Introduction
Atherosclerosis (AS) is the main cause of myocardial
infarction, cerebrovascular accident or peripheral vascular
disease, leading to death from cardiovascular disease (1). AS is considered to be an inflammatory
disease caused by high plasma cholesterol levels and hypertension
(2). Many studies have revealed that
the formation and development of atherosclerotic plaques are
closely related to neovascularization (3,4).
Inflammatory cell aggregation results from the formation of
neovascularization, which may cause plaque hemorrhage and plaque
rupture. Therefore, the reduction of neovascularization in plaque
can be regarded as an important target for the treatment of AS
plaque (5). The formation and
development of atherosclerotic plaque are closely related to the
expression and activity of inflammatory markers. Therefore, the
study of related inflammatory markers has become a hot topic to
study the occurrence and development of atheromatous plaques
(6).
The pathological process of AS is closely related to
the process of inflammation, which is a slow inflammatory process
caused many inflammatory factors in the body, such as interleukin
(IL)-23 and IL-17 (7). IL-17, one of
the ILs, was secreted by Th17 (8).
Th17 is mainly produced in the thymus, some of which are
transformed from other cells (9).
Previous studies suggested that IL-17 could accelerate the progress
of intracranial AS plaques, affecting the formation of thrombus, or
even the stability of plaques (10).
IL-18 is a multipotent proinflammatory cytokine and inflammation
marker, which is related to the occurrence and development of AS
(11). Animal studies (12,13)
suggested that IL-18 is a strong AS-causing factor that promotes
the increase of atherosclerotic plaque areas and enhances
interferon and T lymphocyte in the plaque, suggesting that IL-18
can induce AS and increase the instability of plaques.
To the best of our knowledge, there is currently no
study on the expression and correlation of IL-17 and IL-18 in AS
mice. This study investigated the relationship between the
expression of IL-17 and IL-18 and the stability of plaque, and the
correlation of IL-17 and IL-18 in AS.
Materials and methods
Establishment of experimental animal
models
A total of 60 8-week-old Apo E (Apo E-/-) male mice
(10–15 g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China) [License no. SCXK (Beijing)
2012-0001] and used as the model group. A total of 20 wild male
C57BL/6 mice (10–15 g) were used as the control group. All animals
were raised in Specific Pathogen-Free (SPF) system of Tongji
Medical College, Huazhong University of Science and Technology
(Wuhan, China) [License no. SYXK (E) 2010–0028]. Mice were allowed
to acclimatize for 1 week. Mice in the control group were fed with
the basic diet and mice in the model group were fed with high fat
diet [10% lard, 5% white sugar, 4% cholesterol, 0.5% sodium
cholate, 0.2% propylthiouracil (PTU), 80.3% basic diet]. Vitamin D3
(150,000 U/kg) was injected into abdominal cavity each month.
Feeding conditions were as follows: room temperature at 21±2°C,
humidity at 63±5%, free drinking water from tap water, controlled
indoor lighting from day (8:00-20:00) to night (20:00-8:00) for 20
weeks. The experimental animals were nursed according to protocol
synopsis instructions - institutional animal care and use from the
Institute of Basic Theory for Chinese Medicine, Chinese Academy of
Chinese Medicine Science. Animal studies in the experiment were
approved by the Medical Ethics Committee of The Affiliated Hospital
to Changchun University of Chinese Medicine (Changchun, China).
Laboratory reagents and
instruments
Automatic biochemical analyzer (PUZS-300; Shanghai
Huanxi Medical Instrument Co., Ltd., Shanghai, China), color
Doppler ultrasonic diagnostic apparatus (DC-N2S; Beijing Mindray,
Beijing, China), mouse IL-18 and IL-17 ELISA kits (Shanghai Yanjin
Biological Science and Technology Co., Ltd., Shanghai, China),
automatic quantitative enzyme marker (352; LabSystems Multiskan MS
Helsinki, Finland), centrifuge [KDC-40; Anhui University of Science
and Technology of China (USTC) Innovation Co., Ltd., Huainan,
China], and Olympus microscope; Olympus Co., (Tokyo, Japan) were
used in the present study.
