Introduction
Patients with liver cirrhosis are generally regarded
as immunocompromised (1). Not only
does bacterial infection develop in 14.5 to 34.0% of patients with
liver cirrhosis (2), but
community-acquired infections are also frequently observed in
patients with more advanced liver cirrhosis. Because the spleen is
the largest lymphoid organ in the body with concentrated amounts of
T and B cells, macrophages, and dendritic cells, splenectomy in
patients with cirrhosis has produced concern over decreased tumor
immunity and an elevated risk of infection, such as overwhelming
pneumococcal sepsis (3–5).
Thrombocytopenia has been known to be an important
factor that interferes with treatment of hepatocellular carcinoma
and chronic liver diseases (6). We
previously reported that splenectomy in patients with advanced
cirrhosis corrected thrombocytopenia, improved liver function,
reduced portal venous pressure, and allowed prolonged management of
hepatocellular carcinoma (HCC) and hepatitis C virus (HCV)
infection (7). Other researchers
have also described the clinical benefits of splenectomy in the
management of portal hypertension (8,9), HCC
(10), and living-donor liver
transplantation (11) among patients
with liver cirrhosis. In addition, Zhang et al reported that
synchronous splenectomy and hepatectomy improved the disease-free
survival rate compared with hepatectomy alone (12). Although the clinical merits of
splenectomy for patients with cirrhosis have been reported, the
possible immunological advantages have not yet been fully
explored.
In the present study, we examined the effects of
splenectomy on the neutrophil-to-lymphocyte ratio (NLR), an
indicator of systemic inflammation and cancer outcomes (13,14), and
phenotypes of various immune cells among peripheral blood
mononuclear cells (PBMCs). Immune responses against viral- and
tumor-associated antigens were also assessed and compared before
and after splenectomy. Our results suggested that splenectomy might
ameliorate the impaired immune status in patients with liver
cirrhosis, possibly by reducing suppressive cell fractions and
enhancing the effector cell population and function, which could,
at least in part, explain the mechanisms responsible for clinical
benefits of splenectomy.
Patients and methods
Patients
Eleven patients with liver cirrhosis and
hypersplenism underwent hand-assisted laparoscopic splenectomy at
the Department of Surgery of Kurume University Hospital. Six
patients were male and five were female, with a median age of 65
years (range, 44–75 years). Five and six patients were classified
as having Child-Pugh class A and B liver disease, respectively. Ten
patients were HCV-positive and the remaining patient had alcoholic
cirrhosis. Six patients had HCC. We performed splenectomy in an
attempt to either manage thrombocytopenia caused by hypersplenism
and thus facilitate anti-HCC treatment by improving liver function
(n=6) or to prevent further thrombocytopenia caused by interferon
treatment in patients with HCV (n=5). The patients with HCC
received simultaneous radiofrequency ablation to treat hepatic
lesions. Because prevalence of portal thrombosis after splenectomy
in patients with liver cirrhosis was high (7,15), we
performed prophylactic anticoagulation using danaparoid sodium and
warfarin potassium to prevent portal thrombosis after splenectomy,
as previously reported (7).
Along with careful clinical observations, the white
blood cell (WBC), lymphocyte, neutrophil, and platelet counts were
measured in the peripheral blood before and at 1, 3, and 6 months
after splenectomy. The NLR was defined as the absolute neutrophil
count divided by the absolute lymphocyte count. The study was
conducted in accordance with the provisions of the Declaration of
Helsinki and was approved by the Institutional Review Board at
Kurume University Hospital. Before surgery, possible undesirable
outcomes were explained to all patients involved in the study, and
written informed consent was obtained from all patients.
Immune cell phenotypes
Immune cell phenotypes were determined by flow
cytometry. A total of 30 ml of peripheral blood was serially
obtained immediately before and at 1, 3, and 6 months after
splenectomy. PBMCs were isolated by density gradient centrifugation
with the Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) and
frozen until analysis. After thawing, PBMCs (5×105
cells) were suspended in phosphate-buffered saline (PBS) containing
2% fetal bovine serum (MP Biologicals, Solon, OH, USA) and
incubated for 30 min on ice with the appropriate dilution of
antibodies. For analysis of CD4+ and CD8+ T
cells and their naïve/memory subsets, PBMCs were stained with mouse
anti-human CD4, mouse anti-human CD8, mouse anti-human CCR7, and
mouse anti-human CD45RA monoclonal antibodies (mAbs). Since the
available combination between mAbs and fluorescent dyes was limited
in this multicolor flow cytometry panel, we could not completely
compensate the spectral overlap. Therefore, nonlinear gating lines
were set by using control samples, as previously reported (16). For analysis of gamma-delta (γδ) T,
natural killer T (NKT), and natural killer (NK) cells, PBMCs were
stained with mouse anti-human TCR γδ, mouse anti-human CD3, mouse
anti-human CD16, and mouse anti-human CD56 mAbs. For analysis of
Treg cells, PBMCs were stained with mouse anti-human CD4, mouse
anti-human CD25, and mouse anti-human FoxP3 mAbs using the One Step
Staining Human Treg Flow™ Kit (BioLegend, San Diego, CA, USA). For
analysis of granulocytic myeloid-derived suppressor cells (MDSCs),
PBMCs were stained with mouse anti-human CD11b, mouse anti-human
CD15, and mouse anti-human CD33 mAbs (17). All mAbs were from BioLegend except
for the anti-CD15 mAb (BD Biosciences, San Diego, CA, USA). The
samples were run on a FACSCanto II analyzer (BD Biosciences), and
the frequencies of each of the immune subsets were analyzed using
FlowJo ver. 10 software (Tree Star, Ashland, OR, USA).
Immune responses to antigen
peptides
To evaluate immune cell function, T-cell responses
against viral- or tumor-associated antigen peptides were evaluated
by an interferon gamma (IFN-γ) Enzyme-Linked ImmunoSpot (ELISPOT)
assay with PBMCs before and after splenectomy in the enrolled
patients whose PBMCs were available for analysis. The
viral-associated antigen peptide pool consisted of 23 different HLA
class I-restricted peptides derived from cytomegalovirus,
Epstein-Barr virus, or influenza virus (CEF; Mabtech, Nacka Strand,
Sweden). The CEF peptide pool contained 2 HLA-A1-, 3 HLA-A2-, 3
HLA-A3-, 2 HLA-A11-, 1 HLA-A24-, 1 HLA-A68-, 2 HLA-B7-, 4 HLA-B8-,
2 HLA-B27-, 1 HLA-B35-, and 2 HLA-B44-restrcited peptides. The
tumor-associated antigen peptide pool consisted of 20 different HLA
class I-restricted peptides (KRM-20) as reported previously
(18). The KRM-20 peptide pool
contained 5 HLA-A2-, 9 HLA-A24-, 3 HLA-A3 supertype-, 1 HLA-A24/A3
supertype-, and 2 HLA-A24/A3 supertype/A26-restrcited peptides.
