Introduction
Raynaud's phenomenon (RP) is a disease caused by an
excessive cold-induced vasoconstriction of skin arterioles with an
incidence of ~10% of the general population (1–7).
Patients affected by RP suffer from vasospastic attacks,
ulcerations and puffiness at the level of fingers, nose, toes, and
nipples (7–9). Although attempts have been made to
elucidate and treat RP, definitive or specific treatment options
for this illness are limited and efforts to uncover efficient
medications for this disease needs to be undertaken.
Higenamine is a chemical compound extracted from the
roots of aconite. As a traditional Chinese herb, aconite has been
used solely or in combination with other medicines for the
treatment of patients suffering from heart failure in oriental Asia
for millenaries (10–13). Phase III clinical trials have
conveyed the potential of higenamine-based pharmacological stress
agents in coronary artery diseases (14). Numerous studies have also reported a
multitude of candidate pharmacological properties and a wide range
of medical uses of higenamine (14–18).
Specifically, Higenamine can inhibits apoptosis and protects
gastric smooth muscle cell from death in an in vivo model of
diabetic gastroparesis by promoting the β2-adrenoreceptor
(AR)/phosphatidylionositol 3 kinase (PI3K)/protein kinase B (Akt)
pathway (10). Higenamine was found
to equally protect against ischemia/reperfusion associated cardiac
injury and cardiac cell apoptosis via upregulating the
β2-AR/PI3K/Akt signaling pathway (19). In addition, higenamine can also
protect against arthritis by regulating PI3K/Akt/nuclear factor
erythroid 2-related factor 3 (Nrf2) and heme oxygenase-1 (HO-1)
(20), and decreases intestinal
ischemia-reperfusion injury in vivo by regulating the
Nrf2/HO-1/high mobility group protein B1 signaling pathway
(21). Furthermore, it has been
reported that the combination of higenamine with gingerol inhibits
doxorubicin-induced oxidative stress and apoptotic cell death by
promoting PI3K/Akt signaling in cardiomyocytes (13). However, although higenamine is
effective against the circulatory system, whether it is efficient
in counteracting cold-induced vasoconstriction or plays a role in
clinically-induced hypothermia and the molecular mechanisms
underlying these effects are still unknown.
The present study was designed to investigate the
effects of higenamine on cold-induced vasodilation in vivo
and elucidate the underlying molecular mechanisms. The skin blood
flow was measured to assess the effect of higenamine on
cold-induced vasoconstriction in vivo model, for the first
time to the best of our knowledge. The effect of higenamine on
PI3K/Akt and AMP-activated protein kinase (AMPK)α1/endothelial
nitric oxide synthase (eNOS)/nitric oxide (NO) was also explored as
well as reactive oxygen species (ROS)/α2C-AR and twinfilin-1 (PTK9)
signaling transduction pathways to assess the effect of higenamine
in hypothermic human dermal microvascular endothelial cells
(HDMECs) in vitro for the first time. The present study
provided extensive research evidence on the antispasmodic effect
and mechanism of higenamine on cold-induced skin vasoconstriction.
This will lay a foundation for the modernization of traditional
Chinese medicine and the data exhibited has scientific significance
and clinical value.
Materials and methods
In vivo studies
Grouping
A total of 48 female adult Wistar rats (12 weeks
old; 200–250 g), obtained from the Guangdong Medical Laboratory
Animal Centre (Foshan, China), were housed at 22±2°C and 40–60%
humidity with light/dark cycle of 12-h. The rats had free access to
food and water. Rats were randomly divided into six groups (8 rats
in each group): Low dose higenamine group, medium dose higenamine
group, high dose higenamine group, positive drug control group
(prostaglandin E1; cat. no. 745-65-3; Sigma-Aldrich; Merck KGaA),
model control group and normal control group.
Reagent preparation
Higenamine hydrochloride decoction: 1 mg of
higenamine hydrochloride standard (cat. no. 11041-94-4;
Sigma-Aldrich; Merck KGaA) was dissolved in 30 ml of physiological
saline solution to obtain a decoction at a concentration of 33
µg/ml.
Dosage
According to published studies the experimental
doses of different higenamine subgroups were determined as follows:
The low dose higenamine group (18 µg·kg−1), the medium
dose higenamine group (36 µg·kg−1) and high dose
higenamine group (72 µg·kg−1) (14,22,23). The
dose of prostaglandin E1 (5 µg·kg−1) for the positive
drug control group was determined based on the clinical dosage.
Rats in the model control group were injected with 0.1 ml
physiological saline per rat.
Drug administration
Drug administration was performed by tail vein
injection at room temperature (24°C) based on the above-mentioned
dosages. Briefly, 75% ethanol cotton was used to swab the tail.
Then, the tail was pulled with the left hand and the drugs were
injected with the right hand after exposing the left and right
lateral veins of the tail. During the intravenous administration,
one rat died of a misplaced body position in the rat fixator, which
corresponds to a mortality rate of 2.2%.
Modeling
After drug administration, a cold-induced rat skin
vasoconstriction model was established in vivo. Briefly,
rats were loaded into the rat fixator at room temperature 24°C and
the rat tail was exposed outside the fixator. After adjusting the
rat fixator and maintaining the rat body in a relatively fixed
position, the rats were placed in a more comfortable state. The
tails of rats (except for the normal control group) were placed in
a 10°C water bath for 5 min to establish a cold-induced rat skin
vasoconstriction in vivo model. The tails of rats in the
model control group and administration group were placed in a 10°C
water bath for 5 min after drug administration. The regional blood
flow (RBF) of caudal arterial cortex was measured at 5 min in a
24°C, 10°C water bath and then at 5 min in a 10°C water bath. The
first two measurements were used to compare the caudal RBF
differences between the normal control group and the model group.
The latter two measurements were used to compare the caudal RBF
differences between the administration groups and the model control
group.
RBF measurement
The rats were placed in a rat fixator and the tails
were exposed outside the fixator. The rat fixator was slid forward
and backward to adjust the stopper. In this position, the rat body
was maintained in a relatively fixed position, placing the rat in a
more comfortable state. The RBF of the rats' caudal arterial cortex
was measured by using MoorDRT4 laser-Doppler flowmetry (LDF), the
laser probe was placed in the rat caudal arterial cortex and RBF
was monitored in real time at room temperature (24°C). After
establishing the cold-induced skin vasoconstriction rat model, the
laser probe was placed in the rat tail caudal arterial cortex and
the RBF of rat caudal arterial cortex was monitored in real-time at
10°C using LDF. The RBF measurement was done before and after
higenamine administration.
In vitro studies
Cell culture
HDMECs were purchased from ScienCell Research
Laboratories, Inc. HDMECs were cultured in endothelial medium
supplemented with 5% fetal bovine serum and 1% endothelial cell
growth supplement (both ScienCell Research Laboratories, Inc.) and
placed in an incubator containing 5% CO2 at 37°C in
saturated humidity conditions.
MTT assay for cell proliferation
HDMECs were divided into 6 groups according to
different concentrations (0, 1, 2, 4, 10 and 20 µmol/l) of
higenamine. The HDMECs were collected at logarithmic phase and the
cell suspension was adjusted to 1×105/ml with sterile
PBS. Then, 100 µl suspension was added to each well (four
replicates for each treatment). The culture was performed in an
incubator at 37°C containing 5% CO2 overnight. Next,
cells were treated with different concentrations of higenamine and
incubated at 37°C in 5% CO2 incubator for 30 min.
Subsequently, the culture conditions were switched to 5%
CO2 in an incubator at 28°C and incubated for 30, 60, 90
and 120 min. The MTT assay was used to measure cell proliferation.
