Introduction
Systemic lupus erythematosus (SLE) is a chronic,
archetypal, relapsing-remitting, multisystem autoimmune
inflammatory disease, with an estimated incidence of 5–50 cases per
100,000 people (1). This disease
affects somewhere between 200,000 and 500,000 people in the USA and
more than 1000,000 individuals in China, predominantly young women
in their reproductive years (2). It
is encouraging that the five-year survival rate for SLE has already
surpassed 80% (3,4). However, the mortality still cannot be
ignored, with nearly 10% risk of death within 10 years of diagnosis
(5). Various severe clinical
manifestations severely affect the life quality. Especially, up to
80% of SLE patients are suffering from various different
mucocutaneous symptoms, such as butterfly rash, oral ulcers,
chronic cutaneous lupus erythematosus, and increased
photosensitivity (6).
Over the past decade, targeted therapies for SLE
have lagged behind other autoimmune rheumatic disorders due to the
varying degrees in multiple organ systems (7). But the good news is a range of biologic
agents have shown their potential clinical effects in the
management of SLE (8). Since SLE is
characterized by polyclonal B cell hyper-reactivity that is
considered to be the most important pathogenic event (9). Recently, anti-B cell antibody therapies
have been widely investigated for the treatment of SLE, and have
exhibited encouraging results regarding their efficacy and safety
in clinical trials. Those reported anti-B cell agents mainly
include anti-CD20 antibodies (rituximab, ofatumumab, and
ocrelizumab), anti-CD22 antibody (epratuzumab), and
anti-BAFF-R/BCMA/TACI receptors agents (belimumab and atacicept)
(10). Among them, the effects of
rituximab and belimumab in SLE patients have been discussed in
several meta-analyses (8,11,12). On
the other hand, emerging studies, especially randomized controlled
trials (RCTs), emphasized the efficacy of epratuzumab for the
management of SLE.
As a member of the Siglec protein family, the CD22
membrane receptor is a B-lymphocyte-restricted adhesion molecule
that plays a role in B-cell activation and interaction with T cells
(13). The loss of CD22 function
could contribute to the pathogenesis of autoimmune diseases,
including SLE (14). Epratuzumab is
a humanized monoclonal antibody, which can rapidly induce a marked
decrease of CD22 (>80%) and slight inhibition of other proteins
from the B-cell surface in vitro, including CD19, CD21 and
CD79β (15,16). Initially, epratuzumab was used as a
promising immunotherapy for non-Hodgkin's lymphoma (13). But a few years later, a phase I trial
reported that the total BILAG (British Isles Lupus Activity Group)
score was decreased in 92% SLE participants after 18-week
epratuzumab treatment (17). After
that, more trials with larger samples and longer follow-up were
performed to evaluate the possibilities for improvement in SLE
patients. Wallace et al found that treatment with
epratuzumab was well tolerated in patients with moderately to
severely active SLE, and associated with improvements in disease
activity (18,19). Tsuru and colleagues also demonstrated
the inhibitory effects of epratuzumab on CD22 and B cell count in
SLE patients (20). However, Clowse
et al (21) argued that
epratuzumab did not result in improvements for SLE patients.
Although these results are variable and
controversial, up to now, no quantitatively systematic review has
been conducted in this field. Therefore, meta-analysis is urgently
required to summarize the available evidence to assess the
potential clinical effects of epratuzumab in the management of SLE
(22). The main aim of this
meta-analysis is to synthesize findings from published RCTs
assessing the efficacy and safety in SLE patients.
Materials and methods
Search strategy
We attempted to search the published RCTs that
investigated the effects of epratuzumab for the treatment of SLE
patients. The relevant studies were identified and selected by
searching the electronic databases of PubMed, Embase, and the
Cochrane Library (updated to March 2017). The following terms were
searched: ‘lupus’, ‘systemic lupus’, ‘systemic lupus
erythematosus’, ‘SLE’, ‘epratuzumab’, ‘anti-CD22 antibody’,
‘anti-CD22’, ‘CD22 targeted’ and ‘risk.’ Both the subject words,
such as Medical Subject Heading (MeSH) or Emtree terms, and random
words were used to retrieve the trials comprehensively. Terms were
moderately expanded within each electronic database whenever
necessary. There was no language restriction in the literature
search. With regard to published studies with overlapping data by
the same authors, we selected the most recent or complete study
only.
Inclusion and exclusion
Studies fulfilling the following inclusion criteria
were eligible for this meta-analysis: i) Evaluation of the
epratuzumab treatment for SLE; ii) reported the efficacy or safety
of epratuzumab; and iii) RCTs. The exclusion criteria of our
meta-analysis included i) studies unrelated to SLE; ii) review,
case report, conference abstract, or any other non-RCTs; iii)
interventions without epratuzumab; iv) duplicative or overlapping
publications; and v) trials with fewer than 10 patients. The
inclusion and exclusion criteria were confirmed according to the
results of searching.
Data extraction
The whole process of data extraction was completed
by a single investigator (J.L.) to assure uniformity of data and
then re-evaluated by a second investigator (M.-M.W.). Any
disagreement was resolved by consensus. The following data from
articles that met criteria were abstracted: i) First author's name;
ii) publish year; iii) sample size; iv) age of the participants; v)
percent of women participants; vi) patient characteristics; vii)
interventions (dose and methods of administration); viii) control
condition; ix) follow-up duration (endpoint); and x) outcome
measures for efficacy and safety. It should be noted that we
utilized the data for meta-analysis at the highest dose of
epratuzumab (720–3,600 mg) and the longest endpoint (12 or 48
weeks).
