Introduction
Crohn's Disease (CD) is a chronic recurrent
inflammatory disease affecting the digestive tract. The aetiology
and pathogenesis of CD remains not fully understood. However, the
consensus is that intestinal mucosal immune regulation,
lymphangiogenesis and intestinal mucosal barrier damage are
involved in the development of CD (1,2). A
crucial area of current research has been the identification of
effective drug components for CD treatment. Curcumin, one of the
most popular traditional medicines, is a natural phenolic substance
extracted from the rhizome of Curcuma longa. It has widely
been used as a traditional herb in China and Southeast Asian
countries for thousands of years. Recent studies have confirmed
that curcumin possesses several pharmacological properties
including antioxidant, anti-inflammatory and antitumour abilities;
it also promotes wound healing, is spasmodic, serves as an
anticoagulant and provides liver protection (3–5). Several
clinical and animal experiments have reported that curcumin has
beneficial therapeutic effects on inflammatory bowel disease with
few adverse effects. Therefore, curcumin possesses substantial
potential for clinical application (6–9); however
the effects and mechanism of curcumin in CD treatment remain
unclear. The present study determined suitable curcumin
concentrations for further experimentation in cells using MTT
assay. Then the effects of curcumin on suppressing angiogenesis and
cell invasion were determined. Finally, the mechanisms by which
curcumin can be used to treat CD were investigated by measuring
relative protein expression levels using western blot analysis and
immunofluorescence staining.
Materials and methods
Experimental materials
A total of 12 Male Wistar rats of specific
pathogen-free grade (weight, 300–350 g; 8.0±0.5 week age) were
purchased from Zhejiang Experimental Animal Center (no. 170525003;
animal licence no. SCXK 2014-0001) and supplied by the Experimental
Animals Center of Nanjing University of Chinese Medicine (Nanjing,
China). The animals were housed in controlled conditions
(temperature, 23±2°C; humidity, 45–60%; 12-h light/dark cycle; free
access to water and a standard diet). Adenosine diphosphate (ADP)
was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Foetal bovine serum and RPM 11650 culture medium were purchased
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Curcumin, activated platelets and MTT kit were purchased from
Sigma-Aldrich (Merck KGaA). Total protein and Nuclear Protein
Extraction kits were purchased from Sigma-Aldrich. The vascular
endothelial growth factor (VEGF) ELISA kit (cat. no. 20170320;
Wuhan Huamei Bioengineering Co., Ltd., Wuhan, China), anti-α-smooth
muscle actin (SMA; cat. no. 19245; Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-collagen I (cat. no. ab90395; Abcam,
Cambridge, UK), anti-E-cadherin (cat. no. 3195; Cell Signaling
Technology, Inc.), anti-VEGF (cat. no. 13689-1; Wuhan Sanying
Biotechnology, Wuhan, China), anti-phosphoinositide 3-kinase (PI3K;
cat. no. 21890-1; Wuhan Sanying Biotechnology), anti-phosphorylated
(p-) PI3K (cat. no. 4228; Cell Signaling Technology, Inc.),
anti-protein kinase B (AKT; cat. no. 60203-2; Wuhan Sanying
Biotechnology), anti-p-AKT (cat. no. 4060; Cell Signaling
Technology, Inc.), anti-mammalian target of rapamycin (mTOR; cat.
no. ab32028; Abcam), anti-p-mTOR (cat. no. ab84400; Abcam),
anti-hypoxia inducible factor subunit α (HIF-1α; cat. no. ab51608;
Abcam), anti-laminin subunit beta 1 (LAMB1; cat. no. 109293; Abcam)
and anti-GAPDH (cat. no. 10494-1; Wuhan Sanying Biotechnology) were
purchased from the respective suppliers. This study was approved by
the Ethics Committee of Nanjing University of Chinese Medicine
(approval no. 2017011001).
INMEC isolation and culture
INMECs were isolated following the methods described
in a previous review (10). INMECs,
at fourth or fifth passage, were cultured in the RPM 11650 culture
medium containing 10% FBS and used for the following
experiments.
MTT assay
Cells in the logarithmic growth phase were
inoculated in a 96-well plate and routinely cultured until cell
adherence was observed. Curcumin solution at concentrations of 0,
0.625, 1.25, 2.5, 5, 10, 20 and 40 µM were added to the samples for
4 h. Then the supernatant was removed and 200 µl dimethyl sulfoxide
was added to each well. The samples were placed on a rocking bed at
low-speed oscillation for 1 min. The absorbance values were
measured at 490 nm wavelength and the cell viability of the
different groups was measured. The experiment was repeated three
times.
Cell grouping
According to the MTT assay result, INMECs were
randomly divided into the following groups: Control, platelets and
three different concentrations of curcumin-treated groups (2.5, 5
and 10 µM). The INMECs were cultured at a density of
1×104 cells/well for 4 h. The supernantants of the
different groups were collected following centrifugation to measure
VEGF concentrations then cells were collected for further
experiments. The experiment was repeated three times.
ELISA
INMECs were treated with 25 µM ADP for 24 h at room
temperature, followed by the curcumin treatment of various
concentrations. Culture medium was collected and centrifuged at
10,000 × g for 5 min at 4°C. The VEGF concentrations of the groups
were measured with an ELISA kit at 490 nm according to the
supplier's instructions. Each group was assigned nine wells.
Capillary tube formation
experiment
Matrigel matrix glue was added to a 48-well culture
plate at 37°C for 30 min. INMECs were added to wells at a density
of 1×105 cells/well. Normal serum, normal serum
containing activated platelets and three different curcumin
concentrations (2.5, 5 and 10 µM) + normal serum containing
activated platelets were added to wells and cultured for 12 h. An
inverted microscope was used to observe and photograph capillary
tube formation, and Image J v.6.0 software (National Institutes of
Health, Bethesda, MD, USA) was used to quantitatively analyse
cavity formation length. Each group included three replicates. Five
fields were selected for each well, and the experiment was repeated
three times.