Briefly, 20 µl MTT solution was added to each well and followed by
incubation for an additional 4 h. Then, the culture was stopped and
150 µl of dimethyl sulfoxide (DMSO) was added to each well with
shaking for 10 min at a low-speed shaker to fully dissolve the
crystals. The absorbance (optical density; OD) of each well was
measured by a microplate reader at a measurement wavelength of 490
nm and used to calculate the cell proliferation rate.
NO measurement
After calculating the optimal dosing concentration
and optimal administration time, HDMECs were divided into 4 groups:
Hypothermic higenamine group (higenamine, 20 µmol/l), hypothermic
positive control group (prostaglandin E1, 100 ng/ml), hypothermic
group and normal temperature group. The corresponding drugs were
added to cells in the 4 groups and incubated at 37°C for 30 min.
Next the medium was refreshed and cells in the hypothermia
higenamine group (higenamine, 20 µmol/l), the hypothermia positive
control group (prostaglandin E1, 100 ng/ml) and the hypothermia
group were incubated at 28°C for 120 min while the normal
temperature group was incubated at 37°C for 120 min. The
concentration of NO in the supernatant of each group was determined
using a nitrate reductase assay kit according to the manufacturer's
protocol. The content of NO was calculated using the following
formula: NO (µmol/l) = [(measured OD value-blank OD
value)/(standard OD value-blank OD value)] × standard product
concentration (20 µmol/l) × dilution factor).
ROS measurement
HDMECs were digested with trypsin to prepare a
single cell supernatant and resuspended with 0.5–1 ml ice cold PBS.
Next, the probe solution [2,7-dichlorodihydrofluorescein diacetate
(DCFH-DA); cat. no. 4091-99-0; Sigma-Aldrich; Merck KGaA] was added
directly to the cell suspension to reach a final concentration of
10 µmol/l followed by incubation for 20 min at 37°C. Subsequently,
the cells were washed with fresh serum-free medium to wash away
DCFH-DA so that DCFH-DA could not enter the cells. The fluorescence
intensity (the wavelength of excitation and emission were 488 and
525 nm, respectively) was detected by flow cytometry (BD FACSAria™
III) and the data analysis was performed using BD CellQuest™ Pro
Software (version 5.1; both BD Biosciences). Cells should be
divided into two subpopulations: ROS-negative cells with very low
fluorescence intensity and ROS-positive cells with strong green
fluorescence. It is worth noting that fluorescence intensity is
positively correlated with the intracellular ROS level and can be
used to reflect the level of intracellular ROS (24).
Western blotting
HDMECs were lysed in radioimmunoprecipitation assay
lysis buffer (Roche Diagnostics) according to the standard
protocol. Protein concentrations were detected using the Micro
bicinchoninic acid protein assay kit (Youdi Biotechnology Co.,
Ltd.). Total cell lysate (50 µg) was loaded into each lane and
separated by 12% SDS-PAGE, followed by transfer to PVDF membrane
(EMD Millipore). The membranes were blocked with 3% bovine serum
albumin (cat. no. PRO-422; ProSpec-Tany TechnoGene Ltd.) in
TBS-Tween-20 for 1 h at room temperature. Primary antibodies
including eNOS (cat. no. 32027; 1:500), p-eNOS (cat. no. 9570),
Akt1 (cat. no. 75692), p-Akt1 (cat. no. 12178), AMPKα1 (cat. no.
5832), p-AMPKα1 (cat. no. 2537), α2C-AR (cat. no. ab151618), PTK9
(cat. no. NBP2-37456; all 1:500) and GAPDH (cat. no. 2118; 1:1,000)
were added and incubated overnight at 4°C. Subsequently, after
washing with TBS-Tween-20 three times (×5 min), membranes were
incubated with the horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G secondary antibody (cat. no. 4030-05;
1:1,000; SouthernBiotech) for 1 h at room temperature. Immunoblot
detection and visualization were performed using enhanced
chemiluminescence western blotting detection reagents (SuperSignal™
West Pico PLUS Chemiluminescent Substrate; cat. no. 34577; Thermo
Fisher Scientific, Inc.). Immunoblotting was performed with target
antibodies and protein bands were scanned and quantified using a
ChemiDoc image analysis system (Bio-Rad Laboratories, Inc.). ImageJ
software (version 1.46; National Institute of Health) was used for
densitometry analysis. Except for the PTK9 antibody, which was
bought from Novus Biologicals, LLC and the α2C-AR, which was bought
from Abcam, all primary antibodies were obtained from the Cell
Signaling Technology, Inc.
Statistics
All experiments were performed in triplicate.
Experimental data was expressed as the mean ± standard deviation.
All data were statistically analyzed using GraphPad Prism 6.0
(GraphPad Software). One-way analysis of variance (ANOVA) or
two-way ANOVA were used to compare multiple sets of means where
appropriate. After homogeneity of variance test, the variance was
used together with Dunnett test for pairwise comparisons. The
Wilcoxon rank sum test was used to compare non-normally distributed
data sets in non-parametric tests. Mann-Whitney U method was used
to test the significance of the differences between the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Higenamine exerts antispasmodic
effects on cold-induced cutaneous vasoconstriction in rats
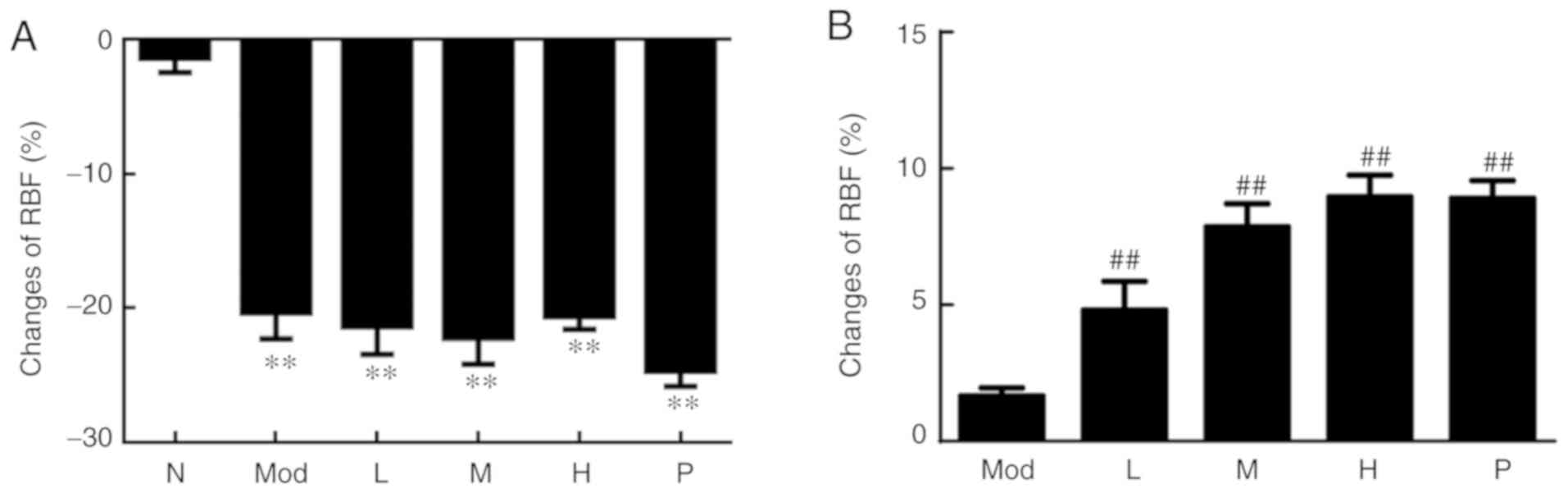
After modeling, as presented in Fig. 1A, the cortical RBF of the rat caudal
artery region in the five groups was significantly decreased
compared with the normal control group (P<0.01), indicating that
the experimental model was successful. After drug administration,
the changes of cortical RBF in the rat caudal artery cortex in the
medium, high dose and the positive control groups (prostaglandin
E1) were compared with the model group. It was observed that the
medium and high doses of higenamine and the positive control group
(prostaglandin E1) had significantly elevated cortical RBF of the
rat caudal artery (P<0.01; Fig.
