Introduction
Sepsis or systemic inflammatory response syndrome is
caused by infection, which is an immune response to pathogens and
immunogenic substances that induce autoimmune injury (1). Sepsis is common in cases of severe
trauma, burns, shock or major surgery. The further development of
sepsis may lead to septic shock and multiple organ dysfunction
syndrome, making sepsis a leading cause of death in patients
admitted to intensive care units (2).
Bacterial infection is the most common cause of
sepsis (2). Bacterial products,
including endotoxin and exotoxins, can directly or indirectly
stimulate various target cells including monocytes,
polymorphonuclear neutrophils or endothelial cells to initiate
inflammation (1). Lipopolysaccharide
(LPS) is a representative endotoxin (3). In the early stages of sepsis, the
alteration of cardiovascular function is associated with patient
prognosis (4). Cardiovascular
complications aggravate sepsis and increase patient mortality
(4,5). Sepsis that is complicated with
cardiovascular dysfunction is commonly referred to as
sepsis-induced cardiomyopathy (SIC). Sepsis-induced myocardial
damage is closely associated with a decreased myocardial energy
supply and weakened myocardial contractility (5). In addition, a large number of
inflammatory factors, including tumor necrosis factor-α (TNF-α),
are also involved in sepsis induced myocardial injury (6,7). In
sepsis, pathogenic microorganisms transmit signals to the nucleus
via cell transduction pathways, triggering a series of
transcriptions and translations (7,8).
Therefore, blocking the pathological process at this stage may
effectively prevent sepsis induced myocardial injury (8).
MicroRNAs (miRNA or miRs) are non-coding RNAs that
regulate the expression of target genes by inhibiting the
translation or degredation of mRNA (9). miRNAs are involved in the regulation of
metabolism, the inflammatory response, heart-associated diseases,
organ transplantation and cancer by regulating mRNA and protein
expression (9,10). Among all miRNAs that regulate the
progression of inflammation, the role of miR-146a is prominent. It
has been demonstrated that miR-146a promotes the release of
inflammatory factors by regulating the toll-like receptor 4
(TLR-4)/NF-κB signaling pathway, which is closely associated with
myocardial damage (11,12). Previous studies have also revealed
that miRNA-146a protects the myocardium from ischemia-reperfusion
injury by suppressing interleukin-1 receptor associated kinase 1
(IRAK1) and TNF receptor associated factor 6 (TRAF6), therefore
attenuating NF-κB activation and inflammatory cytokine production
(13,14). Taganov et al (15) revealed that the stimulation of LPS,
TNF-α or interleukin (IL)-1β upregulates the expression of
NF-κB-dependent miR-146a in human monocyte leukemia cells. The
mutational experiment and luciferase reporter gene assay performed
within the aforementioned study revealed that the target genes of
miR-146a were IRAK1 and TRAF6, which are adaptor protein encoding
genes involved in the TLR-4/NF-κB pathway (15). Activated NF-κB induces the
transcription of a large number of inflammatory genes as well as
the upregulation of miR-146a. However, miR-146a negatively
regulates the NF-κB pathway and inhibits the production of
inflammatory factors by targeting the IRAK1 and TRAF6 signal
transduction proteins upstream of the NF-κB pathway (16). A further study has revealed that
miR-146a binds to the 3′ untranslated region (UTR) of IRAK1 and
TRAF6, inhibiting their expression at the transcriptional level and
alleviating sepsis-induced cardiac dysfunction by inhibiting
certain inflammatory factors, including IL-6, IL-8, IL-1β and TNF-α
(17).
The TLR-4/NF-κB signaling pathway serves a crucial
regulatory role in sepsis-induced cardiomyopathy. Blocking this
pathway may therefore be an important method for treating SIC
(18,19). The current study aimed to assess the
association between the TLR-4/NF-κB pathway and levels of
inflammatory cytokines by detecting changes of miR-146a expression
in the myocardium of mice with SIC. The effect of the TLR-4/NF-κB
pathway on sepsis-induced cardiomyopathy was also investigated.
Materials and methods
Animals
A total of 60 healthy male Sprague Dawley (SD) rats
(Shandong Laboratory Animal Centre; age, 6–8 weeks; weight, 180–200
g) were housed in an specific pathogen free laboratory animal room
at 20±2°C with a 60–70% relative humidity, under a 12 h light/dark
cycle. All rats recieved free access to food and water. SD rats
were equally divided into four groups using a random number table,
with 15 rats in each group. Groups included a control group
(control), a septic model group (LPS), a miR-146a agonist group
(agonist) and a miR-146a inhibitor group (inhibitor). The current
study was approved by the Animal Ethics Committee of Kunming
Medical University Animal Center (Kunming, China).
Rat model with septic
cardiomyopathy
Rats in the LPS group received an intraperitoneal
injection of 0.2 µl/g of water and a further injection of 7.5 mg/kg
LPS (cat. no. L26331; Shanghai Abcone Co., Ltd.) 24 h later. At the
same time point of LPS injection, in control group rats, an equal
volume of water or physiological saline was injected to control
rats. In the miR-146a agonist and inhibitor group, rats were
injected with 0.2 µl/g miR-146a agonist (Shanghai Tuoran Biological
Technology co., Ltd.) or inhibitor (Shanghai Tuoran Biological
Technology co., Ltd.) in the tail vein 24 h prior to LPS
injection.
Sample collection
24 h after LPS injection, 2 ml of rat blood was
collected from the internal iliac vein. Blood was maintained at
room temperature for 15 min and subsequently centrifuged at 1,000 ×
g for 15 min at 4°C. The upper serum was aspirated and stored at
−80°C. After blood was taken, rats were sacrificed and hearts were
extracted and washed with pre-cooled physiological saline. Half of
the heart tissue was frozen in a −80°C refrigerator and the other
half were soaked with 4% paraformaldehyde at room temperature for
24 h and then embedded in paraffin for pathological
examination.