Transwell invasion assay
Transwell chambers in a 24-well plate were used for
the Transwell invasion assay. For each sample, the surface of the
membrane was evenly spread with Dulbecco's modified Eagle's medium
(DMEM)-diluted Matrigel containing a diluted growth factor
(Matrigel:DMEM, 1:4, v/v) and maintained in an incubator (37°C, 5%
CO2) for 30 min prior to cell seeding. Then 100 µl of
INMEC suspension (1×105/ml) in the logarithmic growth
phase was inoculated into the upper chamber and 600 µl of serum
free-DMEM was added. Each group was assigned three wells. Normal
culture medium, activated platelets, activated platelets +2.5 µM
curcumin, activated platelets +5 µM curcumin and activated
platelets +10 µM curcumin were added to the wells and cultured for
24 h. Cells were stained with 0.1% crystal violet (Beyotime
Institute of Biotechnology) at room temperature for 5 min, The
chamber was then removed, medium discarded and samples were washed
twice with PBS. Tweezers were used to place the filter membrane
onto a slide plate, and five fields were randomly selected under an
inverted light microscope to observe and count the number of
migratory cells (magnification, ×100).
Western blot analysis
INMECs were collected from the groups and the total
proteins or nuclear proteins were extracted using a total protein
extraction kit or nucleus protein kit respectively. The protein
concentrations were quantified by bicinchoninic acid assay. In
brief, 50 µg protein samples were loaded/lane to perform
electrophoresis with 12% SDS-PAGE then transferred to
polyvinylidene difluoride membranes. Following blocking of the
membranes with 5% skimmed milk at 37°C for 1 h, the membranes were
incubated with primary antibodies (α-SMA, 1:1,000; collagen I,
1:1,000; E-cadherin, 1:2,000; VEGFR, 1:1,000; PI3K, 1:1,000;
p-PI3K, 1:1,000; AKT, 1:1,000; p-AKT, 1:1,000; mTOR, 1:1,000;
p-mTOR, 1:1,000; HIF-1α, 1:1,000; LamB1, 1:2,000 and GAPDH, 1:500).
The samples were incubated overnight at 4°C and washed thrice with
PBS. Membranes were incubated with HRP-marked second antibody
(1:2,000; cat. no. ab205718; Abcam, Cambridge, UK) at room
temperature for 1 h. Enhanced chemiluminescence reagent was used to
visualise protein bands and band intensity were analysed using
Image J v6.0 software (National Institutes of Health). GAPDH was
used as a reference in this experiment.
Immunofluorescence staining
INMECs were inoculated into 48-well plates, and the
corresponding treatments were added for 12 h. Then cells were
washed with PBS, fixed with 2% polyoxymethylene for 30 min and
washed again with PBS (30 sec; three times) at room temperature.
The cells were permeated with 1% Triton X-100 then 5% bovine serum
albumin was used to block samples at room temperature for 1 h.
HIF-1α antibody (1:1,000; cat. no. ab51608; Abcam) was added and
samples were incubated overnight at 4°C then washed with PBS.
Fluorescein-conjugated secondary antibody (1:1,000; cat. no.
ab205718) was added to samples at room temperature for 2 h then
DAPI was used to stain the nuclei and buffered glycerol was added
to mount samples. HIF-1α staining was observed under a laser
confocal microscope (magnification, ×100) following 1 h.
Statistical analysis
Data were presented as mean ± standard deviation.
SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for data
analysis. A one-way analysis of variance with a post hoc Dunnett's
t-test was used to assess differences amongst the groups. P<0.05
was considered to indicate statistical significance.
Results
Cell viability following treatment
with different curcumin concentrations
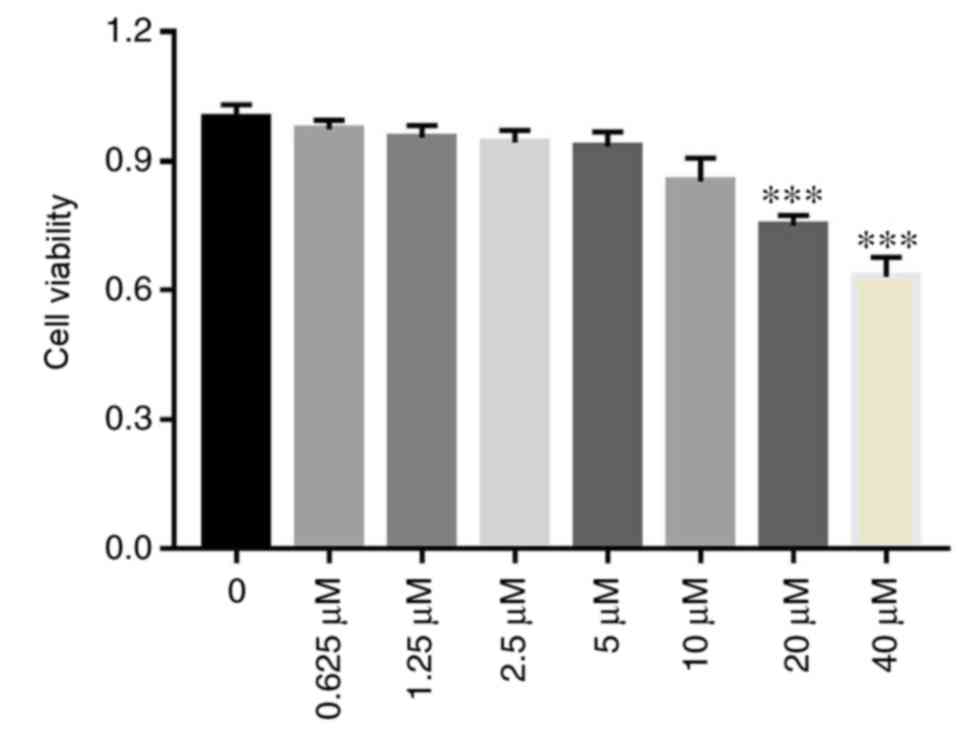
There were no significant differences in cell
viability amongst the 0, 0.625, 1.25, 2.5, 5 and 10 µM curcumin
concentration groups (P>0.05; Fig.
