad
Laboratories, Inc.).
Co-culture conditions
Direct co-cultures were established by adding VSMCs
to normal arterial decellularized scaffolds or calcified arterial
decellularized scaffolds in 24-well plates. Briefly, normal and
calcified arterial decellularized scaffolds were perfused with 2%
agarose solution at 37°C, chilled at 4°C and sectioned into 5×5 mm
squared sections. Following removal of agarose, the arterial
decellularized scaffolds were stored in PBS at 4°C. VSMCs (A-10;
American Type Culture Collection, Manassas, VA, USA) were suspended
at a concentration of 1×107/ml. A total of 10 µl of cell
suspension was added onto the arterial decellularized scaffold
slices and incubated for 30 min at room temperature. The slices
were transferred to 24-well plates. Cells were cultured in
high-glucose Dulbecco's modified Eagle medium (Sigma-Aldrich; Merck
KGaA) supplemented with 10% fetal bovine serum (Royacel) at 37°C in
an incubator containing 5% CO2 for 9 days. The media
were changed every 3 days. Each co-culture experiment was performed
three times. The mixed culture system of VSMC-arterial
decellularized scaffolds was constructed. Specimens were obtained
at 2, 5, 10, 15 and 21 days following co-culture. Subsequently, the
expression of α-SMA and OPN were detected.
Western blot analysis
The assay was performed using the following primary
antibodies: Rabbit polyclonal anti-α-SMA (cat. no. ab72583; Abcam),
anti-OPN (cat. no. ab8448; Abcam), and anti-GAPDH (cat. no. AG019;
Beyotime Institute of Biotechnology). Glass plates (1.5 mm) were
washed and dried for use. Total protein was extracted with RIPA and
was quantified using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology) and 30 µg protein/lane was separated via SDS-PAGE on
a 10% gel. After electrophoresis, proteins on the gel were
transferred to a PVDF membrane, which was incubated with 5% skim
milk powder for 1 h at room temperature. All primary antibodies
were incubated overnight at 4°C, with a dilution ratio of 1:1,000,
and then washed with TBST 3 times (5 min each time). Secondary
antibody (HRP-labeled Goat Anti-Rabbit IgG; 1:2,000; cat. no.
A0208; Beyotime Institute of Biotechnology) was added for 1 h at