1B). These results indicated that higenamine improves
cold-induced vasoconstriction.
Effect of higenamine on PI3K/Akt and
AMPK/eNOS/NO signaling pathways in hypothermic HDMECs
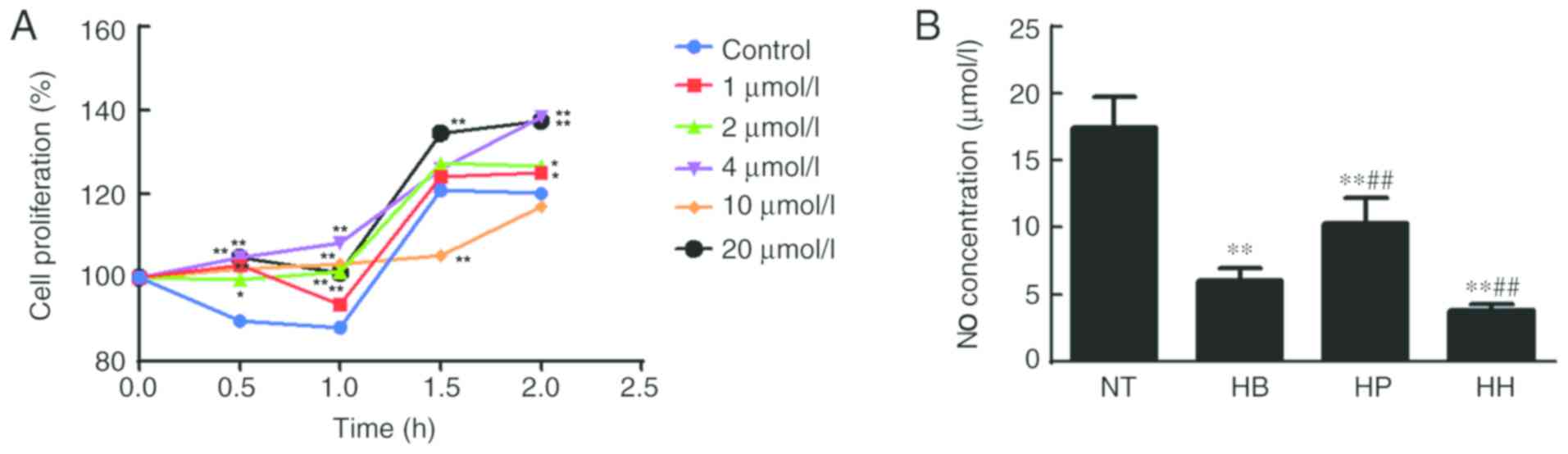
The MTT assay was used to detect the effect of
higenamine on the proliferation of hypothermic HDMECs. Compared
with the control group, the results shown in Fig. 2A indicated that 1, 2, 4 and 20 µmol/l
of higenamine significantly increased cell proliferation at the
time point of 0.5 h compared with the control group (P<0.01,
except for 2 µmol/l at P<0.05). At 1 h, similar results were
obtained (P<0.01). Moreover, at 1.5 h, 20 µmol/l of higenamine
significantly increased while 10 µmol/l of higenamine significantly
inhibited cell proliferation compared with the control group
(P<0.01). At 2 h, 1, 2 (P<0.05), 4 and 20 µmol/l (P<0.01)
of higenamine significantly increased cell proliferation ompared
with the control group. The treatment concentration of 20 µmol/l
and the incubation time of 120 min were the best concentration and
incubation time, respectively. Therefore, these conditions were
used for subsequent studies.
The concentration of NO in the supernatant of HDMEC
cultures was detected by the nitrate reductase method. The results
(Fig. 2B) demonstrated that the NO
concentration in the hypothermia group was significantly decreased
compared with the normal temperature group (P<0.05) but was not
reversed by treatment with higenamine. This suggested that the
production of NO in hypothermic HDMECs was suppressed and that cold
may impair the production and function of NO in HDMECs. No
statistically significant difference in NO concentration was found
between the higenamine group and hypothermia group, indicating that
higenamine could not reverse the NO inhibition caused by
hypothermia in HDMECs. Compared with the hypothermia group, the NO
concentration in the positive control group was significantly
increased (P<0.01), suggesting that prostaglandin E1 moderated
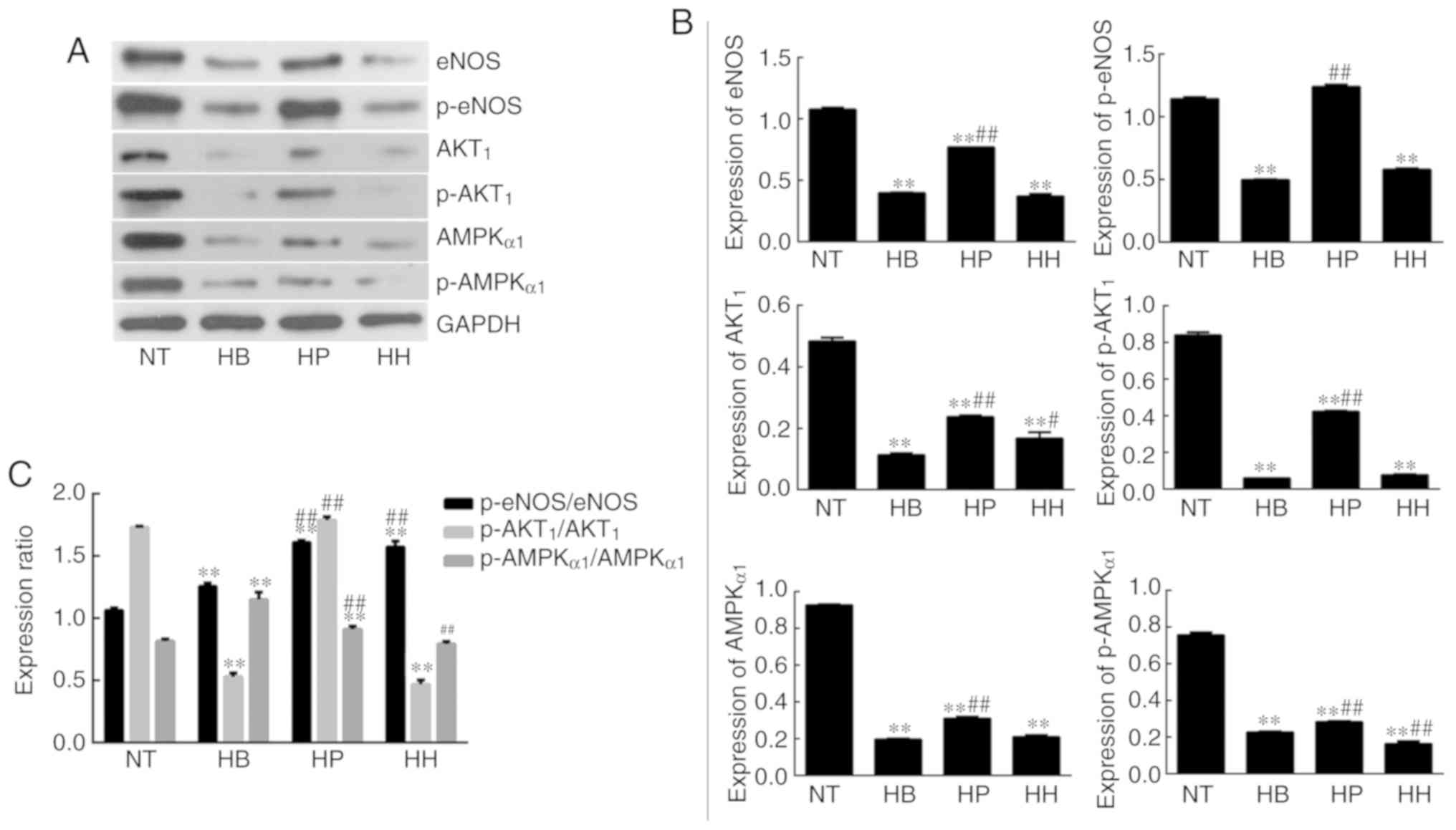
the inhibition of NO production in hypothermic HDMECs. Western
blotting analysis demonstrated that the expression levels of eNOS,
p-eNOS, Akt1, p-Akt1, AMPKα1 and p-AMPKα1 were significantly
downregulated in the hypothermia group compared with the normal
temperature group (P<0.01; Fig.