1). However, cell viability of the 20 and 40 µM curcumin
concentration groups significantly decreased compared with the 0 µM
curcumin concentration group (P<0.001; Fig. 1). The results demonstrated that the
0, 0.625, 1.25, 2.5, 5 and 10 µM curcumin concentrations were
suitable for INMECs in this in vitro study.
VEGF concentration decreases with
increasing curcumin concentration
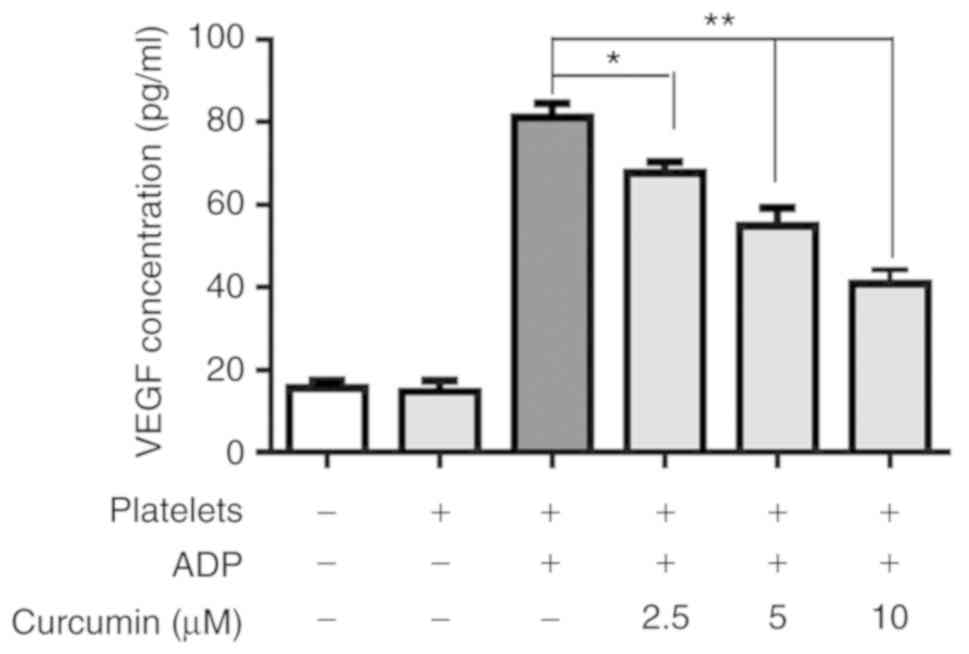
Compared with the control group, the VEGF
concentration of the platelets group was significantly upregulated
(P<0.001; Fig. 2), indicating
that activated platelets stimulated INMECs to secrete VEGF. With
curcumin supplementation, the VEGF concentrations of the different
curcumin-treated groups were significantly suppressed compared with
the platelets group (P<0.05; Fig.
2). Furthermore, there was a significant difference in terms of
VEGF concentration amongst the different curcumin
concentration-treated groups (Fig.
2).
Branch point numbers decrease with
increasing curcumin concentrations
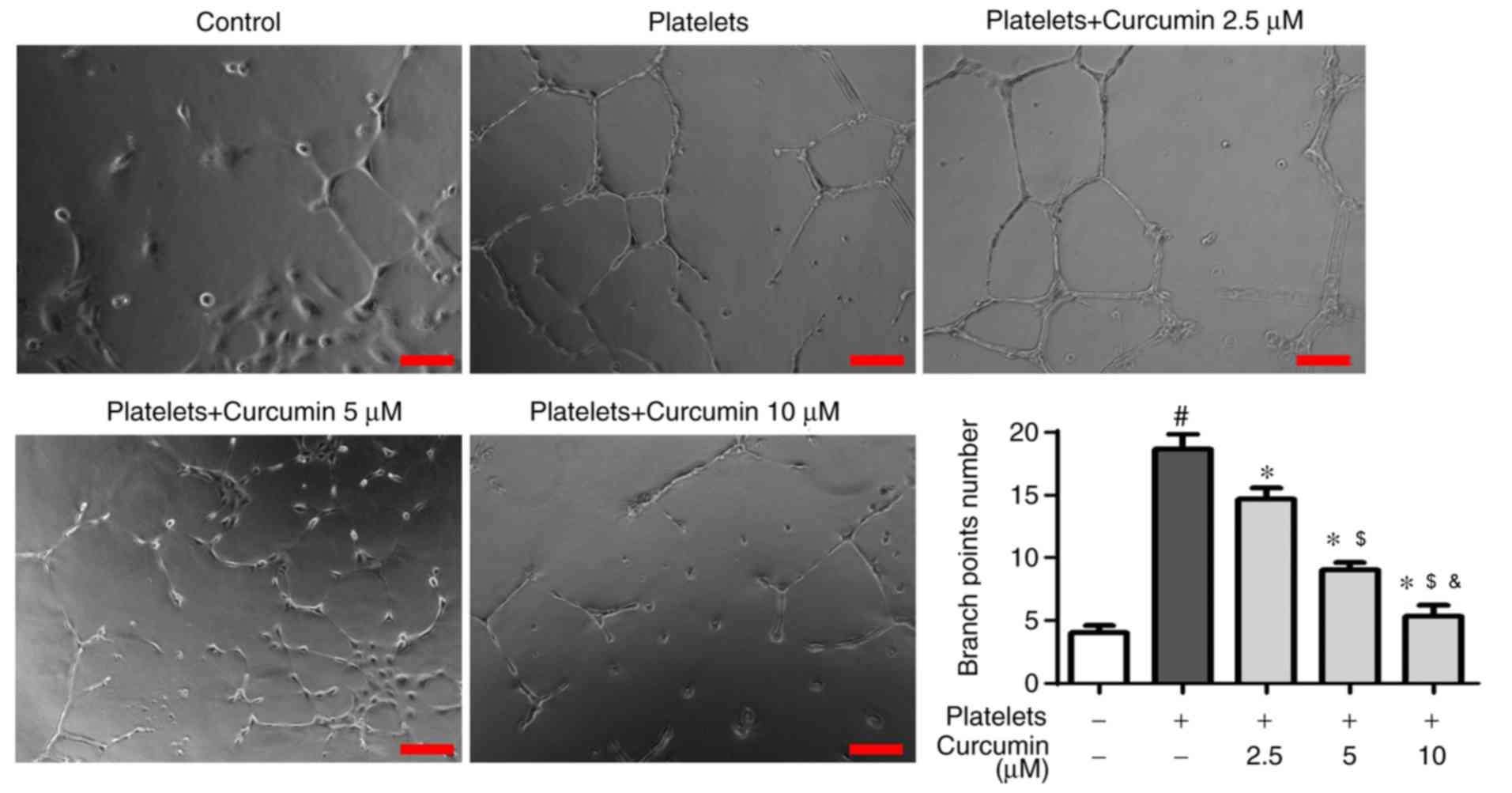
There was a significantly higher branch point number
in the platelets group compared with the control group (P<0.05;
Fig. 3), suggesting that activated
platelets induced angiogenesis in INMECs. However, there were
significantly fewer branch points in the curcumin-treated groups
than in the platelets group (P<0.05; Fig. 3) with curcumin concentration
exhibiting a dose-dependent association (P<0.05; Fig. 3).