room temperature, then samples were washed 3 times with TBST.
Visualization was performed with ECL (BeyoECL Star kit, Beyotime
Institute of Biotechnology) for 2–5 min followed by exposure to a
chemiluminescence imager (ChemiDoc™ XRS+; Bio-Rad
Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) detection of α-SMA and OPN
Total cellular RNA from VSMC co-cultured with
scaffolds was purified using TRIzol reagent (Thermo Fisher
Scientific, Inc.), and complementary DNA (cDNA) was synthesized
using a ReverTra Ace® qPCR RT kit (Toyobo Life Science)
at 37°C for 15 min and at 95°C for 5 min. cDNA samples were placed
immediately on ice and transferred to −20°C prior to subsequent
qPCR detection. The following 10-µl reaction system was used for
qPCR: 0.1 µl cDNA template, 0.2 µl of each primer, 4.5 µl of 0.1%
DEPC water and 5 µl of SYBR Green qPCR premix (Takara Biotechnology
Co., Ltd.). The following thermocycling conditions were used:
Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5
sec and 60°C for 30 sec. Using GAPDH as an internal reference gene,
2−ΔΔCq was used to calculate the expression level of
each gene of interest (24). The
following primer sequences were used: α-SMA forward,
5′-AGGGACTAATGGTTGGAATGG-3′ and reverse,
5′-CAATCTCACGCTCGGCAGTAG-3′; OPN, forward,
5′-CTGATGCTACAGACGAGGAC-3′ and reverse,
5′-CAATCTCACACTATCAATCACATCGGAAT-3′; GAPDH forward,
5′-GCAAGTTCAACGGCACAG-3′ and reverse,
5′-CGCCAGTAGACTCCACGAC-3′.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 11.0; SPSS, Inc. Chicago, IL, USA). Results are
presented as the mean ± standard error of the mean. Significant
differences was estimated using one-way analysis of variance
followed by Student-Newmann-Keuls multiple comparison tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Rat model of vascular
calcification
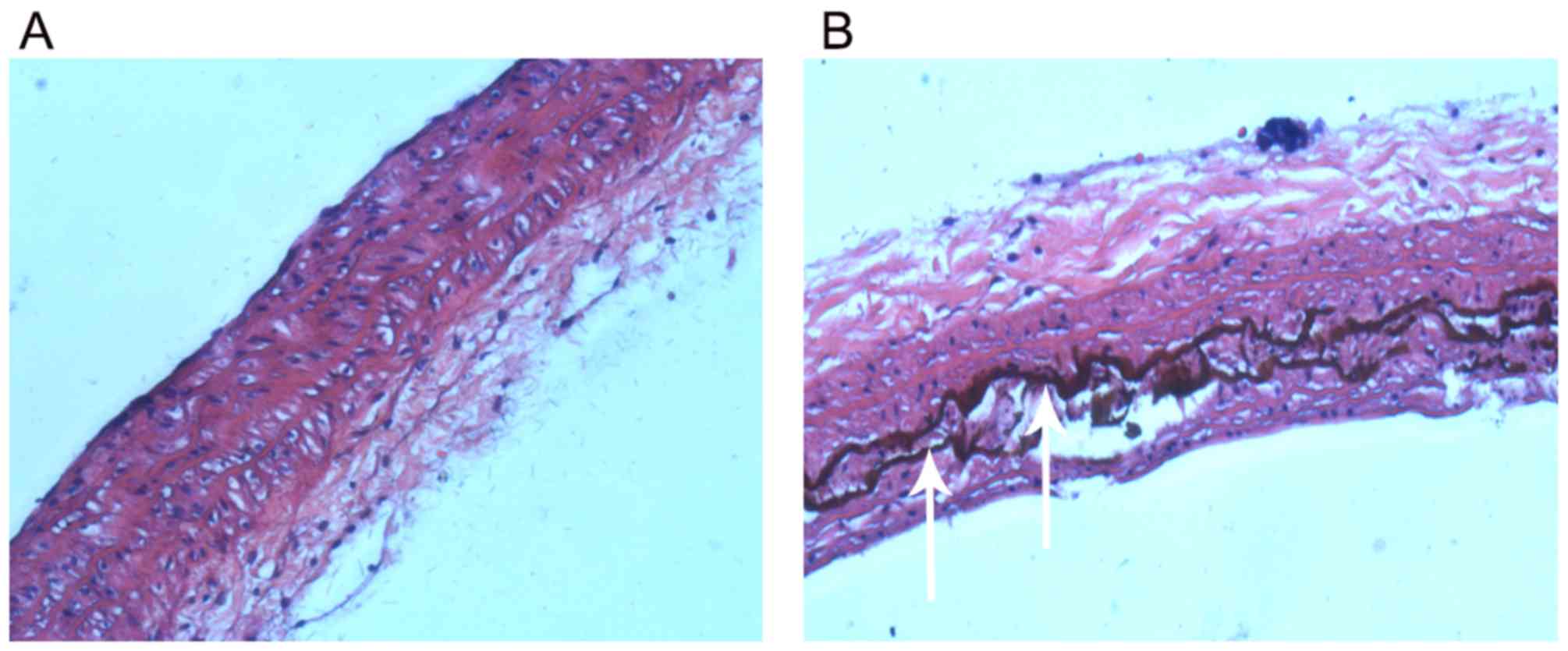
Von Kossa staining results indicated that there was
no calcification in the aorta of normal rats (Fig. 1A). However, there were strongly
positively stained, black calcified nodules in the thoracic aorta
of rats with vascular calcification (Fig. 1B), indicating the successful
construction of the rat model of vascular calcification.