3). This indicated that hypothermia inhibits the PI3K/Akt and
AMPK/eNOS signaling pathways in HDMECs. Compared with the
hypothermia group, the expression of Akt1 was significantly
increased (P<0.01) by treatment with higenamine. This suggested
that higenamine stimulates the PI3K/Akt signaling pathway in
hypothermic HDMECs. Compared with the hypothermia group, p-Akt1,
eNOS, p-eNOS, AMPKα1, p-AMPKα1 were downregulated by higenamine
(P<0.05), suggesting that higenamine inhibits the AMPK/eNOS
signaling pathway in hypothermic HDMECs.
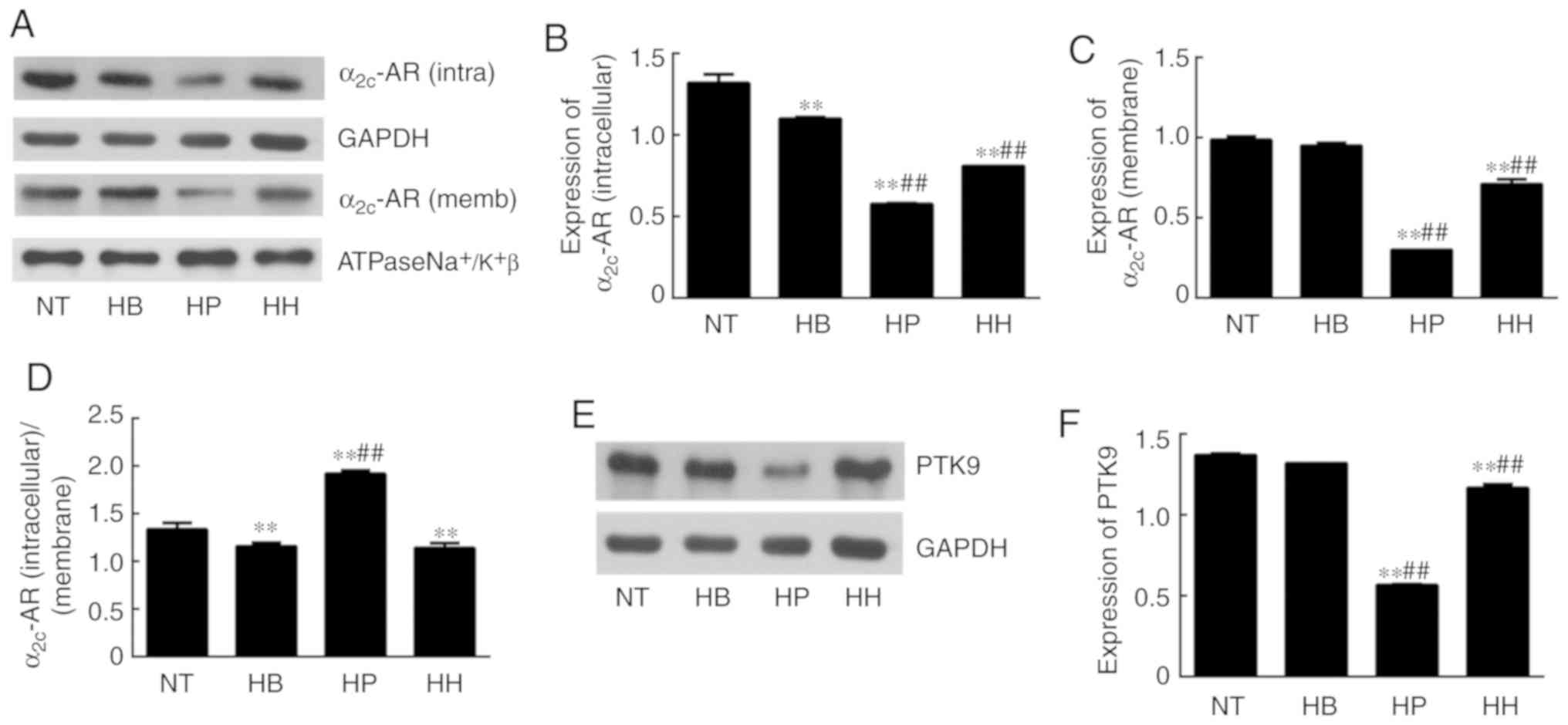
Effect of higenamine on ROS/α2C-AR and
PTK9 signaling pathways in hypothermic HDMECs
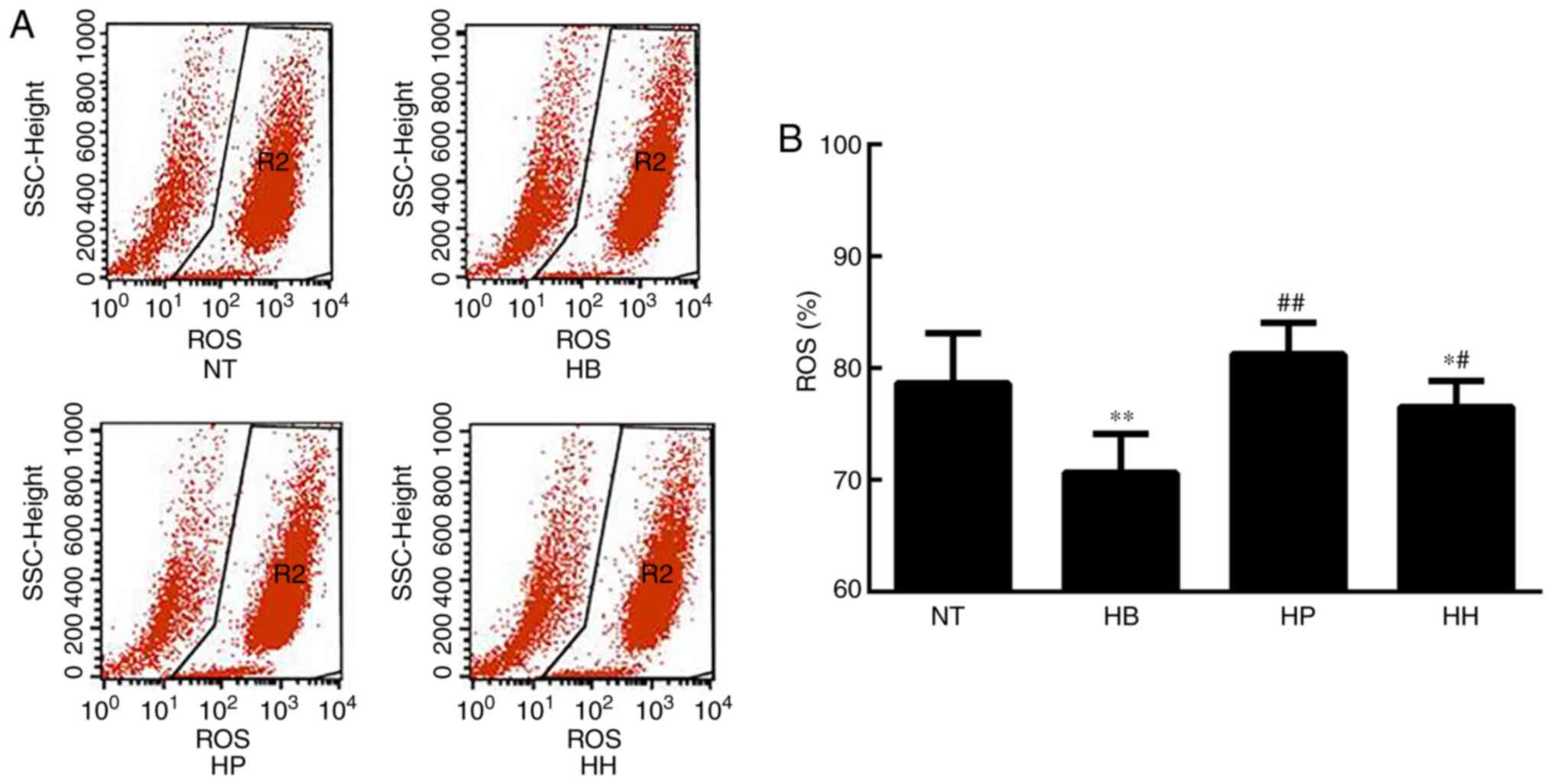
ROS analysis demonstrated that the level of ROS was
significantly elevated in the hypothermic group compared with the
normal temperature group (P<0.01; Fig. 4). Since ROS is an indicator of
cellular oxidative stress, it was suggested that hypothermia
induced oxidative stress in HDMECs. Compared with the hypothermic
group, ROS levels were suppressed by treatment with higenamine and
the positive control drug, suggesting that higenamine as well as
prostaglandin E1 inhibited the accumulation of ROS (Fig. 4). This result suggested that
higenamine prevented oxidative stress. To further elucidate the
molecular mechanisms involved, western blotting analysis was
performed and the results demonstrated that compared with the
normal temperature group, the expression of α2C-AR (intracellular)
was significantly downregulated in the positive control group
(P<0.01), but the expression of α2C-AR (membrane) was
significantly increased in the hypothermia group (P<0.05;
Fig. 5) while the expression of
α2C-AR (membrane) was downregulated by the positive control drug
(P<0.01). Compared with the hypothermia group, the ratio of
α2C-AR (intracellular)/α2C-AR (membrane) was significantly
decreased in higenamine group (P<0.01). The ratio of α2C-AR
(intracellular)/α2C-AR (membrane) was significantly decreased,
suggesting that α2C-AR in hypothermic HDMECs translocated
intracellularly to the membrane. This suggested that hypothermia
may induce α2C-AR translocation, which transfers intracellularly to
transmembrane and enhances the contraction of vascular smooth
muscle. Compared with the normal temperature group, the expression
of PTK9 was upregulated in the hypothermia group (P<0.05) and
downregulated in the higenamine group (P<0.05). This implied
that the expression of PTK9 was upregulated in cold-induced HDMECs
but reversed by treatment with higenamine. As PTK9 is associated
with actin binding, it was postulated that the activity of actin in
cold-treated HDMECs was increased and that the contraction of
smooth muscle was enhanced by hypothermic treatment. Compared with
the hypothermia group, the expression of α2C-AR (intracellular or
membrane) was significantly downregulated in the higenamine and the
positive control group (P<0.05). The ratio of α2C-AR
(intracellular)/α2C-AR (membrane) ratio significantly increased in
the two groups (P<0.01). This indicated that higenamine and
prostaglandin E1 can suppress the expression of intracellular and
membrane α2C-AR, and prevent the translocation of α2C-AR from the
cytoplasm to the membrane. Overall, compared with the normal
temperature group, the expression of ROS/α2C-AR and PTK9 was
increased in the hypothermia group (Fig.