Cell invasion decreases with
increasing curcumin concentration
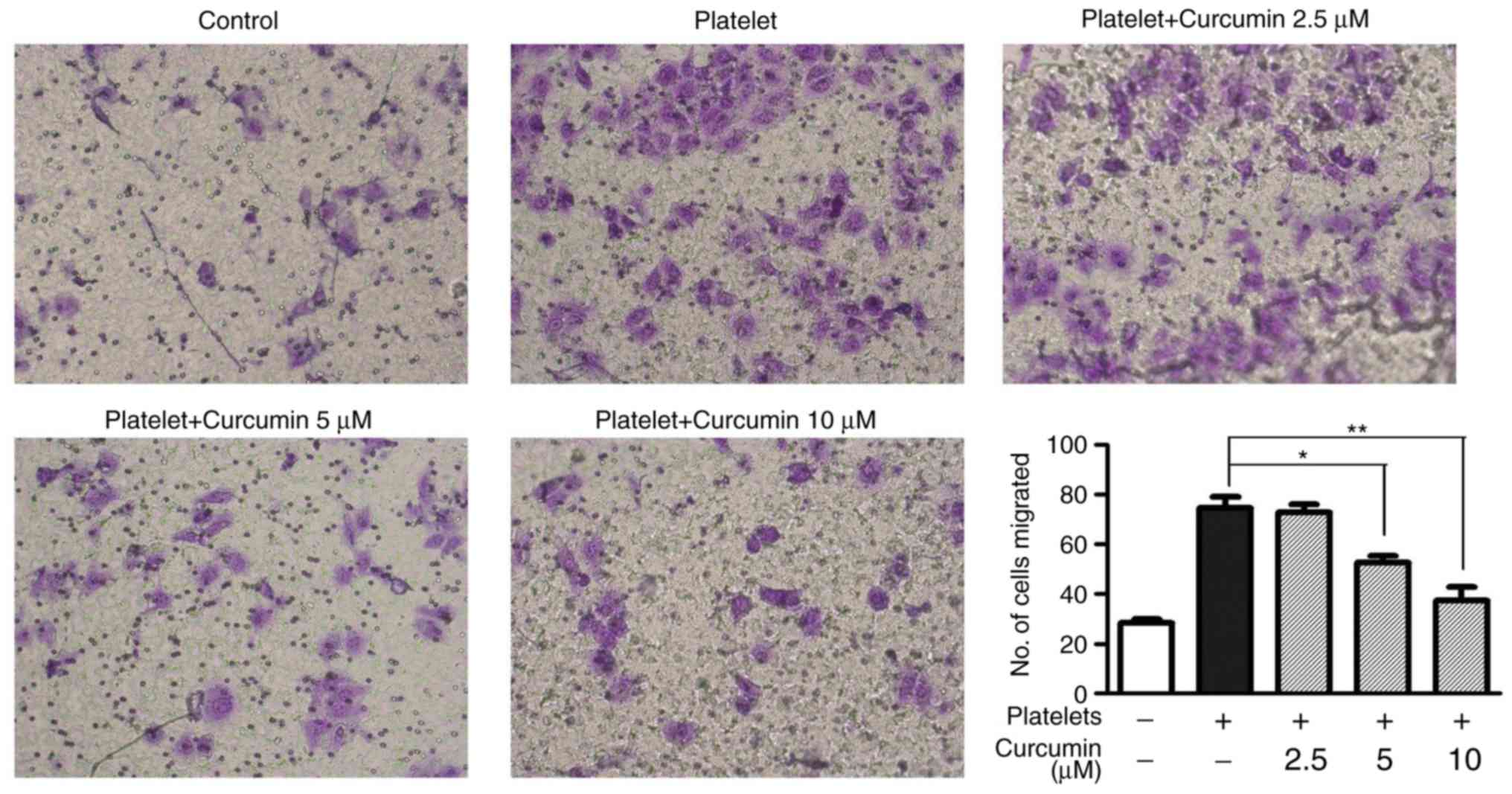
Compared with the platelets group, the 5 µM
(P<0.05; Fig. 4) and 10 µM
(P<0.01; Fig. 4) curcumin-treated
groups had significantly decreased cell invasion. These results
suggested that 5 and 10 µM curcumin concentrations suppressed the
number of migrated INMECs in vitro.