Serum extraction
The weight of mice was recorded and observed each
week. Mice were fasted before death and weighed. Blood was
collected from the orbit and kept at room temperature for 1 h, and
centrifuged at 2,500 × g for 15 min at 4°C to obtain the
supernatant and then put into EP tube to get the serum sample,
which was stored in a refrigerator at −80°C to detect serum IL-17,
IL-18 and blood lipids.
Determination of blood lipids
Blood lipids in the separated serum was tested by
PUZS-300 automatic biochemical analyzer, including total
cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL),
high-density lipoprotein (HDL) and blood glucose levels (Glu).
Ultrasonic testing
The transducer frequency of color Doppler ultrasonic
diagnostic apparatus was 7.5 MHz after the animals were sacrificed.
The plaque was scanned along the carotid artery. Intima-media
thickness (IMT) was the vertical distance between lumen intima to
media and adventitia. IMT >1.5 mm indicates the formation of AS
plaque. Plaques were divided into hard, flat, ulcer and mixed
plaques based on the size, shape and echo, among which stable
plaques include hard and flat plaques, unstable plaques include
soft, ulcer and mixed plaques. The severity of the plaque was
estimated by semi-quantitative method as follows: unilateral plaque
≤2.0 mm indicates grade I. Unilateral plaque >2.0 mm or
bilateral plaque with one side ≤2.0 mm indicates grade II.
Bilateral plaque >2.0 mm indicates grade III.
Detection of IL-18 and IL-17
The concentration of IL-17 and IL-18 in serum of
mice in the model and the control groups was determined by ELISA
according to the instructions. A total of 10 standard wells were
placed on the enzyme label plate, 100 µl of standard and 50 µl of
standard diluent were blended in wells 1 and 2, then100 µl mixed
liquor was taken from wells 1 and 2 and added to wells 3 and 4 and
50 µl of standard diluent was added. Then 50 µl of the mixed liquor
in well 3 and 4 was discarded and 50 µl was added to wells 5 and 6,
and 50 µl of standard diluent was added. Each of the 10 wells after
the dilution was 50 µl. The blank wells and the tested samples were
set (the blank control wells without the enzyme-labeled reagents
and samples were the same as the above steps). The tested wells
were added with 40 µl of the sample diluent and 10 µl diluent (5
times sample dilution). The well wall was not touched but shaken
gently when operating, and was incubated in a water bath or
incubator at 37°C for 30 min. The sealed film was uncovered and the
liquid was discarded using blotting paper. Each well was filled
with scrubbing solution. After 30 sec, this step was repeated 5
times and the well was patted dry. Then 50 µl of the enzyme
labeling reagent was added to each well and the mixture was
incubated at 37°C for 30 min except for the blank wells and 50 µl
of substrate A and B was added to each well. The color was
developed at 37°C for 15 min and 50 µl of the stop solution was
added to each well and zeroed with a blank well. The OD value
(optical density value) of each well was measured at a wavelength
of 450 nm in 25 min. The levels of IL-18 and IL-17 in the serum
were calculated.