Decellularized arterial biological
scaffold
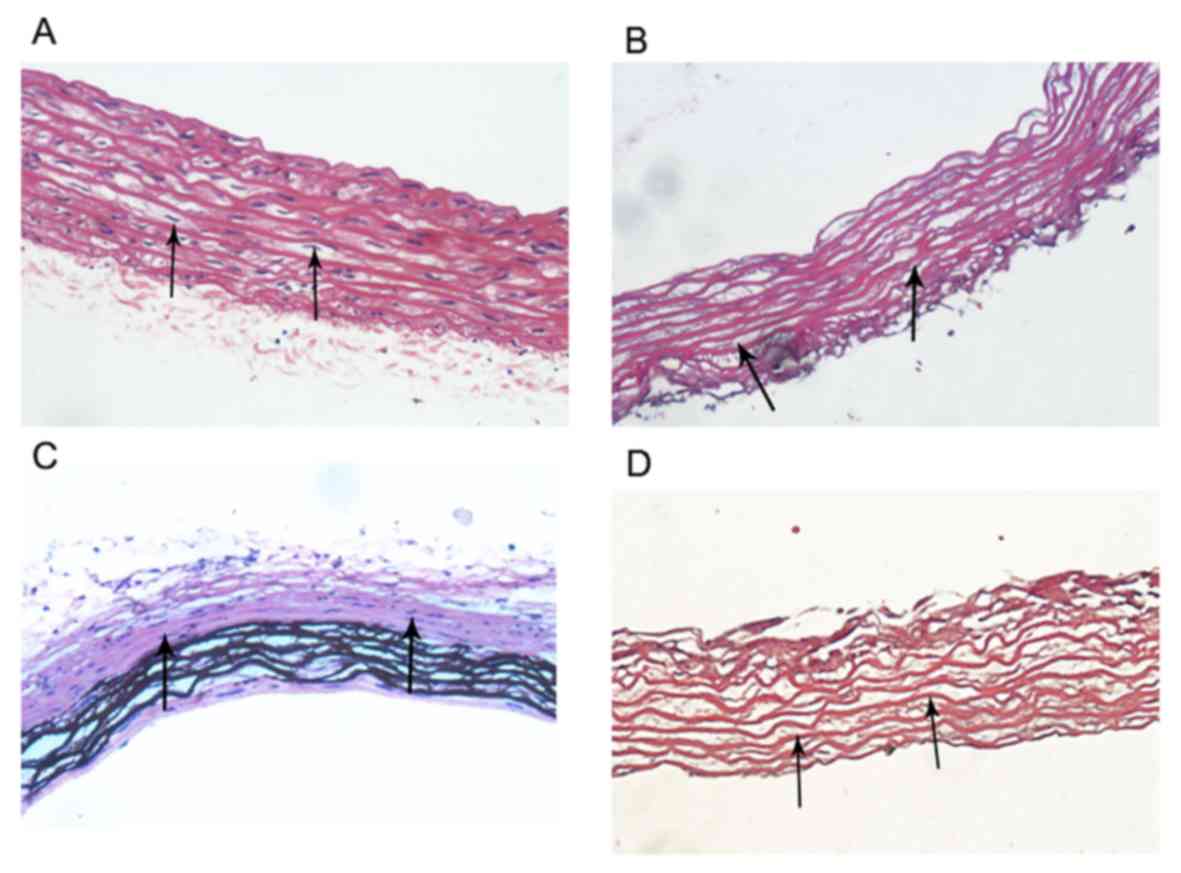
There were numerous of nuclei both in normal
arteries and calcified arteries (Fig. 2A
and C; black arrows); however, no nuclei were found in the
normal arterial decellularized scaffolds and calcified arterial
decellularized scaffolds, and the elastic fiber structures were