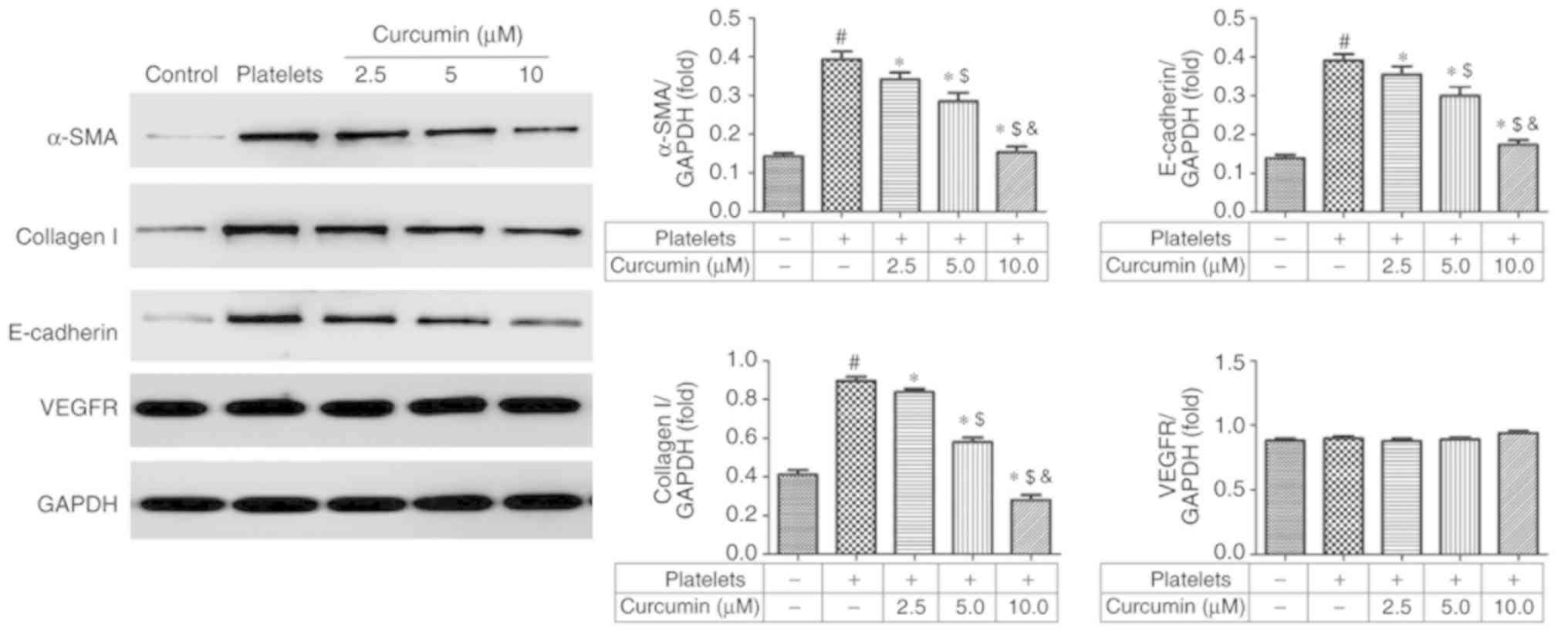
α-SMA, collagen I, E-cadherin and
VEGFR protein expression levels following curcumin treatment
Compared with the control group, the platelets group
exhibited significantly upregulated α-SMA, collagen I and
E-cadherin protein expression levels (P<0.05; Fig. 5). However, α-SMA, collagen I and
E-cadherin protein expression levels were significantly suppressed
in a dose-dependent manner in the curcumin-treated groups compared
with the platelets group (P<0.05; Fig. 5). There were no significant
differences in VEGFR protein levels amongst the different groups
(P>0.05; Fig. 5).
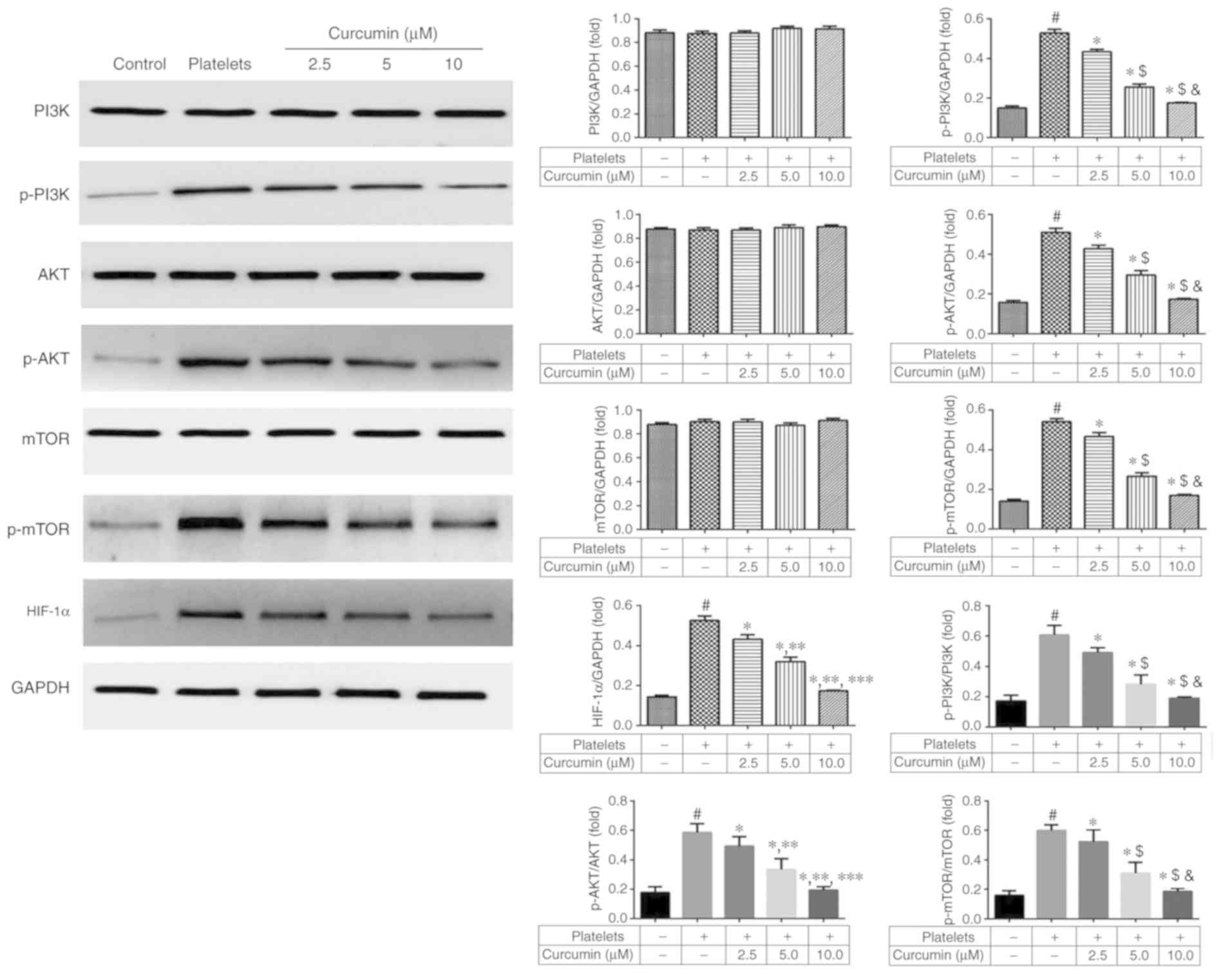
PI3K/AKT/mTOR pathway relative to
HIF-1α protein expression levels following curcumin treatment
There were no significant differences in PI3K, AKT
and mTOR protein expression levels amongst the five groups
(P>0.05; Fig. 6). The p-PI3K,
p-AKT, p-mTOR and HIF-1α protein expression levels were
significantly suppressed in the curcumin-treated groups, in a
dose-dependent manner, compared with the platelets group
(P<0.05; Fig. 6). These results
suggested that curcumin affects HIF-1α by regulating
phosphorylation of the PI3K/AKT/mTOR pathway.