PBMCs (1×105 cells/well) were incubated in round 96-well
microculture plates (Thermo Fisher Scientific, Rochester, NY, USA)
with 200 µl of medium (OpTmizer T Cell Expansion SFM; Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
(MP Biologicals), interleukin 2 (20 IU/ml; AbD Serotec, Kidlington,
UK), and the peptide pools (CEF, 2 µg/ml; KRM-20, 10 µg/ml) for 7
days. After incubation, the cells (1–2×104 cells/well)
were harvested and tested for their ability to produce IFN-γ in
response to the corresponding peptide pool (CEF, 2 µg/ml; KRM-20,
10 µg/ml). Antigen-specific IFN-γ secretion after 18 h of
incubation was determined by the ELISPOT assay in accordance with
the manufacturer's instructions (Mabtech), and spots were counted
by an ELISPOT reader (CTL ImmunoSpot S5 Series; Cellular
Technology, Ltd., Shaker Heights, OH, USA). Antigen-specific T-cell
responses were evaluated by the difference between the numbers of
spots produced in response to each corresponding peptide pool and
those produced without it.
Statistical analysis
Data are expressed as mean or median with standard
deviation. The paired Student's t-tests was used for comparison of
percentages of lymphocytes and neutrophils in the WBC counts,
neutrophil-to-lymphocyte ratios and immune cell phenotypes
determined by flow cytometry. Since these analyses were
exploratory, but not confirmatory, paired Student's t-tests was
employed without considering adjustment of multiplicity. The
Wilcoxon signed rank test was used for comparison of spot numbers
of antigen-specific T cells determined by ELISPOT assay. A
two-sided significance level of 5% was considered statistically
significant in all analyses. Statistical analyses were performed
using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Clinical outcomes
The average weight of the excised spleen was 522±211
g. The average blood loss volume was 82±187 ml, and the average
operation time was 235±74 min. Postoperative complications were
portal thrombosis (n=1), ascites (n=1), bacterial colitis (n=1),
and fever (n=1). Portal thrombosis was treated with anticoagulants.
Bacterial infection and ascites were successfully treated with
antibiotics and diuretics, respectively. No patients had fatal
complications. Patients were discharged at 14±2.6 days.
The average platelet count before splenectomy was
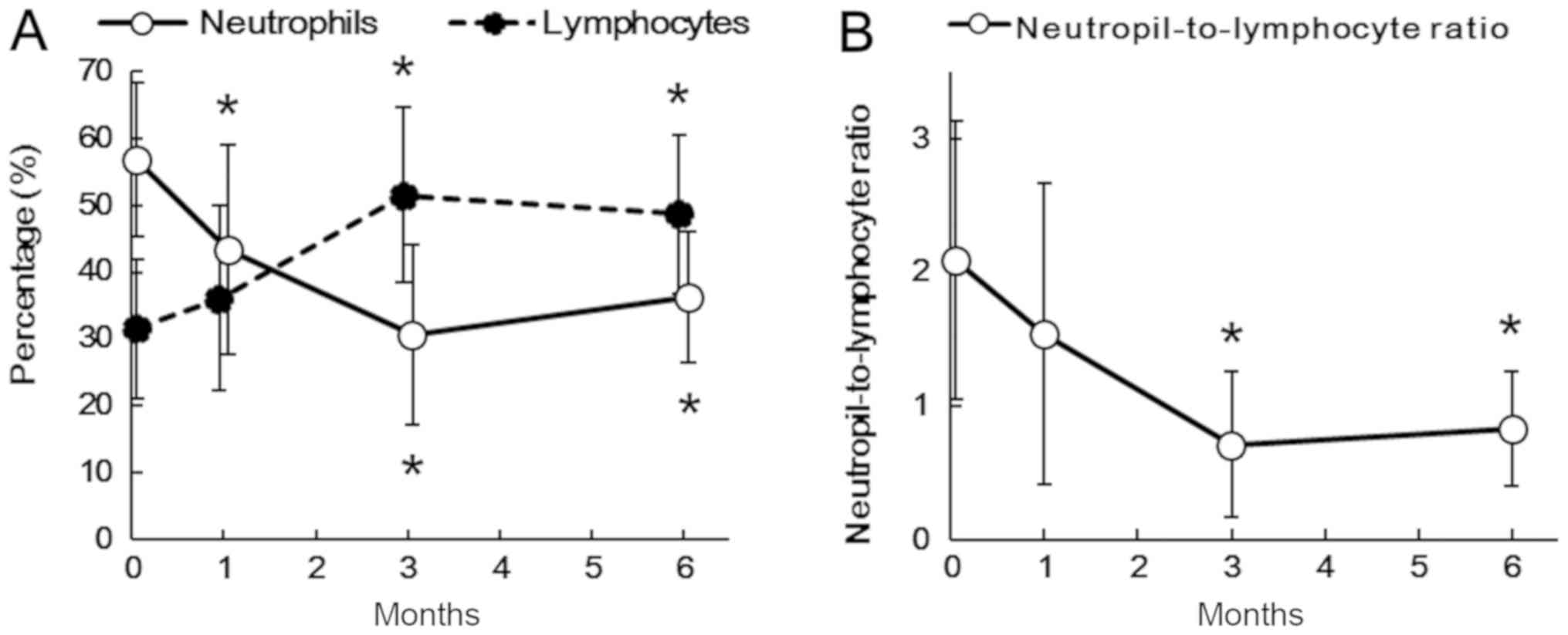
4.9±1.6×104/mm3, and the average WBC count
was 2633±947/mm3. Although the neutrophil count was
significantly higher only at 1 month after splenectomy (P=0.039),
the lymphocyte count remained at higher levels at 1 month
(P=0.004), 3 months (P<0.001), and 6 months (P<0.001). The
percentage of neutrophils in WBC was significantly higher than that
of lymphocytes in WBC before splenectomy (P<0.05). In contrast
to a significant decrease in the percentages of neutrophils in WBC
at 1 month (P=0.033), 3 months (P<0.001), and 6 months (P=0.001)
after splenectomy, those of lymphocytes in WBC significantly
increased at 3 months (P<0.001) and 6 months (P=0.005; Fig. 1A). The NLR significantly decreased at
3 months (P<0.001) and 6 months (P=0.008; Fig. 1B).