complete and arranged in an orderly manner (Fig. 2B and D; black arrows). Furthermore,
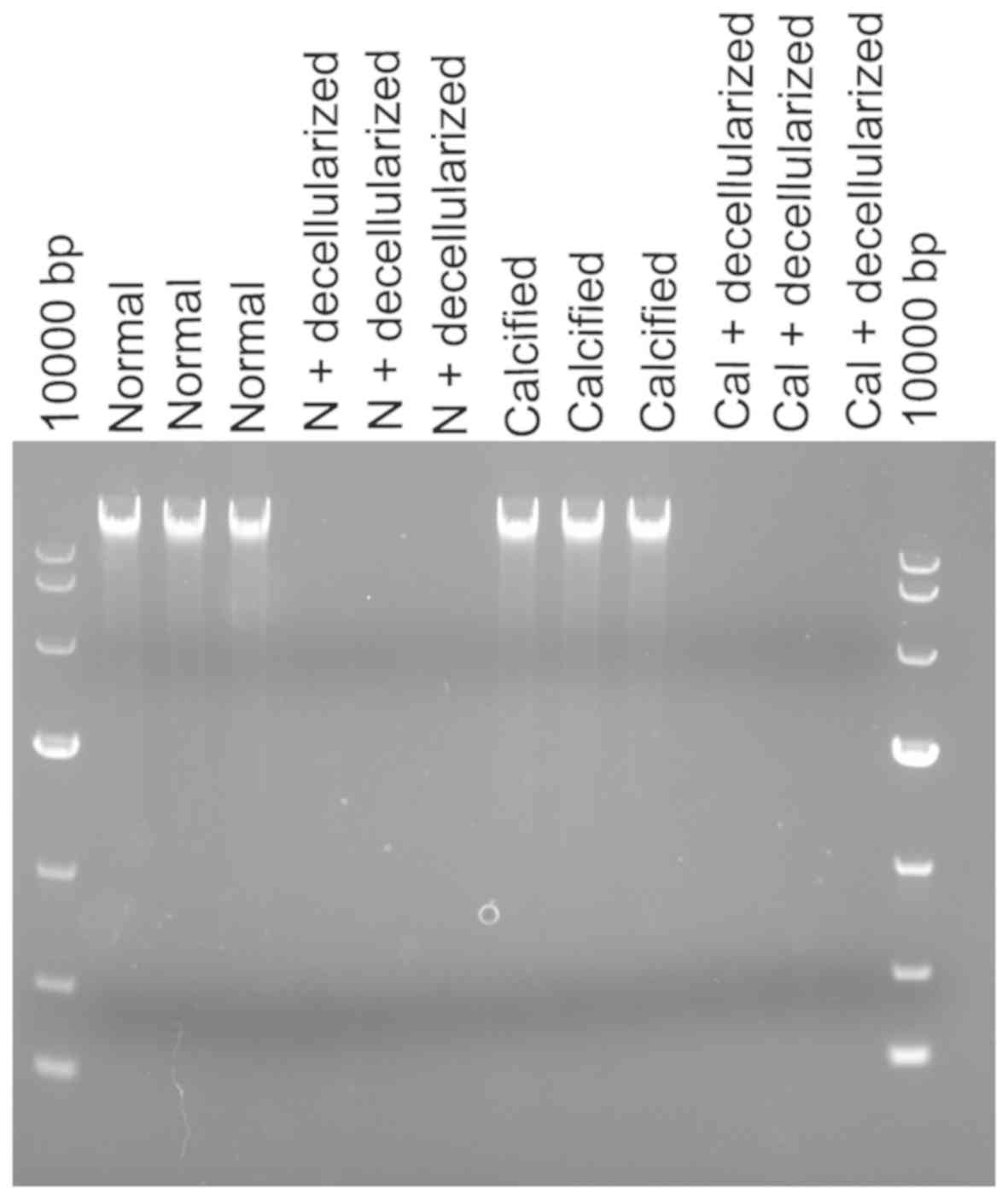
there was no DNA band detected in the normal arterial
decellularized scaffolds and calcified arterial decellularized
scaffolds according to agarose gel electrophoresis results
(Fig. 3), indicating the successful
construction of decellularized biological scaffolds.