Hematoxylin and eosin (HE)
staining
Myocardial tissues were fixed in 4% paraformaldehyde
at room temperature for 24 h. Tissues were then cut into 4 µm
slices after washing with PBS, dehydration with a descending
alcohol series, waxing and embedding in paraffin. Slices were
placed in a dish with warm water to unfold and were subsequently
placed on a glass slide for patching. After holding at room
temperature for 60 min, sections were observed with a light
microscope (magnification, ×400) after HE staining (hematoxylin, 5
min at room temperature and eosin, 3 min at room temperature).
Detection of cell apoptosis
Myocardial tissues were dehydrated, embedded and the
sections were routinely dewaxed, washed, hydrated and fixed as
aforementioned. The procedure followed strictly in accordance with
the TUNEL Apoptosis kit manufacturers protocol (Merck KGaA). Each
paraffin section was randomly selected from five fields
(magnification, ×400) and at least 100 cells were counted in each
field of view with a light microscope (magnification, ×400). The
number of apoptotic cells (TUNEL positive cells) and the total
number of cells were counted respectively. Apoptotic index=number
of apoptotic cells/total cell number ×100.
Determination of serum myocardial
injury markers
Rat serum was warmed to room temperature. Cardiac
troponin I (cTnI; cat. no. ab246529) and B-type natriuretic peptide
(BNP; cat. no. ab108815) content were detected using an ELISA assay
(Abcam). Creatine kinase myocardial bound (CK-MB) and myoglobin
(Mb) content were detected using the chemiluminescence method (cat.
no. E-CL-R0722c and E-CL-R0132c; Ke Lei Biological Technology Co.,
Ltd.).
Western blot analysis
Myocardial tissues were washed in a pre-cooled
physiological saline at 4°C. After the addition of RIPA lysate
(Beyotime Institute of Biotechnology), tissues were homogenized and
the supernatant was centrifuged at 5,000 × g for 10 min at 4°C.
Total protein content was determined using a BCA Assay kit (cat.
no. p0011; Beyotime Institute of Biotechnology). The expression of
synaptic plasticity-associated proteins including NF-κB, TLR-4,
TNF-α and intercellular adhesion molecule-1 (ICAM-1) were detected
via western blot analysis. Protein (10 µl) was loaded and separated
by 12% SDS-PAGE electrophoresis, which was transferred to a PVDF
membrane (Roche Diagnostics). Membranes were subsequently blocked
using 5% skimmed milk with TBST at 25°C for 1 h and incubated with
the following primary antibodies obtained from Abcam at 4°C
overnight: NF-κB (1:500; cat. no. ab19870), TLR-4 (1:500; cat. no.
ab95562), TNF-α (1:500; cat. no. ab9755), ICAM-1 (1:500; cat. no.
ab171123) and β-actin (1:500; cat. no. ab8226). The membrane was
then incubated with Goat Anti-Rabbit IgG H&L secondary
antibodies (1:1,000; cat. no. ab150077; Abcam) at 25°C for 2 h. The
signal on the membrane was detected using an enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.) and
Quantity One (version 4.0; Bio-Rad Laboratories, Inc.) was used for
quantification.
Reverse transcription-quantitative
(RT-q) PCR assay
The total RNA of rat myocardial tissue was extracted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed to synthesize cDNA using the Takara PrimeScript
RT Master Mix kit (Takra Bio, Inc.) according to manafacturers
protocol. The sequences of miR-146a, TNF-α, IL-1α, IL-1β and GADPH
mRNA were obtained from Gene Bank (https://cipotato.org/genebankcip/). Primers were
designed using Primer premier 5.0 software (Primers). The following
primer sequences were used: miR-146a forward,
5′-GAACTGAATTCCATGGGTTGTGT-3′ and reverse,
5′-GCCCACGATGACAGAGAGATCC-3′; TNF-α forward,
5′-AGTCCGGGCAGGTCTACTTT-3′ and reverse, 5′-GCACCTCAGGGAAGAGTCTG-3′;
IL-1α forward, 5′-AAGTTTGTCATGAATGATTCCCTC-3′ and reverse,
5′-GTCTCACTACCTGTGATGATGAGT-3′; IL-1β forward,
5′-ATAAGCCCACTCTACACCTCTGA-3′ and reverse,
5′-ATTGGCCCTGAAAGGAGAGAGA-3′; GADPH forward,
5′-GGCATCCCATAGAGGCGAAGTC-3′ and reverse,
5′-ACGCCGGACTTGATGAGGCGAT-3′. The average Cq values of the target
gene and the reference gene were determined by RT-qPCR. qPCR was
performed using SYBR® Green Master Mix (Takara Bio,
Inc.) according to the manufacturer's protocol; amplification was
performed under the recommended parameters: Initial denaturation at
95°C for 5 min, followed by 40 cycles of 95°C for 5 sec, 60°C for
15 sec and 72°C for 15 sec, and a final extension at 94°C for 15
sec. The relative quantitative method was used to calculate the
relative expression of the sample and the amount of the gene of the
treatment group relative to the blank group was calculated using
the 2−ΔΔCq method (20).
Statistical analysis
Data analysis was performed using SPSS 17.0
statistical software (SPSS Inc.) and the results of all
experimental data were expressed as mean ± standard deviation. If
the experimental data was consistent with the homogeneity of
variance, one-way ANOVA followed by a least significant difference
test were performed to compare the differences between groups. If
the experimental data variance was not with the homogeneity of
variance, the Kruskal-Wallis non-parametric test and the LSD method
were used to compare the differences between groups. P<0.05 was
considered to indicate a statistically significant result.