Changes in CD4+ and
CD8+ T cells and their naïve/memory subsets
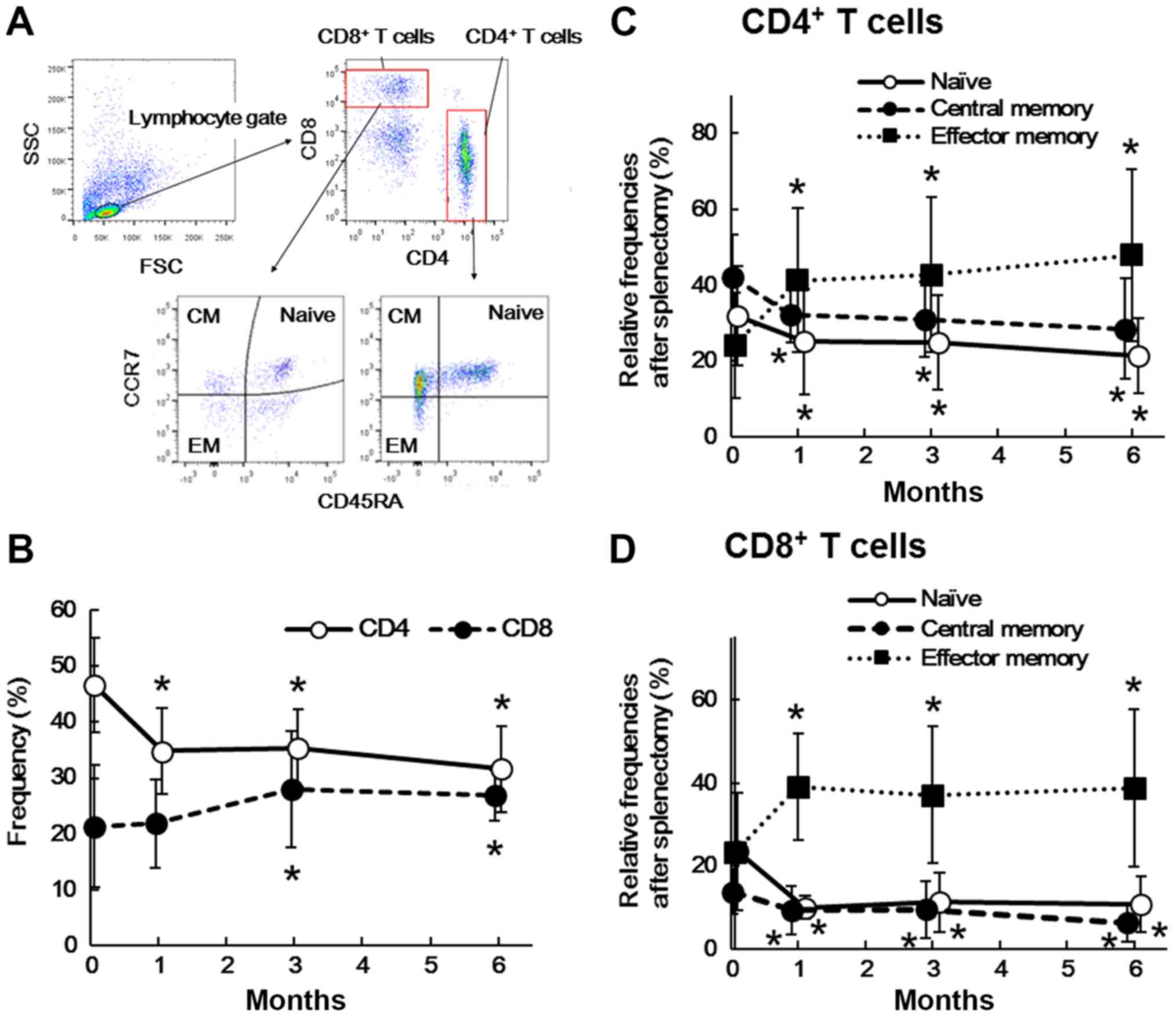
Fig. 2A shows a
representative flow cytometry profile of CD4+ T cells,
CD8+ T cells, and their naïve/memory subsets in PBMCs
before and after splenectomy. As shown in Fig. 2B, the frequency of CD4+ T
cells significantly decreased at 1 month (P=0.003), 3 months
(P=0.001), and 6 months (P=0.016) after splenectomy, while that of
CD8+ T cells significantly increased at 3 months
(P=0.004) and 6 months (P=0.014). In addition, the frequencies of
the naïve (CCR7+CD45RA+) and central memory
(CCR7+CD45RA−) subset of CD4+ T
cells significantly decreased at 1 month (P<0.001 and P=0.022,
respectively), 3 months (P=0.001 and P=0.012, respectively), and 6
months (P<0.001 and P=0.023, respectively) after splenectomy. In
contrast, that of the effector memory subset
(CCR7−CD45RA−) significantly increased at 1
month (P=0.001), 3 months (P<0.001), and 6 months (P=0.001;
Fig. 2C).
Similarly, in CD8+ T cells, the
frequencies of the naïve (CCR7+CD45RA+) and
central memory (CCR7+CD45RA−) subset
significantly decreased at 1 month (P=0.007 and P=0.017,
respectively), 3 months (P=0.010 and P=0.018, respectively), and 6
months (P=0.008 and P=0.008, respectively) after splenectomy,
whereas that of the effector memory subset
(CCR7−CD45RA−) significantly increased after
1 month (P=0.003), 3 months (P=0.017), and 6 months (P=0.011;
Fig. 2D).
Changes in γδ T, NKT, and NK
cells
We also investigated the frequencies of γδ T, NKT,
and NK cells among PBMCs before and after splenectomy. Fig. 3A shows a representative flow
cytometry profile of γδ T, NKT and NK cells. As shown in Fig. 3B, the frequency of γδ T cells
(CD3+ TCR γδ+) significantly increased at 1
month (P=0.039) and 3 months (P=0.008) after splenectomy. In the
same manner, the frequency of NKT cells
(CD3+CD56+) tended to increase after 1 month
(P=0.064) and 3 months (P=0.007; Fig.
3C). However, NK cells (CD3−D56+) showed
a significant increase after 1 month (P=0.010), but not at any
other time points (3 months, P=0.134; 6 months, P=0.210; Fig. 3D). The frequency of the
CD56dimCD16+ NK cell subset tended to
increase at 1 month (P=0.052), 3 months (P=0.017), and 6 months
(P=0.070) after splenectomy, whereas that of the
CD56brightCD16dim/− NK cell subset tended to
decrease after 1 month (P=0.023), 3 months (P=0.068), and 6 months
(P=0.069; Fig. 3E). We observed
significant changes in γδ T, NKT, and NK cells only at the earlier
two time points (1 and 3 months), suggesting that they might
represent a transient, but not continuous, response to
splenectomy.