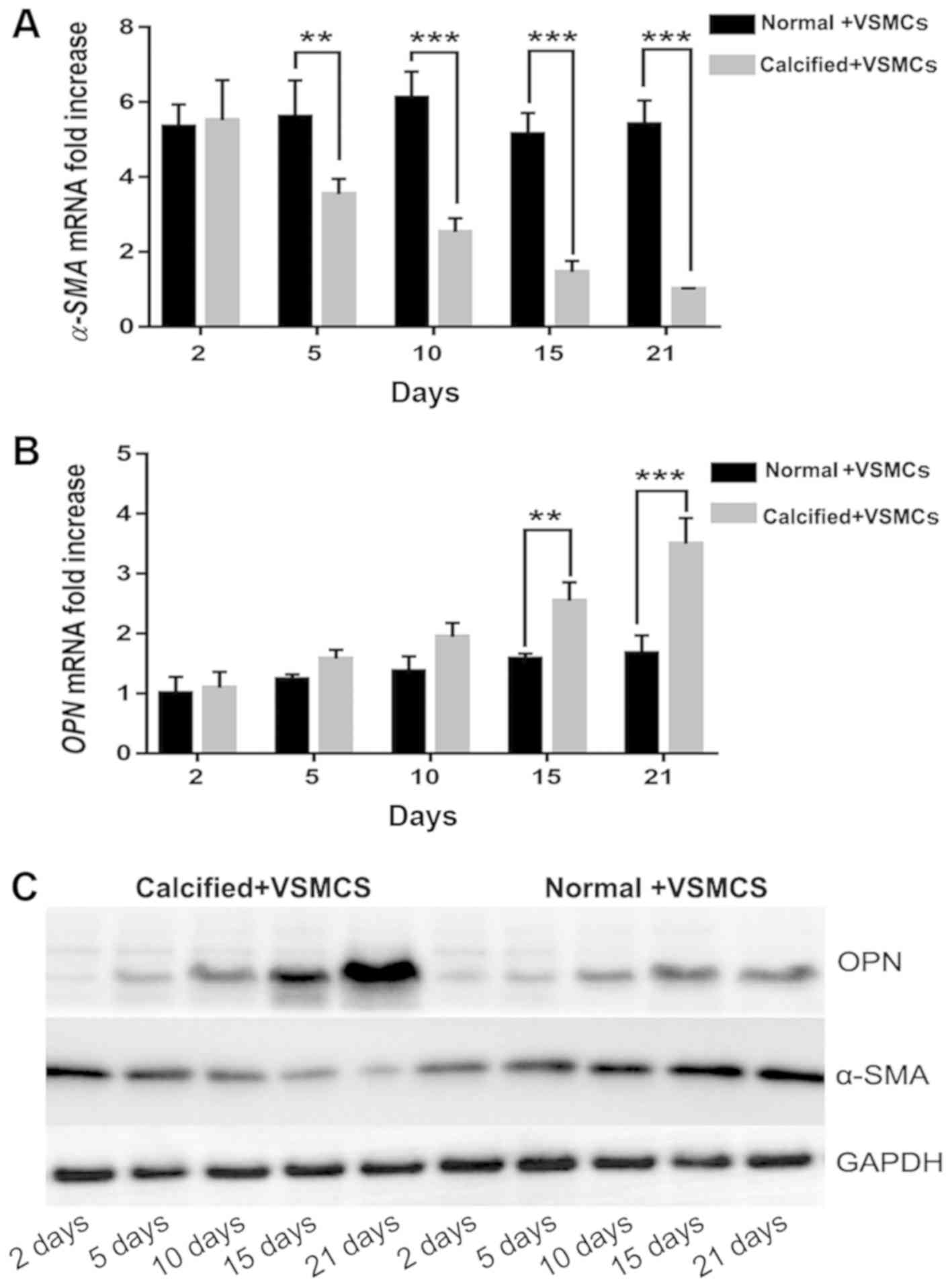
Expression of α-SMA and OPN
The expression of α-SMA mRNA was significantly
increased in the normal decellularized arterial scaffold + VSMCs
co-culture group compared with the calcified decellularized
scaffold+VSMCs co-culture group on days 5, 10, 15 and 21 following
establishment of the co-culture systems (P<0.05 and P<0.001;
Fig. 4). Notably, the expression of
OPN mRNA in the AR co-culture group was increased when compared
with the normal co-culture group. This difference was statistically
significant on days 15 and 21 following establishment of the
co-culture systems (P<0.001; Fig.