5F), indicating that hypothermia can induce an oxidative stress
response in the HDMECs, and initiate the translocation of the
original α2C-AR from the cytoplasm to the membrane. Compared with
the hypothermia group, higenamine treatment inhibited the
activation of ROS/α2C-AR and PTK9 signaling pathways in the
hypothermic HDMECs, suggesting that higenamine may inhibit the
effect of cold-induced vasoconstriction.
Discussion
Previously, pharmacological studies have
demonstrated that higenamine has a vasodilating effect (14,19,23), but
its underlying mechanisms are not fully understood. This study was
aimed to investigate the antispasmodic effect of higenamine on
cold-induced vasoconstriction and the underlying mechanisms. An
in vivo model of cold-induced cutaneous vasoconstriction in
rats was established. The results indicated that cold-decreased the
RBF of rat caudal arterial cortex, but the medium and high doses of
higenamine reversed these effects. Although certain studies have
reported the vasodilatory effect of higenamine, these previous
studies have only focused on isolated aortas (23,25) and
there have been no reports on medial arteries and arterioles. In
the present study, it was demonstrated that establishing a
cold-induced rat skin vasoconstriction in vivo model reduced
the RBF of the rat caudal arterial cortex. Studies have
demonstrated that in an animal model of cold blood stasis syndrome,
microcirculatory flow decreases the diameter of the
microvasculature and the flow velocity and flow rate would be
impaired and suppressed (26–28). The
RBF reduction in the cold-induced cutaneous vasoconstriction model
is similar to that in the cold blood stasis syndrome model.
Higenamine can be found in a variety of medicinal
herbs such as Aconite, Asarum, Galangal and Citrus
aurantium. However, whether its vasodilation effect is related
to cold has not been reported so far. In the present study,
increasing doses of higenamine gradually increased the change in
RBF, indicating that the effect of higenamine on cold-induced skin
vasoconstriction in rats may be positively correlated with the
dose. The in vivo studies of cardiovascular pathophysiology
in the past mainly focused on the positive inotropic effects in the
heart and cardiac electrophysiological effects of higenamine. The
primary method is to measure the blood pressure and heart rate of
the model (29,30). These studies also suggested the
effect of higenamine on the cardiac pump function and
electrophysiology.
Vascular endothelial cells (VECs) play an important
role in regulating vascular homeostasis. The main functions of VECs
are as follows: Endothelial cell barrier, secretion of multiple
vasoactive substances and regulation of vascular tone (31). VECs secrete active substances that
promote vasodilation and vasoconstriction (32). Whether the vasodilatory effect of
higenamine on cold-induced vasoconstriction is
endothelium-dependent is a major problem to be discussed. Few
studies have been conducted on the effects of cold on microvascular
endothelial cells. The HDMECs were used for the investigation of
the mechanisms of cold-induced vasoconstriction. Previous studies
indicated that the PI3K/Akt/mTOR signaling pathway and the
AMPK/eNOS/NO signaling pathway play a key role in metabolism and is
associated with vasodilation and increased blood flow (33–36).