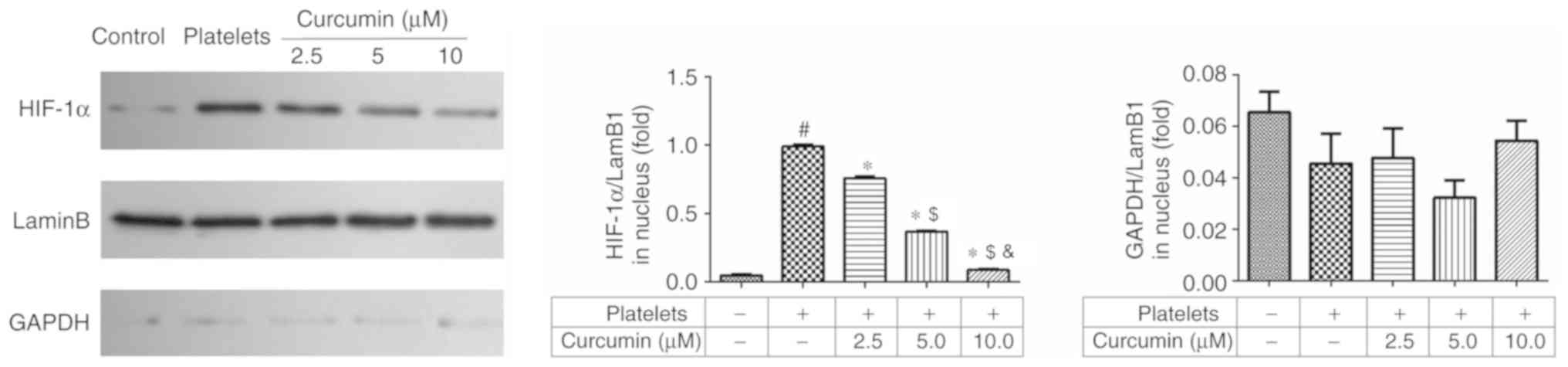
HIF-1α and LamB1 protein expression
levels in the nucleus following curcumin treatment
Compared with the control group, the platelets group
significantly upregulated HIF-1α protein expression levels in the
nucleus (P<0.05; Fig. 7).
Curcumin treatment significantly suppressed HIF-1α protein
expression levels in the 5.0 and 10.0 µM curcumin-treated groups
(P<0.05; Fig. 7). In addition,
there was a significant difference between the 5 and 10 µM
curcumin-treated groups (P<0.05; Fig.
7).
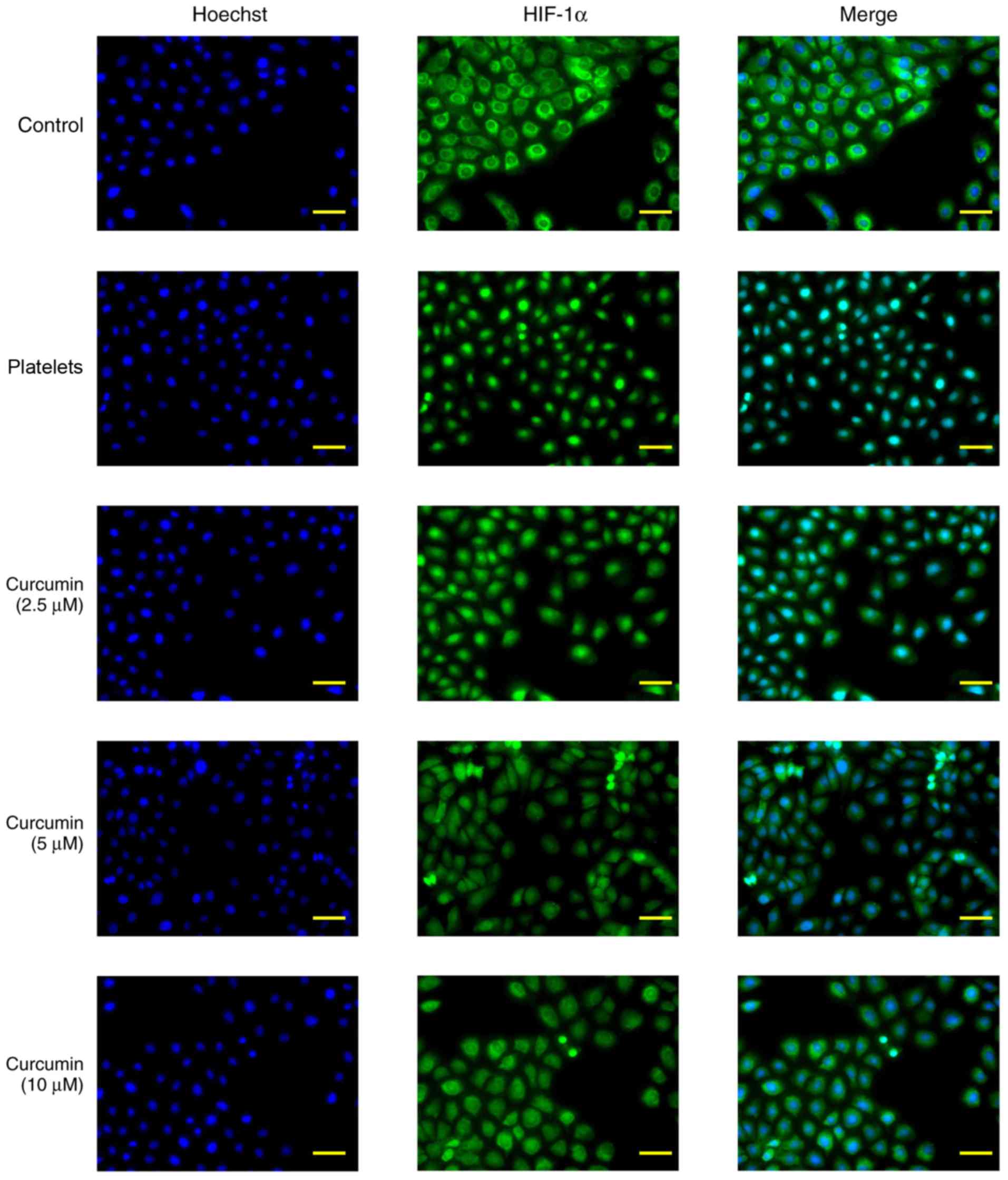
HIF-1α protein nuclear
translocation
The immunofluorescence staining results determined
that HIF-1α protein expression levels increased following
stimulation with activated platelets (Fig. 8). By contrast, curcumin suppressed
HIF-1α protein expression levels and decreased HIF-1α protein
nuclear translocation in vitro (Fig. 8).
Discussion
The most commonly used drugs for the treatment of CD
include aminosalicylic acid, glucocorticoids, immunosuppressive
agents, inflammatory transmitter inhibitors, targeted biological
immunotherapy and antibiotics (11).
In the treatment of CD, traditional Chinese medicine has been
proven to be multi-channel medicines and can be used for long-term
treatment of patients, with fewer adverse events (12,13).
During CD development, INMECs produce blood vessels induced by
activated platelets (14–16). Curcumin is a traditional natural drug
extract that exhibits anti-inflammatory, antitumour and
immunoregulation properties (17–19).
However, the treatment effects of curcumin on CD remain unclear.
Firstly, the present study evaluated the safety of curcumin on
INMECs. MTT assay results demonstrated that curcumin concentrations
below 20 µM did not cause significant toxicity. The effects and
mechanisms of curcumin on angiogenesis and the invasion of INMECs
induced by activated platelet stimulation were then investigated.
The results demonstrated that VEGF concentration was significantly
reduced, branch point number was decreased and number of migrated
cells were decreased with curcumin treatment in a dose-dependent
manner. Finally, the relative protein expression levels were
investigated to determine the mechanism of action.
Previous studies demonstrated that α-SMA, collagen
I, E-cadherin and VEGFR have key roles in cell migration (20–24). The
present study demonstrated that curcumin suppressed INMEC
angiogenesis and invasion by regulating α-SMA, collagen I and
E-cadherin protein expression levels. By contrast, curcumin did not
have significant effects on VEGFR protein expression levels. α-SMA,
collagen I and E-cadherin genes are downstream of the HIF-1α
signalling pathway, therefore the upstream signalling pathway was
investigated to determine the effects of curcumin in
vitro.
PI3K/AKT/mTOR/HIF-1α pathway stimulation is a
crucial component in cell angiogenesis and migration, and can