Assessment of quality
We independently graded the strength of evidence for
each outcome in accordance with the Cochrane criteria (23) (J.L. and Q.S.). This quality
assessment system was composed of the adequacy of sequence
generation, allocation concealment, blinding, addressing of
dropouts (incomplete outcome data), selective outcome reporting,
and other potential sources of bias. An assessment of ‘yes’
indicated a low risk of bias, while ‘no’ indicated a high risk of
bias. Labeling an item as ‘unclear’ indicated an unclear or unknown
risk of bias (23). Ethical approval
or additional consent from participants was not provided since this
study was a systematic review of published studies. Ethical
approval or additional consent from patients was not provided
because the present study was a literature review of previously
reported studies.
Statistical analysis
All data analysis was performed with the Review
Manager 5.3 software from the Cochrane Collaboration. Unadjusted
risk ratios (RRs) with a 95% confidence interval (CI) were
calculated for binary outcomes. For continuous variables,
standardized mean differences (SMDs) and 95% CIs were calculated by
measuring the post-treatment difference between the mean of the
epratuzumab treatment and the mean of control condition, divided by
the pooled standard deviation (SD). When necessary, the mean and SD
were estimated from the median, range, and the size of a sample
according to Hozo's formulas (24).
The effect of heterogeneity between studies rather than sampling
error was quantified by using an I2 statistic as well as
a P-value. If I2 ≥50% or P-value ≤0.1, a random effect
model was adopted. Otherwise, a fixed effect model was used. Funnel
plots were not performed to evaluate publication bias due to the
limited number of included studies. P-value ≤0.5 was considered as
the significance level.
Results
Study selection
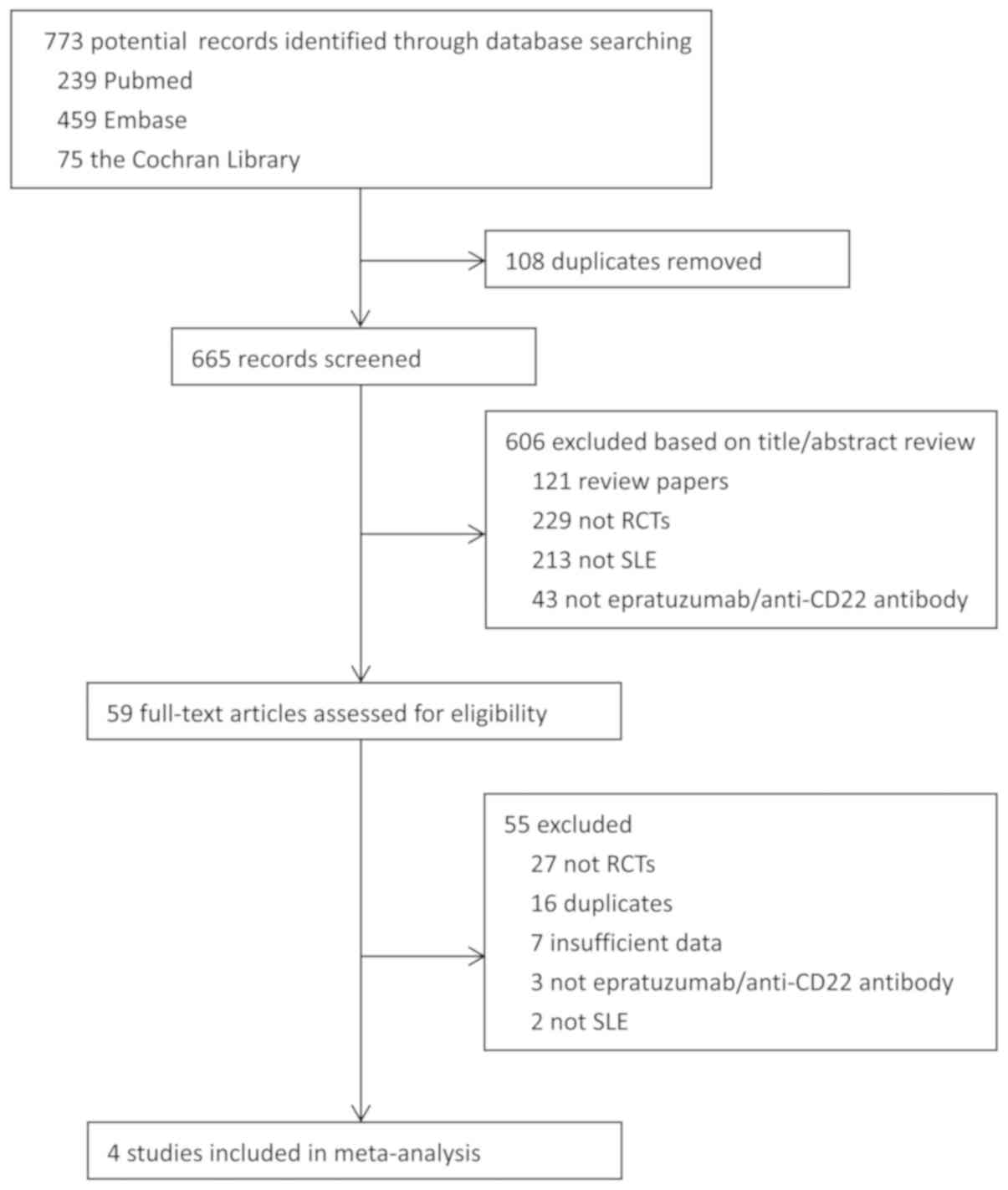
The process of selection is shown in a PRISMA flow
diagram (Fig. 1). Our search
returned 773 publications and abstracts, of which 108 were
identified as duplicates using Endnote X7 software and excluded. A
total of 600 studies were removed based on title or abstract
review. The remaining publications were further identified by
reading the full text. After careful assessment, four articles
(five trials) met the inclusion criteria and were selected for
meta-analysis (18–21). Specifically, three studies resulted
eligible to evaluate effectiveness (18,19,21), and
four resulted eligible to evaluate safety (18–21).