Results
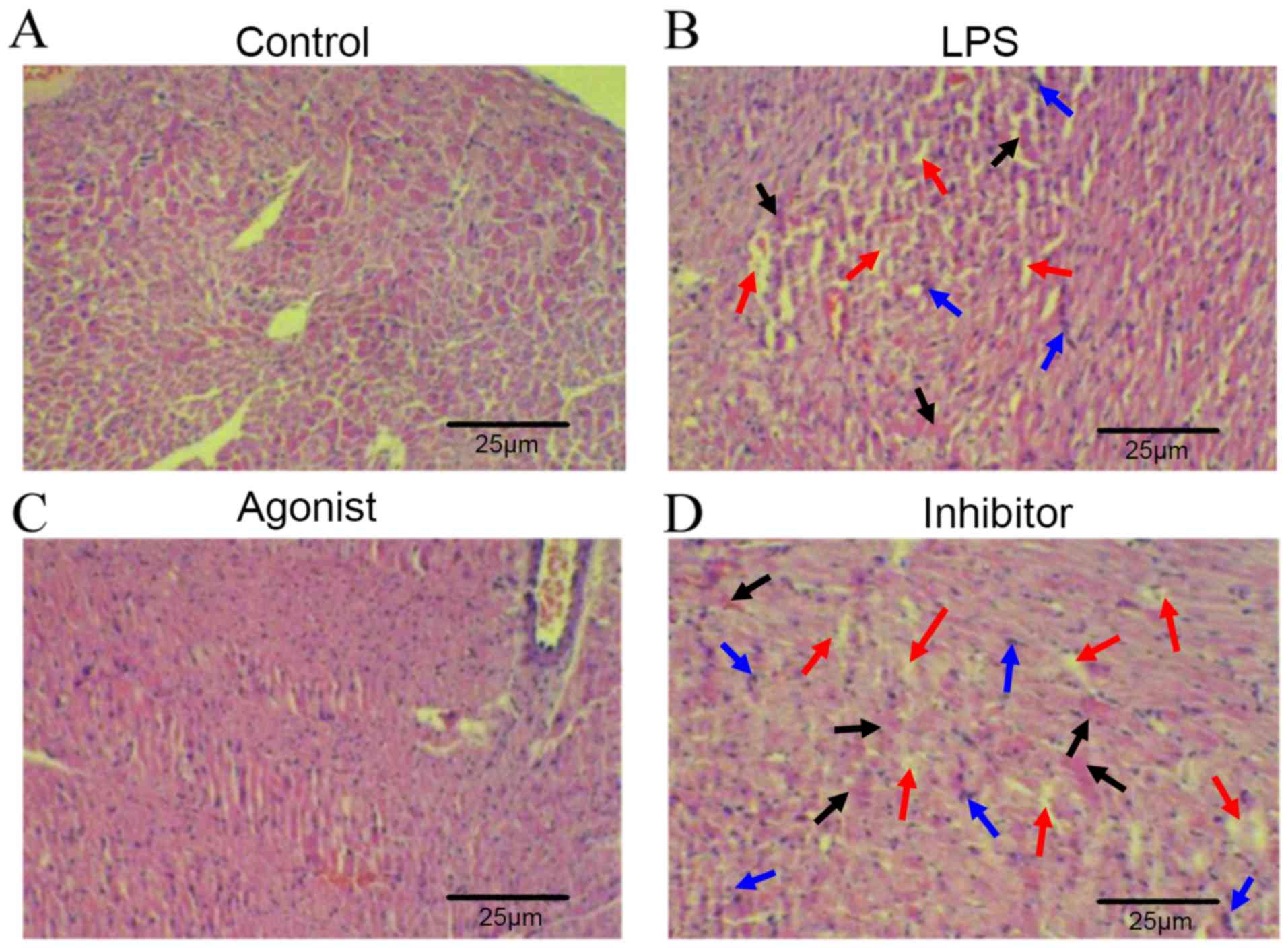
Pathological changes of myocardial
tissue in four groups
After analyzing the myocardial pathological tissue
sections, the results revealed that myocardial interstitial tissue
in the LPS group were edematous, with degenerated cardiomyocytes
(black arrow), loosely arranged cells (red arrow) and broken nuclei
(blue arrow; Fig. 1A and B).
Compared with the LPS group, cardiomyocytes in the miR-146a agonist
group were neatly arranged and there was no swelling of the cells
and no obvious fragmentation of the nucleus (Fig. 1B and C). However, these cardiomyocyte
observations in the microRNA-146a inhibition group were aggravated
compared with the LPS group (Fig.
1B-D).
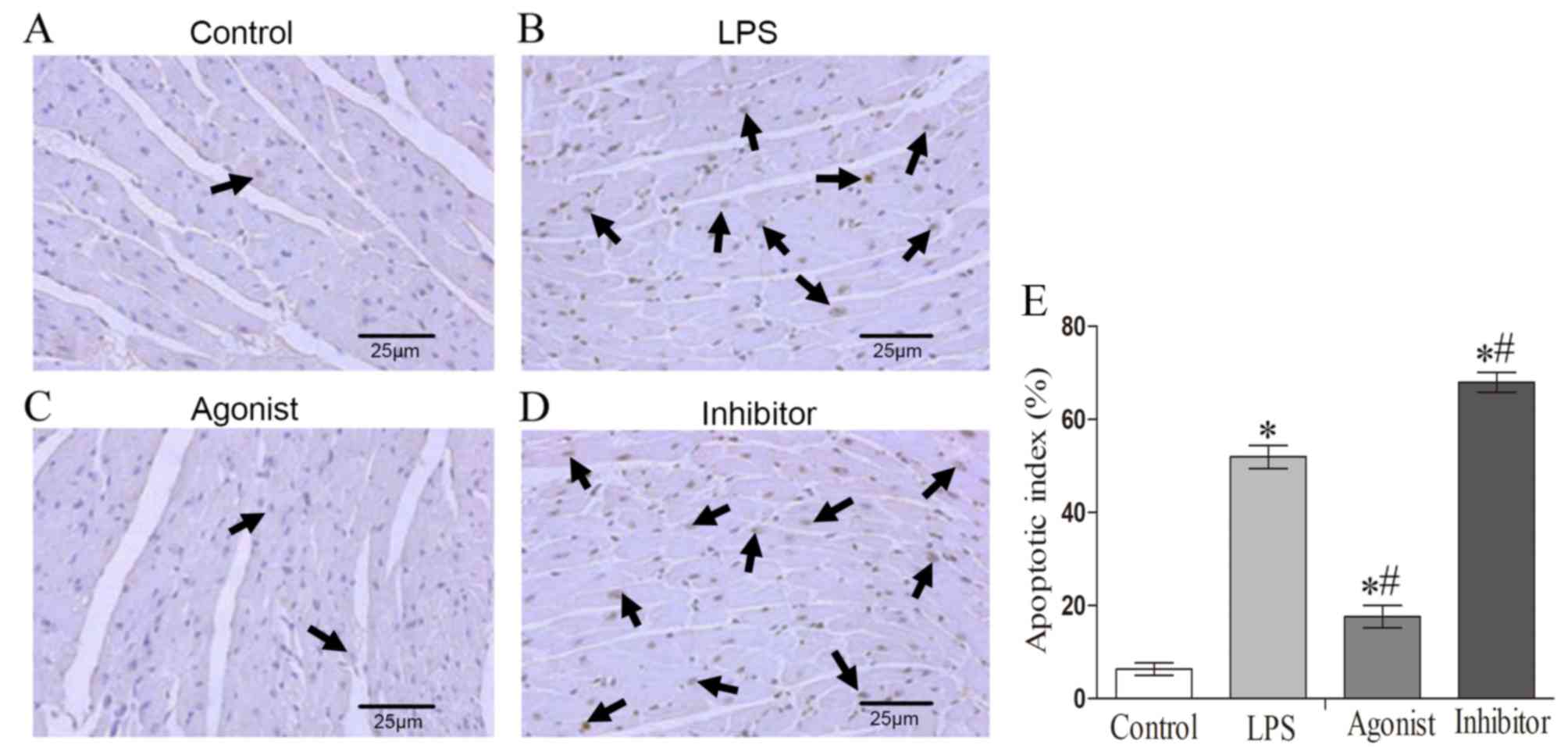
Detection of the cardiomyocyte
apoptosis
Cells that exhibited brown karyon staining were
deemed to be apoptotic cells. Among the four rat groups, the
control group exhibited fewer apoptotic cells (Fig. 2A). The proportion of apoptosis in