4). Western blot analysis revealed similar results. The results
indicated that the phenotype of VSMCs was regulated by vascular
calcification-induced AR may via downregulation of the expression
of α-SMA and upregulating the expression of OPN.
Discussion
AR is a pathological manifestation of vascular
disease complicated by hypertension, diabetes and other chronic
diseases (7). AR is an important
pathophysiological process in the development of vascular
calcification (7). Notably, arterial
calcification primarily occurs in the intima and middle membrane of
the large artery and middle artery wall, which increases the
probability of acute and chronic cardiac and cerebrovascular
events, such as acute myocardial infarction, stroke, aneurysm
rupture and bleeding, and seriously endangers the health and safety
of individuals (1,6). Therefore, an improved understanding of
the pathogenesis of the AR process could provide useful information
for the prevention and treatment of complications caused by
vascular calcification. The AR process includes SMC migration and
proliferation, and ECM production (7). However, little is known with regard to
the changes that occur in ECM composition and SMCs during vascular
calcification, even though >50% of the neointimal hyperplasia
consists of ECM proteins (7).
The mechanical properties of the ECM serve a
critical role in regulating signaling pathways that underlie cell
adhesion, migration, proliferation and differentiation (18,19).
Hypertension and other factors lead to changes in the proportion of
ECM components in blood vessels, including an increase of collagen
and fibrous adhesive protein, degradation of elastin and abnormal
expression of matrix proteins, such as osteopontin (3), which lead to the thickening of the
arterial wall, stenosis of the lumen and a decrease in vascular
compliance (3). ECM is primarily
composed of collagen and elastin and serves various functions that
are essential for maintaining structural integrity of the
cardiovascular wall (25). ECM
remodeling is one of the underlying mechanisms in atherosclerotic
cardiovascular disease, including as atherosclerosis, aneurysm
formation and restenosis (7). The
effect of ECM scaffolds in supporting tissue regeneration is mainly
associated with two major characteristics: The maintained 3D
structure and the bioactive components. The natural 3D structure of
ECM provides structural support and tensile strength, attachment
sites for cell surface receptors, and a reservoir for signaling
factors that modulate angiogenesis, cell migration, cell
proliferation, and orientation (26).