Study characteristics
The characteristics of the included trials are
summarized in Table I. In brief, all
studies were published from 2013 to 2017. A total of 1,921 subjects
were included in the four eligible RCTs, comprising the epratuzumab
group and the control group. The largest study had a population
size of 1,584 subjects (21), while
the smallest study recruited 20 subjects (20). It is worth mentioning that two RCTs
(EMBODY 1 and EMBODY 2), 793 patients and 791 patients,
respectively, were included in the largest study (21). Therefore, EMBODY 1 and EMBODY 2 were
analyzed respectively in the meta-analysis. All studies recruited
moderate-to-severe SLE participants aged ≥18 years. The percentage
of female patients is more than 90%. A wide range of dosages of
epratuzumab (100–3,600 mg every week or every other week) was used
in the included trials. Only the highest dosage in the original
trial was utilized for analysis. The control conditions were
placebo (20) or placebo + standard
of care (SOC) (18,19,21).
Duration of studies ranged between 12 weeks (19,20) and
48 weeks (18,21). All studies were designed as
randomized, double-blind, placebo-controlled trials.
 | Table I.General characteristics of the
studies included in the meta-analysis. |
Table I.
General characteristics of the
studies included in the meta-analysis.
| Author, year | Sample size | Age (years), %
women | Patient
characteristics | Interventions | Control
condition | Follow-up,
duration | Outcome
measures | (Refs.) |
|---|
| Wallace et al,
(2013) | 90 | ≥18, 94.4 | Moderate/severe
flaring SLE | Epratuzumab
(360–720 mg/m2) + SOC | Placebo + SOC | 48 weeks | BILAG, AE,
SAE, | (18) |
| Wallace et al,
(2014) | 227 | ≥18, 95.2 | Moderate/severe
flaring SLE | Epratuzumab
(100–3,600 mg, every other week) + SOC | Placebo + SOC | 12 weeks | BILAG, AE, SAE,
SLEDAI-2K, B-cell count, CD22 | (19) |
| Tsuru et al,
(2015) | 20 | 18–65, 90.0 | Moderate-to-severe
SLE | Epratuzumab
(100–1,200 mg, every or every other week) | Placebo | 12 weeks | AE, SAE,
pharmacokinetic parameters, CD20, CD22, CD3 | (20) |
| Clowse et al,
(2017) | 1,584 | ≥18, 90.2 | Moderate-to-severe
active SLE | Epratuzumab (600
mg, every week; 1,200 mg, every other week) | Placebo + SOC | 48 weeks | BICLA, BILAG,
SLEDAI-2K, CD19, CD3, dsDNA, C3, C4, AE | (21) |
Study quality
Overall, three trials (75%) had adequate sequence
generation and adequate concealed allocation (19–21). All
selected studies used blinding and reported the complete and
unselective outcomes. Nevertheless, other potential sources of bias
were sufficiently addressed by some included trials. Although
randomization has been conducted before grouping, the baseline
condition of one trial was not very consistent (19). One study had a high drop-out rate
(>30%), which may be resulted from the long follow-up duration
(21). Table II presents the summary details on
the risk of bias among included trials.
 | Table II.Risk of bias assessment in the
studies considered for meta-analysis. |
Table II.
Risk of bias assessment in the
studies considered for meta-analysis.
| Author, year | Random sequence
generation | Allocation
concealment | Blinding | Incomplete outcome
data | Selective
reporting | Free of other
bias | (Refs.) |
|---|
| Wallace et al,
2013 | U | U | L | L | L | L | (18) |
| Wallace et al,
2014 | L | L | L | L | L | H | (19) |
| Tsuru et al,
2015 | L | L | L | L | L | L | (20) |
| Clowse et al,
2017 | L | L | L | L | L | H | (21) |
Efficacy of epratuzumab for SLE
Since not every included trial reported follow-up
effects and there were also differences in the follow-up time, the
present meta-analysis aimed to evaluate the immediate
post-treatment effects of epratuzumab. Some tools can be used as
the efficacy variables for SLE, such as British Isles Lupus
Assessment Group index (BILAG), the BILAG-based Combined Lupus
Assessment (BICLA), SLE Disease Activity Index 2000 (SLEDAI-2K),
Systemic Lupus International Collaborating Clinics American College
of Rheumatology (SLICC ACR), and Safety of Estrogens in Systemic
Lupus Erythematosus National Assessment-Systemic Lupus
Erythematosus Disease Activity Index (SELENA-SLEDAI). Any measured
parameters for disease activity were pooled in the meta-analysis if
they were conducted not less than 2 trials. Therefore, BICLA
response, BILAG score and SLEDA-2K score were analyzed in the
review.