Akt/PKB phosphorylates eNOS at serine-1177 (Ser1177) and mediates
the non-genetic rapid activation of eNOS and the AMPK-mediated
signaling pathway also phosphorylates eNOS at the serine-1177
(Ser1177) site, causing vasodilation (37). However, it is rarely reported whether
this signaling pathway is associated with cold-induced VED. This
study found that the expression of PI3K/Akt and AMPK/eNOS/NO
signaling pathways were downregulated in hypothermic HDMECs,
demonstrating that cold can downregulate these signaling pathways
in vascular endothelial cells. RP is mainly induced by cold and the
pathogenesis of RP is not yet fully understood. RP is associated
with excessive contraction of the terminal artery and previous
studies have suggested that VED is the main pathological
manifestation of RP (38–40). However, the mechanism of VED in RP is
not fully understood. Downregulation of PI3K/Akt and AMPK/eNOS/NO
signaling pathways in hypothermic HDMECs suggests that the
pathogenesis of cold induced VED in RP may be related to the
downregulation of PI3K/Akt and AMPK/eNOS/NO signaling pathways.
Studies have demonstrated that in the model of heart
failure induced by doxorubicin, higenamine exerts antioxidative
stress, cell apoptosis and protects cardiomyocytes by activating
the PI3K/Akt signaling pathway (13,19,41). The
results of the present study demonstrated that in the hypothermia
group, the expression of Akt1 was increased while the expression of
p-Akt1 was downregulated in the higenamine group. The results of
the present study suggested that higenamine may improve the
inhibition of PI3K/Akt signaling pathway in HDMECs at low
temperature and the vasodilating effect of higenamine may have an
endothelium-dependent effects.
The AMPK/eNOS/NO signaling pathway plays an
important regulatory role in energy metabolism. NO is a
vasodilating factor produced by endothelial cells, which mainly
regulates vascular tone. In this study, it was demonstrated that
the NO concentration and the expression levels of eNOS, p-NOS,
AMPKα1, and p-AMPKα1 in the hypothermia positive control group
(prostaglandin E1) were upregulated compared with the hypothermia
group. This suggested that prostaglandin E1 can ameliorate the
inhibition of the AMPK/eNOS/NO signaling pathway in hypothermic
HDMECs and promote NO production in endothelial cells. In contrast,
the NO concentration and the expression of eNOS, p-NOS, AMPKα1 and
p-AMPKα1 in the hypothermia higenamine group was downregulated
compared with the hypothermia group. This suggested that higenamine
exacerbates the inhibition of the AMPK/eNOS/NO signaling pathway in
HDMECs induced by hypothermia but inhibits NO production in
endothelial cells. The PI3K/Akt and AMPK/eNOS/NO signaling pathways
have significant regulatory functions in energy metabolism.
Higenamine had an effect on the inhibition of the PI3K/Akt
signaling pathway and NO production in cold-induced endothelial
cells, but had no effect on the inhibition of AMPK/eNOS/NO
signaling pathway. Therefore, the endothelium-dependent
vasodilation effect of higenamine remains to be further
studied.
Cold-induced vasoconstriction is associated with
oxidative stress. Studies have found that cold induces the
production and activation of ROS in endothelial cells, and promotes
inflammatory responses in vivo (42). ROS levels are indicators of cellular
oxidative stress. Under oxidative stress conditions, ROS production
is increased and leads to subsequent changes in membrane lipids,
proteins, and nucleic acids. Oxidative damage to these biomolecules
is associated with aging and various pathological events, including
atherosclerosis, tumorigenesis, ischemia-reperfusion injury and
neurodegenerative diseases. In this experiment, it was demonstrated
that the ROS level in the hypothermia group was increased compared
with the normal temperature group. Compared with the hypothermia
group, ROS level was decreased in the higenamine and positive
control groups. Studies have found that cold causes a rapid
increase of ROS in skin vascular smooth muscle cells (VSMCs),
activates the Rho/Rho-kinase signaling pathway, leading to
translocation of smooth muscle cell α2C-AR from the trans-Golgi
apparatus to the extracellular membrane, therefore promoting distal
vasoconstriction (43–45). Cold-induced vasoconstriction was also
found to be associated with the production of ROS in the
mitochondria through redox signaling in VSMCs, which activates
RhoA/Rho kinase signaling and causes the original intracellular
stationary α2C-ARs to migrate to the cell surface, resulting in
contraction of vascular smooth muscle (46–48). In
addition, it was reported that hypothermic stimulation can increase
PTK activity in patients with RP and promote PTK phosphorylation.
Furspan et al (49) found
that increased PTK phosphorylation in RP patients mediates
vasoconstriction caused by cold stimulation. In the present study,
ROS/α2C-AR and PTK9 signaling pathways in HDMECs were investigated.
Compared with the normal temperature group, the expression of
α2C-AR (membrane) was upregulated in the hypothermia group and the
ratio of α2C-AR (intracellular)/α2C-AR (the membrane) was decreased
in the higenamine and the hypothermia groups, suggesting that the
membrane α2C-AR production of HDMEC was increased and the ratio of
α2C-AR (intracellular)/α2C-AR (cell membrane) was decreased. This
data demonstrated that α2C-AR was translocated from the cytoplasm
to the membrane in the hypothermic HDMECs, indicating that cold
induced the α2C-AR translocation and enhanced the vascular smooth
muscle contraction. Compared with the normal temperature group, the
expression of PTK9 was downregulated in the hypothermia group,
suggesting that cold-induced upregulation of PTK9 expression in
HDMECs was associated with actin binding. ROS/α2C-AR and PTK9
signaling pathways, oxidative stress, α2C-AR translocation, and
smooth muscle contraction were induced by hypothermia in HDMECs.
The study found that the expression of α2C-AR (membrane) in the
hypothermia higenamine group was decreased compared with normal
temperature group. Compared with the hypothermia group, the
expression of α2C-AR (intracellular) in the hypothermia higenamine
group was also decreased. The ratio of α2C-AR
(intracellular)/α2C-AR (membrane) was increased in the higenamine
group, suggesting that the expression of intracellular and membrane
α2C-AR was inhibited and the translocation of α2C-AR from the
cytoplasm to the membrane was prevented. These observations
suggested that higenamine has an inhibitory effect on the
activation of ROS/α2C-AR and PTK9 signaling pathways in
cold-induced HDMECs and inhibits cold-induced vasoconstriction.
Studies indicate that clinical-induction of
hypothermia can improve the neurological function of unconscious
subjects following unexpected cardiac arrest by preserving heart
and brain stability (50–53). Recently, it has been reported that
early application of mild hypothermia therapy not only improves the
neurological outcome, but also increases the survival probability
of patients postdischarge (54). The
present study's findings indicated that higenamine and its
regulated pathways could play a significant role in the mild
hypothermia therapy. The analysis of temperature from a range of
mild hypothermia therapies is of great significance in clinical
application and is also important for in-depth elucidation in
further studies. The present study has some limitations that need
to be addressed in future studies. Firstly, the results of the
present study demonstrated that the vasodilating effect of
higenamine may not be related to the AMPK/eNOS/NO signaling
pathway, which requires an in-depth investigation. Secondly,
studies have demonstrated that higenamine can downregulate the
expression of iNOS mRNA induced by lipopolysaccharide and has
anti-inflammatory effects (55–57). In
this study, it was found that the expression of eNOS/NO was
downregulated by higenamine in hypothermic HDEMCs. Therefore,
whether there is an inhibitory effect of higenamine on different
types of NOS requires further study. Finally, as an emerging drug,
the research on the dosage and pharmacokinetics of higenamine in
vitro and in vivo are encouraged.