cardiomyocyte apoptosis in the LPS group was increased compared
with the control group (Fig. 2A and
B). However, in the miR-146a agonist group, the number of
apoptotic cells were lower compared with the LPS group (Fig. 2B and C), while the opposite result
was observed in the miR-146a inhibition group (Fig. 2B-D). Quantitative analysis revealed
that the apoptotic index of the LPS group was increased
significantly compared with control group. The apoptotic index was
lower in miR-146a agonist group, but higher in the miR-146a
inhibition group (Fig. 2E).
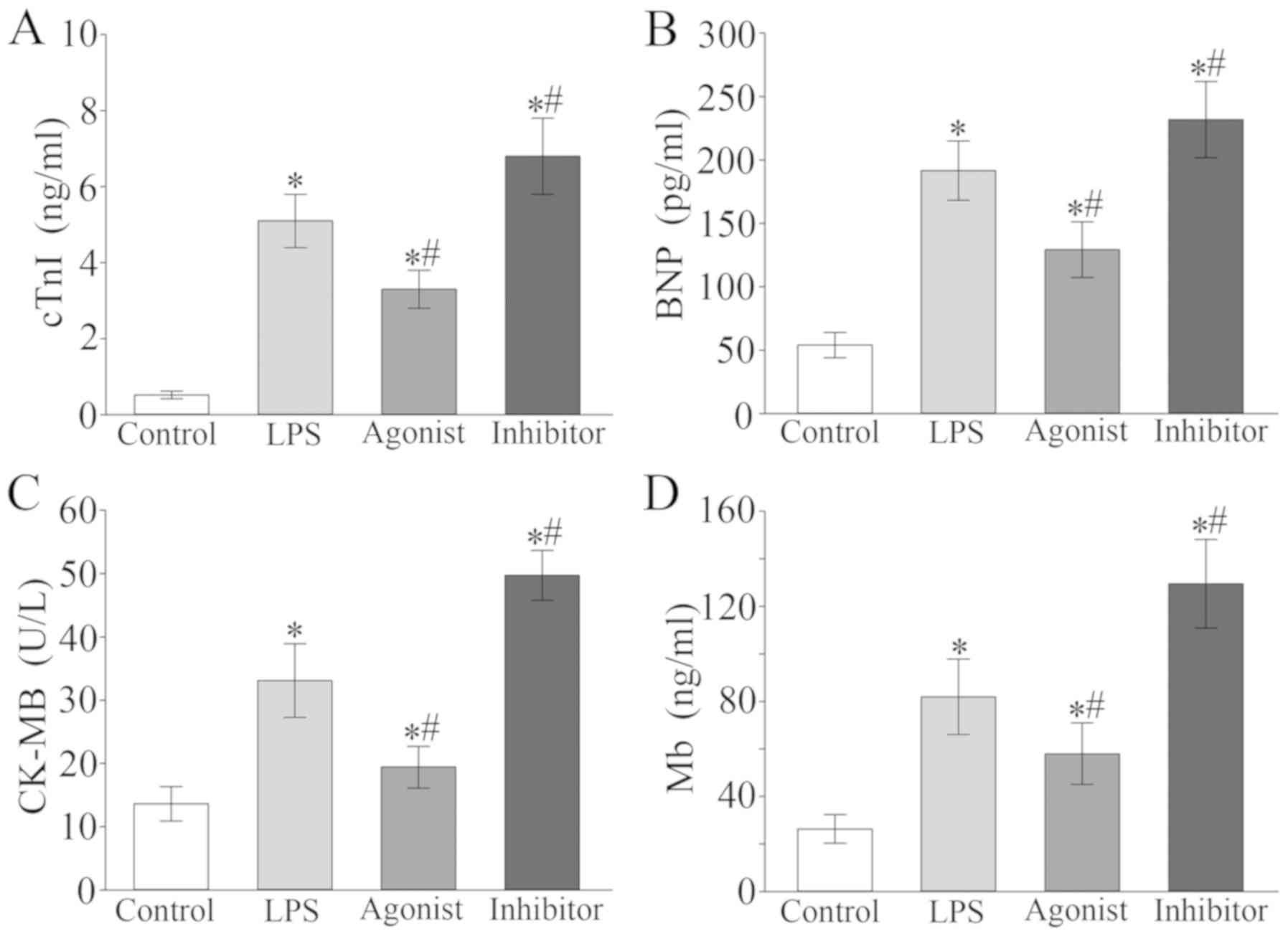
Expression of the serum myocardial
injury markers
The results of the current study revealed that the
serum levels of cTnI, BNP, CK-MB and Mb in the LPS group were
significantly higher than those in the control (P<0.05; Fig. 3). In addition, compared with the LPS
group, levels of the aforementioned markers were significantly
decreased in the miR-146a agonist group, but increased in the
miR-146a inhibitor group (P<0.05; Fig. 3).