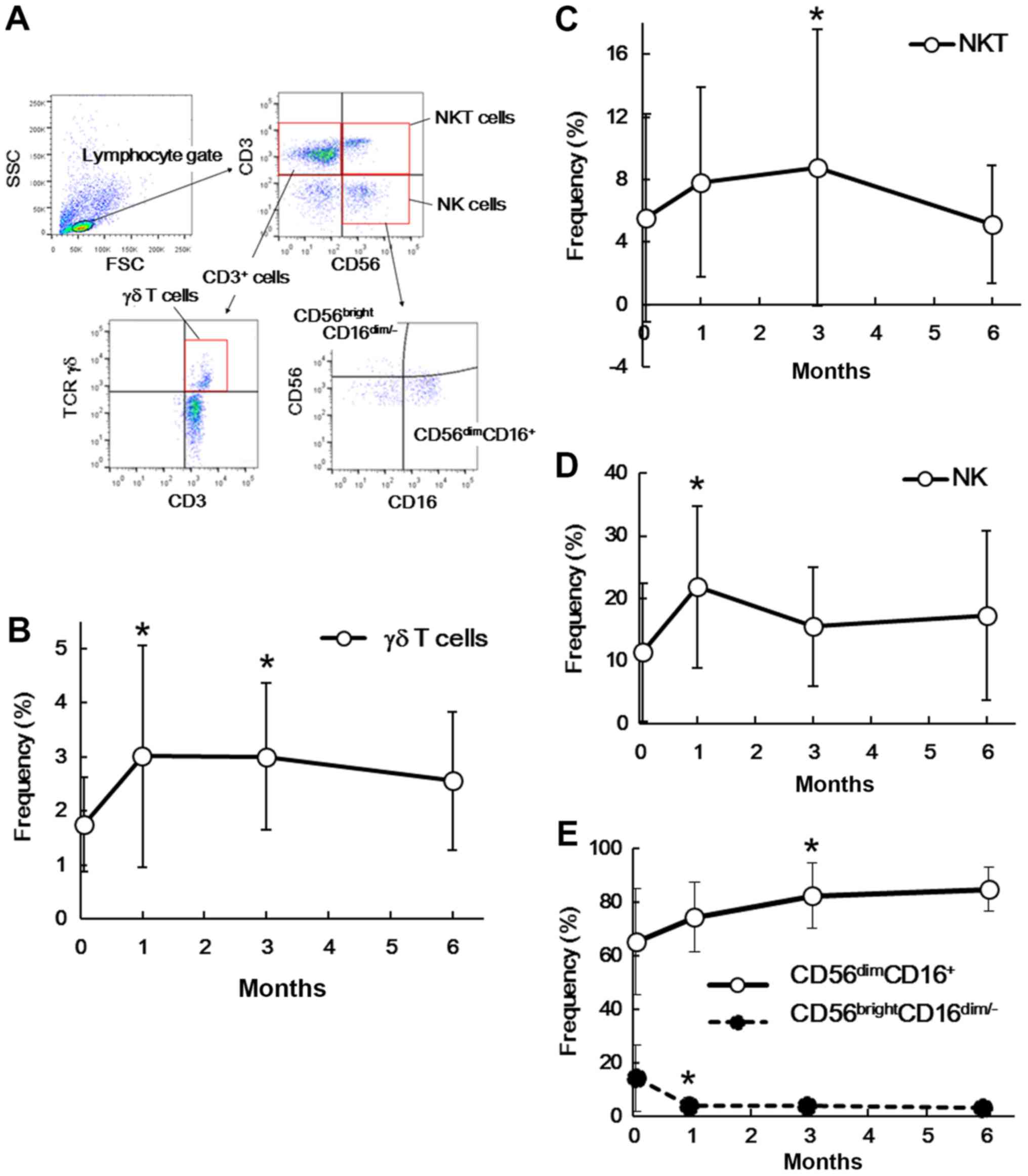 | Figure 3.Alterations in γδ T, NKT and NK cells
after splenectomy. (A) Representative flow cytometry profile of γδ
T, NKT and NK cells. Frequencies of (B) γδ T cells, (C) NKT cells,
(D) NK cells or (E) NK cell subsets defined by CD16 and CD56
expressions before and after splenectomy. Data are presented as the
mean ± standard deviation. The frequencies at the indicated time
points after splenectomy were compared with those before
splenectomy. *P<0.05 by paired Student's t-test compared with
the data before splenectomy. NKT, natural killer T; NK, natural
killer; CD, cluster of differentiation; SSC, side scatter; FSC,
forward scatter; TCR, T-cell receptor. |
Changes in Treg cells and MDSC
The frequencies of inhibitory immune cell subsets,
including Treg cells and MDSCs, were further examined in PBMCs
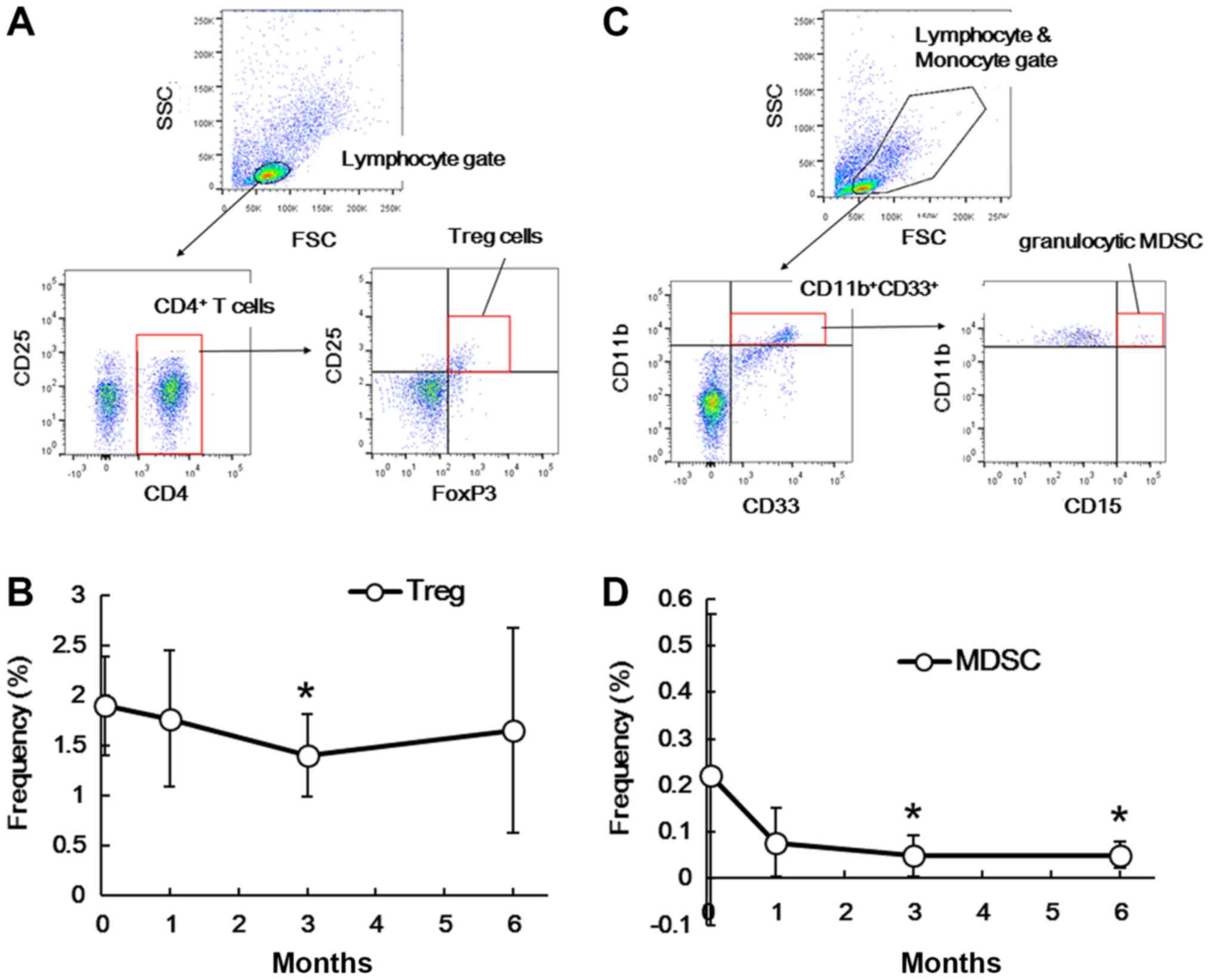
before and after splenectomy. Fig.