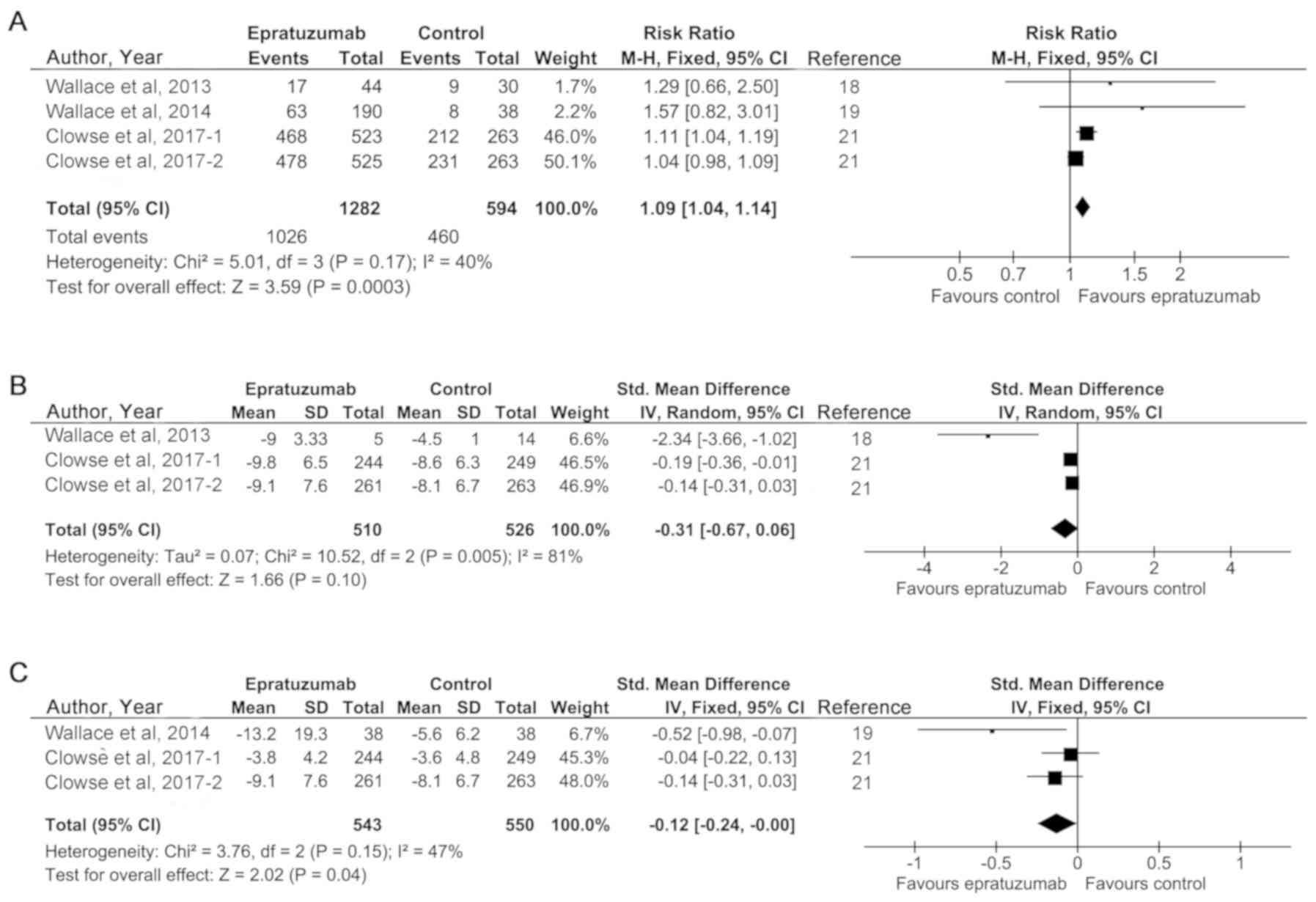
The results showed that there were not
significant heterogeneities in BICLA response and SLEDA-2K score,
as was evident from I2<50%
Thus, fixed effect models were adopted in these
analyses. However, in the analysis of BILAG score, a significant
heterogeneity was in existence and a random effect model was used.
As shown in Fig. 2A, pooling trials
that reported BICLA response got an RR of 1.09 (95% CI, 1.04 to
1.14), which was a significant effect in favor of epratuzumab
(P=0.0003). However, the mean effect size for BILAG score was
slightly but not markedly in favor of epratuzumab (SMD=−0.31; 95%
CI, −0.67 to 0.06; P=0.10) (Fig.
2B). The results of SLEDA-2K score indicated that epratuzumab
could significantly improve SLE disease activity (SMD=−0.12; 95%
CI, −0.24 to 0.00; P=0.04) (Fig.
2C). These findings show that epratuzumab has greater
therapeutic effectiveness than placebo control condition in SLE
patients. Subgroup meta-analyses of various ethnicities, treatment
durations, and drug concentrations were not performed due to the
limited amount of RCTs.
