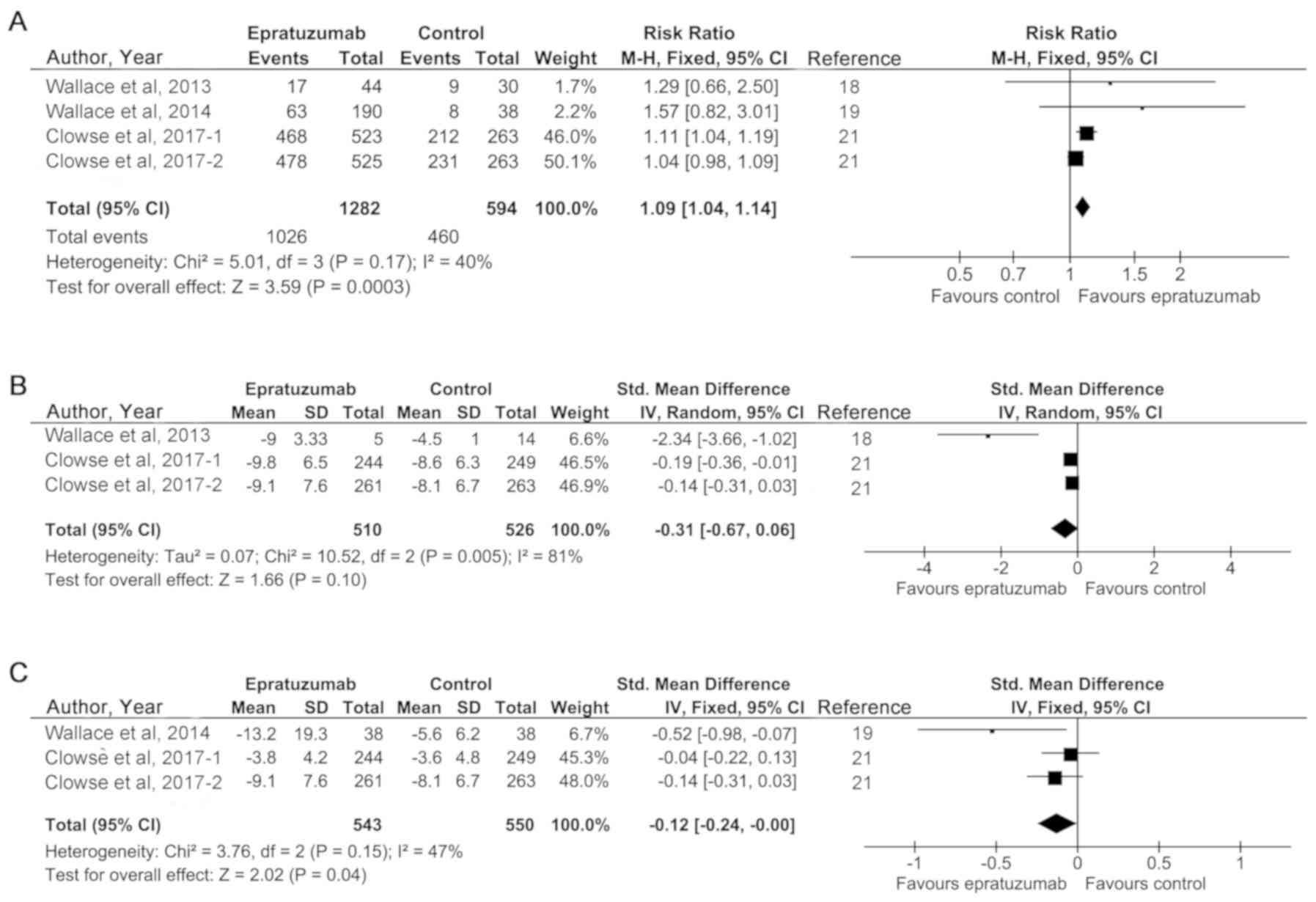
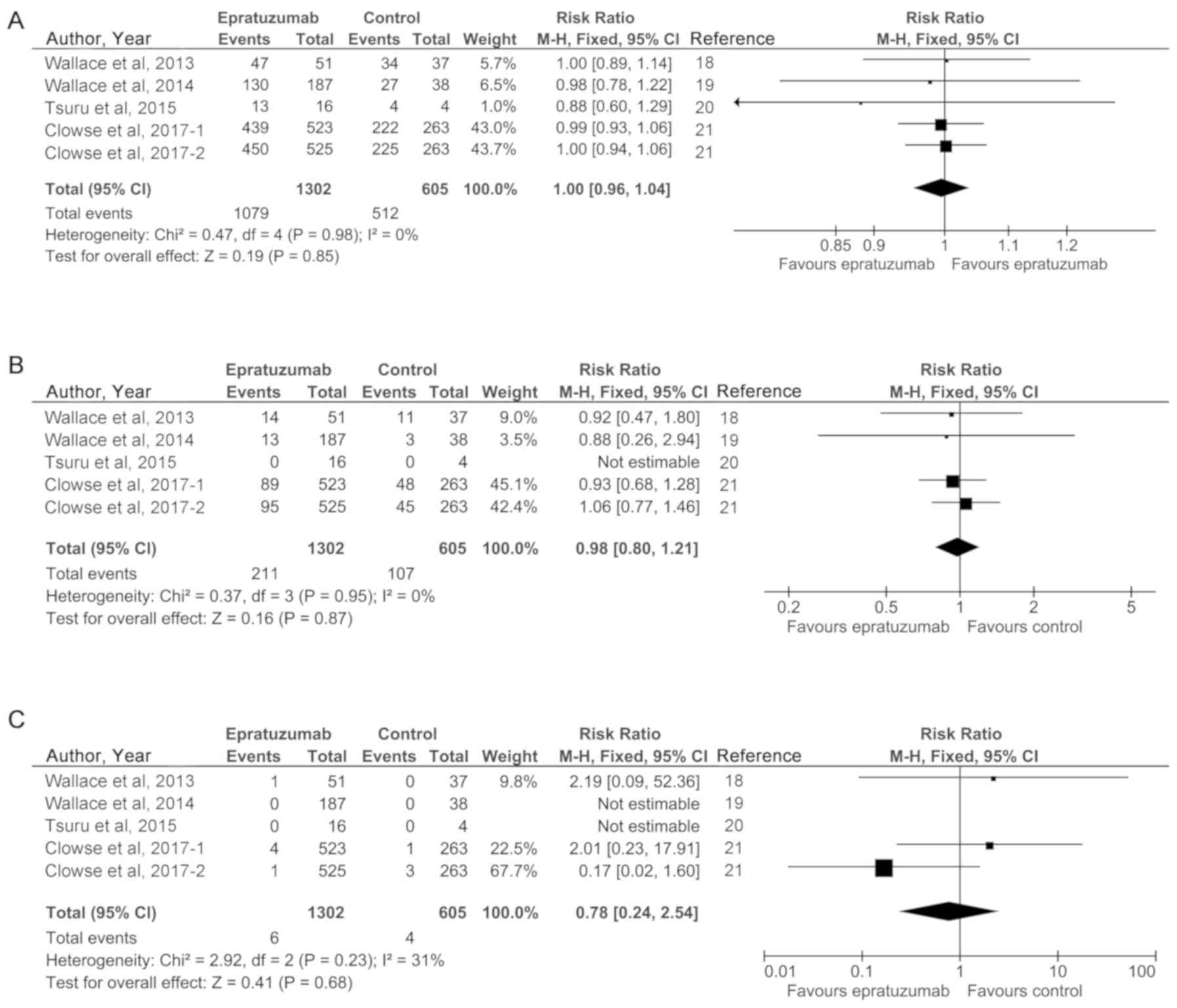
|
1
|
Uramoto KM, Michet CJ Jr, Thumboo J, Sunku
J, O'Fallon WM and Gabriel SE: Trends in the incidence and
mortality of systemic lupus erythematosus, 1950–1992. Arthritis
Rheum. 42:46–50. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim SS, Bayakly AR, Helmick CG, Gordon C,
Easley KA and Drenkard C: The incidence and prevalence of systemic
lupus erythematosus, 2002–2004: The Georgia Lupus Registry.
Arthritis Rheumatol. 66:357–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin H, Wei JC, Tan CY, Liu YY, Li YH, Li
FX, Deng DH, Yan B, Liu Y and Zhao Y: Survival analysis of
late-onset systemic lupus erythematosus: A cohort study in China.