4A shows a representative flow cytometry profile of Treg cells.
As shown in Fig. 4B, the frequency
of Treg cells (CD4+CD25+FoxP3+)
among PBMCs transiently decreased 3 months (P=0.045) after
splenectomy, but not at the other time points (1 month, P=0.782; 6
months, P=0.416). Fig. 4C shows a
representative flow cytometry profile of granulocytic MDSCs. As
shown in Fig. 4D, the frequency of
granulocytic MDSCs
(CD11b+CD15+CD33+) was
significantly lower at 3 months (P=0.022) and 6 months (P=0.045)
after splenectomy. In contrast, the frequency of monocytic MDSCs
(CD14+HLA-DR−/low) was not significantly
affected (data not shown).
Evaluation of T-cell responses to
viral- and tumor-associated antigens
To evaluate the effects of splenectomy on immune
function, we examined T-cell responses to viral-associated (n=8)
and tumor-associated (n=5) antigens using an IFN-γ ELISPOT assay
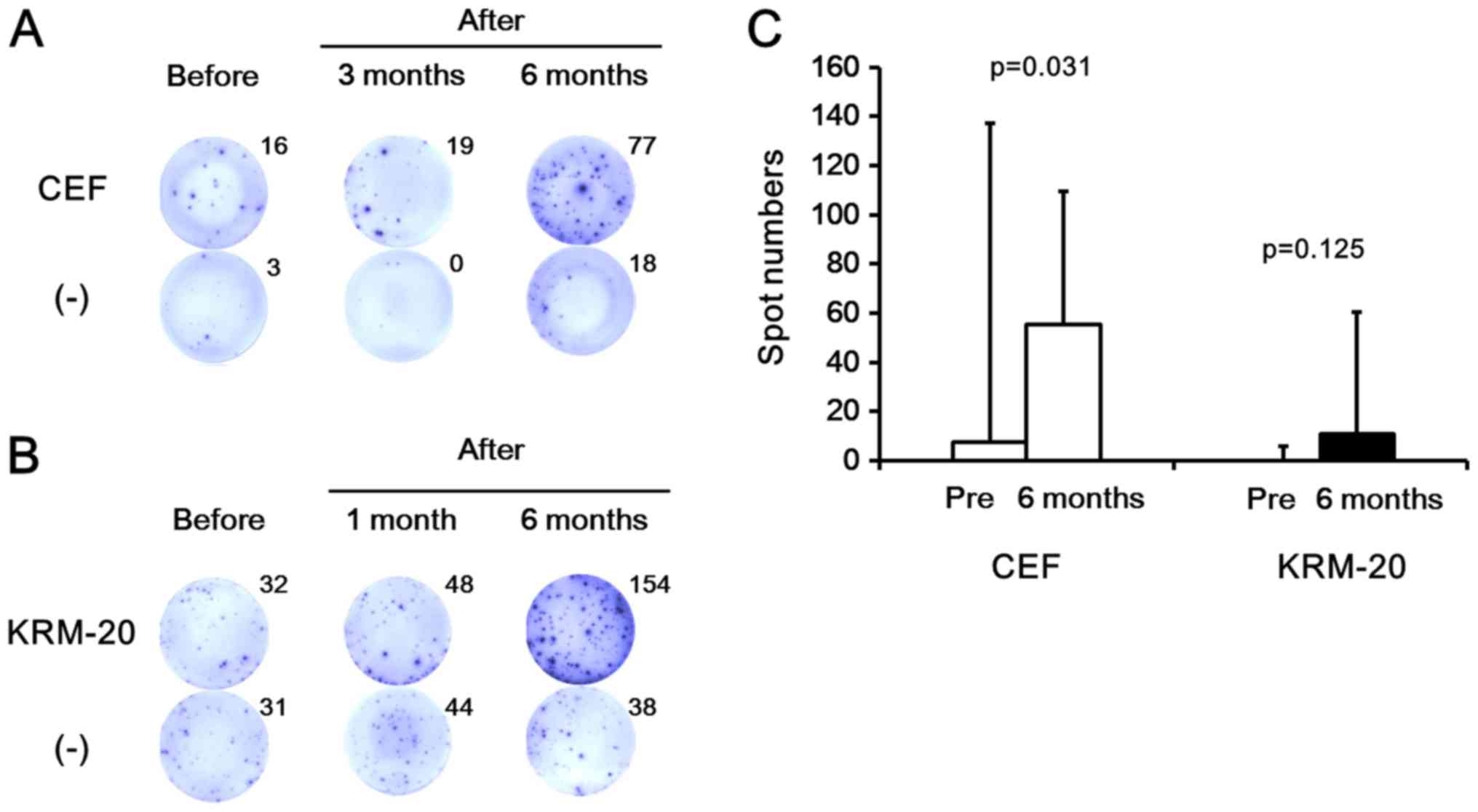
with PBMCs before and after splenectomy. Fig. 5A and B show representative results of
the assay in response to both viral- and tumor-associated antigens.
In five of eight patients with liver cirrhosis, PBMCs after
splenectomy showed higher numbers of IFN-γ-producing cells after
stimulation with the viral-associated antigen peptide pool (CEF)
compared with those before splenectomy. As shown in Fig. 5C, the spot numbers of CEF-specific
cells after 6 months of splenectomy were significantly higher than
those before splenectomy (P=0.031). In addition, when PBMCs were
stimulated with the tumor-associated antigen peptide pool (KRM-20),
more IFN-γ-producing T cells were detected after splenectomy in two
of five patients with cirrhosis. As shown in Fig. 5C, the spot numbers of KRM-20-specific
cells after 6 months of splenectomy tended to be higher than those
before splenectomy, although not statistically significant
(P=0.125), possibly due to the small sample size and the high
standard deviation.
Discussion
The current study demonstrated that splenectomy
significantly increased the frequencies of immune cells with
effector properties such as CD8+ T cells, γδ T cells,
NKT cells, and NK cells, but significantly decreased those with
suppressive properties such as Treg cells and MDSCs. In addition,
the immune responses against viral- and tumor-associated antigens
were augmented after splenectomy. These findings suggest that
splenectomy might ameliorate the impaired immune status in
cirrhotic patients with hypersplenism.