Higenamine may reverse the inhibition of the
PI3K/Akt signaling pathway in hypothermic HDMECs, however it may
not have regulatory roles in the inhibition of the AMPK/eNOS/NO
signaling pathway. The ROS/α2C-AR and PTK9 signaling pathways were
upregulated in the hypothermic HDMECs. Cold can induce the
oxidative stress in the vascular smooth muscle and strengthen the
contraction function. Higenamine inhibits the activation of
ROS/α2C-AR and PTK9 signaling pathways in hypothermic HDMECs and
may play an important role in inhibiting the oxidative stress in
cold-induced vasoconstriction. The results of the present study
will boost the modernization of traditional Chinese medicine and
has clinical and scientific relevance.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81874404).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
GP Zhao designed the study. JH Guan, HM Lin, MJ Xie,
MN Huang, D Zhang, SS Ma and WY Bian carried out experiments. HM
Lin, JH Guan and MJ Xie analyzed data. JH Guan, HM Lin and GP Zhao
interpreted results of experiments. JH Guan, HM Lin, MJ Xie, MN
Huang and D Zhang prepared the figures. HM Lin and JH Guan wrote
the manuscript. All authors contributed equally. All authors read
and approved the final manuscript.
Ethics approval
Animal studies were performed in accordance with the
declaration of Helsinki and approved by the institutional Ethics
Committee of the School of traditional Chinese medicine, Jinan
University, Guangzhou (China).
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
al-Awami M, Schillinger M and Minar E:
Vasospasm of the scrotum-a manifestation of Raynaud's phenomenon?
VASA. 33:87–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barr WG and Fahey PJ: Reduction of
pulmonary capillary blood volume following cold exposure in
patients with Raynaud's phenomenon. Chest. 94:1195–1199. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belch JJ, Land D, Park RH, McKillop JH and
MacKenzie JF: Decreased oesophageal blood flow in patients with
Raynaud's phenomenon. Br J Rheumatol. 27:426–430. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brouwer RM, Wenting GJ and Schalekamp MA:
Acute effects and mechanism of action of ketanserin in patients
with primary Raynaud's phenomenon. J Cardiovasc Pharmacol.
15:868–876. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown S: Diagnosis and management of
patients with Raynaud's phenomenon. Nurs Stand. 26:41–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engelhart M and Seibold JR: The effect of
local temperature versus sympathetic tone on digital perfusion in
Raynaud's phenomenon. Angiology. 41:715–723. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fardoun MM, Nassif J, Issa K, Baydoun E
and Eid AH: Raynaud's Phenomenon: A brief review of the underlying
mechanisms. Front Pharmacol. 7:4382016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hughes M and Herrick AL: Raynaud's
phenomenon. Best Pract Res Clin Rheumatol. 30:112–132. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuryliszyn-Moskal A, Kita J and Hryniewicz
A: Raynaud's phenomenon: New aspects of pathogenesis and the role
of nailfold videocapillaroscopy. Reumatologia. 53:87–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
An X, Long C, Deng X, Tang A, Xie J, Chen
L and Wang Z: Higenamine inhibits apoptosis and maintains survival
of gastric smooth muscle cells in diabetic gastroparesis rat model
via activating the beta2-AR/PI3K/AKT pathway. Biomed Pharmacother.
95:1710–1717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bai G, Yang Y, Shi Q, Liu Z, Zhang Q and
Zhu YY: Identification of higenamine in Radix Aconiti Lateralis
Preparata as a beta2-adrenergic receptor agonist1. Acta Pharmacol
Sin. 29:1187–1194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao YX, Yu GR, Xu JM, Xu YQ, Bian YT and
Zheng DS: Effect of acute higenamine administration on
bradyarrhythmias and HIS bundle. A clinical study of 14 cases and
animal experiment on dogs. Chin Med J (Engl). 95:781–784.
1982.PubMed/NCBI
|
|
13
|
Chen YL, Zhuang XD, Xu ZW, Lu LH, Guo HL,
Wu WK and Liao XX: Higenamine combined with [6]-gingerol suppresses
doxorubicin-triggered oxidative stress and apoptosis in
cardiomyocytes via upregulation of PI3K/Akt pathway. Evid Based
Complement Alternat Med. 2013:9704902013.PubMed/NCBI
|
|
14
|
Zhang N, Lian Z, Peng X, Li Z and Zhu H:
Applications of Higenamine in pharmacology and medicine. J
Ethnopharmacol. 196:242–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stajić A, Anđelković M, Dikić N, Rašić J,
Vukašinović-Vesić M, Ivanović D and Jančić-Stojanović B:
Determination of higenamine in dietary supplements by UHPLC/MS/MS
method. J Pharm Biomed Anal. 146:48–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang N, Qu K, Wang M, Yin Q, Wang W, Xue
L, Fu H, Zhu H and Li Z: Identification of higenamine as a novel
alpha1-adrenergic receptor antagonist. Phytother Res. 33:708–717.
2019. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Geng J, Jiang M, Li C, Han Y and
Jiang J: The cardiac electrophysiology effects of higenamine in
guinea pig heart. Biomed Pharmacother. 109:2348–2356. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohen PA, Travis JC, Keizers PHJ, Boyer FE
and Venhuis BJ: The stimulant higenamine in weight loss and sports
supplements. Clin Toxicol (Phila). 57:125–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu MP, Zhang YS, Zhou QM, Xiong J, Dong YR
and Yan C: Higenamine protects ischemia/reperfusion induced cardiac
injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT
signaling pathway. Pharmacol Res. 104:115–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duan W, Chen J, Wu Y, Zhang Y and Xu Y:
Protective effect of higenamine ameliorates collagen-induced
arthritis through heme oxygenase-1 and PI3K/Akt/Nrf-2 signaling
pathways. Exp Ther Med. 12:3107–3112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Zhu C, Wang G, Xu R and Zhu Y:
Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal
ischemia-reperfusion injury in mice. Inflamm Res. 64:395–403. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee YS, Kang YJ, Kim HJ, Park MK, Seo HG,
Lee JH, Yun-Choi HS and Chang KC: Higenamine reduces apoptotic cell
death by induction of heme oxygenase-1 in rat myocardial
ischemia-reperfusion injury. Apoptosis. 11:1091–1100. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang KC, Chong WS and Lee IJ: Different
pharmacological characteristics of structurally similar
benzylisoquinoline analogs, papaverine, higenamine, and GS 389, on
isolated rat aorta and heart. Can J Physiol Pharmacol. 72:327–334.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo D, Xu Z, Hu X, Zhang F, Bian H, Li N,
Wang Q, Lu Y, Zheng Q and Gu J: URI prevents potassium
dichromate-induced oxidative stress and cell death in gastric
cancer cells. Am J Transl Res. 8:5399–5409. 2016.PubMed/NCBI
|
|
25
|
Chang KC, Lim JK and Park CW:
Pharmacological evaluation of GS-389, a novel
tetrahydroisoquinoline analog related to higenamine, on vascular
smooth muscle. Life Sci. 51:67–74. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao EW, Deng JG, Du ZC, Yan K, Zheng ZW,
Wang Q, Huang LZ, Bao CH, Deng XQ, Lu XY and Tang ZL: Experimental
study on two-way application of traditional Chinese medicines
capable of promoting blood circulation and removing blood stasis
with neutral property in cold and hot blood stasis syndrome I.
Zhongguo Zhong Yao Za Zhi. 37:3302–3306. 2012.(In Chinese).
PubMed/NCBI
|
|
27
|
Ryan TJ and Copeman PW: Microvascular
pattern and blood stasis in skin disease. Br J Dermatol.
81:563–573. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ning SY, Jiang BP, Xu L, Fang TH and Wu
MH: Effect of Liangxuehuayu Recipe on hemorheology in rats with
blood stasis syndrome. Asian Pac J Trop Med. 5:935–938. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura I, Makino M, Takamura Y, Islam MA
and Kimura M: Positive chronotropic and inotropic effects of
higenamine and its enhancing action on the aconitine-induced
tachyarrhythmia in isolated murine atria. Jpn J Pharmacol.