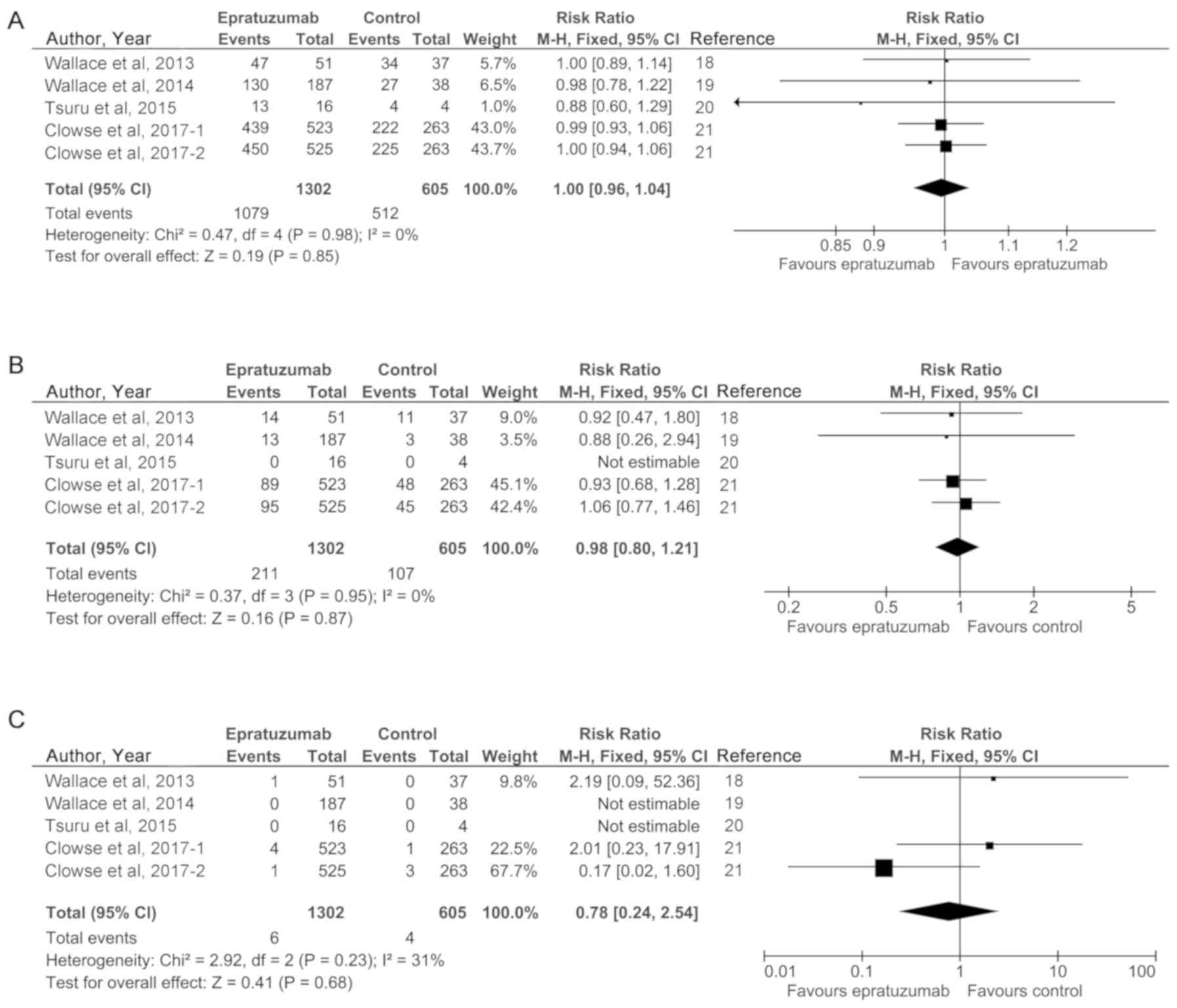
Safety of epratuzumab for SLE
The safety of epratuzumab therapy on SLE disease
activity was reported in four randomized controlled studies (five
trials). Here, we conducted the meta-analyses of adverse event
(AE), serious adverse event (SAE) and death to reflect the safety
of epratuzumab treatment. The most common AEs in patients included
headache, nausea, upper respiratory tract infection, and so on. An
absence of heterogeneity among the studies included in these
analyses was observed (I2<50%). Therefore, fixed
effect models were used in the safety analyses. Statistical
analyses revealed the absence of statistically significant
differences between the epratuzumab group and control group,
indicating the excellent safety profile of this drug for SLE
treatment. In detail, the pooled results were (RR=1.00; 95% CI,
0.96 to 1.04; P=0.85) for AE, (RR=0.98; 95% CI, 0.80 to 1.21;
P=0.87) for SAE, and (RR=0.78; 95% CI, 0.24 to 2.54; P=0.68) for
death. These results are presented in Fig. 3A-C, respectively.
Discussion
SLE is a multiorgan autoimmune disease influenced by
various interdependent cellular pathways and complex genetics
(25,26). B-lineage cells can induce specific
immune responses whereby their antigen-presenting effect, and
respond to antigenic challenge as effector cells of the humoral
immune response. Therefore, they are regarded as the pivotal
regulator involved in the pathogenesis of SLE (27). Besides anti-CD20 therapy, increasing
studies have focused on the safety and activity of humanized
anti-CD22 antibody epratuzumab for SLE since the initial clinical
trial conducted by Dörner et al in 2006 (17).