induce α-SMA, collagen I and E-cadherin protein activation
(25–30). The present study identified that
curcumin affected phosphorylation of the PI3K/AKT/mTOR pathway;
when suppressed, HIF-1α total and nuclear protein expression
decreased. HIF-1α nuclear translocation is an essential part of the
association between the PI3K/AKT/mTOR pathway and downstream genes
(α-SMA, collagen I and E-cadherin). HIF-1α nuclear translocation
was observed by immunofluorescence staining with the results
demonstrating that HIF-1α nuclear translocation was inhibited by
curcumin supplementation. In conclusion, curcumin was identified to
have a dose-dependent inhibitory effect on INMEC angiogenesis and
invasion induced by activated platelets, likely via inhibiting
PI3K/AKT/mTOR pathway activation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81603622) and the
Natural Science Foundation of Jiangsu Province of China (grant no.
BK20161319).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX was responsible for drafting the manuscript, as
well as the acquisition, analysis and interpretation of data. ZXX
collected data. SY interpreted the data. JL was responsible for
drafting the manuscript. HC contributed to the study conception.
LM, XGS and LT designed and supervised the current study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics committee of
Nanjing University of Chinese Medicine (approval no.
2017011001).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baumgart DC and Sandborn WJ: Cronhn's
disease. Lancet. 380:1590–1605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu ZJ, Yadav PK, Su JL, Wang JS and Fei
K: Potential role of Th17 cells in the pathogenesis of inflammatory
bowel disease. World J Gastroenterol. 15:5784–5788. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramsewak RS, DeWitt DL and Nair MG:
Cytotoxicity, antioxidant and anti-inflammatory activities of
curcumins I–III from Curcuma longa. Phytomedicine.
7:303–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiavascular, pulmonary, metabolic,
autoimmune and neoplastic disease. Int J Biochem Cell Biol.
41:40–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yadav VR, Suresh S, Devi K and Yadav S:
Effect of cyclodextrin complexation of curcumin on its solubility
and antiangiogenic and anti-inflammatory activity in rat colitis
model. AAPS PharmSciTech. 10:752–762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salh B, Assi K, Templeman V, Parhar K,
Owen D, Gómez-Muñoz A and Jacobson K: Curcumin attenuates
DNB-induced murine colitis. Am J Physiol Gastrointest Liver
Physiol. 285:G235–G243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venkataranganna MV, Rafiq M, Gopumadhavan
S, Peer G, Babu UV and Mitra SK: NCB-02 (standardized Curcumin
preparation) protects dinitrochlorobenzene-induced colitis through
down-regulation of NFkappa-B and iNOS. World J Gastroenterol.