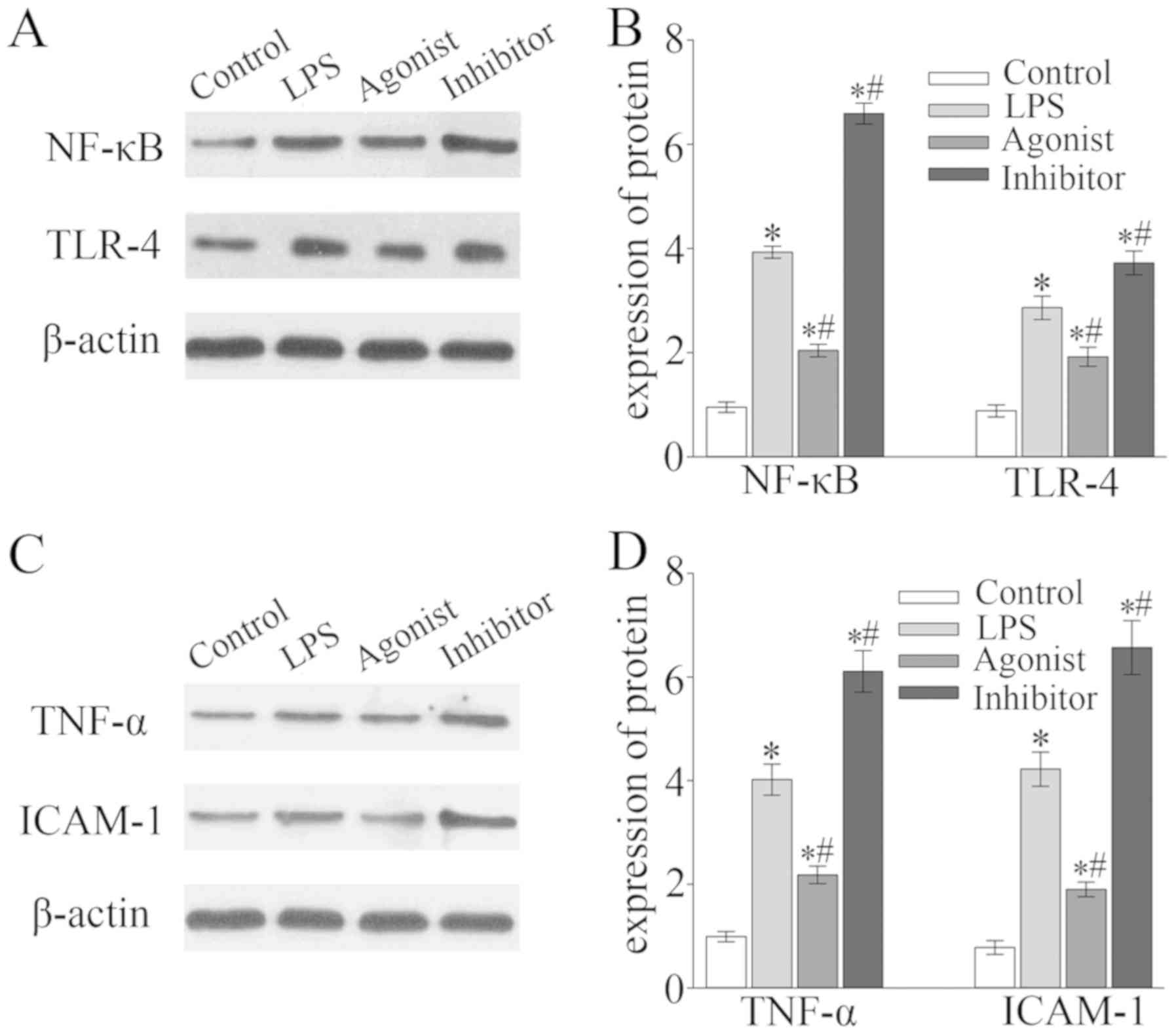
Expression of myocardial
tissue-associated proteins
The expression of NF-κB, TLR-4, TNF-α and ICAM-1 in
rat myocardium tissue of the LPS group and the miR-146a inhibitor
group was significantly increased compared with the control group
and expression was significantly increased in the inhibitor group
compared with the LPS group (P<0.05; Fig. 4). In the miR-146a agonist group, the
expression of NF-κB, TLR-4, TNF-α and ICAM-1 was also significantly
upregulated when compared with the control group, but the
expression was significantly lower than that of the LPS group
(P<0.05; Fig. 4).