Statistical analysis
The experimental data were analyzed by SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). The
measurement data were expressed by mean ± standard deviation and
compared by paired t-test between the two groups. One-way ANOVA
test was used for comparison among groups. LSD method was used for
comparison between two groups. Pearson's was used for the
correlation between IL-17 and IL-18. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of blood lipid levels
between AS and normal mice
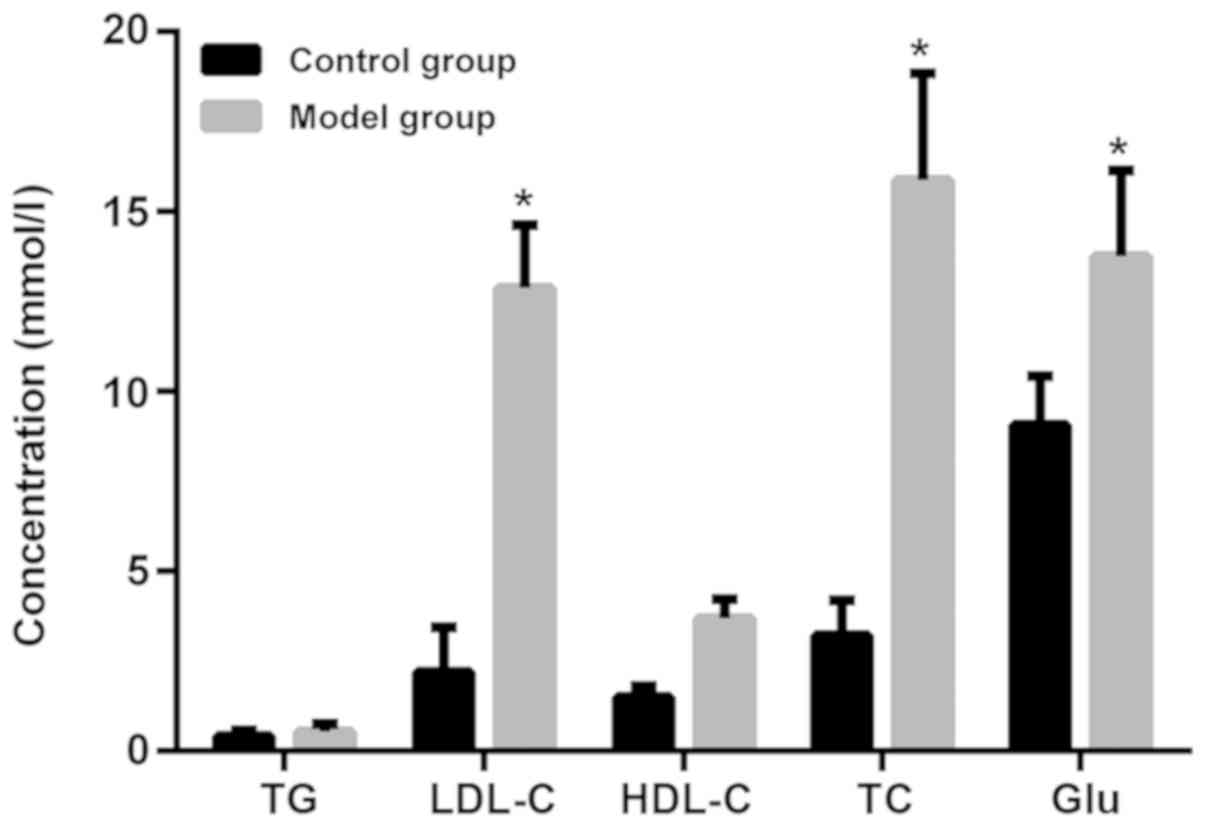
As shown in Fig. 1,
the expression levels of LDL-cholesterol (LDL-C), TC, Glu in the
model group induced by high-fat diet were significantly higher than
those in the control group (P<0.05). There was no significant
difference in TG and HDL-cholesterol (HDL-C) between the model and
the control groups (P>0.05).