66:75–80. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Z, Liu X, Tao Z, Shi R, Zhang X, Yao
Z, Liu Y, Zhu K and Chen B: Effects of higeramine on hemodynamics
and its tolerability and safety, an experimental study. Zhonghua Yi
Xue Za Zhi. 82:352–355. 2002.(In Chinese). PubMed/NCBI
|
|
31
|
Michiels C: Endothelial cell functions. J
Cell Physiol. 196:430–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brandes RP, Schmitz-Winnenthal FH, Félétou
M, Gödecke A, Huang PL, Vanhoutte PM, Fleming I and Busse R: An
endothelium-derived hyperpolarizing factor distinct from NO and
prostacyclin is a major endothelium-dependent vasodilator in
resistance vessels of wild-type and endothelial NO synthase
knockout mice. Proc Natl Acad Sci USA. 97:9747–9752. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang PC, Ng YF, Ho S, Gyda M and Chan SW:
Resveratrol and cardiovascular health-promising therapeutic or
hopeless illusion? Pharmacol Res. 90:88–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu J, Han Y, Wang J, Liu Y, Zheng S, Zhou
L, Jose PA and Zeng C: Irisin lowers blood pressure by improvement
of endothelial dysfunction via AMPK-Akt-eNOS-NO pathway in the
spontaneously hypertensive rat. J Am Heart Assoc. 5:e0034332016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamawaki H, Kuramoto J, Kameshima S, Usui
T, Okada M and Hara Y: Omentin, a novel adipocytokine inhibits
TNF-induced vascular inflammation in human endothelial cells.
Biochem Biophys Res Commun. 408:339–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fujimura N, Jitsuiki D, Maruhashi T,
Mikami S, Iwamoto Y, Kajikawa M, Chayama K, Kihara Y, Noma K, Goto
C and Higashi Y: Geranylgeranylacetone, heat shock protein
90/AMP-activated protein kinase/endothelial nitric oxide
synthase/nitric oxide pathway, and endothelial function in humans.
Arterioscler Thromb Vasc Biol. 32:153–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thors B, Halldórsson H and Thorgeirsson G:
Thrombin and histamine stimulate endothelial nitric-oxide synthase
phosphorylation at Ser1177 via an AMPK mediated pathway independent
of PI3K-Akt. FEBS Lett. 573:175–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu W, Liu JY, Yin ZY, Long CL and Wang H:
The characteristics of vascular endothelial injuries induced by
extreme environmental factors. Zhongguo Ying Yong Sheng Li Xue Za
Zhi. 29:494–500. 2013.PubMed/NCBI
|
|
39
|
Turton EPL, Kent PJ and Kester RC:
VASCULAR REVIEW: The aetiology of Raynaud's phenomenon. Cardiovasc
Surgery. 6:431–440. 1998. View Article : Google Scholar
|
|
40
|
Matucci-Cerinic M, Kahaleh B and Wigley
FM: Review: Evidence that systemic sclerosis is a vascular disease.
Arthritis Rheum. 65:1953–1962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu
W, Zhu Y, Man Y, Wang S and Li J: Astragalus polysaccharide
suppresses doxorubicin-induced cardiotoxicity by regulating the
PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev.
2014:6742192014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Awad EM, Khan SY, Sokolikova B, Brunner
PM, Olcaydu D, Wojta J, Breuss JM and Uhrin P: Cold induces
reactive oxygen species production and activation of the NF-kappa B
response in endothelial cells and inflammation in vivo. J Thromb
Haemost. 11:1716–1726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maeng J, Sheverdin V, Shin H, Ha I, Bae
SS, Yang-Yen HF and Lee K: Up-regulation of Rhoa/Rho kinase pathway
by translationally controlled tumor protein in vascular smooth
muscle cells. Int J Mol Sci. 15:10365–10376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jeyaraj SC, Unger NT, Eid AH, Mitra S,
Paul El-Dahdah N, Quilliam LA, Flavahan NA and Chotani MA: Cyclic
AMP-Rap1A signaling activates RhoA to induce α(2c)-adrenoceptor
translocation to the cell surface of microvascular smooth muscle
cells. Am J Physiol Cell Physiol. 303:C499–C511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bailey SR, Eid AH, Mitra S, Flavahan S and
Flavahan NA: Rho kinase mediates cold-induced constriction of
cutaneous arteries: Role of alpha2C-adrenoceptor translocation.
Circ Res. 94:1367–1374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eid AH, Chotani MA, Mitra S, Miller TJ and
Flavahan NA: Cyclic AMP acts through Rap1 and JNK signaling to
increase expression of cutaneous smooth muscle
alpha2C-adrenoceptors. Am J Physiol Heart Circ Physiol.
295:H266–H272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Honda M, Suzuki M, Nakayama K and Ishikawa
T: Role of alpha2C-adrenoceptors in the reduction of skin blood
flow induced by local cooling in mice. Br J Pharmacol. 152:91–100.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jantschak F, Popp AM, Hofmann RA, Villalón
CM, Centurión D and Pertz HH: Postjunctional α2C-adrenoceptors
mediate vasoconstriction in rat tail artery: Influence of
precontraction and temperature on vasoreactivity. Naunyn
Schmiedebergs Arch Pharmacol. 382:487–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Furspan PB, Chatterjee S, Mayes MD and
Freedman RR: Cooling-induced contraction and protein tyrosine
kinase activity of isolated arterioles in secondary Raynaud's
phenomenon. Rheumatology (Oxford). 44:488–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leonov Y, Sterz F, Safar P, Radovsky A,
Oku K, Tisherman S and Stezoski SW: Mild cerebral hypothermia
during and after cardiac arrest improves neurologic outcome in
dogs. J Cereb Blood Flow Metab. 10:57–70. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Arbour RB: Brain death: Assessment,
controversy, and confounding factors. Critical Care Nurse.
33:27–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Globus MY, Alonso O, Dietrich WD, Busto R
and Ginsberg MD: Glutamate release and free radical production
following brain injury: Effects of posttraumatic hypothermia. J
Neurochem. 65:1704–1711. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Geocadin RG, Koenig MA, Jia X, Stevens RD
and Peberdy MA: Management of brain injury after resuscitation from
cardiac arrest. Neurol Clin. 26487–506. (ix)2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bradley SM, Liu W, McNally B, Vellano K,
Henry TD, Mooney MR, Burke MN, Brilakis ES, Grunwald GK, Adhaduk M,
et al: Temporal trends in the use of therapeutic hypothermia for
out-of-hospital cardiac arrest. JAMA Netw Open. 1:e1845112018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee HY, Lee JS, Kim EJ, Han JW, Lee HW,
Kang YJ and Chang KC: Inhibition of lipopolysaccharide-induced
inducible nitric oxide (iNOS) mRNA expression and nitric oxide
production by higenamine in murine peritoneal macrophages. Arch
Pharm Res. 22:55–59. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kang YJ, Lee YS, Lee GW, Lee DH, Ryu JC,
Yun-Choi HS and Chang KC: Inhibition of activation of nuclear
factor kappaB is responsible for inhibition of inducible nitric
oxide synthase expression by higenamine, an active component of
aconite root. J Pharmacol Exp Ther. 291:314–320. 1999.PubMed/NCBI
|
|
57
|
Park JE, Kang YJ, Park MK, Lee YS, Kim HJ,
Seo HG, Lee JH, Hye Sook YC, Shin JS, Lee HW, et al: Enantiomers of
higenamine inhibit LPS-induced iNOS in a macrophage cell line and
improve the survival of mice with experimental endotoxemia. Int
Immunopharmacol. 6:226–233. 2006. View Article : Google Scholar : PubMed/NCBI
|