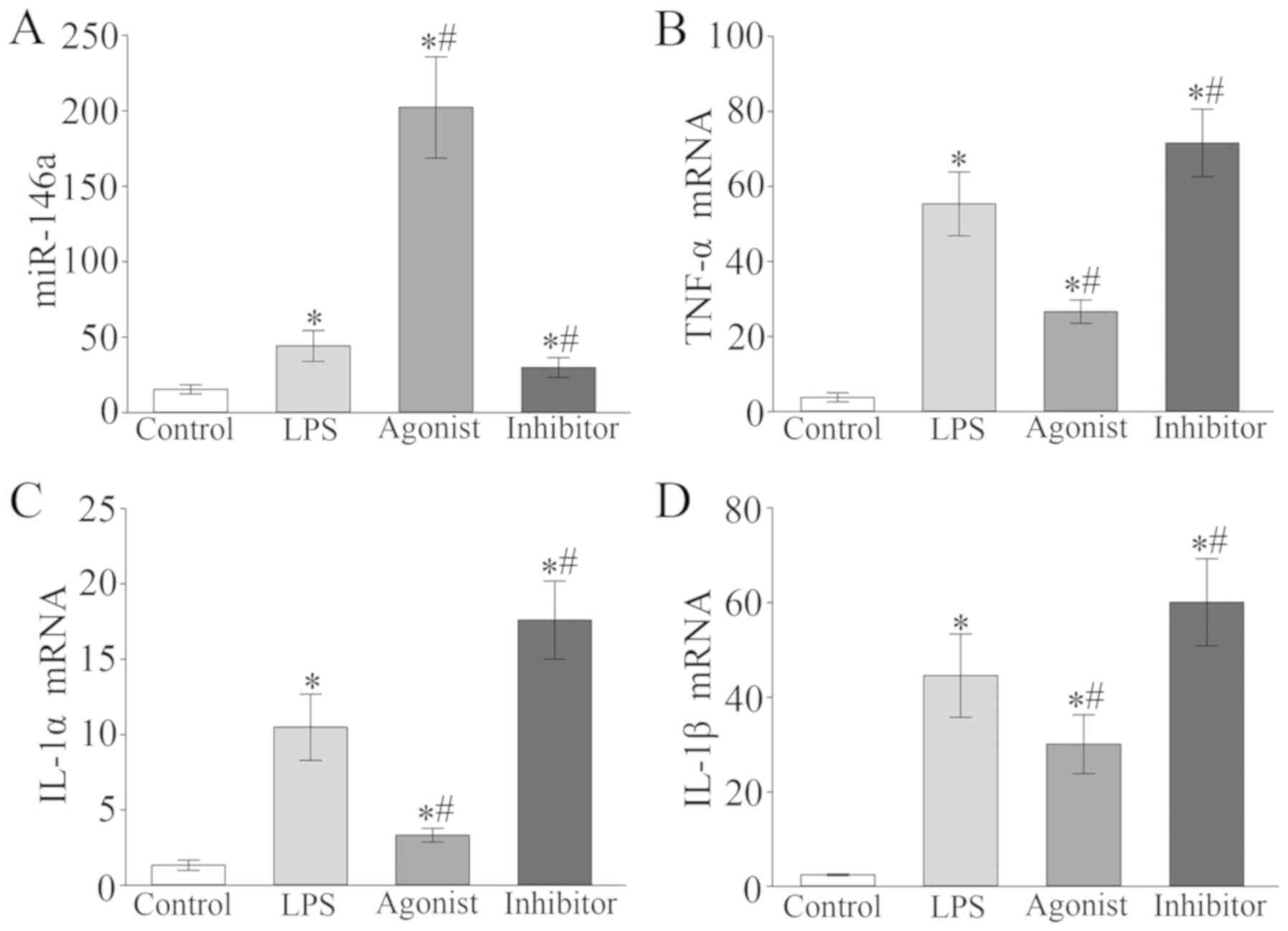
Expression of myocardial
tissue-associated mRNAs
The mRNA expression of miR-146a, TNF-α, IL-1α and
IL-1β in the myocardial tissue of the model group were
significantly higher compared with the control group (P<0.05;
Fig. 5). In the miR-146a agonist
group, miR-146a expression significantly increased, but the
expression of TNF-α, IL-1α and IL-1β significantly decreased
compared with the LPS group (P<0.05; Fig. 5). The expression of miR-146a in the
miR-146a inhibition group was significantly downregulated while
TNF-α, IL-1α and IL-1β expression was upregulated when compared
with the LPS group (P<0.05; Fig.
5).
Discussion
The excessive stimulation of the inflammatory
response is significant in the pathogenesis of SIC. Previous
studies have revealed that TLR-4 mediates the activation of the
Myd88-dependent NF-κB signaling pathway and releases downstream
inflammatory factors including TNF-α, IL-6, IL-8, IL-1β, which
serve a role in the subsequent sepsis cascade reaction (20–22).
After invasion of the body, the pathogenic microorganism promotes
the cascadal release of inflammatory factors via cell membrane and
intracellular signaling pathways, which are important in the
pathogenesis of sepsis-induced myocardial damage (23,24).
Therefore, the TLR-4/NF-κB signaling pathway is an important link
in sepsis-induced myocardial damage. When sepsis occurs, LPS in the
cell wall of Gram-negative bacteria can transduce extracellular
signals by activating the intrinsic immune recognition of TLR-4,
sequentially activating IRAK1 and TRAF6. NF-κB subsequently
translocates rapidly from the cytoplasm to the nucleus, initiating
the transcription of target genes and releasing downstream
inflammatory factors including TNF-α and IL-6 (25–27).
TNF-α and IL-6 inhibit the myocardium by inhibiting Ca2+
transport, regulating the nitric oxide pathway, degrading key
contractile proteins, affecting mitochondrial function and
activating intracellular signal transduction which leads to the
myocardial dysfunction observed in sepsis (28,29).
miRNAs are a class of endogenous regulatory
non-coding RNAs present in eukaryotes and are ~20–25 nucleotides in
length (30). Mature miRNAs are
produced from a long primordial transcript that can be cut by a
series of nucleases (10). The
target mRNA is recognized by base-pair pairing and is subsequently
silenced or repressed depending on the degree of mIRNA
complementation (31). miRNAs
including miRNA-125b, miRNA-150, miRNA-155, miRNA-223, miRNA-132
and miRNA-146a, serve a significant role in the regulation of
inflammatory responses by inhibiting the expression of
inflammation-associated genes (32–37). A
previous study has demonstrated that activated miR-146a is rapidly
upregulated in human monocytes and certain targets signaling
molecules including IRAK1 and TRAF6, indicating that miRNA-146a may
be involved in the negative feedback regulation of the inflammatory
signaling pathway (38). Consistent
with this, miR-146a promotes toxin tolerance and cross-tolerance in
the body by regulating monocyte production of TNF-α (38). miR-146a is the first regulator of the
miRNA family that has been revealed to be associated with
inflammation, which makes it an early diagnostic marker for sepsis
with high specificity and sensitivity (38,39). In
recent years, it has been demonstrated that miRNA-146a regulates
the TLR-4/NF-κB signaling pathway and downstream inflammatory
factors via negative feedback regulation (40).