Changes of body mass index (BMI) in
mice
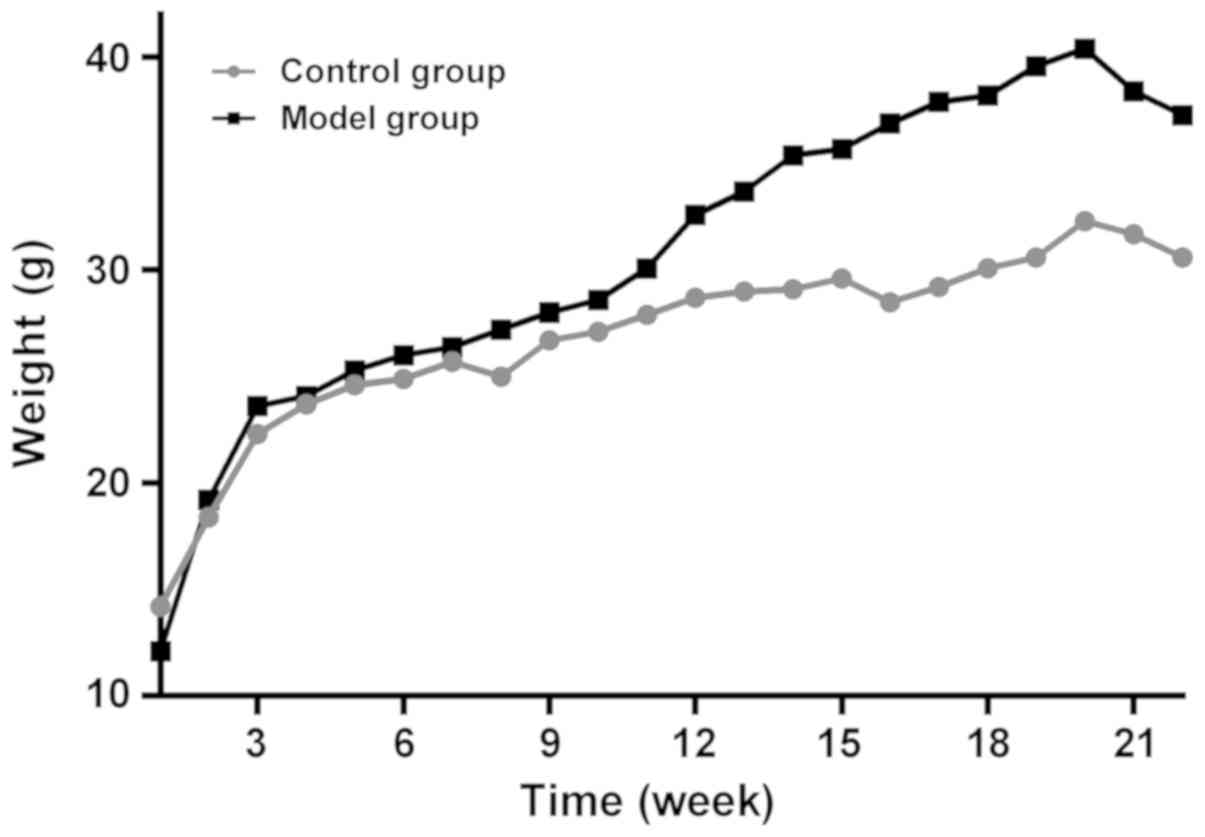
As shown in Fig. 2,
there was no significant difference in initial weight between the
model and the control groups (P>0.05). The body weight of the
two groups increased with feeding time. The body weight of the two
groups decreased at the 21st week. Weight in the model group was
significantly higher than that in the control group at the 16th
week (P<0.05) until the end of the experiment.
Comparison of IL-17 and IL-18
expression in serum of AS and normal mice
The expression of IL-17 and IL-18 in the model group
was significantly higher than that in the control group (t=6.903,
11.02, P<0.05; Table I).
 | Table I.Comparison of IL-17 and IL-18
expression in serum of AS mice and normal mice (mean ± standard
deviation). |
Table I.
Comparison of IL-17 and IL-18
expression in serum of AS mice and normal mice (mean ± standard
deviation).
| Group | Cases | IL-17 (µg/l) | IL-18 (pg/ml) |
|---|
| Control | 20 | 23.13±4.32 | 37.76±5.39 |
| Model | 60 | 37.23±8.76 | 61.23±8.98 |
| t |
|
6.903 | 11.02 |
| P-value |
| <0.001 |
<0.001 |
Comparison of IL-17 and IL-18
concentrations of non-plaque mice in the model group
The concentration of IL-17 and IL-18 in the
non-plaque group was significantly lower than that in the stable
plaque and unstable plaque groups (P<0.05). The concentration of
IL-17 and IL-18 in the stable plaque group was significantly lower
than that in the unstable plaque group (P<0.05; Table II).
 | Table II.Comparison of IL-17 and IL-18
concentrations of non-plaque mice in the model group (mean ±
standard deviation). |
Table II.