Epratuzumab can evoke CD22 phosphorylation and
restrictedly bind to B-lymphomas and B-lymphocytes, consistent with
the expression of CD22 (28,29). In a rodent model, epratuzumab takes a
pivotal role in regulating the signaling transduction pathways in B
cells, while the number and functions of B cells do not change
significantly (30). It has been
proved that the epratuzumab takes its immunosuppressive action via
inhibiting the production of some proinflammatory cytokines in B
cells, such as IL-6 and TNF-α (31).
In the past few years, several systematic reviews have evaluated
the potential clinic effects of epratuzumab as a biologic therapy
in SLE, and shown the great promise with safe profiles and quality
of life (32–34). However, a recent phase III trial
demonstrated that epratuzumab was not effective in SLE (35). For the included RCTs, some RCTs
reported the positive effects of epratuzumab in SLE patients
(18,19), while Clowse and the colleagues argued
that epratuzumab treatment with SOC did not lead to improvements in
response rates over that observed in the placebo treatment with SOC
(21).
Since there are some controversially reported data
about epratuzumab therapy in SLE patients, the aim of the present
work was to comprehensively determine the efficacy and safety of
epratuzumab in patients with SLE. Using an exhaustive search
strategy, we identified four high-quality clinic studies (five
RCTs) to evaluate the intervention of epratuzumab for SLE. This
meta-analysis demonstrates that epratuzumab may contribute to the
improvements in SLE disease activity. Specifically, compared to
control groups, epratuzumab significantly increased the BICLA
response and reduced the SLEDA-2K score. Although BILAG score did
not reach to statistically significant change in epratuzumab group,
the BILAG score did decrease markedly compared with control group
(SMD=−0.31; 95% CI, −0.67 to 0.06; P=0.10). This result may be
affected by the large heterogeneity, which can hardly be explored
due to the limited trials. On the other hand, epratuzumab is
relatively safe compared with control group. The incidence of AEs,
SAEs, and deaths was similar between epratuzumab and control group
(all P-values >0.05), and no additional safety signals were
found in the original studies after epratuzumab treatment.
Aaccording to Wallace et al (19), treatment with epratuzumab 2,400 mg
cumulative dose was well tolerated in SLE patients, and associated
with improvements in disease activity.
It should also be noted that the dosage and
administration frequency of epratuzumab are quite variable in
different included trials, ranging from 100 to 3,600 mg and once to
twice a week. In the present meta-analysis, we chose the highest
dosage to summarize the effects of epratuzumab. If we focus on
Fig. 2, we can find that the
treatment outcomes in the studies of Wallace et al (19) are much better than other studies.
After analyzing the study design among these trials, we find that
infusion of 600 mg epratuzumab once a week for four consecutive
weeks (cumulative dose 2,400 mg) may be an acceptable therapeutic
strategy of epratuzumab for SLE patients.
To ensure the high quality, the present
meta-analysis only identified RCTs. As shown in Table I, all included RCTs were published in
recent five years (2013–2017). Since women were more susceptible to
SLE than men (36,37), the included female patients accounted
for more than 90%. The inclusion criteria and exclusion criteria of
these RCTs were similar, although a great diversity of diagnostic
methods were used, including ACR, BILAG index, SLEDAI-2K, SLEDAI.
All participants were more than 18 years old and suffering from
moderate-to-severe SLE. Most studies had low drop-out rates except
one (21). All the RCTs were phase
I/II blinding trials, recruiting various populations including
Caucasian, Black, Asian, Hispanic/Latino and Mixed race. We can
also found that the heterogeneities among various data are so small
as to be ignorable in most comparisons except BILAG score
(I2=81%, P=0.005). Therefore, take together, the
included studies are of high quality, and the findings of
meta-analysis are reliable, valid, and robust.
Although the results of this study suggest some
promising clinical benefits of epratuzumab therapy for SLE, some
limitations that may reduce our ability to draw conclusions from
these findings should also be acknowledged. First of all, different
studies reported different outcomes to reflect the efficacy of
epratuzumab, which make them difficult to blend in the
meta-analysis. Especially, SLE-associated laboratory parameters
(such as C3, C4 levels, and dsDNA) (38) cannot be used to conduct a
meta-analysis. Further studies in this field should take the same
measurement criteria to detect the efficacy and safety for SLE,
such as SLEDAI-2K and BILAG-2004. Besides, the number of published
RCTs included in our meta-regression analysis would affect the
results of this study. The relatively small samples limit the
ability of the Egger's test and funnel plot to show publication
bias and may lead to false positive and negative conclusions
(39). Furthermore, most
participants were divided in either epratuzumab + SOC group or
placebo + SOC group. Nevertheless, one trial did not clearly state
the control condition (20). The
different condition of the control group may also affect our
conclusion. Finally, the different dosages of epratuzumab
(100–3,600 mg) and endpoints of treatment (12–48 weeks) may also
affect the meta-analysis results.
Taken together, our meta-analysis demonstrates that
anti-CD22 antibody epratuzumab may be an effective option for the
treatment of moderately-to-severely active SLE. And epratuzumab
appears to have an acceptable safety profile in these patients.