The results of the current study demonstrate that in
the LPS group, the expression of cTnI, BNP, CK-MB, Mb and NF-κB,
the protein expression of TLR-4, TNF-α and ICAM-1 and the mRNA
expression of miR-146a, TNF-α, IL-1α and IL-1β were significantly
higher compared with the control group. Compared with the LPS
group, miR-146a levels in the miR-146a agonist group significantly
increased, while the expression of cTnI, BNP, CK-MB, Mb and
NF-κB-associated proteins and the mRNA expression of TNF-α, IL-1α
and IL-1β were downregulated. The results indicated that the
further upregulation of miR-146a in heart tissue may produce a
negative feedback regulatory response within the TLR-4/NF-κB
signaling pathway, thereby inhibiting the inflammatory cascade and
reducing the release of inflammatory factors which directly impair
tissue damage and therefore improve SIC. The expression of
miRNA-146a in the miRNA-146a inhibition group was significantly
lower compared with the LPS group, while the expression of cTnI,
BNP, CK-MB, Mb and NF-κB-associated proteins were significantly
higher than those in the LPS group. These results indicated that in
the case of miR-146a inhibition, the NF-κB signaling pathway was
activated, the expression of downstream inflammatory factors was
increased and myocardial damage was therefore more severe in rats.
It was thus demonstrated that miR-146a may regulate the TLR-4/NF-κB
signaling pathway and downstream inflammatory factors via negative
feedback mechanisms. This is an important signal transduction
mechanism and a key regulatory pathway in the pathogenesis of
SIC.
In summary, the TLR-4/NF-κB, signaling pathway is an
important signal transduction mechanism and a key regulatory
pathway in SIC. miR-146a may regulate the TLR-4/NF-κB signaling
pathway and downstream inflammatory factors via negative feedback
mechanisms, so as to inhibit the inflammatory response and improve
sepsis-induced cardiac dysfunction. However, blood and specimens
were only collected 24 h after LPS injection in the current study.
The role of miR-146a in sepsis-induced cardiac dysfunction over a
longer time period is still unknown. Investigation over extended
experimental time and further study into the mechanisms of miR-146a
in sepsis-induced cardiac dysfunction is therefore required.
In conclusion, miR-146a may regulate the TLR-4/NF-κB
signaling pathway via negative feedback mechanisms, thereby
improving inflammation and cardiac dysfunction in sepsis-induced
cardiomyopathy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JX and WF designed the study, performed the
experiments and wrote the manuscript. JX, LZ and XF established the
animal models. XD and ZZ collected the data. JX and LZ analyzed the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Kunming Medical University Animal Center (Kunming,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tønnesen E and Larsen K: Severe sepsis and
septic shock. Ugeskr Laeger. 176:V031302002014.(In Danish).
PubMed/NCBI
|
|
2
|
Sharawy N and Lehmann C: New directions
for sepsis and septic shock research. J Surg Res. 194:520–527.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Z and Robinson RA: The role of
proteomics in understanding biological mechanisms of sepsis.
Proteomics Clin Appl. 8:35–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sato R and Nasu M: A review of
sepsis-induced cardiomyopathy. J Intensive Care. 3:482015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato R, Kuriyama A, Takada T, Nasu M and
Luthe SK: Prevalence and risk factors of sepsis-induced
cardiomyopathy: A retrospective cohort study. Medicine (Baltimore).
95:e50312016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hobai IA, Edgecomb J, LaBarge K and
Colucci WS: Dysregulation of intracellular calcium transporters in
animal models of sepsis-induced cardiomyopathy. Shock. 43:3–15.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romero-Bermejo FJ, Ruiz-Bailen M,
Gil-Cebrian J and Huertos-Ranchal MJ: Sepsis-induced
cardiomyopathy. Curr Cardiol Rev. 7:163–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv X and Wang H: Pathophysiology of
sepsis-induced myocardial dysfunction. Mil Med Res. 3:302016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Espín-Pérez A, Krauskopf J, Chadeau-Hyam
M, van Veldhoven K, Chung F, Cullinan P, Piepers J, van Herwijnen
M, Kubesch N, Carrasco-Turigas G, et al: Short-term transcriptome
and microRNAs responses to exposure to different air pollutants in
two population studies. Environ Pollut. 242:182–190. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bernardo BC, Ooi JY, Lin RC and McMullen
JR: miRNA therapeutics: A new class of drugs with potential
therapeutic applications in the heart. Future Med Chem.
7:1771–1792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou F, Wang W, Xing Y, Wang T, Xu X and
Wang J: NF-κB target microRNAs and their target genes in
TNFα-stimulated HeLa cells. Biochim Biophys Acta. 1839:344–354.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu ZW, Liu YF, Wang S and Li B: miRNA-146a
induces vascular smooth muscle cell apoptosis in a rat model of
coronary heart disease via NF-κB pathway. Genet Mol Res.
14:18703–18712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Ha T, Liu L, Zou J, Zhang X,
Kalbfleisch J, Gao X, Williams D and Li C: Increased expression of
microRNA-164a decreases myocardial ischemia/reperfusion injury.
Cardiovasc Res. 97:432–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L,
Kalbfleisch JH, Gao X, Kao RL, Williams DL and Li C: Attenuation of
cardiac dysfunction in polymicrobial sepsis by microRNA-146a is
mediated via targeting of IRAK1 and TRAF6 expression. J Immunol.
195:672–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun W, Wang Q, Guo Y, Zhao Y, Wang X,
Zhang Z, Deng G and Guo M: Selenium suppresses inflammation by
inducing microRNA-146a in Staphylococcus aureus-infected mouse
mastitis model. Oncotarget. 8:110949–110964. 2017.PubMed/NCBI
|
|
17
|
Wu ZW, Liu YF, Wang S and Li B:
Corrigendum miRNA-146a induces vascular smooth muscle cell
apoptosis in a rat model of coronary heart disease via NF-κB
pathway-Genet. Mol. Res. 14(4): 18703–18712, Genet Mol Res 15.