The NLR is one of the markers used to identify
systemic inflammation, which is associated with the development,
progression, and prognosis of various benign and malignant
diseases. A higher NLR reflects increased production and secretion
of pro-inflammatory cytokines and vascular endothelial growth
factor by neutrophils in the circulation, resulting in vascular
invasion, seeding, and growth of malignant cells (13,14,19,20). For
example, an elevated NLR has been reported to correlate with the
tumor burden, recurrence, and survival in patients with colorectal
cancer, colorectal liver metastasis, HCC, gastric cancer, renal
cancer, and pancreatic cancer (13,14,19–21). The
NLR has also been found to reflect the severity of other diseases,
including sepsis, cardiovascular abnormalities, and diabetes
mellitus (22). The present study
showed that the NLR was significantly lower at 3 and 6 months after
splenectomy. Similarly, reduction of the NLR, which was correlated
with better prognosis, was observed after splenectomy in our
previous study of patients with HCC (7).
Patients with cirrhosis have a higher
CD4+/CD8+ T cell ratio because of an increase
in CD4+ T cell frequency together with a decrease in
CD8+ T cell frequency (3). Since CD8+ T cells play
crucial roles in anti-infection and anti-tumor immune responses,
patients with liver cirrhosis are considered to be
immunocompromised in part due to reduced CD8 cells, which might
lead to higher incidences of bacterial infection and HCC
development/recurrence (3,4). The current study demonstrated that
splenectomy lowered the CD4+/CD8+ T-cell
ratio by decreasing the CD4+ T-cell frequency and
increasing the CD8+ T-cell frequency, which may
potentially enhance the anti-infection and anti-tumor immune
responses. In addition, we detected an increase in effector memory
T-cell subsets and a reduction in naïve and central memory subsets
after splenectomy. Naïve T cells initiate immune responses to new
antigens, while memory T cells (central memory and effector memory
cells) respond to previously encountered antigens (23). Central memory cells are restricted to
the lymphoid tissue and blood, whereas effector memory cells
broadly migrate between peripheral tissues, blood, and the spleen,
and provide immediate protection against pathogens (24). Such changes in naïve/memory T-cell
subsets in this study might have resulted from accelerated
antigen-specific immune responses after splenectomy (23,25,26).
Indeed, in the current study, T-cell-mediated IFN-γ production in
response to viral- and tumor-associated antigens was shown to
increase after splenectomy in some patients with cirrhosis.
Similarly, it has been suggested that splenectomy in patients with
cirrhosis might improve their impaired immune status by increasing
IFN-γ production and reducing programmed cell death protein 1
(PD-1)-expression in peripheral CD4 T cells (27,28).
This is the first study to show a decrease in MDSCs
after splenectomy in patients with cirrhosis. Recent studies have
shown that MDSCs are closely associated with suppressive immune
conditions (29) and that a high
MDSC count before treatment is a negative prognostic factor for
patients with HCC undergoing hepatic arterial infusion chemotherapy
(30). In tumor-bearing animal
models, immature myeloid cells, including MDSCs, were found to
accumulate in the spleen, the removal of which inhibited the growth
and progression of liver cancer and prolonged animal survival
(31). Because MDSCs also inhibit
cytotoxic T-cell activation (25,26,31), the
reduced number of MDSCs in our patients after splenectomy might
enhance immune competence.
The current study also demonstrated for the first
time that CD4+CD25+FoxP3+ Treg
cells decreased after splenectomy in the peripheral circulation of
patients with cirrhosis. Treg cells are another subset of immune
suppressors that play an important role in the development and
progression of various diseases such as autoimmune disease, viral
infection, and cancer (29). In a
previous study, the frequency of Treg cells was elevated in
patients with HCV+ liver cirrhosis (32). A higher frequency of Treg cells was
shown to be significantly associated with enhanced tumor growth of
HCC in patients undergoing intra-arterial chemotherapy (33). The spleen is also reportedly a major
source of Treg cells in patients with splenomegaly (34). MDSCs, the numbers of which decreased
after splenectomy in this study, promote de novo development and
induction of Treg cells (29).
Considering all of these findings, splenectomy appears to enhance
the immune response through reduction of Treg cells and MDSCs in
the spleen and peripheral blood.
In contrast, NK cells play an important role in
anti-viral and anti-tumor defenses. NK cells perform various
effector functions, including production of IFN-γ, activation of
tumor antigen-specific CD8+ T cells, and initiation of
perforin-mediated cytolysis of tumor cells (35,36).
Other researchers have reported that in patients with
HCV+ liver cirrhosis, NK cells decreased in number and
were correlated with HCC development (37), and that HCV downregulated NK cell
function via viral serine protease NS3 (38). In the current study, the frequency of
NK cells significantly increased 1 month after splenectomy. In
particular, the number of cells in the CD56dim NK cell
subset, which exhibited more powerful cytotoxic activity than
CD56bright NK cells (39), was significantly elevated after
splenectomy. Splenectomy in HCV-positive patients with cirrhosis
augmented the activity of NK cells (36). A significant increase in both the
percentage and activity of NK cells after splenectomy has also been
reported by other researchers (40).
Because MDSCs reportedly suppress the production of IFN-γ by NK
cells (41), it is possible that
reduction of MDSCs after splenectomy may increase the NK cell
number and function to augment protective immunity.
Recently, oral thrombopoietin receptor agonists have
been reported to stimulate thrombopoiesis, and potentially improve
pancytopenia in cirrhotic patients (42,43).
They transiently increase platelet counts for a limited period
(42,43), while splenectomy in cirrhotic
patients improve hypersplenism, including increase of platelet and
white blood cell counts, for a long period (7,8). In
addition, splenectomy not only has the clinical benefits, such as
improvement of liver function and reduction of portal venous
pressure (7–11), but also may contribute to
immunological benefits, based on our study and others (27,28). It
would be interesting to examine immune status after treatment with
oral thrombopoietin receptor agonists and compare it with that
after splenectomy in cirrhotic patients.
Partial splenic embolization (PSE) via catheter
intervention is also known to be an effective alternative to
splenectomy that is less invasive than surgery. Interestingly,
Matsukiyo et al recently reported that PSE not only improve
leukopenia and thrombocytopenia but also induce activation of
CD4+ T cells, including Th1 and Th2 cells, in patients
with cirrhosis and thrombocytopenia (44). Because the current study did not
address Th1 and Th2 cytokine expression and their balance in
CD4+ T cells, it would be interesting to examine them
before and after splenectomy and compare them with those after PSE
in cirrhotic patients in future studies. Matsukiyo et al
also demonstrated that Treg cells, which were identified as
CD4+CD25highCD127low, did not
significantly change after PSE (44), whereas we showed that Treg cells,
which were identified as
CD4+CD25+FoxP3+, decreased after
splenectomy in patients with cirrhosis. Such discrepancy might be
explained by the different definition of Treg cells, as previously
reported (45).
In summary, the current study suggested that
splenectomy might ameliorate the impaired immune status of patients
with cirrhosis, possibly by reducing suppressive cell fractions and
enhancing the effector cell population and function. To our
knowledge, this is the first study to comprehensively examine the
effects of splenectomy on the NLR as well as the immunological
phenotypes and responses in peripheral blood. However, this study
has several limitations. First, this was a small study comprising
patients with different disease states. Second, although changes in
the immune cell phenotypes were precisely characterized, the immune
functions of each cell subset were not examined in detail. Third,
the enrolled patients were followed only for a limited period of
time after splenectomy. Further studies with more patients and
longer follow-ups are required.