Elevated extracellular calcium and phosphate levels
impact the survival and phenotype of VSMCs, which results in
cellular adaptations and damage that ultimately promote
calcification (8). As a result, the
normal contractile VSMCs are converted into synthetic VSMCs
(9), with significantly decreased
α-SMA expression and significantly increased OPN expression
(10). The current experimental
results were also consistent with that conclusion. In the current
study, the results indicated that the expression of α-SMA in the
normal + VSMCs co-culture group was significantly increased,
compared with the calcified + VSMCs co-culture group. α-SMA is a
specific phenotypic marker of VSMCs (27). In the current study, in the normal +
VSMCs co-culture group, the scaffolds did not affect the α-SMA
expression of VSMCs. However, in the calcified + VSMCs co-culture
group, the scaffolds reduced the expression of α-SMA of VSMCs and
this phenomenon was more pronounced with time. Furthermore, OPN is
considered as a biomarker of VSMC phenotype switch and an active
player in the progression of vascular remodeling diseases such as
atherosclerosis (28). In the
cureent study, the expression of OPN in the calcified + VSMCs
co-culture group was significantly increased, compared with the
normal + VSMCs co-culture group. The normal decellularized arterial
scaffolds did not affect the expression of OPN in the normal +
VSMCs co-culture group; while in the calcified + VSMCs co-culture
group, the decellularized arterial scaffolds increased the
expression of OPN, and this phenomenon was more pronounced with
time.
To the best of our knowledge, the present study is
the first to investigate the impact of normal and calcified
decellularized arterial scaffolds on VSMCs. By constructing 3D
arterial decellularized biological scaffolds and combining these
with VSMCs, the impact of the changes in ECM on the VSMC phenotype
could be studied. This study provides a novel platform for VSMC
in vitro culture and associated disease mechanism
research.
In a further study, the mechanisms of VSMC
transformation induced by calcified arterial decellularized
scaffolds and normal scaffolds will be investigated. Furthermore,
the components and structures of the arterial decellularized
biological scaffolds that can be used as possible therapeutic
targets in vascular calcification will be explored.
In conclusion, arterial decellularized scaffolds may
affect the transformation of VSMCs by downregulating the expression
of α-SMA and upregulating the expression of OPN. Future studies
should be performed to investigate the overexpression of α-SMA or
knockdown of OPN to verify our hypothesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Natural Science Foundation (grant no. Q15H170003) and Zhejiang
Provincial Health Department Fund (grant no. 2017KY168).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LU and ZW wrote the first draft of the manuscript.
HX studied the literature and conceived the study. SW, PW, XT and
CX all performed in the experiments. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First People's Hospital of Taizhou.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Demer LL and Tintut Y: Vascular
calcification: pathobiology of a multifaceted disease. Circulation.
117:2938–2948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kay AM, Simpson CL and Stewart JA Jr: The
role of AGE/RAGE signaling in diabetes-mediated vascular
calcification. J Diabetes Res. 2016:68097032016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pugliese G, Iacobini C, Blasetti Fantauzzi
C and Menini S: The dark and bright side of atherosclerotic
calcification. Atherosclerosis. 238:220–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evrard S, Delanaye P, Kamel S, Cristol JP
and Cavalier E; SFBC/SN Joined Working Group on Vascular
Calcifications, : Vascular calcification: From pathophysiology to
biomarkers. Clin Chim Acta. 438:401–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harper E, Forde H, Davenport C, Rochfort
KD, Smith D and Cummins PM: Vascular calcification in type-2
diabetes and cardiovascular disease: Integrative roles for OPG,
RANKL and TRAIL. Vascul Pharmacol. 82:30–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan TH, Huang XQ and Tan HM: Vascular
fibrosis in atherosclerosis. Cardiovasc Pathol. 22:401–407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yahagi K, Kolodgie FD, Lutter C, Mori H,
Romero ME, Finn AV and Virmani R: Pathology of human coronary and
carotid artery atherosclerosis and vascular calcification in
diabetes mellitus. Arterioscler Thromb Vasc Biol. 37:191–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kapustin AN, Chatrou ML, Drozdov I, Zheng
Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT,
Alvarez-Hernandez D, et al: Vascular smooth muscle cell
calcification is mediated by regulated exosome secretion. Circ Res.
116:1312–1323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alexander MR and Owens GK: Epigenetic
control of smooth muscle cell differentiation and phenotypic
switching in vascular development and disease. Annu Rev Physiol.