Further RCTs with large sample size and long follow-up duration are
needed to verify the effects of epratuzumab. We also expect that
the comparisons between epratuzumab and other anti-B cell therapies
can be focused to select the better one. It is also important to
explore the efficacy of combination therapy, which has been shown
to be effective in non-Hodgkin's lymphoma (40).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and MMW contributed to the study design, data
extraction, quality assessment, analysis and interpretation of
data, and drafting of the manuscript. QS contributed to the study
design, quality assessment and analysis, interpretation of data and
revising the article. XHG contributed to the study design, data
extraction, and analysis and interpretation of data. LS and YL
contributed to the conception of the study, study design and
revising the article. All authors proofread and approved the
submitted version of the article.
Ethics approval and consent to
participate
This study was conducted based on published data. No
ethics approval and consent to participate was required for this
study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Uramoto KM, Michet CJ Jr, Thumboo J, Sunku
J, O'Fallon WM and Gabriel SE: Trends in the incidence and
mortality of systemic lupus erythematosus, 1950–1992. Arthritis
Rheum. 42:46–50. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim SS, Bayakly AR, Helmick CG, Gordon C,
Easley KA and Drenkard C: The incidence and prevalence of systemic
lupus erythematosus, 2002–2004: The Georgia Lupus Registry.
Arthritis Rheumatol. 66:357–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin H, Wei JC, Tan CY, Liu YY, Li YH, Li
FX, Deng DH, Yan B, Liu Y and Zhao Y: Survival analysis of
late-onset systemic lupus erythematosus: A cohort study in China.
Clin Rheumatol. 31:1683–1689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan JH, Hoh SF, Win MT, Chan YH, Das L and
Arkachaisri T: Childhood-onset systemic lupus erythematosus in
Singapore: Clinical phenotypes, disease activity, damage, and
autoantibody profiles. Lupus. 24:998–1005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cervera R, Khamashta MA, Font J,
Sebastiani GD, Gil A, Lavilla P, Mejía JC, Aydintug AO,
Chwalinska-Sadowska H, de Ramón E, et al: Morbidity and mortality
in systemic lupus erythematosus during a 10-year period: A
comparison of early and late manifestations in a cohort of 1,000
patients. Medicine (Baltimore). 82:299–308. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stockinger T, Richter L, Kanzler M,
Melichart-Kotik M, Pas H, Derfler K, Schmidt E and Rappersberger K:
Systemic lupus erythematosus: Unusual cutaneous manifestations.
Hautarzt. 67:970–981. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallace DJ: The evolution of drug
discovery in systemic lupus erythematosus. Nat Rev Rheumatol.
11:616–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borba HH, Wiens A, de Souza TT, Correr CJ
and Pontarolo R: Efficacy and safety of biologic therapies for
systemic lupus erythematosus treatment: Systematic review and
meta-analysis. BioDrugs. 28:211–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jovancevic B, Lindholm C and Pullerits R:
Anti B-cell therapy against refractory thrombocytopenia in SLE and
MCTD patients: Long-term follow-up and review of the literature.
Lupus. 22:664–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faurschou M and Jayne DR: Anti-B cell
antibody therapies for inflammatory rheumatic diseases. Annu Rev
Med. 65:263–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei LQ, Liang YG, Zhao Y, Liang HT, Qin DC
and She MC: Efficacy and safety of belimumab plus standard therapy
in patients with systemic lupus erythematosus: A meta-analysis.
Clin Ther. 38:1134–1140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan L, Han F and Chen JH: Efficacy and
safety of rituximab therapy for systemic lupus erythematosus: A
systematic review and meta-analysis. J Zhejiang Univ Sci B.
13:731–744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leonard JP and Link BK: Immunotherapy of
non-Hodgkin's lymphoma with hLL2 (epratuzumab, an anti-CD22
monoclonal antibody) and Hu1D10 (apolizumab). Semin Oncol.
29:81–86. 2002. View Article : Google Scholar
|
|
14
|
Dörner T, Shock A and Smith KG: CD22 and
autoimmune disease. Int Rev Immunol. 31:363–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rossi EA, Goldenberg DM, Michel R, Rossi
DL, Wallace DJ and Chang CH: Trogocytosis of multiple B-cell
surface markers by CD22 targeting with epratuzumab. Blood.
122:3020–3029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dorner T, Shock A, Goldenberg DM and
Lipsky PE: The mechanistic impact of CD22 engagement with
epratuzumab on B cell function: Implications for the treatment of
systemic lupus erythematosus. Autoimmun Rev. 14:1079–1086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dörner T, Kaufmann J, Wegener WA, Teoh N,
Goldenberg DM and Burmester GR: Initial clinical trial of
epratuzumab (humanized anti-CD22 antibody) for immunotherapy of
systemic lupus erythematosus. Arthritis Res Ther. 8:R742006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wallace DJ, Gordon C, Strand V, Hobbs K,
Petri M, Kalunian K, Houssiau F, Tak PP, Isenberg DA, Kelley L, et
al: Efficacy and safety of epratuzumab in patients with
moderate/severe flaring systemic lupus erythematosus: Results from
two randomized, double-blind, placebo-controlled, multicentre
studies (ALLEVIATE) and follow-up. Rheumatology (Oxford).