Comparison of IL-17 and IL-18
concentrations of non-plaque mice in the model group (mean ±
standard deviation).
| Group | Cases | IL-17 (μg/l) | IL-18 (pg/ml) |
|---|
| Stable plaque | 21 | 32.87±6.76 | 56.09±7.34 |
| Unstable plaque | 28 |
38.36±9.76a |
67.32±8.67a |
| Non-plaque | 11 |
26.28±6.09a,b |
40.98±6.23a,b |
| F |
| 8.954 | 46.29 |
| P-value |
|
0.0004 |
<0.001 |
Expression of IL-17 and IL-18 in AS
mice with different degrees of inflammation
According to grades of AS, the levels of IL-17 and
IL-18 at grade I were significantly lower than those at grades II
and III (P<0.05). The levels of IL-17 and IL-18 at grade II were
significantly lower than those at grade III (P<0.05; Table III).
 | Table III.Expression of IL-17 and IL-18 in
different degrees of inflammation in AS mice (mean ± standard
deviation). |
Table III.
Expression of IL-17 and IL-18 in
different degrees of inflammation in AS mice (mean ± standard
deviation).
| Severity | Cases | IL-17 (µg/l) | IL-18 (pg/ml) |
|---|
| I | 18 | 27.12±5.98 | 41.76±6.32 |
| II | 30 |
33.76±7.99a |
56.13±7.22a |
| III | 12 |
40.01±10.34a,b |
69.34±9.13a,b |
| F |
| 9.638 | 51.76 |
| P-value |
| 0.0002 | <0.001 |
Correlation of IL-17 and IL-18
expression in serum
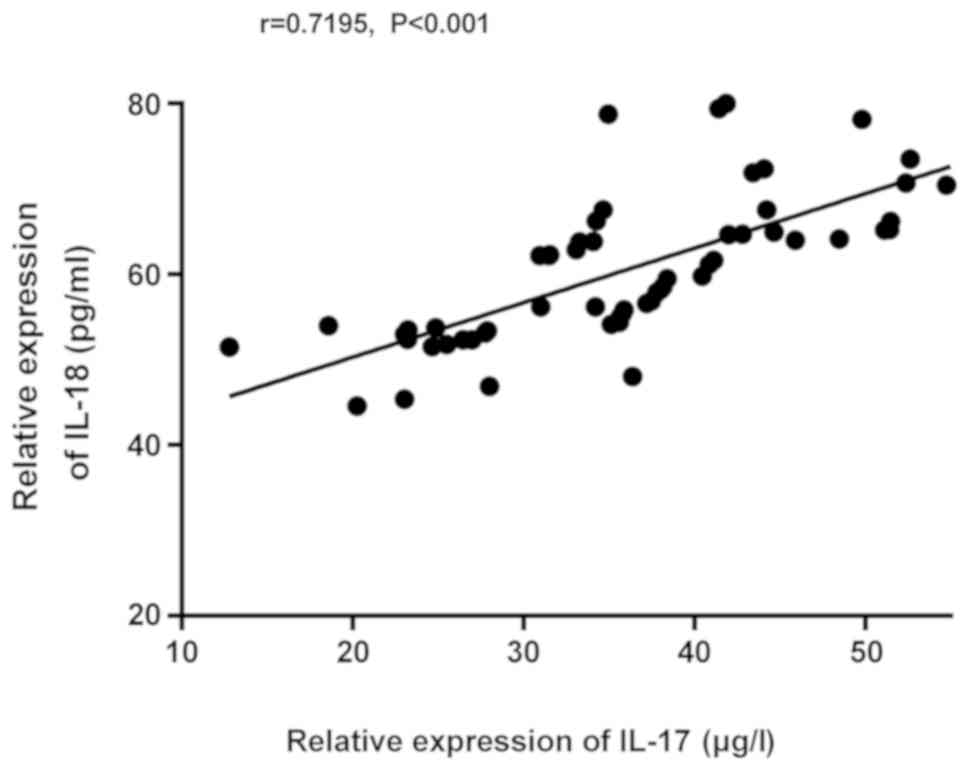
Based on the correlation of IL-17 and IL-18
expression in the model group, the expression of IL-18 increased
with the expression of IL-17, indicating that the expression of
IL-17 was positively correlated with that of IL-18 (r=0.7195,
P<0.001; Fig. 3).