Acknowledgements
The authors would like to thank Ms Yuri
Kasama-Kawaguchi (Kurume University, Kurume, Japan) for her
technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TO and TS planned and designed the study. YH, TO, TS
and TY performed the experiments and data analysis. YH, TO and KO
treated the patients and acquired the clinical data and samples.
KI, HT and KO provided scientific advice and interpreted the data.
YH, TO and TS interpreted the data and drafted the manuscript. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
The present study was conducted in accordance with
the provisions of the Declaration of Helsinki and was approved by
the Institutional Review Board at Kurume University Hospital
(Kurume, Japan). Before surgery, possible undesirable outcomes were
explained to all patients involved in the study, and written
informed consent was obtained from them.
Patient consent for publication
Not applicable.
Competing interests
Kyogo Itoh has stock ownership of BrightPath
Biotherapeutics Co., Ltd., and received research funding from Taiho
Pharmaceutical Co., Ltd. Takuto Yamashita is an employee of
BrightPath Biotherapeutics Co., Ltd. The other authors declare that
they have no competing interests.
References
|
1
|
Planas R, Ballesté B, Alvarez MA, Rivera
M, Montoliu S, Galeras JA, Santos J, Coll S, Morillas RM and Solà
R: Natural history of decompensated hepatitis C virus-related
cirrhosis. A study of 200 patients. J Hepatol. 40:823–830. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borzio M, Salerno F, Piantoni L, Cazzaniga
M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P,
Fornaciari G, et al: Bacterial infection in patients with advanced
cirrhosis: A multicentre prospective study. Dig Liver Dis.
33:41–48. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nomura Y, Kage M, Ogata T, Kondou R,
Kinoshita H, Ohshima K and Yano H: Influence of splenectomy in
patients with liver cirrhosis and hypersplenism. Hepatol Res.
44:E100–E109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wada Y, Nakashima O, Kutami R, Yamamoto O
and Kojiro M: Clinicopathological study on hepatocellular carcinoma
with lymphocytic infiltration. Hepatology. 27:407–414. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okabayashi T and Hanazaki K: Overwhelming
postsplenectomy infection syndrome in adults-a clinically
preventable disease. World J Gastroenterol. 14:176–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawaguchi T, Kuromatsu R, Ide T, Taniguchi
E, Itou M, Sakata M, Abe M, Sumie S and Sata M: Thrombocytopenia,
an important interfering factor of antiviral therapy and
hepatocellular carcinoma treatment for chronic liver diseases.
Kurume Med J. 56:9–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogata T, Okuda K, Sato T, Hirakawa Y,
Yasunaga M, Horiuchi H, Nomura Y, Kage M, Ide T, Kuromatsu R, et
al: Long-term outcome of splenectomy in advanced cirrhotic patients
with hepatocellular carcinoma and thrombocytopenia. Kurume Med J.
60:37–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashizume M, Tomikawa M, Akahoshi T,
Tanoue K, Gotoh N, Konishi K, Okita K, Tsutsumi N, Shimabukuro R,
Yamaguchi S and Sugimachi K: Laparoscopic splenectomy for portal
hypertension. Hepatogastroenterology. 49:847–852. 2002.PubMed/NCBI
|
|
9
|
Anegawa G, Kawanaka H, Uehara H, Akahoshi
T, Konishi K, Yoshida D, Kinjo N, Hashimoto N, Tomikawa M,
Hashizume M and Maehara Y: Effect of laparoscopic splenectomy on
portal hypertensive gastropathy in cirrhotic patients with portal
hypertension. J Gastroenterol Hepatol. 24:1554–1558. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu K, Lei P, Yao Z, Wang C, Wang Q, Xu S,
Xiong Z, Huang H, Xu R, Deng M and Maehara Y: Laparoscopic RFA with
splenectomy for hepatocellular carcinoma. World J Surg Oncol.
14:1962016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshizumi T, Taketomi A, Soejima Y,
Ikegami T, Uchiyama H, Kayashima H, Harada N, Yamashita Y, Kawanaka
H, Nishizak T and Maehara Y: The beneficial role of simultaneous
splenectomy in living donor liver transplantation in patients with
small-for-size graft. Transpl Int. 21:833–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang XY, Li C, Wen TF, Yan LN, Li B, Yang
JY, Wang WT and Jiang L: Synchronous splenectomy and hepatectomy
for patients with hepatocellular carcinoma and hypersplenism: A
casecontrol study. World J Gastroenterol. 21:2358–2366. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halazun KJ, Hardy MA, Rana AA, Woodland DC
IV, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, BrownRS Jr and
Emond JC: Negative impact of neutrophil-lymphocyte ratio on outcome
after liver transplantation for hepatocellular carcinoma. Ann Surg.
250:141–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aino H, Sumie S, Niizeki T, Kuromatsu R,
Tajiri N, Nakano M, Satani M, Okamura S, Shimose S, Miyahara K and
Torimura T: The systemic inflammatory response as a prognostic
factor for advanced hepatocellular carcinoma with extrahepatic
metastasis. Mol Clin Oncol. 5:83–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kinjo N, Kawanaka H, Akahoshi T, Tomikawa
M, Yamashita N, Konishi K, Tanoue K, Shirabe K, Hashizume M and
Maehara Y: Risk factors for portal venous thrombosis after
splenectomy in patients with cirrhosis and portal hypertension. Br
J Surg. 97:910–916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roederer M: Spectral compensation for flow
cytometry: Visualization artifacts, limitations and caveats.
Cytometry. 45:194–205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dumitru CA, Moses K, Trellakis S, Lang S
and Brandau S: Neutrophils and granulocytic myeloid-derived
suppressor cells: Immunophenotyping, cell biology and clinical
relevance in human oncology. Cancer Immunol Immunother.
61:1155–1167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noguchi M, Arai G, Matsumoto K, Naito S,
Moriya F, Suekane S, Komatsu N, Matsueda S, Sasada T, Yamada A, et
al: Phase I trial of a cancer vaccine consisting of 20 mixed
peptides in patients with castration-resistant prostate cancer:
Dose-related immune boosting and suppression. Cancer Immunol
Immunother. 64:493–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang H, Li B, Zhang A, Lu W, Xiang C and
Dong J: Prognostic significance of neutrophil-to-lymphocyte ratio
in colorectal liver metastasis: A systematic review and
meta-analysis. PLoS One. 18:e01594472016. View Article : Google Scholar
|
|
20
|
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ and
Yuan SG: Prognostic significance of neutrophil to lymphocyte ratio
in pancreatic cancer: A meta-analysis. World J Gastroenterol.