74:13–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu LH, Huang L, Zhang X, Zhang P, Zhang
SM, Guan H, Zhang Y, Zhu XY, Tian S, Deng K and Li H: Mindin
regulates vascular smooth muscle cell phenotype and prevents
neointima formation. Clin Sci (Lond). 129:129–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruiz-Ortega M, Lorenzo O, Rupérez M,
Esteban V, Suzuki Y, Mezzano S, Plaza JJ and Egido J: Role of the
renin-angiotensin system in vascular diseases: Expanding thefield.
Hypertension. 38:1382–1387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB. 18:816–827. 2004. View Article : Google Scholar
|
|
13
|
Leask A, Holmes A and Abraham DJ:
Connective tissue growth factor: A new and important player in the
pathogenesis of fibrosis. Curr Rheumatol Rep. 4:136–142. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amălinei C, Căruntu ID, Giuşcă SE and
Bălan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.PubMed/NCBI
|
|
15
|
Eweida AM and Marei MK: Naturally
occurring extracellular matrix scaffolds for dermal regeneration:
Do they really need cells? Biomed Res Int. 2015:8396942015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lindberg K and Badylak SF: Porcine small
intestinal submucosa (SIS): A bioscaffold supporting in vitro
primary human epidermal cell differentiation and synthesis of
basement membrane proteins. Burns. 27:254–266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Badylak SF, Taylor D and Uygun K:
Whole-organ tissue engineering: Decellularization and
recellularization of three-dimensional matrix scaffolds. Annu Rev
Biomed Eng. 15:27–53. 2011. View Article : Google Scholar
|
|
18
|
Gessner RC, Hanson AD, Feingold S, Cashion
AT, Corcimaru A, Wu BT, Mullins CR, Aylward SR, Reid LM and Dayton
PA: Functional ultrasound imaging for assessment of extracellular
matrix scaffolds used for liver organoid formation. Biomaterials.
34:9341–9351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren H, Shi X, Tao L and Xiao J: Evaluation
of two decellularization methods in the development of a
whole-organ decellularized rat liver scaffold. Liver Int.
33:448–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sazonova OV, Isenberg BC, Herrmann J, Lee
KL, Purwada A, Valentine AD, Buczek-Thomas JA, Wong JY and Nugent
MA: Extracellular matrix presentation modulates vascular smooth
muscle cell mechanotransduction. Matrix Biol. 41:36–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Butler DL, Goldstein SA and Guilak F:
Functional tissue engineering: The role of biomechanics. J Biomech
Eng. 122:570–575. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steppan J, Bergman Y, Viegas K, Armstrong
D, Tan S, Wang H, Melucci S, Hori D, Park SY, Barreto SF, et al:
Tissue transglutaminase modulates vascular stiffness and function
through crosslinking-dependent and crosslinking-independent
functions. J Am Heart Assoc. 6(pii): e0041612017.PubMed/NCBI
|
|
23
|
Srivastava R, Zhang J, Go GW, Narayanan A,
Nottoli TP and Mani A: Impaired LRP6-TCF7L2 activity enhances
smooth muscle cell plasticity and causes coronary artery disease.
Cell Rep. 13:746–13759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lacolley P, Regnault V, Segers P and
Laurent S: Vascular smooth muscle and arterial stiffening:
Relevance in development, aging, and disease. Physiol Rev.
97:1555–1617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Badylak SF: The extracellular matrix as a
scaffold for tissue reconstruction. Semin Cell Dev Biol.
13:377–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han YL, Yan CH, Liu HW, Hu Y, Kang J, Wang
X and Shaohua L: Overexpression of the cellular repressor of
E1A-stimulated-genes regulate the rat primary VSMCs differentiation
in vitro. Prog Biochem Biophys. 31:1099–1105. 2004.
|
|
28
|
Lee SJ, Baek SE, Jang MA and Kim CD:
Osteopontin plays a key role in vascular smooth muscle cell
proliferation via EGFR-mediated activation of AP-1 and C/EBPβ
pathways. Pharmacol Res. 108:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|