Discussion
The occurrence and development of AS is a chronic
inflammatory process, among which inflammatory cells and mediators
are involved (14). Many studies
suggested that the rupture and shedding of vulnerable
atherosclerotic plaques were independent risk factors for acute
cerebral infarction (15,16). Therefore, the prevention and
treatment of cardiovascular disease is to identify plaque at
earlier stages (17). Studies
indicated that unstable plaques were soft plaques composed of
lipids, with ulcers or bleeding plaque on the surface, which were
prone to acute ischemia (18).
As a proinflammatory cytokine, IL-17 is secreted by
Th17 cells, which activates macrophages, vascular smooth and
endothelial cells, resulting in inflammatory cytokines and the
formation of AS (19). Previous
findings have shown that the downregulation of IL-17 expression was
associated with the intraperitoneal injection of anti-IL-17
antibodies. IL-17 antibody inhibits the formation of plaques in Apo
E-/- model mice, playing an important role in the formation of AS
plaques (20). IL-18, not only
promoted the proliferation of T cells and increased the activity of
T cells and NK cells, but also promoted the production of cytokines
such as tumour necrosis factor (TNF) TNF-α, IL-1, and IL-8 and the
reduction of IL-10. The excessive secretion of IL-18 caused
invasive inflammatory cells, or even damaged cells (21).
Lipid metabolism disorder is considered to be the
cause and pathological basis in the formation of AS. Excessive
lipid in the plasma will enter the intima of the artery after
injury and was accumulated in the artery wall. Deposit in the
subintimal space leads to lipid deposition and early
atherosclerotic lesions (22). The
expression levels of LDL-C, TC and Glu in the model group induced
by high-fat diet were significantly higher than those in the normal
control group. There was no significant difference in TG and HDL-C
between the model and the control groups. Aubin et al
(23) indicated that no changes of
TC, LDL-C, HDL-C and TG were found in plasma of SD rats after 8
weeks of high fat feeding, which was consistent with the results of
HDL-C and TG in this study. However, the levels of LDL-C, TC and
Glu in the model group were higher than those in normal control
group, which might be related to longer feeding time. There was no
significant difference in initial weight between the model and the
control groups. The body weight of the two groups increased with
feeding time. The body weight decreased in the two groups at the
21st week. Weight of the mice in the model group were significantly
higher than those in the control group at the 16th week. Sun et
al (24) studied on the changes
of BMI in mice screened for atherosclerotic animal models. Mallat
et al (25) suggested that
the expression of IL-18 mRNA was higher in atherosclerotic plaques
compared with normal arteries. The study indicated that the
expression of IL-17 and IL-18 in serum of the model group were
significantly higher than that in the normal control group
(P<0.05). Underhill et al (26) showed that the levels of serum IL-23,
IL-17 and CRP in the acute cerebral infarction group were higher
than those in the control group (P<0.05). Some studies have
suggested that the reduction of IL-17 expression was related to
intraperitoneal injection of anti-IL-17 antibodies. IL-17 antibody
inhibited the formation of plaques in Apo E-/- model mice, playing
an important role in the formation of AS plaques (20). Mallat et al (27) indicated that IL-18 mRNA and related
proteins were expressed in human carotid atherosclerotic plaques.
The expression of IL-18 in unstable plaques was stronger than that
in stable plaques, indicating that IL-18 was related to the
stability of the plaque. The concentration of IL-17 and IL-18 in
the non-plaque group was significantly lower than that in the
stable plaque and unstable plaque groups. The concentration of
IL-17 and IL-18 in the stable plaque group was significantly lower
than that in the unstable plaque group. IL-17 and IL-18 are
involved in the early inflammatory response of atherosclerotic
plaque, which have an impact on the development and instability of
plaque. Animal models (27)
indicated that the inhibition of the process of AS plaque and the
stable development of plaques can be achieved by inhibiting IL-18
antibodies. According to different grades of AS, the expression of
IL-17 and IL-18 in the model group at grade I was significantly
lower than that at grades II and III (P<0.05), while the
expression of IL-17 and IL-18 at grade II was significantly lower
than that at grade III (P<0.05). IL-17 and IL-18 antibodies play
an important role in the development of AS. The higher the level of
IL-17 and IL-18 antibodies, the more severe the AS of patients. The
expression of IL-18 increased with the expression of IL-17,
indicating that the expression of IL-17 was positively correlated
with that of IL-18 (r=0.7195, P<0.001). IL-17 and IL-18 may be
interrelated inflammatory factors in the formation and development
of atherosclerotic plaque. There is a correlation between IL-18 and
P-selectin in coronary atherosclerotic plaques (28). There is no report on research on the
correlation between IL-17 and IL-18 expression in AS.