13:1103–1107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanai H, Iida T, Takeuchi K, Watanabe F,
Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M,
et al: Curcumin maintenance therapy for ulcerative colitis:
Randomized, multicenter, double-blind, placebo-controlled trial.
Clin Gastroenterol Hepatol. 4:1502–1506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holt PR, Katz S and Kirshoff R: Curcumin
therapy in inflammatory bowel disease: A pilot study. Dig Dis Sci.
50:2191–2193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kohn EA, Du Z, Sato M, Van Schyndle CM,
Welsh MA, Yang YA, Stuelten CH, Tang B, Ju W, Bottinger EP and
Wakefield LM: A novel approach for the generation of genetically
modified mammary epithelial cell cultures yields new insights into
TGFβ signaling in the mammary gland. Breast Cancer Res. 12:R832010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomollón F: Developments in the treatment
of inflammatory bowel disease: 2014 overview. Gastroenterol
Hepatol. 37 (Suppl 3):S14–S21. 2014.(In Spanish). View Article : Google Scholar
|
|
12
|
Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ
and Wu JC: Systematic review: The efficacy of herbal therapy in
inflammatory bowel disease. Aliment Pharmacol Ther. 38:854–863.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rahimi R, Nikfar S and Abdollahi M:
Induction of clinical response and remission of inflammatory bowel
disease by use of herbal medicines: A meta-analysis. World J
Gastroenterol. 19:5738–5749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leonetti D, Reimund JM, Tesse A, Viennot
S, Martinez MC, Bretagne AL and Andriantsitohaina R: Circulating
microparticles from Crohn's disease patients cause endothelial and
vascular dysfunctions. PLoS One. 8:e730882013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danese S, Katz JA, Saibeni S, Papa A,
Gasbarrini A, Vecchi M and Fiocchi C: Activated platelets are the
source of elevated levels of soluble CD40 ligand in the circulation
of inflammatory bowel disease patients. Gut. 52:1435–1441. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scaldaferri F, Lancellotti S, Pizzoferrato
M and De Cristofaro R: Haemostatic system in inflammatory bowel
diseases: New players in gut inflammation. World J Gastroenterol.
17:594–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reddy PH, Manczak M, Yin X, Grady MC,
Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M,
et al: Protective effects of indian spice curcumin against
amyloid-β in Alzheimer's disease. J Alzheimers Dis. 61:843–866.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh N, Sachdev A and Gopinath P:
Polysaccharide functionalized single walled carbon nanotubes as
nanocarriers for delivery of curcumin in lung cancer cells. J
Nanosci Nanotechnol. 18:1534–1541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Masuelli L, Granato M, Benvenuto M,
Mattera R, Bernardini R, Mattei M, d'Amati G, D'Orazi G, Faggioni
A, Bei R and Cirone M: Chloroquine supplementation increases the
cytotoxic effect of curcumin against Her2/neu overexpressing breast
cancer cells in vitro and in vivo in nude mice while counteracts it
in immune competent mice. Oncoimmunology. 6:e13561512017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu KH, Ho CT, Chen ZF, Chen LC, Whang-Peng
J, Lin TN and Ho YS: The apple polyphenol phloretin inhibits breast
cancer cell migration and proliferation via inhibition of signals
by type 2 glucose transporter. J Food Drug Anal. 26:221–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Wang Y, Zhang J, Zhong J and Yang
R: COL1A1 promotes metastasis in colorectal cancer by regulating
the WNT/PCP pathway. Mol Med Rep. 17:5037–5042. 2018.PubMed/NCBI
|
|
22
|
Guo H, Zhang X, Chen Q, Bao Y, Dong C and
Wang X: miR-132 suppresses the migration and invasion of lung
cancer cells by blocking USP9X-induced epithelial-mesenchymal
transition. Am J Transl Res. 10:224–234. 2018.PubMed/NCBI
|
|
23
|
Ogunbolude Y, Dai C, Bagu ET, Goel RK,
Miah S, MacAusland-Berg J, Ng CY, Chibbar R, Napper S, Raptis L, et
al: FRK inhibits breast cancer cell migration and invasion by
suppressing epithelial-mesenchymal transition. Oncotarget.
8:113034–113065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Tong D, Guo Q, Wang X, Wu F, Li Q,
Yang J, Zhao L, Qin Y, Liu Y and Huang C: HOXD3 targeted by
miR-203a suppresses cell metastasis and angiogenesis through VEGFR
in human hepatocellular carcinoma cells. Sci Rep. 8:24312018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi YH, Jin GY, Li LC and Yan GH:
Inhibition of protein kinase C delta attenuates allergic airway
inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF
pathway. PLoS One. 8:e817732013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Tan J, Xie H, Wang J, Meng X and
Wang R: HIF-1α regulates EMT via the Snail and β-catenin pathways
in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol
Med. 20:688–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Zhu L, Fang J, Ge Z and Li X:
LRG1 modulates epithelial-mesenchymal transition and angiogenesis
in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res.
35:292016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baumann B, Hayashida T, Liang X and
Schnaper HW: Hypoxia-inducible factor-1α promotes
glomerulosclerosis and regulates COL1A2 expression through
interactions with Smad3. Kidney Int. 90:797–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe T, Yasue A and Tanaka E:
Hypoxia-inducible factor-1α is required for transforming growth
factor-β1-induced type I collagen, periostin and α-smooth muscle
actin expression in human periodontal ligament cells. Arch Oral
Biol. 59:595–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang N, Liang Y, Yang P and Ji F: Propofol
suppresses LPS-induced nuclear accumulation of HIF-1α and tumor
aggressiveness in non-small cell lung cancer. Oncol Rep.
37:2611–2619. 2017. View Article : Google Scholar : PubMed/NCBI
|