Clin Rheumatol. 31:1683–1689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan JH, Hoh SF, Win MT, Chan YH, Das L and
Arkachaisri T: Childhood-onset systemic lupus erythematosus in
Singapore: Clinical phenotypes, disease activity, damage, and
autoantibody profiles. Lupus. 24:998–1005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cervera R, Khamashta MA, Font J,
Sebastiani GD, Gil A, Lavilla P, Mejía JC, Aydintug AO,
Chwalinska-Sadowska H, de Ramón E, et al: Morbidity and mortality
in systemic lupus erythematosus during a 10-year period: A
comparison of early and late manifestations in a cohort of 1,000
patients. Medicine (Baltimore). 82:299–308. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stockinger T, Richter L, Kanzler M,
Melichart-Kotik M, Pas H, Derfler K, Schmidt E and Rappersberger K:
Systemic lupus erythematosus: Unusual cutaneous manifestations.
Hautarzt. 67:970–981. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallace DJ: The evolution of drug
discovery in systemic lupus erythematosus. Nat Rev Rheumatol.
11:616–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borba HH, Wiens A, de Souza TT, Correr CJ
and Pontarolo R: Efficacy and safety of biologic therapies for
systemic lupus erythematosus treatment: Systematic review and
meta-analysis. BioDrugs. 28:211–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jovancevic B, Lindholm C and Pullerits R:
Anti B-cell therapy against refractory thrombocytopenia in SLE and
MCTD patients: Long-term follow-up and review of the literature.
Lupus. 22:664–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faurschou M and Jayne DR: Anti-B cell
antibody therapies for inflammatory rheumatic diseases. Annu Rev
Med. 65:263–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei LQ, Liang YG, Zhao Y, Liang HT, Qin DC
and She MC: Efficacy and safety of belimumab plus standard therapy
in patients with systemic lupus erythematosus: A meta-analysis.
Clin Ther. 38:1134–1140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan L, Han F and Chen JH: Efficacy and
safety of rituximab therapy for systemic lupus erythematosus: A
systematic review and meta-analysis. J Zhejiang Univ Sci B.
13:731–744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leonard JP and Link BK: Immunotherapy of
non-Hodgkin's lymphoma with hLL2 (epratuzumab, an anti-CD22
monoclonal antibody) and Hu1D10 (apolizumab). Semin Oncol.
29:81–86. 2002. View Article : Google Scholar
|
|
14
|
Dörner T, Shock A and Smith KG: CD22 and
autoimmune disease. Int Rev Immunol. 31:363–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rossi EA, Goldenberg DM, Michel R, Rossi
DL, Wallace DJ and Chang CH: Trogocytosis of multiple B-cell
surface markers by CD22 targeting with epratuzumab. Blood.
122:3020–3029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dorner T, Shock A, Goldenberg DM and
Lipsky PE: The mechanistic impact of CD22 engagement with
epratuzumab on B cell function: Implications for the treatment of
systemic lupus erythematosus. Autoimmun Rev. 14:1079–1086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dörner T, Kaufmann J, Wegener WA, Teoh N,
Goldenberg DM and Burmester GR: Initial clinical trial of
epratuzumab (humanized anti-CD22 antibody) for immunotherapy of
systemic lupus erythematosus. Arthritis Res Ther. 8:R742006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wallace DJ, Gordon C, Strand V, Hobbs K,
Petri M, Kalunian K, Houssiau F, Tak PP, Isenberg DA, Kelley L, et
al: Efficacy and safety of epratuzumab in patients with
moderate/severe flaring systemic lupus erythematosus: Results from
two randomized, double-blind, placebo-controlled, multicentre
studies (ALLEVIATE) and follow-up. Rheumatology (Oxford).
52:1313–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wallace DJ, Kalunian K, Petri MA, Strand
V, Houssiau FA, Pike M, Kilgallen B, Bongardt S, Barry A, Kelley L
and Gordon C: Efficacy and safety of epratuzumab in patients with
moderate/severe active systemic lupus erythematosus: Results from
EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled,
multicentre study. Ann Rheum Dis. 73:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuru T, Tanaka Y, Kishimoto M, Saito K,
Yoshizawa S, Takasaki Y, Miyamura T, Niiro H, Morimoto S, Yamamoto
J, et al: Safety, pharmacokinetics, and pharmacodynamics of
epratuzumab in Japanese patients with moderate-to-severe systemic
lupus erythematosus: Results from a phase 1/2 randomized study. Mod
Rheumatol. 26:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clowse ME, Wallace DJ, Furie RA, Petri MA,
Pike MC, Leszczyński P, Neuwelt CM, Hobbs K, Keiserman M, Duca L,
et al: Efficacy and safety of epratuzumab in moderately to severely
active systemic lupus erythematosus: Results from two phase III
randomized, double-blind, placebo-controlled trials. Arthritis
Rheumatol. 69:362–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao S and Huang S: Association between
vitamin D receptor gene BsmI, FokI, ApaI and TaqI polymorphisms and
the risk of systemic lupus erythematosus: A meta-analysis.