This study investigated the expression of IL-17 and
IL-18 in animal models through detecting animal models, which could
be used as indicators of the severity of disease. However, the
specific mechanism of IL-17 and IL-18 in the formation of
atherosclerotic plaques is not clarified. The reduction of
pro-inflammatory factors and increase of anti-inflammatory factors
will need further study.
In conclusion, serum IL-17 and IL-18 played an
important role in the formation and development of atherosclerotic
plaques and were closely related to the stability and severity of
plaques. The expression of IL-17 and IL-18 was positively
correlated. Future studies are to focus on ?
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT conceived and designed the study, collected,
analyzed and interpreted the experiment data, drafted this study,
and revised the manuscript critically for important intellectual
content. XT read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Hospital to Changchun University of Chinese Medicine
(Changchun, China).
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ramji DP and Davies TS: Cytokines in
atherosclerosis: Key players in all stages of disease and promising
therapeutic targets. Cytokine Growth Factor Rev. 26:673–685. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McLaren JE, Michael DR, Ashlin TG and
Ramji DP: Cytokines, macrophage lipid metabolism and foam cells:
Implications for cardiovascular disease therapy. Prog Lipid Res.
50:331–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross JS, Stagliano NE, Donovan MJ,
Breitbart RE and Ginsburg GS: Atherosclerosis: a cancer of the
blood vessels? Am J Clin Pathol. 116 (Suppl):S97–S107.
2001.PubMed/NCBI
|
|
4
|
Chen CH and Walterscheid JP: Plaque
angiogenesis versus compensatory arteriogenesis in atherosclerosis.
Circ Res. 99:787–789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doyle B and Caplice N: Plaque
neovascularization and antiangiogenic therapy for atherosclerosis.
J Am Coll Cardiol. 49:2073–2080. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoving S, Heeneman S, Gijbels MJ, te Poele
JA, Russell NS, Daemen MJ and Stewart FA: Single-dose and
fractionated irradiation promote initiation and progression of
atherosclerosis and induce an inflammatory plaque phenotype in
ApoE(−/-) mice. Int J Radiat Oncol Biol Phys. 71:848–857. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong Y, Ye F, You W and Wu ZM:
Correlation between serum inflammatory cytokine levels and fibrous
cap thickness of fibrofatty plaque in coronary culprit lesions.
Zhonghua Xin Xue Guan Bing Za Zhi. 45:566–571. 2017.(In Chinese).
PubMed/NCBI
|
|
8
|
Taleb S, Tedgui A and Mallat Z: IL-17 and
Th17 cells in atherosclerosis: Subtle and contextual roles.
Arterioscler Thromb Vasc Biol. 35:258–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu S and Cao X: Interleukin-17 and its
expanding biological functions. Cell Mol Immunol. 7:164–174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaffen SL: Recent advances in the IL-17
cytokine family. Curr Opin Immunol. 23:613–619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Armstrong EJ, Morrow DA and Sabatine MS:
Inflammatory biomarkers in acute coronary syndromes: part III:
biomarkers of oxidative stress and angiogenic growth factors.
Circulation. 113:e289–e292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tenger C, Sundborger A, Jawien J and Zhou
X: IL-18 accelerates atherosclerosis accompanied by elevation of
IFN-gamma and CXCL16 expression independently of T cells.