2016. View Article : Google Scholar
|
|
18
|
Li J, Xie C, Zhuang J, Li H, Yao Y, Shao C
and Wang H: Resveratrol attenuates inflammation in the rat heart
subjected to ischemia-reperfusion: Role of the TLR4/NF-κB signaling
pathway. Mol Med Rep. 11:1120–1126. 2015.PubMed/NCBI
|
|
19
|
Karra R, Knecht AK, Kikuchi K and Poss KD:
Myocardial NF-κB activation is essential for zebrafish heart
regeneration. Proc Natl Acad Sci USA. 112:13255–13260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang SF, Zhuang TF, Si YM, Qi KY and Zhao
J: Coriolus versicolor mushroom polysaccharides exert
immunoregulatory effects on mouse B cells via membrane Ig and TLR-4
to activate the MAPK and NF-κB signaling pathways. Mol Immunol.
64:144–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim E, Kim HC, Lee S, Ryu HG, Park YH, Kim
JH, Lim YJ and Park HP: Dexmedetomidine confers neuroprotection
against transient global cerebral ischemia/reperfusion injury in
rats by inhibiting inflammation through inactivation of the
TLR-4/NF-κB pathway. Neurosci Lett. 649:20–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bi W, Zhu L, Jing X, Zeng Z, Liang Y, Xu
A, Liu J, Xiao S, Yang L, Shi Q, et al: Rifampicin improves
neuronal apoptosis in LPS-stimulated co-cultured BV2 cells through
inhibition of the TLR-4 pathway. Mol Med Rep. 10:1793–1799. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cimolai MC, Alvarez S, Bode C and Bugger
H: Mitochondrial mechanisms in septic cardiomyopathy. Int J Mol
Sci. 16:17763–17778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Q, Pan X, Wang L, Wang X and Xiong D:
The protective role of neuregulin-1: A potential therapy for
sepsis-induced cardiomyopathy. Eur J Pharmacol. 788:234–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park H, Huang X, Lu C, Cairo MS and Zhou
X: MicroRNA-146a and microRNA-146b regulate human dendritic cell
apoptosis and cytokine production by targeting TRAF6 and IRAK1
proteins. J Biol Chem. 290:2831–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei J, Wang J, Zhou Y, Yan S, Li K and Lin
H: MicroRNA-146a contributes to SCI recovery via regulating TRAF6
and IRAK1 expression. Biomed Res Int. 2016:40134872016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang W and Kong L, Ni Q, Lu Y, Ding W,
Liu G, Pu L, Tang W and Kong L: miR-146a ameliorates liver
ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS
One. 9:e1015302014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hiram R, Rizcallah E, Sirois C, Sirois M,
Morin C, Fortin S and Rousseau E: Resolvin D1 reverses reactivity
and Ca2+ sensitivity induced by ET-1, TNF-α, and IL-6 in the human
pulmonary artery. Am J Physiol Heart Circ Physiol. 307:H1547–H1558.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiram R, Rizcallah E, Marouan S, Sirois C,
Sirois M, Morin C, Fortin S and Rousseau E: Resolvin E1 normalizes
contractility, Ca2+ sensitivity and smooth muscle cell migration
rate in TNF-α- and IL-6-pretreated human pulmonary arteries. Am J
Physiol Lung Cell Mol Physiol. 309:L776–L788. 2015.PubMed/NCBI
|
|
31
|
Dumache R, Rogobete AF, Bedreag OH,
Sarandan M, Cradigati AC, Papurica M, Dumbuleu CM, Nartita R and
Sandesc D: Use of miRNAs as biomarkers in sepsis. Anal Cell Pathol
(Amst). 2015:1867162015.PubMed/NCBI
|
|
32
|
Landskroner-Eiger S, Moneke I and Sessa
WC: miRNAs as modulators of angiogenesis. Cold Spring Harb Perspect
Med. 3:a0066432013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou S, Zhang P, Liang P and Huang X: The
expression of miR-125b regulates angiogenesis during the recovery
of heat-denatured HUVECs. Burns. 41:803–811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng J, Yang Y, Zhang P, Wang F, Ma Y, Qin
H and Wang Y: miR-150 functions as a tumour suppressor in human
colorectal cancer by targeting c-Myb. J Cell Mol Med. 18:2125–2134.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Slezak-Prochazka I, Kluiver J, de Jong D,
Smigielska-Czepiel K, Kortman G, Winkle M, Rutgers B, Koerts J,
Visser L, Diepstra A, et al: Inhibition of the miR-155 target NIAM
phenocopies the growth promoting effect of miR-155 in B-cell
lymphoma. Oncotarget. 7:2391–2400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meloche J, Le Guen M, Potus F, Vinck J,
Ranchoux B, Johnson I, Antigny F, Tremblay E, Breuils-Bonnet S,
Perros F, et al: miR-223 reverses experimental pulmonary arterial
hypertension. Am J Physiol Cell Physiol. 309:C363–C372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hadar A, Milanesi E, Walczak M,
Puzianowska-Kuźnicka M, Kuźnicki J, Squassina A, Niola P, Chillotti
C, Attems J, Gozes I and Gurwitz D: SIRT1, miR-132 and miR-212 link
human longevity to Alzheimer's Disease. Sci Rep. 8:84652018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bae SC and Lee YH: MiR-146a levels in
rheumatoid arthritis and their correlation with disease activity: A
meta-analysis. Int J Rheum Dis. 21:1335–1342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu Q, Song J, Ding B, Cui Y, Liang J and
Han S: miR-146a promotes cervical cancer cell viability via
targeting IRAK1 and TRAF6. Oncol Rep. 39:3015–3024. 2018.PubMed/NCBI
|
|
40
|
Zhang QB, Qing YF, Yin CC, Zhou L, Liu XS,
Mi QS and Zhou JG: Mice with miR-146a deficiency develop severe
gouty arthritis via dysregulation of TRAF 6, IRAK 1 and NALP3
inflammasome. Arthritis Res Ther. 20:452018. View Article : Google Scholar : PubMed/NCBI
|