52:1313–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wallace DJ, Kalunian K, Petri MA, Strand
V, Houssiau FA, Pike M, Kilgallen B, Bongardt S, Barry A, Kelley L
and Gordon C: Efficacy and safety of epratuzumab in patients with
moderate/severe active systemic lupus erythematosus: Results from
EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled,
multicentre study. Ann Rheum Dis. 73:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuru T, Tanaka Y, Kishimoto M, Saito K,
Yoshizawa S, Takasaki Y, Miyamura T, Niiro H, Morimoto S, Yamamoto
J, et al: Safety, pharmacokinetics, and pharmacodynamics of
epratuzumab in Japanese patients with moderate-to-severe systemic
lupus erythematosus: Results from a phase 1/2 randomized study. Mod
Rheumatol. 26:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clowse ME, Wallace DJ, Furie RA, Petri MA,
Pike MC, Leszczyński P, Neuwelt CM, Hobbs K, Keiserman M, Duca L,
et al: Efficacy and safety of epratuzumab in moderately to severely
active systemic lupus erythematosus: Results from two phase III
randomized, double-blind, placebo-controlled trials. Arthritis
Rheumatol. 69:362–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao S and Huang S: Association between
vitamin D receptor gene BsmI, FokI, ApaI and TaqI polymorphisms and
the risk of systemic lupus erythematosus: A meta-analysis.
Rheumatol Int. 34:381–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahebkar A, Rathouska J, Derosa G,
Maffioli P and Nachtigal P: Statin impact on disease activity and
C-reactive protein concentrations in systemic lupus erythematosus
patients: A systematic review and meta-analysis of controlled
trials. Autoimmun Rev. 15:344–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hozo SP, Djulbegovic B and Hozo I:
Estimating the mean and variance from the median, range, and the
size of a sample. BMC Med Res Methodol. 5:132005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morawski PA and Bolland S: Expanding the B
cell-centric view of systemic lupus erythematosus. Trends Immunol.
38:373–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teruel M and Alarcόn-Riquelme ME: The
genetic basis of systemic lupus erythematosus: What are the risk
factors and what have we learned. J Autoimmun. 74:161–175. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dörner T and Lipsky PE: Beyond
pan-B-cell-directed therapy-new avenues and insights into the
pathogenesis of SLE. Nat Rev Rheumatol. 12:645–657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallace DJ and Goldenberg DM: Epratuzumab
for systemic lupus erythematosus. Lupus. 22:400–405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carnahan J, Wang P, Kendall R, Chen C, Hu
S, Boone T, Juan T, Talvenheimo J, Montestruque S, Sun J, et al:
Epratuzumab, a humanized monoclonal antibody targeting CD22:
Characterization of in vitro properties. Clin Cancer Res.
9:3982S–3990S. 2003.PubMed/NCBI
|
|
30
|
Özgör L, Brandl C, Shock A and Nitschke L:
Epratuzumab modulates B-cell signaling without affecting B-cell
numbers or B-cell functions in a mouse model with humanized CD22.
Eur J Immunol. 46:2260–2272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fleischer V, Sieber J, Fleischer SJ, Shock
A, Heine G, Daridon C and Dörner T: Epratuzumab inhibits the
production of the proinflammatory cytokines IL-6 and TNF-α, but not
the regulatory cytokine IL-10, by B cells from healthy donors and
SLE patients. Arthritis Res Ther. 17:1852015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao V and Gordon C: Evaluation of
epratuzumab as a biologic therapy in systemic lupus erythematosus.
Immunotherapy. 6:1165–1175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al Rayes H and Touma Z: Profile of
epratuzumab and its potential in the treatment of systemic lupus
erythematosus. Drug Des Devel Ther. 8:2303–2310. 2014.PubMed/NCBI
|
|
34
|
Antoniu S: Epratuzumab for systemic lupus
erythematosus. Expert Opin Biol Ther. 14:1045–1047. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Onuora S: Systemic lupus erythematosus:
Epratuzumab not effective in phase III trials. Nat Rev Rheumatol.
12:6222016. View Article : Google Scholar
|
|
36
|
Achour A, Mankaï A, Thabet Y, Sakly W,
Braham F, Kechrid C, Bahri F, Bouajina E, Chouchène S, Haddad O and
Ghedira I: Systemic lupus erythematosus in the elderly. Rheumatol
Int. 32:1225–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rahman A and Isenberg DA: Systemic lupus
erythematosus. N Engl J Med. 358:929–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim KL, Jones AC, Brown NS and Powell RJ:
Urine neopterin as a parameter of disease activity in patients with
systemic lupus erythematosus: Comparisons with serum sIL-2R and
antibodies to dsDNA, erythrocyte sedimentation rate, and plasma C3,
C4 and C3 degradation products. Ann Rheum Dis. 52:429–435. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong H, Ni CX, Liu YZ, Zhang Y, Su WJ,
Lian YJ, Peng W and Jiang CL: Mindfulness meditation for insomnia:
A meta-analysis of randomized controlled trials. J Psychosom Res.
89:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Strauss SJ, Morschhauser F, Rech J, Repp
R, Solal-Celigny P, Zinzani PL, Engert A, Coiffier B, Hoelzer DF,
Wegener WA, et al: Multicenter phase II trial of immunotherapy with
the humanized anti-CD22 antibody, epratuzumab, in combination with
rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J
Clin Oncol. 24:3880–3886. 2006. View Article : Google Scholar : PubMed/NCBI
|