Rheumatol Int. 34:381–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahebkar A, Rathouska J, Derosa G,
Maffioli P and Nachtigal P: Statin impact on disease activity and
C-reactive protein concentrations in systemic lupus erythematosus
patients: A systematic review and meta-analysis of controlled
trials. Autoimmun Rev. 15:344–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hozo SP, Djulbegovic B and Hozo I:
Estimating the mean and variance from the median, range, and the
size of a sample. BMC Med Res Methodol. 5:132005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morawski PA and Bolland S: Expanding the B
cell-centric view of systemic lupus erythematosus. Trends Immunol.
38:373–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teruel M and Alarcόn-Riquelme ME: The
genetic basis of systemic lupus erythematosus: What are the risk
factors and what have we learned. J Autoimmun. 74:161–175. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dörner T and Lipsky PE: Beyond
pan-B-cell-directed therapy-new avenues and insights into the
pathogenesis of SLE. Nat Rev Rheumatol. 12:645–657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallace DJ and Goldenberg DM: Epratuzumab
for systemic lupus erythematosus. Lupus. 22:400–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carnahan J, Wang P, Kendall R, Chen C, Hu
S, Boone T, Juan T, Talvenheimo J, Montestruque S, Sun J, et al:
Epratuzumab, a humanized monoclonal antibody targeting CD22:
Characterization of in vitro properties. Clin Cancer Res.
9:3982S–3990S. 2003.PubMed/NCBI
|
|
30
|
Özgör L, Brandl C, Shock A and Nitschke L:
Epratuzumab modulates B-cell signaling without affecting B-cell
numbers or B-cell functions in a mouse model with humanized CD22.
Eur J Immunol. 46:2260–2272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fleischer V, Sieber J, Fleischer SJ, Shock
A, Heine G, Daridon C and Dörner T: Epratuzumab inhibits the
production of the proinflammatory cytokines IL-6 and TNF-α, but not
the regulatory cytokine IL-10, by B cells from healthy donors and
SLE patients. Arthritis Res Ther. 17:1852015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao V and Gordon C: Evaluation of
epratuzumab as a biologic therapy in systemic lupus erythematosus.
Immunotherapy. 6:1165–1175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al Rayes H and Touma Z: Profile of
epratuzumab and its potential in the treatment of systemic lupus
erythematosus. Drug Des Devel Ther. 8:2303–2310. 2014.PubMed/NCBI
|
|
34
|
Antoniu S: Epratuzumab for systemic lupus
erythematosus. Expert Opin Biol Ther. 14:1045–1047. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Onuora S: Systemic lupus erythematosus:
Epratuzumab not effective in phase III trials. Nat Rev Rheumatol.
12:6222016. View Article : Google Scholar
|
|
36
|
Achour A, Mankaï A, Thabet Y, Sakly W,
Braham F, Kechrid C, Bahri F, Bouajina E, Chouchène S, Haddad O and
Ghedira I: Systemic lupus erythematosus in the elderly. Rheumatol
Int. 32:1225–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rahman A and Isenberg DA: Systemic lupus
erythematosus. N Engl J Med. 358:929–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim KL, Jones AC, Brown NS and Powell RJ:
Urine neopterin as a parameter of disease activity in patients with
systemic lupus erythematosus: Comparisons with serum sIL-2R and
antibodies to dsDNA, erythrocyte sedimentation rate, and plasma C3,
C4 and C3 degradation products. Ann Rheum Dis. 52:429–435. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong H, Ni CX, Liu YZ, Zhang Y, Su WJ,
Lian YJ, Peng W and Jiang CL: Mindfulness meditation for insomnia:
A meta-analysis of randomized controlled trials. J Psychosom Res.
89:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Strauss SJ, Morschhauser F, Rech J, Repp
R, Solal-Celigny P, Zinzani PL, Engert A, Coiffier B, Hoelzer DF,
Wegener WA, et al: Multicenter phase II trial of immunotherapy with
the humanized anti-CD22 antibody, epratuzumab, in combination with
rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J
Clin Oncol. 24:3880–3886. 2006. View Article : Google Scholar : PubMed/NCBI
|