7:2807–2815. 2015. View Article : Google Scholar
|
|
21
|
Min KW, Kwon MJ, Kim DH, Son BK, Kim EK,
Oh YH and Wi YC: Persistent elevation of postoperative
neutrophil-to-lymphocyte ratio: A better predictor of survival in
gastric cancer than elevated preoperative neutrophil-to-lymphocyte
ratio. Sci Rep. 25:139672017. View Article : Google Scholar
|
|
22
|
Mozos I, Malainer C, Horbańczuk J, Gug C,
Stoian D, Luca CT and Atanasov AG: Inflammatory markers for
arterial stiffness in cardiovascular diseases. Front Immunol.
8:10582017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mueller SN, Gebhardt T, Carbone FR and
Heath WR: Memory T cell subsets, migration patterns and tissue
residence. Annu Rev Immunol. 31:137–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Groot F, van Capel TM, Schuitemaker J,
Berkhout B and de Jong EC: Differential susceptibility of naïve,
central memory and effector memory T cells to dendritic
cell-mediated HIV-1 transmission. Retrovirology. 3:522006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ugel S, Peranzoni E, Desantis G, Chioda M,
Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S and
Bronte V: Immune tolerance to tumor antigens occurs in a
specialized environment of the spleen. Cell Rep. 2:628–639. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levy L, Mishalian I, Bayuch R, Zolotarov
L, Michaeli J and Fridlender ZG: Splenectomy inhibits non-small
cell lung cancer growth by modulating anti-tumor adaptive and
innate immune response. Oncoimmunology. 4:e9984692015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hashimoto N, Shimoda S, Kawanaka H,
Tsuneyama K, Uehara H, Akahoshi T, Kinjo N, Taketomi A, Shirabe K,
Akashi K, et al: Modulation of CD4+ T cell responses following
splenectomy in hepatitis C virus-related liver cirrhosis. Clin Exp
Immunol. 165:243–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sumida K, Shimoda S, Iwasaka S, Hisamoto
S, Kawanaka H, Akahoshi T, Ikegami T, Shirabe K, Shimono N, Maehara
Y, et al: Characteristics of splenic CD8+ T cell exhaustion in
patients with hepatitis C. Clin Exp Immunol. 174:172–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: Myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizukoshi E, Yamashita T, Arai K,
Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K and Kaneko
S: Myeloid-derived suppressor cells correlate with patient outcomes
in hepatic arterial infusion chemotherapy for hepatocellular
carcinoma. Cancer Immunol Immunother. 65:715–725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Long X, Wang J, Zhao JP, Liang HF, Zhu P,
Cheng Q, Chen Q, Wu YH, Zhang ZG, Zhang BX and Chen XP: Splenectomy
suppresses growth and metastasis of hepatocellular carcinoma
through decreasing myeloid-derived suppressor cells in vivo. J
Huazhong Univ Sci Technolog Med Sci. 36:667–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang CH, Jeng WJ, Ho YP, Teng W, Chen WT,
Chen YC, Lin SM, Chiu CT, Sheen IS and Lin CY: Increased regulatory
T cells in patients with liver cirrhosis correlated with
hyperbilirubinemia and predict bacterial complications. J
Gastroenterol Hepatol. 30:775–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagai H, Mukouzu T, Matsui D, Kanekawa T,
Matsui T, Kanayama M, Wakui N, Momiyama K, Shinohara M, Ishii K, et
al: Host Immunity influences the efficacy of combined
intra-arterial chemotherapy for advanced hepatocellular carcinoma
in liver cirrhosis patients. Hepatogastroenterology. 61:741–746.
2014.PubMed/NCBI
|
|
34
|
Romano A, Hou X, Sertorio M, Dessein H,
Cabantous S, Oliveira P, Li J, Oyegue S, Arnaud V, Luo X, et al:
Foxp3+regulatory T cells in hepatic fibrosis and splenomegaly
caused by schistosoma japonicum: The spleen may be a major source
of tregs in subjects with splenomegaly. PLoS Negl Trop Dis.
10:e00043062016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bedard M, Salio M and Cerundolo V:
Harnessing the power of invariant natural killer T cells in cancer
immunotherapy. Front Immunol. 18:18292017. View Article : Google Scholar
|
|
36
|
Sekiguchi T, Nagamine T, Takagi H and Mori
M: Reduction of virus burden-induced splenectomy in patients with
liver cirrhosis related to hepatitis C virus infection. World J
Gastroenterol. 7:2089–2094. 2006. View Article : Google Scholar
|
|
37
|
Kawarabayashi N, Seki S, Hatsuse K, Ohkawa
T, Koike Y, Aihara T, Habu Y, Nakagawa R, Ami K, Hiraide H and
Mochizuki H: Decrease of CD56(+)T cells and natural killer cells in
cirrhotic livers with hepatitis C may be involved in their
susceptibility to hepatocellular carcinoma. Hepatology. 32:962–969.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang CM, Yoon JC, Park JH and Lee JM:
Hepatitis C virus impairs natural killer cell activity via viral
serine protease NS3. PLoS One. 14:e01757932017. View Article : Google Scholar
|
|
39
|
Poli A, Michel T, Thérésine M, Andrès E,
Hentges F and Zimmer J: CD56bright natural killer (NK) cells: An
important NK cell subset. Immunology. 126:458–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J, Han W, Gao Z, Wang Y, Wu L, Zhang
J, Lai W and Wang Z: Elevation of CD16+CD56+
NK-cells and down-regulation of serum interleukin-21 (IL-21) and
IL-1α after splenectomy in relapsed hemophagocytic
lymphohistiocytosis of unknown cause. Hematology. 22:477–483.
2017.PubMed/NCBI
|
|
41
|
Goh CC, Roggerson KM, Lee HC, Golden-Mason
L, Rosen HR and Hahn YS: Hepatitis C virus-induced myeloid-derived
suppressor cells suppress NK cell IFN-γ production by altering
cellular metabolism via arginase-1. J Immunol. 196:2283–2292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kawaguchi T, Komori A, Seike M, Fujiyama
S, Watanabe H, Tanaka M, Sakisaka S, Nakamuta M, Sasaki Y, Oketani
M, et al: Efficacy and safety of eltrombopag in Japanese patients
with chronic liver disease and thrombocytopenia: A randomized,
open-label, phase II study. J Gastroenterol. 47:1342–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sakamaki A, Watanabe T, Abe S, Kamimura K,
Tsuchiya A, Takamura M, Kawai H, Yamagiwa S and Terai S:
Lusutrombopag increases hematocytes in a compensated liver
cirrhosis patient. Clin J Gastroenterol. 10:261–264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsukiyo Y, Nagai H, Matsui T and
Igarashi Y: Host immunological effects of partial splenic
embolization in patients with liver cirrhosis. J Immunol Res.
2018:17463912018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santegoets SJ, Dijkgraaf EM, Battaglia A,
Beckhove P, Britten CM, Gallimore A, Godkin A, Gouttefangeas C, de
Gruijl TD, Koenen HJ, et al: Monitoring regulatory T cells in
clinical samples: Consensus on an essential marker set and gating
strategy for regulatory T cell analysis by flow cytometry. Cancer
Immunol Immunother. 64:1271–1286. 2015. View Article : Google Scholar : PubMed/NCBI
|