Arterioscler Thromb Vasc Biol. 25:791–796. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whitman SC, Ravisankar P and Daugherty A:
Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/-)
mice through release of interferon-gamma. Circ Res. 90:E34–E38.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crea F and Liuzzo G: Pathogenesis of acute
coronary syndromes. J Am Coll Cardiol. 61:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu D, Hippe DS, Underhill HR,
Oikawa-Wakayama M, Dong L, Yamada K, Yuan C and Hatsukami TS:
Prediction of high-risk plaque development and plaque progression
with the carotid atherosclerosis score. JACC Cardiovasc Imaging.
7:366–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kadoglou NP, Lambadiari V, Gastounioti A,
Gkekas C, Giannakopoulos TG, Koulia K, Maratou E, Alepaki M,
Kakisis J, Karakitsos P, et al: The relationship of novel
adipokines, RBP4 and omentin-1, with carotid atherosclerosis
severity and vulnerability. Atherosclerosis. 235:606–612. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abbas A, Gregersen I, Holm S, Daissormont
I, Bjerkeli V, Krohg-Sørensen K, Skagen KR, Dahl TB, Russell D,
Almås T, et al: Interleukin 23 levels are increased in carotid
atherosclerosis: possible role for the interleukin 23/interleukin
17 axis. Stroke. 46:793–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tacke F, Alvarez D, Kaplan TJ, Jakubzick
C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et
al: Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1
to accumulate within atherosclerotic plaques. J Clin Invest.
117:185–194. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Boer OJ, van der Meer JJ, Teeling P,
van der Loos CM, Idu MM, van Maldegem F, Aten J and van der Wal AC:
Differential expression of interleukin-17 family cytokines in
intact and complicated human atherosclerotic plaques. J Pathol.
220:499–508. 2010.PubMed/NCBI
|
|
20
|
Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang
P, Guo C, Wang Q, Wang X, Ma C, et al: A critical function of Th17
proinflammatory cells in the development of atherosclerotic plaque
in mice. J Immunol. 185:5820–5827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kharitonenkov A, Dunbar JD, Bina HA,
Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF,
Knierman MD, et al: FGF-21/FGF-21 receptor interaction and
activation is determined by betaKlotho. J Cell Physiol. 215:1–7.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Zhao B, Cui Y, Huang Y, Huang C,
Huang J, Han L and Lao L: Effects of shenque moxibustion on
behavioral changes and brain oxidative state in apolipoprotein
e-deficient mice. Evid Based Complement Alternat Med.
2015:8048042015.PubMed/NCBI
|
|
23
|
Aubin MC, Lajoie C, Clément R, Gosselin H,
Calderone A and Perrault LP: Female rats fed a high-fat diet were
associated with vascular dysfunction and cardiac fibrosis in the
absence of overt obesity and hyperlipidemia: therapeutic potential
of resveratrol. J Pharmacol Exp Ther. 325:961–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Q, Wang A, Jin X, Natanzon A, Duquaine
D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, et al:
Long-term air pollution exposure and acceleration of
atherosclerosis and vascular inflammation in an animal model. JAMA.
294:3003–3010. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mallat Z, Corbaz A, Scoazec A, Besnard S,
Lesèche G, Chvatchko Y and Tedgui A: Expression of interleukin-18
in human atherosclerotic plaques and relation to plaque
instability. Circulation. 104:1598–1603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Underhill HR, Hatsukami TS, Cai J, Yu W,
DeMarco JK, Polissar NL, Ota H, Zhao X, Dong L, Oikawa M, et al: A
noninvasive imaging approach to assess plaque severity: the carotid
atherosclerosis score. AJNR Am J Neuroradiol. 31:1068–1075. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mallat Z, Corbaz A, Scoazec A, Graber P,
Alouani S, Esposito B, Humbert Y, Chvatchko Y and Tedgui A:
Interleukin-18/interleukin-18 binding protein signaling modulates
atherosclerotic lesion development and stability. Circ Res.
89:E41–E45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakamura I, Hasegawa K, Wada Y, Hirase T,
Node K and Watanabe Y: Detection of early stage atherosclerotic
plaques using PET and CT fusion imaging targeting P-selectin in low
density lipoprotein receptor-deficient mice. Biochem Biophys Res
Commun. 433:47–51. 2013. View Article : Google Scholar : PubMed/NCBI
|